A Photochemical Vapor Generation Method for the Determination of Hg and Pb in Imitation Jewelry by Inductively Coupled Plasma Optical Emission Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Sample Preparation
2.2. Detection of Hg and Pb by PVG-ICP-OES
2.3. Statistical Analysis
3. Results
3.1. Reactor for Photochemical Vapor Generation
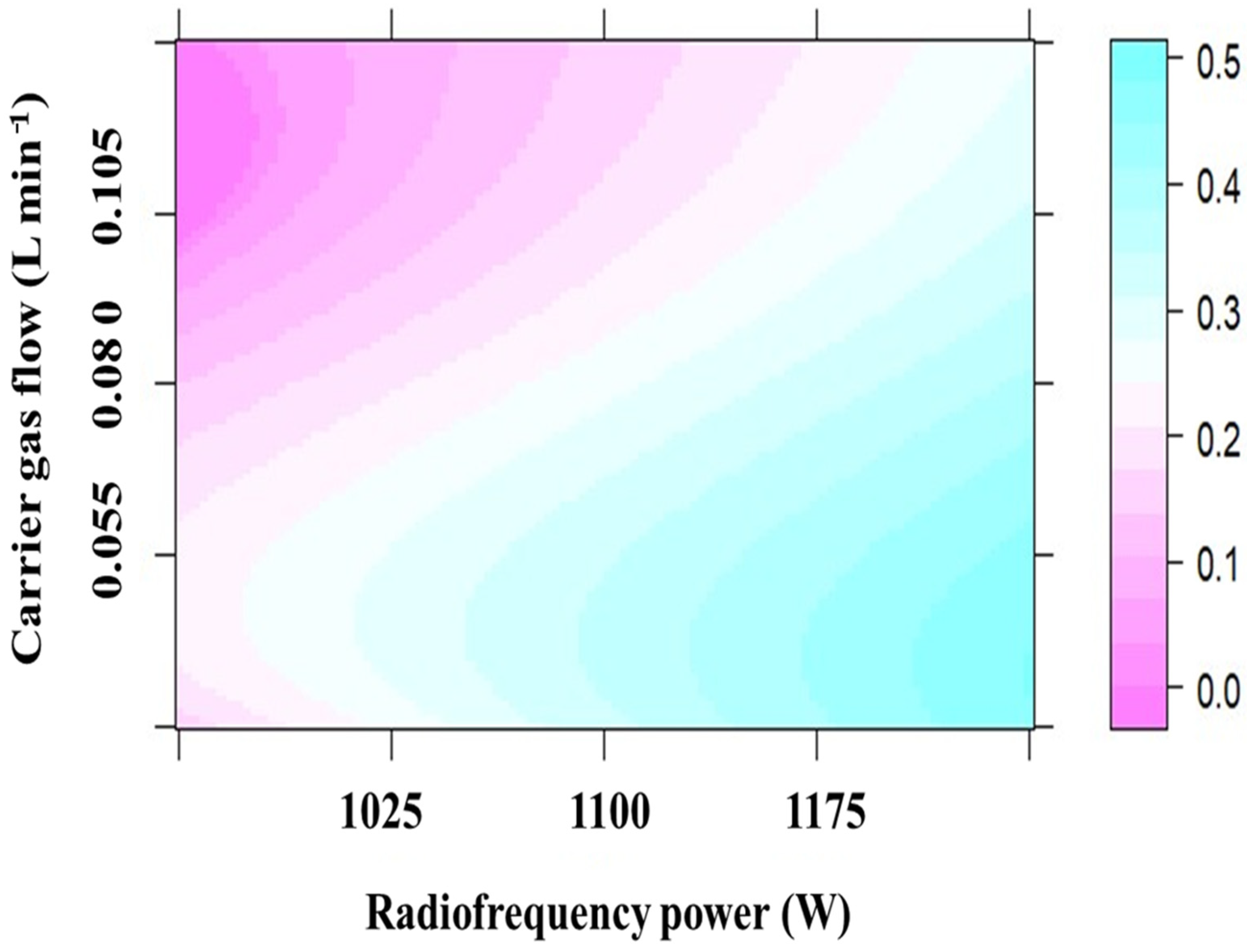
3.2. Radio Frequency Power and Carrier Gas Flow Rate for Hg and Pb Detection by PVG-ICP-OES
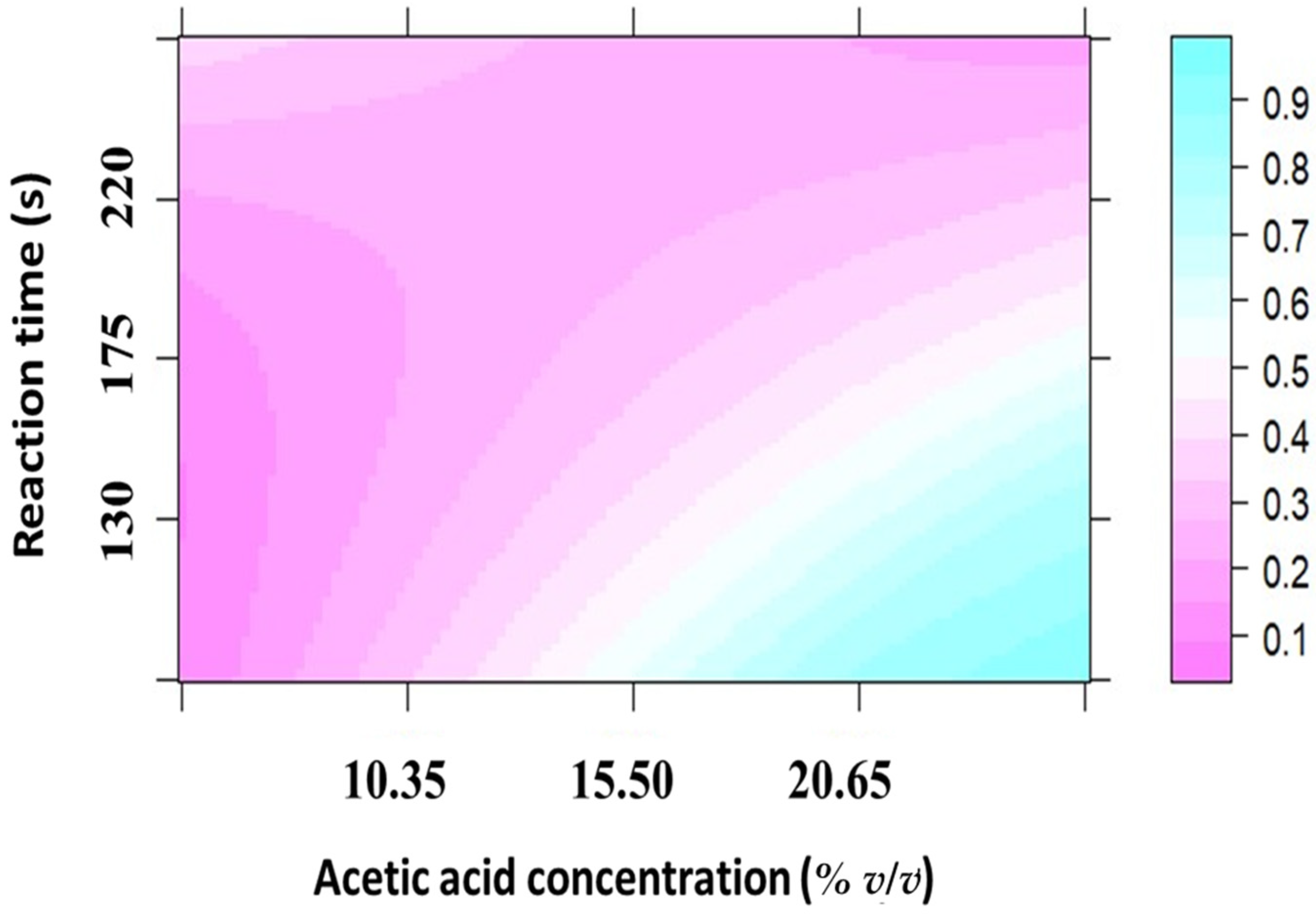
3.3. Acetic Acid Concentration and Reaction Time for the Photochemical Vapor Generation of Hg and Pb in Solutions from Imitation Jewelries
