Detection of Ammonia Nitrogen in Neutral Aqueous Solutions Based on In Situ Modulation Using Ultramicro Interdigitated Array Electrode Chip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
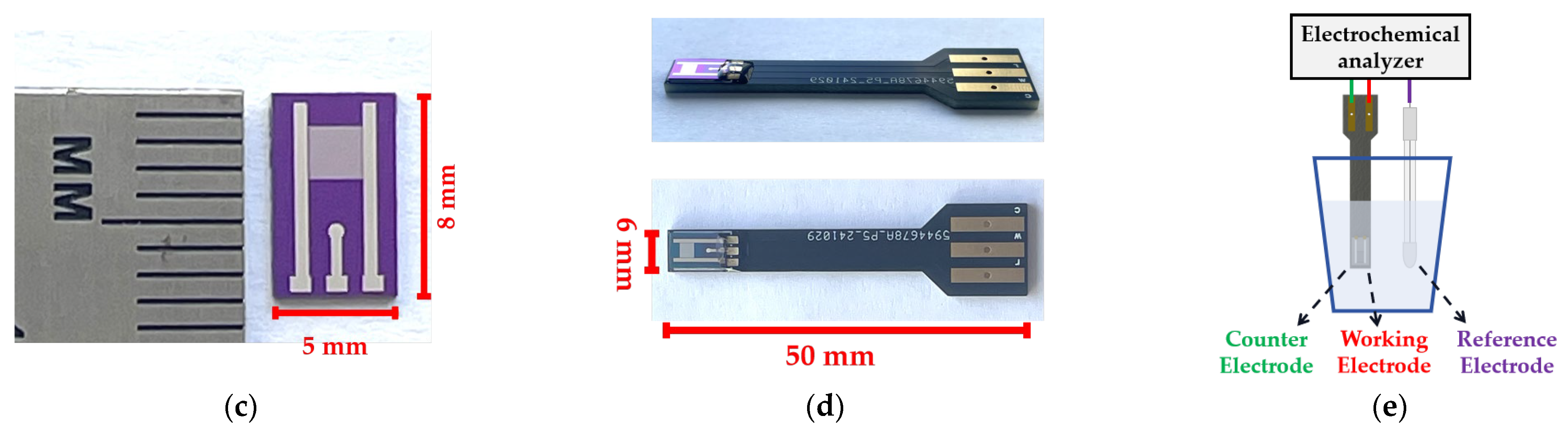
2.2. Design and Fabrication of the UIAE Chip
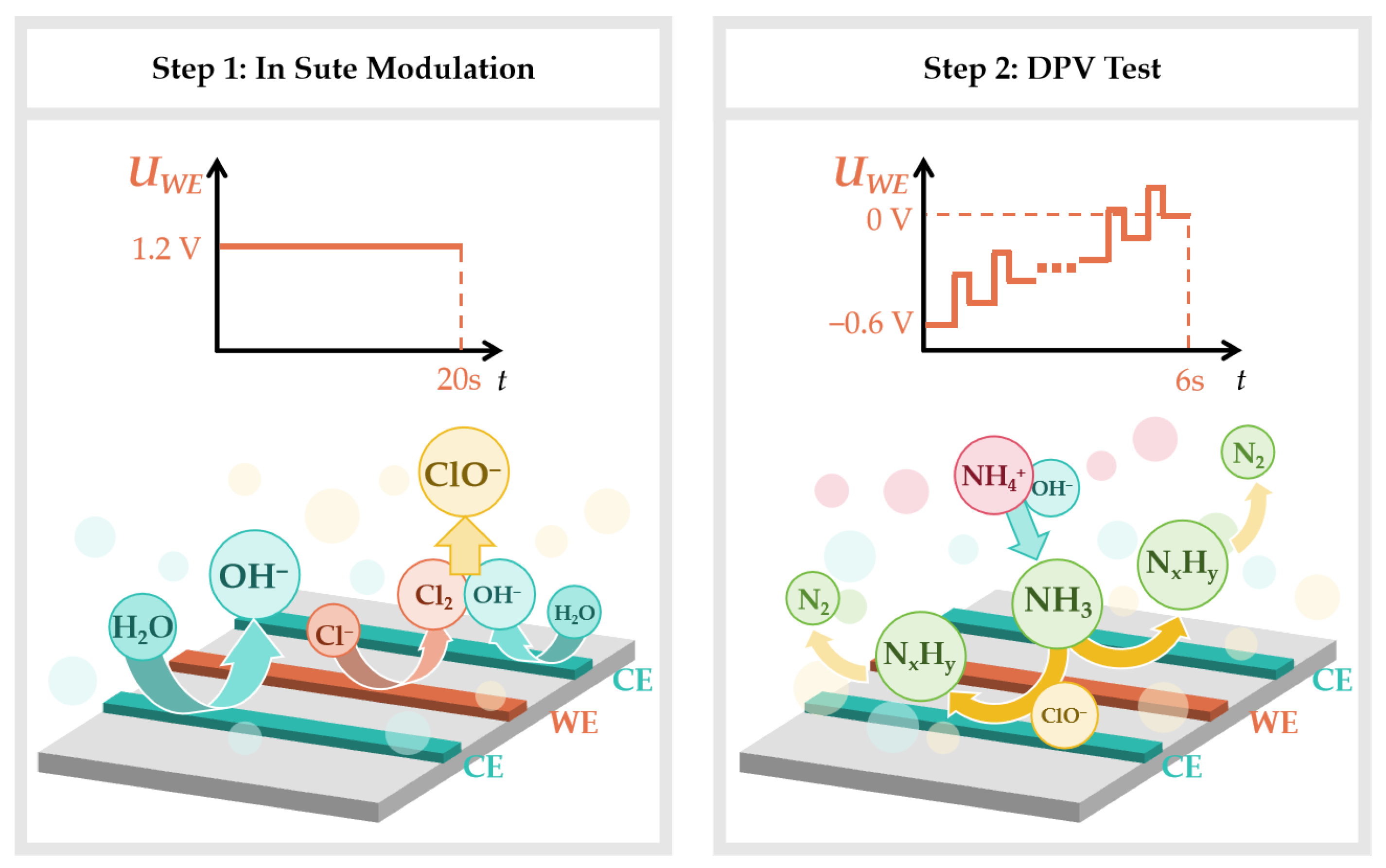
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Detection of NH3-N with UIAEs in an Alkaline Environment
3.2. In Situ Modulation Performance of UIAEs in a Neutral Environment
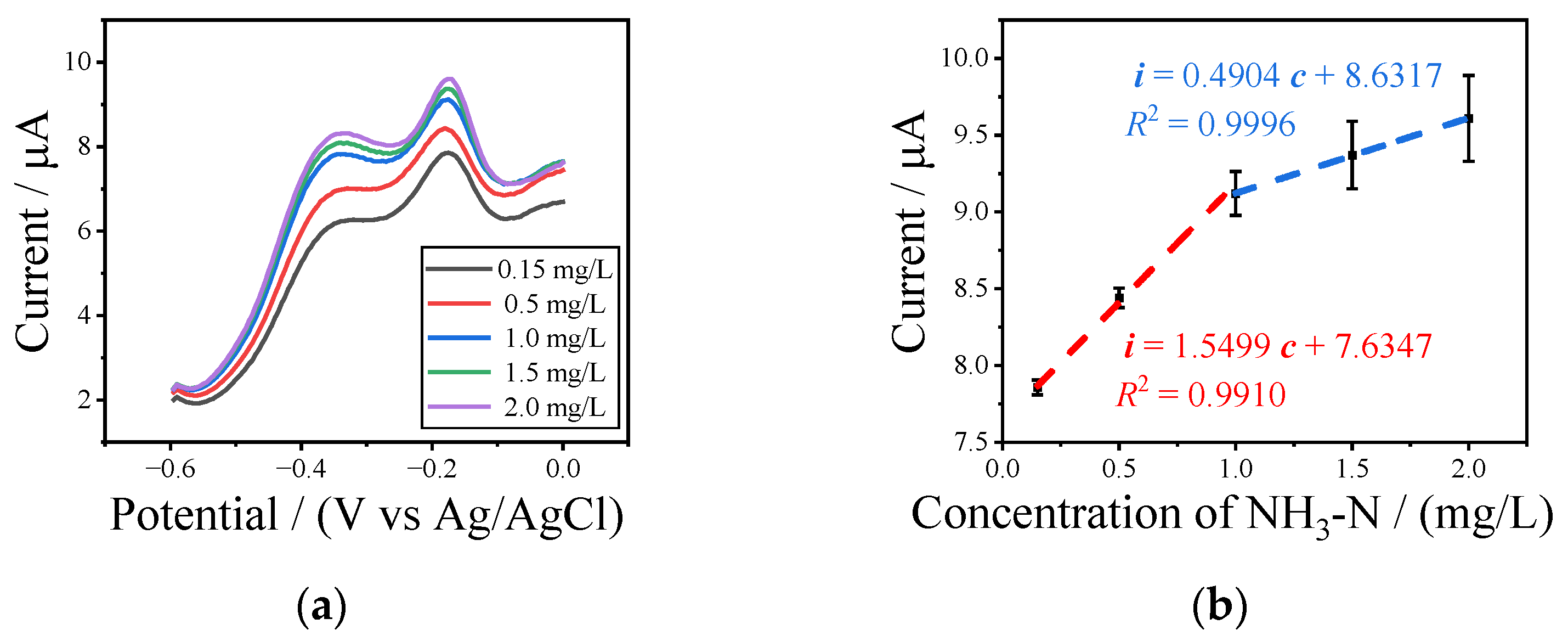
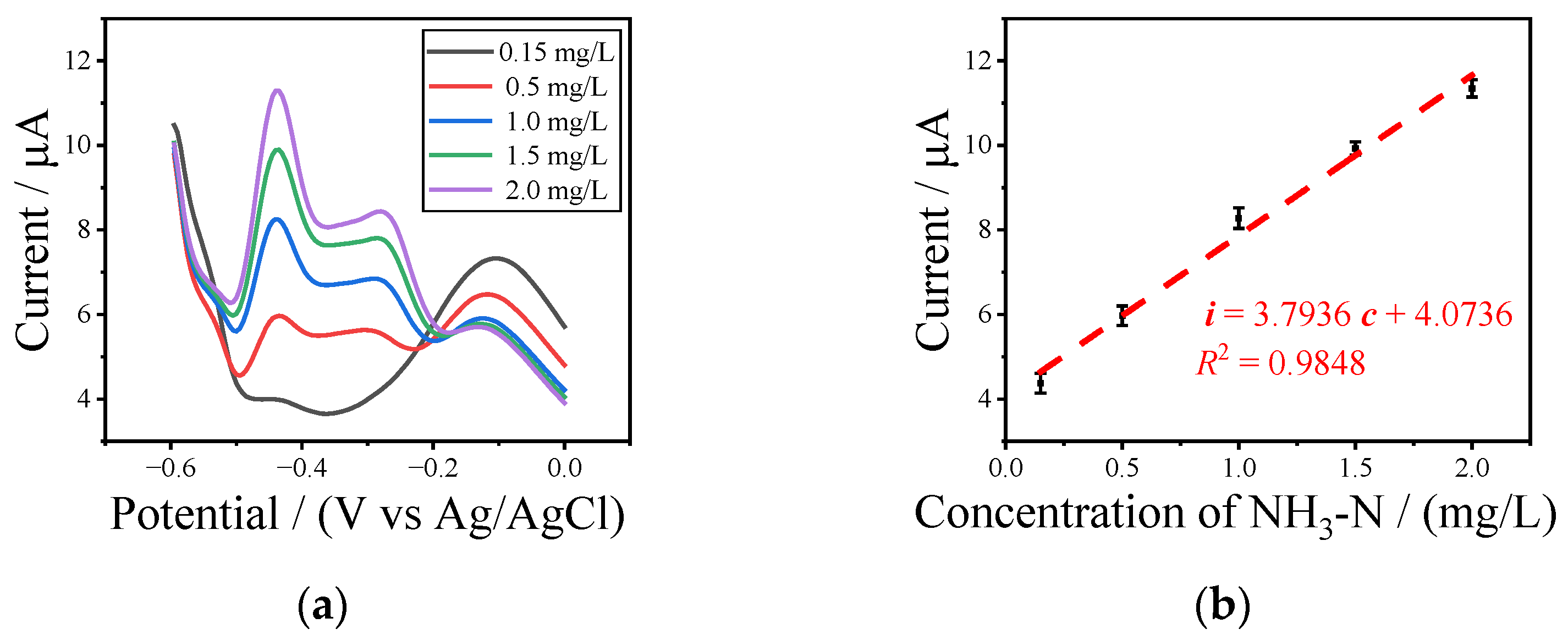
3.3. Detection of NH3-N with UIAEs in a Neutral Environment
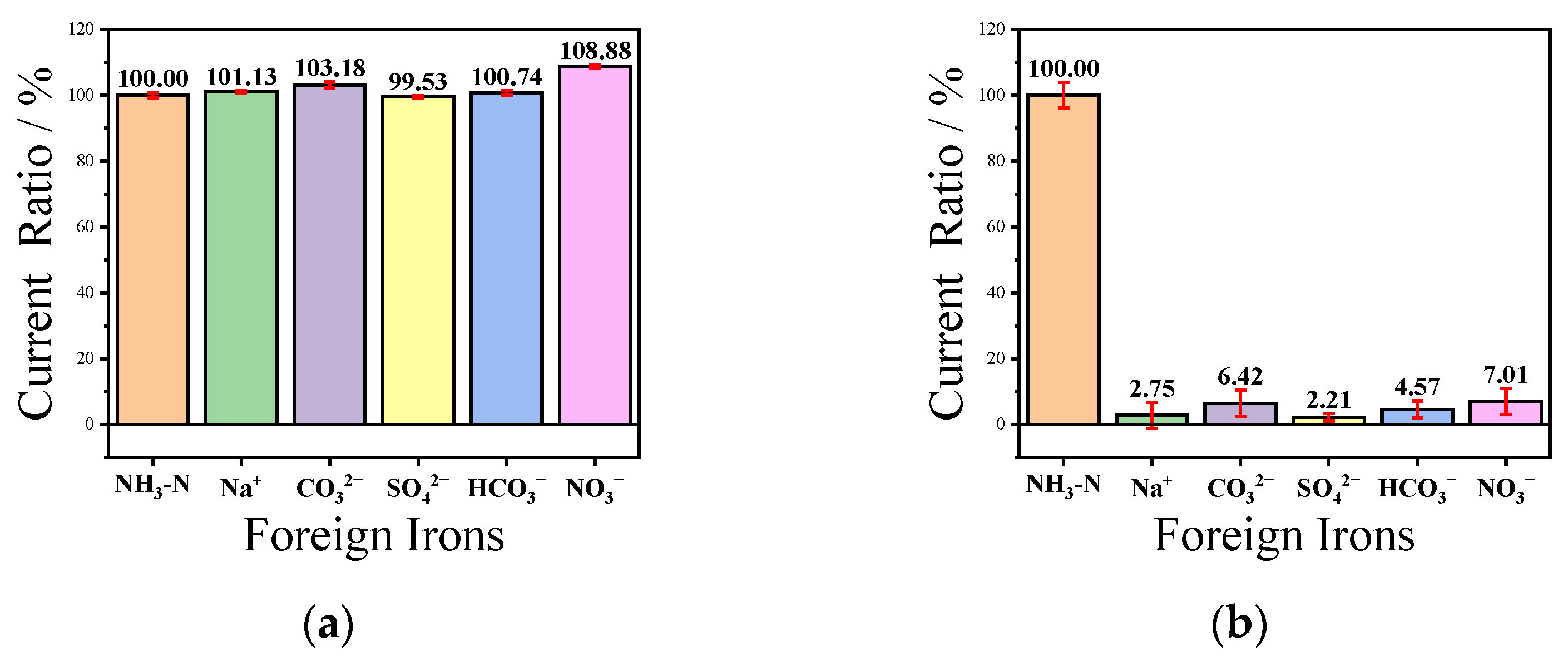
3.4. Anti-Interference Ability and Selectivity
3.5. Repeatability and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, Y.; Mao, Y.; Kang, Z.; Luo, Q. Application of an Ammonium Ion-Selective Electrode for the Real-Time Measurement of Ammonia Nitrogen Based on Ph and Temperature Compensation. Measurement 2019, 137, 98–101. [Google Scholar] [CrossRef]
- Gerischer, H.; Mauerer, A. Untersuchungen Zur Anodischen Oxidation Von Ammoniak an Platin-Elektroden. J. Electroanal. Chem. 1970, 25, 421–433. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Solla-Gullón, J.; Rodrı́guez, P.; Herrero, E.; Montiel, V.; Feliu, J.M.; Aldaz, A. Shape-Dependent Electrocatalysis: Ammonia Oxidation on Platinum Nanoparticles with Preferential (100) Surfaces. Electrochem. Commun. 2004, 6, 1080–1084. [Google Scholar] [CrossRef]
- Elnabawy, A.O.; Herron, J.A.; Karraker, S.; Mavrikakis, M. Structure Sensitivity of Ammonia Electro-Oxidation on Transition Metal Surfaces: A First-Principles Study. J. Catal. 2021, 397, 137–147. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, Y.; Xu, H.; Nie, P. Gold Nanoparticle-Modified Electrodes for Electrochemical Sensing of Ammonia Nitrogen in Water. ACS Appl. Nano Mater. 2023, 7, 577–593. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, G.; Zhao, X.; He, D.; Zhao, C.; Suo, H. Electrochemical Detection of Ammonia in Water Using Nicu Carbonate Hydroxide-Modified Carbon Cloth Electrodes: A Simple Sensing Method. Sensors 2024, 24, 4824. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, G.; Zhang, Q.; He, D.; Zhao, C.; Suo, H. Sensitive Electrochemical Detection of Ammonia Nitrogen Via a Platinum-Zinc Alloy Nanoflower-Modified Carbon Cloth Electrode. Sensor 2024, 24, 915. [Google Scholar] [CrossRef]