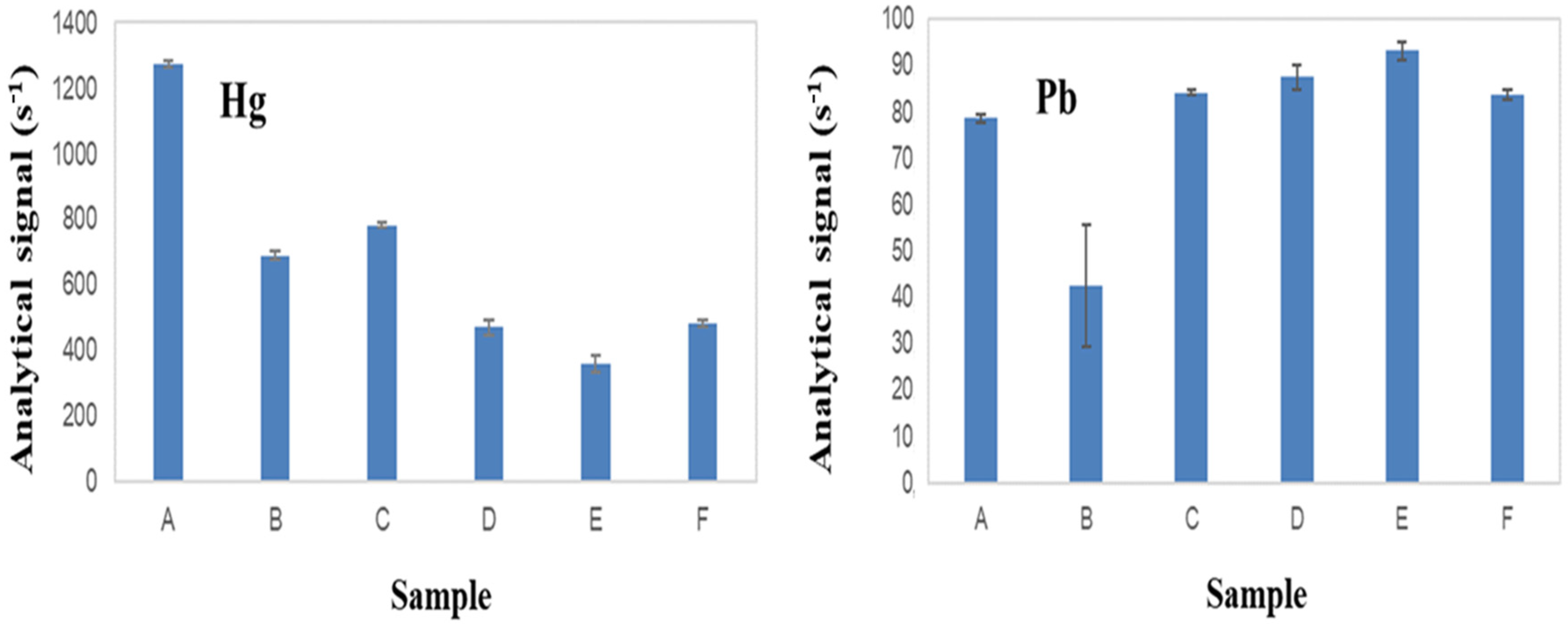
3.4. Suppression or Enhancement of Photochemical Vapor Generation of Hg and Pb by the Presence of Concomitants
3.5. Analytical Figures of Merit for the Determination of Hg and Pb in Imitation Jewelries by PVG-ICP-OES
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guney, M.; Zagury, G. Bioaccessibility of As, Cd, Cu, Ni, Pb, and Sb in Toys and Low-Cost Jewelry. Environ. Sci. Technol. 2014, 48, 1238–1246. [Google Scholar] [CrossRef]
- Tenuta, L.; Testa, S.; Antinarelli Freitas, F.; Cappellieri, A. Sustainable Materials for Jewelry: Scenarios from a Design Perspective. Sustainability 2024, 16, 1309. [Google Scholar] [CrossRef]
- Pouzar, M.; Zvolská, M.; Jarolím, O.; Audrlická Vavrušová, L. The Health Risk of Cd Released from Low-Cost Jewelry. Int. J. Environ. Res. Public Health 2017, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- INMETRO. Inmetro Estabelece Limites para Cádmio e Chumbo em Bijuterias e Joias. Available online: https://www.gov.br/inmetro/pt-br/centrais-de-conteudo/noticias/inmetro-estabelece-limites-para-cadmio-e-chumbo-em-bijuterias-e-joias (accessed on 25 August 2021).