- Blume, S.O.P.; Ben-Mrad, R.; Sullivan, P.E. Modelling the Capacitance of Multi-Layer Conductor-Facing Interdigitated Electrode Structures. Sens. Actuators B Chem. 2015, 213, 423–433. [Google Scholar] [CrossRef]
- Yagati, A.K.; Park, J.; Cho, S. Reduced Graphene Oxide Modified the Interdigitated Chain Electrode for an Insulin Sensor. Sensors 2016, 16, 109. [Google Scholar] [CrossRef]
- Liu, N.; Gao, Y. Recent Progress in Micro-Supercapacitors with in-Plane Interdigital Electrode Architecture. Small 2017, 13, 1701989. [Google Scholar] [CrossRef]
- Kosri, E.; Ibrahim, F.; Thiha, A.; Madou, M. Micro and Nano Interdigitated Electrode Array (Idea)-Based Mems/Nems as Electrochemical Transducers: A Review. Nanomaterials 2022, 12, 4171. [Google Scholar] [CrossRef] [PubMed]
- Pastre, A.; Boé, A.; Rolland, N.; Bernard, R. All-Solid-State Interdigitated Micro-Supercapacitors Based on Porous Gold Electrodes. Sensors 2023, 23, 619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Tong, J.; Li, Y.; Sun, J.; Bian, C.; Xia, S. Palladium-Gold Modified Ultramicro Interdigital Array Electrode Chip for Nitrate Detection in Neutral Water. Micromachines 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Patella, B.; O’Sullivan, B.; Daly, R.; Lovera, P.; Inguanta, R.; O’Riordan, A. Simultaneous Detection of Copper, Lead and Mercury in River Water with in-Situ Ph Control Using Electrochemical Stripping Techniques, Tyndall National Institute: Cork, Ireland, 2020; unpublished work.
- Seymour, I.; O’Sullivan, B.; Lovera, P.; Rohan, J.F.; O’Riordan, A. Electrochemical Detection of Free-Chlorine in Water Samples Facilitated by in-Situ Ph Control Using Interdigitated Microelectrodes. Sens. Actuators B Chem. 2020, 325, 128774. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Patella, B.; Daly, R.; Seymour, I.; Robinson, C.; Lovera, P.; Rohan, J.; Inguanta, R.; O’Riordan, A. A Simulation and Experimental Study of Electrochemical Ph Control at Gold Interdigitated Electrode Arrays. Electrochim. Acta 2021, 395, 139113. [Google Scholar] [CrossRef]
- Seymour, I.; O’Sullivan, B.; Lovera, P.; Rohan, J.F.; O’Riordan, A. Elimination of Oxygen Interference in the Electrochemical Detection of Monochloramine, Using in Situ Ph Control at Interdigitated Electrodes. ACS Sens. 2021, 6, 1030–1038. [Google Scholar] [CrossRef]
- Wasiewska, L.A.; Seymour, I.; Patella, B.; Inguanta, R.; Burgess, C.M.; Duffy, G.; O’Riordan, A. Reagent Free Electrochemical-Based Detection of Silver Ions at Interdigitated Microelectrodes Using in-Situ Ph Control. Sens. Actuators B Chem. 2021, 333, 129531. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y. Ammonia Removal in Electrochemical Oxidation: Mechanism and Pseudo-Kinetics. J. Hazard. Mater. 2009, 161, 1010–1016. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Waite, T.D. Active Chlorine Mediated Ammonia Oxidation Revisited: Reaction Mechanism, Kinetic Modelling and Implications. Water Res. 2018, 145, 220–230. [Google Scholar] [CrossRef]
- Li, Q.; Liu, G.H.; Du, H.; Xian, G.; Qi, L.; Wang, H. Synergistic Mechanisms between Chlorine-Mediated Electrochemical Advanced Oxidation and Ultraviolet Light for Ammonia Removal. J. Environ. Manag. 2024, 352, 120057. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Bai, J.; Li, L.; Chen, S.; Zhou, T.; Wang, J.; Xia, L.; Xu, Q.; Zhou, B. Extremely Efficient Decomposition of Ammonia N to N2 Using Clo from Reactions of Ho and Hocl Generated in Situ on a Novel Bifacial Photoelectroanode. Environ. Sci. Technol. 