- Jurowski, K. The Toxicological Assessment of Hazardous Elements (Pb, Cd and Hg) in Low-Cost Jewelry for Adults from Chinese E-Commerce Platforms: In Situ Analysis by Portable X-ray Fluorescence Measurement. J. Hazard. Mater. 2023, 460, 132167. [Google Scholar] [CrossRef] [PubMed]
- Weidenhamer, J.D.; Clement, M.L. Widespread Lead Contamination of Imported Low-Cost Jewelry in the US. Chemosphere 2007, 67, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Hamann, D.; Thyssen, J.P.; Hamann, C.R.; Hamann, C.; Menné, T.; Johansen, J.D.; Spiewak, R.; Maibach, H.; Lundgren, L.; Lidén, C. Jewellery: Alloy Composition and Release of Nickel, Cobalt and Lead Assessed with the EU Synthetic Sweat Method. Contact Dermat. 2015, 73, 231–238. [Google Scholar] [CrossRef]
- FENÍCIA. Inmetro e Receita Identificam Presença de Metais Pesados em Joias e Bijuterias Apreendidas em Itaguaí. 25 November 2019. Available online: https://feniciacomex.com.br/2019/11/25/inmetro-e-receita-identificam-presenca-de-metais-pesados-em-joias-e-bijuterias/ (accessed on 1 August 2022).
- Staufer, T.; Grüner, F. Review of Development and Recent Advances in Biomedical X-ray Fluorescence Imaging. Int. J. Mol. Sci. 2023, 24, 10990. [Google Scholar] [CrossRef]
- de Gois, J.S.; Costas-Rodríguez, M. Chapter 2—Types of Nebulizers in Plasma-Based Techniques: How to Choose an Appropriate Nebulizer. In Analytical Nebulizers: Fundamentals and Applications; Canals, A., Aguirre, A.M., Ahmadi, M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2023; Volume 1, pp. 11–24. [Google Scholar]
- de Gois, J.S.; Maranhão, T.d.A.; Oliveira, F.J.S.; Frescura, V.L.A.; Curtius, A.J.; Borges, D.L.G. Analytical Evaluation of Nebulizers for the Introduction of Acetic Acid Extracts Aiming at the Determination of Trace Elements by Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B 2012, 77, 35–43. [Google Scholar] [CrossRef]
- Sturgeon, R.E. Photochemical vapor generation: A radical approach to analyte introduction for atomic spectrometry. J. Anal. At. Spectrom. 2017, 32, 2319–2340. [Google Scholar] [CrossRef]
- Tateishi, I.; Furukawa, M.; Katsumata, H.; Kaneco, S. Metal and Molecular Vapor Separation Analysis for Direct Determination of Mn and Cu by Atomic Absorption Detection, Free of Background Absorption. Sustain. Chem. 2022, 3, 475–481. [Google Scholar] [CrossRef]
- Senila, M. Recent Advances in the Determination of Major and Trace Elements in Plants Using Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2024, 29, 3169. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Fang, J.; Sun, R.; Li, X.; Wang, Y. Determination of Gold in Geological Samples by Chelate Vapor Generation at Room Temperature Coupled with Atomic Fluorescence Spectrometry. Spectrochim. Acta Part B 2022, 194, 106579. [Google Scholar] [CrossRef]
- He, Y.; Hou, X.; Zheng, C.; Sturgeon, R.E. Critical Evaluation of the Application of Photochemical Vapor Generation in Analytical Atomic Spectrometry. Anal. Bioanal. Chem. 2007, 388, 769–774. [Google Scholar] [CrossRef]
- Sánchez Morales, R.; Sáenz-López, P.; de las Heras Perez, M.A. Green Chemistry and Its Impact on the Transition towards Sustainable Chemistry: A Systematic Review. Sustainability 2024, 16, 6526. [Google Scholar] [CrossRef]
- Rodríguez-Maese, R.; Rodríguez-Saldaña, V.; Leal, L.O. Automation Systems in Pb Analysis: A Review on Environmental Water and Biological Samples. Water 2025, 17, 565. [Google Scholar] [CrossRef]
- Wu, E.M.-Y.; Kuo, S.-L. Characteristics of Photochemical Reactions with VOCs Using Multivariate Statistical Techniques on Data from Photochemical Assessment Monitoring Stations. Atmosphere 2022, 13, 1489. [Google Scholar] [CrossRef]
- The R Foundation for Statistical Computing. R Version 4.0.2 (2020-06-22)—”Taking Off Again”. Available online: https://www.r-project.org/ (accessed on 7 March 2016).
- Roth, T. qualityTools: Statistics in Quality Science. R Package Version 1.55. 2016. Available online: https://www.rdocumentation.org/packages/qualityTools/versions/1.55 (accessed on 7 March 2016).