2019, 53, 6945–6953. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Y.; Bai, J.; Li, J.; Li, L.; Zhou, T.; Chen, S.; Wang, J.; Rahim, M.; Guan, X.; et al. Efficient Degradation of N-Containing Organic Wastewater Via Chlorine Oxide Radical Generated by a Photoelectrochemical System. Chem. Eng. J. 2020, 392, 123695. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, W.; Bai, J.; Li, J.; Wang, J.; Zhou, T.; Guan, X.; Zhou, B. Highly Efficient Removal of Total Nitrogen and Dissolved Organic Compound in Waste Reverse Osmosis Concentrate Mediated by Chlorine Radical on 3d Co3o4 Nanowires Anode. J. Hazard. Mater. 2022, 424, 127662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Cong, A.; Tong, J.; Bian, C. Ultramicro Interdigitated Array Electrode Chip with Optimized Construction for Detection of Ammonia Nitrogen in Water. Micromachines 2023, 14, 629. [Google Scholar] [CrossRef]
- Kaloyeros, A.E.; Pan, Y.; Goff, J.; Arkles, B. Review—Silicon Nitride and Silicon Nitride-Rich Thin Film Technologies: State-of-the-Art Processing Technologies, Properties, and Applications. ECS J. Solid State Sci. Technol. 2020, 9, 063006. [Google Scholar] [CrossRef]
- Wang, G.; Gao, J.; Sun, B.; He, D.; Zhao, C.; Suo, H. Enhanced Ammonia Sensitivity Electrochemical Sensors Based on Ptcu Alloy Nanoparticles in-Situ Synthesized on Carbon Cloth Electrode. J. Electroanal. Chem. 2022, 922, 116721. [Google Scholar] [CrossRef]
- Wang, G.; Ma, G.; Gao, J.; He, D.; Zhao, C.; Suo, H. Enhanced Sensitivity of Electrochemical Sensors for Ammonia-Nitrogen Via in-Situ Synthesis Ptni Nanoleaves on Carbon Cloth. Sensors 2024, 24, 387. [Google Scholar] [CrossRef]
- Ning, Y.-F.; Yan, P.; Chen, Y.-P.; Guo, J.-S.; Shen, Y.; Fang, F.; Tang, Y.; Gao, X. Development of a Pt Modified Microelectrode Aimed for the Monitoring of Ammonium in Solution. Int. J. Environ. Anal. Chem. 2017, 97, 85–98. [Google Scholar] [CrossRef]



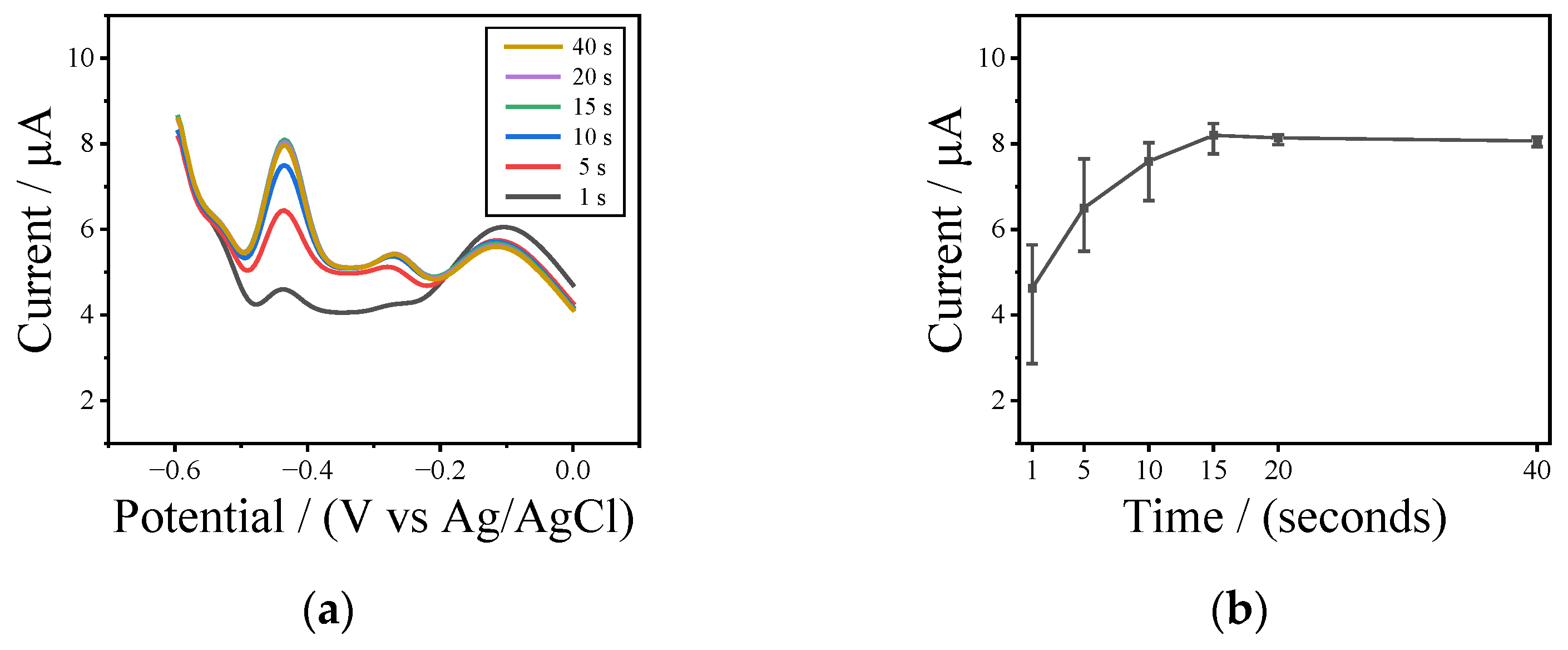

| Electrode Type | Substrate Solution | Method | Detection Range (µM) | Sensitivity (µA µM−1 mm−2) | Detection Limit (µM) | RSD (%) | Ref |
|---|---|---|---|---|---|---|---|
| AuNPs/CC@G | 0.1 M KOH | CV | 1 × 10−3–10,000 | 6.4477 | 9.84 × 10−4 | 1.11 | [5] |
| PtCu/CC | 1 M KOH | DPV | 0.5–40 | 0.0940 | 8.6 × 10−3 | 0.60 | [27] |
| 40–500 | 0.0078 | ||||||
| PtNi/CC | 1 M KOH | DPV | 0.5–150 | 0.0783 | 2.4 × 10−2 | 1.85 | [28] |
| 150–500 | 0.0095 | ||||||
| PtZn NFs/CC | 1 M KOH | DPV | 1–100 | 0.4300 | 2.78 × 10−2 | 4.68 | [7] |
| 100–400 | 0.1178 | ||||||
| Pt NPs-Pt Wire | 0.1 M Na2SO4 (pH = 3) | LSV | 45–64,000 | 0.0662 | 45 | / | [29] |
| Pt UIAE | 0.2 M Na2HPO4 (pH = 10) | DPV | 10.7–142.9 | 0.0098 | 1.77 | 2.90 | [25] |
| Pt UIAE | 1 M NaOH | DPV | 10.7–71.45 | 0.0273 | / | / | This work |
| Pt UIAE | 0.2 M KCl (pH = 7.5) | DPV with modulation | 10.7–142.9 | 0.0664 | 3.44 | 1.98 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Qiu, N.; Zhang, Z.; Li, Y.; Bian, C. Detection of Ammonia Nitrogen in Neutral Aqueous Solutions Based on In Situ Modulation Using Ultramicro Interdigitated Array Electrode Chip. Chemosensors 2025, 13, 138. https://doi.org/10.3390/chemosensors13040138
Liu Y, Qiu N, Zhang Z, Li Y, Bian C. Detection of Ammonia Nitrogen in Neutral Aqueous Solutions Based on In Situ Modulation Using Ultramicro Interdigitated Array Electrode Chip. Chemosensors. 2025; 13(4):138. https://doi.org/10.3390/chemosensors13040138
Chicago/Turabian StyleLiu, Yuqi, Nan Qiu, Zhihao Zhang, Yang Li, and Chao Bian. 2025. "Detection of Ammonia Nitrogen in Neutral Aqueous Solutions Based on In Situ Modulation Using Ultramicro Interdigitated Array Electrode Chip" Chemosensors 13, no. 4: 138. https://doi.org/10.3390/chemosensors13040138
APA StyleLiu, Y., Qiu, N., Zhang, Z., Li, Y., & Bian, C. (2025). Detection of Ammonia Nitrogen in Neutral Aqueous Solutions Based on In Situ Modulation Using Ultramicro Interdigitated Array Electrode Chip. Chemosensors, 13(4), 138. https://doi.org/10.3390/chemosensors13040138