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008; ISBN 978-0-387-75968-5. [Google Scholar]
- Cezário, P.S.d.O.; do Nascimento, M.C.L.; Luna, A.S.; de Gois, J.S. Application of a Lab-Made Ternary Fe-Cr-Al Coil Vaporizer Coupled to ICP OES for Boron Determination in Powdered Food after the Sample Preparation in Alkaline Media. Microchem. J. 2020, 157, 104875. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- De Quadros, D.P.C.; Borges, D.L.G. Direct analysis of alcoholic beverages for the determination of cobalt, nickel, and tellurium by inductively coupled plasma mass spectrometry following photochemical vapor generation. Microchem. J. 2014, 116, 244–248. [Google Scholar] [CrossRef]
- Linhart, O.; Smolejova, J.; Cerveny, V.; Hranicek, J.; Novakova, E.; Resslerova, T.; Rychlovsky, P. Determination of As by UV-photochemical generation of its volatile species with AAS detection. Monatsh. Chem. 2016, 147, 1447–1454. [Google Scholar] [CrossRef]
- Ingle, J.D.; Crouch, S.R. Spectrochemical Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 1988. [Google Scholar]
- Francisco, B.B.A.; Rocha, A.A.; Grinberg, P.; Sturgeon, R.E.; Cassella, R.J. Determination of inorganic mercury in petroleum production water by inductively coupled plasma optical emission spectrometry following photochemical vapor generation. J. Anal. At. Spectrom. 2016, 31, 751–758. [Google Scholar] [CrossRef]
- Dos Santos, E.J.; Herrmann, A.B.; Dos Santos, A.B.; Baika, L.M.; Sato, C.S.; Tormen, L.; Sturgeon, R.E.; Curtius, A.J. Determination of thimerosal in human and veterinarian vaccines by photochemical vapor generation coupled to ICP OES. J. Anal. At. Spectrom. 2010, 25, 1627–1632. [Google Scholar] [CrossRef]
- de Jesus, A.; Sturgeon, R.E.; Liu, J.; Silva, M.M. Determination of mercury in gasoline by photochemical vapor generation coupled to graphite furnace atomic absorption spectrometry. Microchem. J. 2014, 117, 100–105. [Google Scholar] [CrossRef]
- Greda, K.; Welna, M.; Szymczycha-Madeja, A.; Pohl, P. Sensitive determination of Ag, Bi, Cd, Hg, Pb, Tl, and Zn by inductively coupled plasma optical emission spectrometry combined with the microplasma-assisted vapor generation. Talanta 2022, 249, 123694. [Google Scholar] [CrossRef] [PubMed]

| Analyte | Parameter | Value |
|---|---|---|
| Hg | Slope (L mg−1 s−1) | 42.7 ± 1.9 |
| Intercept (s−1) | 411.9 ± 17.8 | |
| R2 | 0.9941 | |
| LOD (mg g−1) | 0.2 | |
| LOQ (mg g−1) | 0.7 | |
| Pb | Slope (L mg−1 s−1) | 1.957 ± 0.006 |
| Intercept (s−1) | 110.01 ± 0.04 | |
| R2 | 0.9999 | |
| LOD (mg g−1) | 0.3 | |
| LOQ (mg g−1) | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, F.P.; de Gois, J.S. A Photochemical Vapor Generation Method for the Determination of Hg and Pb in Imitation Jewelry by Inductively Coupled Plasma Optical Emission Spectrometry. Chemosensors 2025, 13, 144. https://doi.org/10.3390/chemosensors13040144
Braga FP, de Gois JS. A Photochemical Vapor Generation Method for the Determination of Hg and Pb in Imitation Jewelry by Inductively Coupled Plasma Optical Emission Spectrometry. Chemosensors. 2025; 13(4):144. https://doi.org/10.3390/chemosensors13040144
Chicago/Turabian StyleBraga, Fernanda P., and Jefferson Santos de Gois. 2025. "A Photochemical Vapor Generation Method for the Determination of Hg and Pb in Imitation Jewelry by Inductively Coupled Plasma Optical Emission Spectrometry" Chemosensors 13, no. 4: 144. https://doi.org/10.3390/chemosensors13040144
APA StyleBraga, F. P., & de Gois, J. S. (2025). A Photochemical Vapor Generation Method for the Determination of Hg and Pb in Imitation Jewelry by Inductively Coupled Plasma Optical Emission Spectrometry. Chemosensors, 13(4), 144. https://doi.org/10.3390/chemosensors13040144







