Luminescent Pyrene-Derivatives for Hg2+ and Explosive Detection
Abstract
1. Introduction
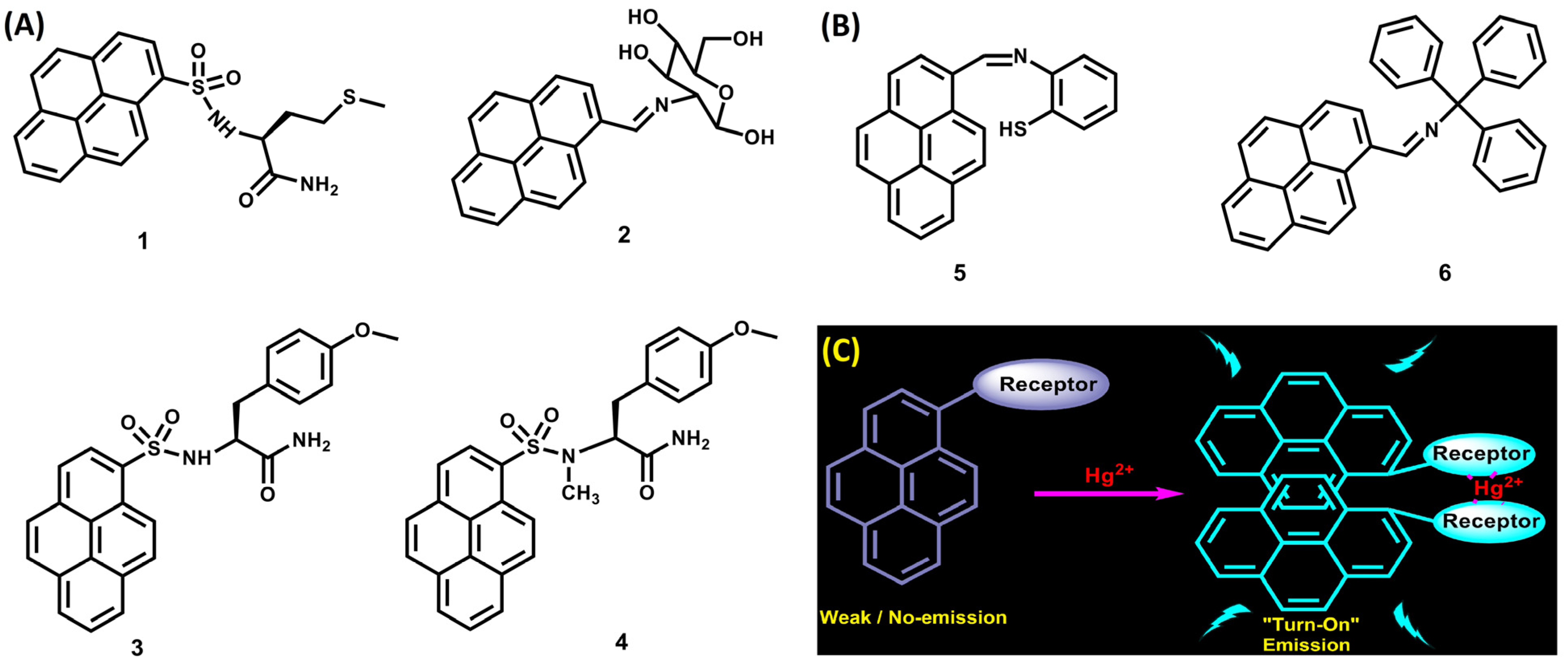
2. Pyrene Derivatives for Hg2+ Detection via PL for “Turn On” Responses
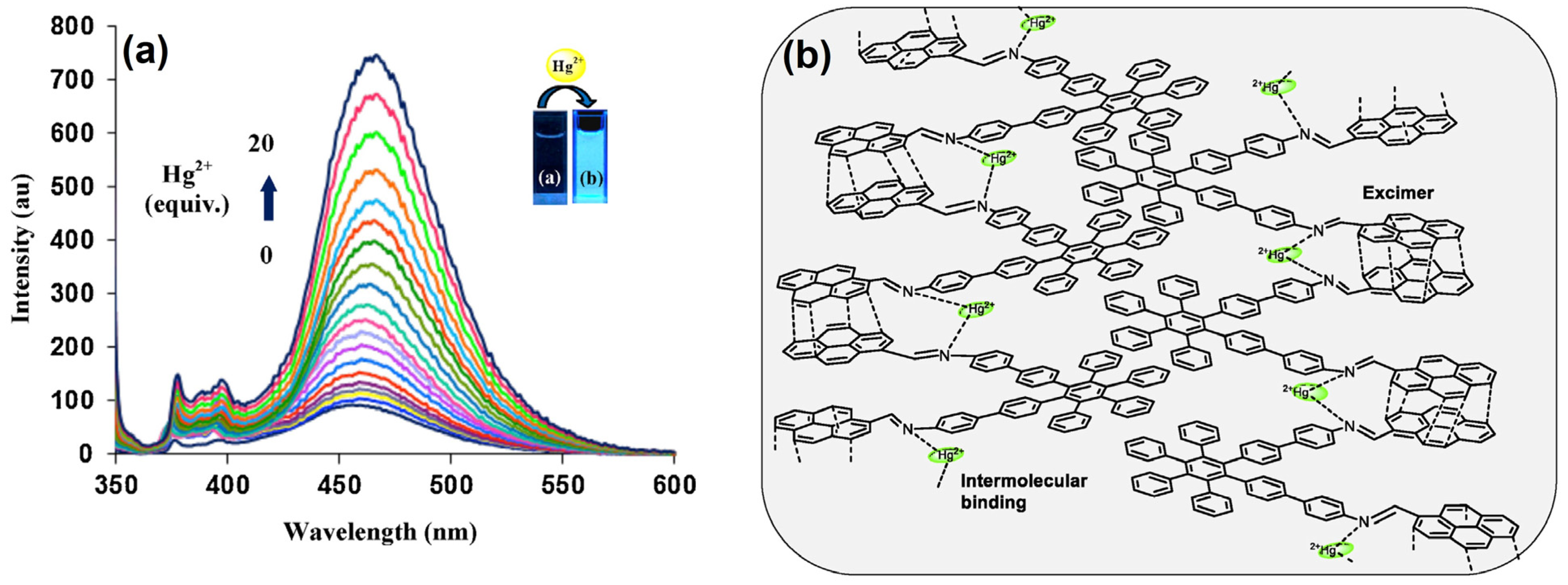
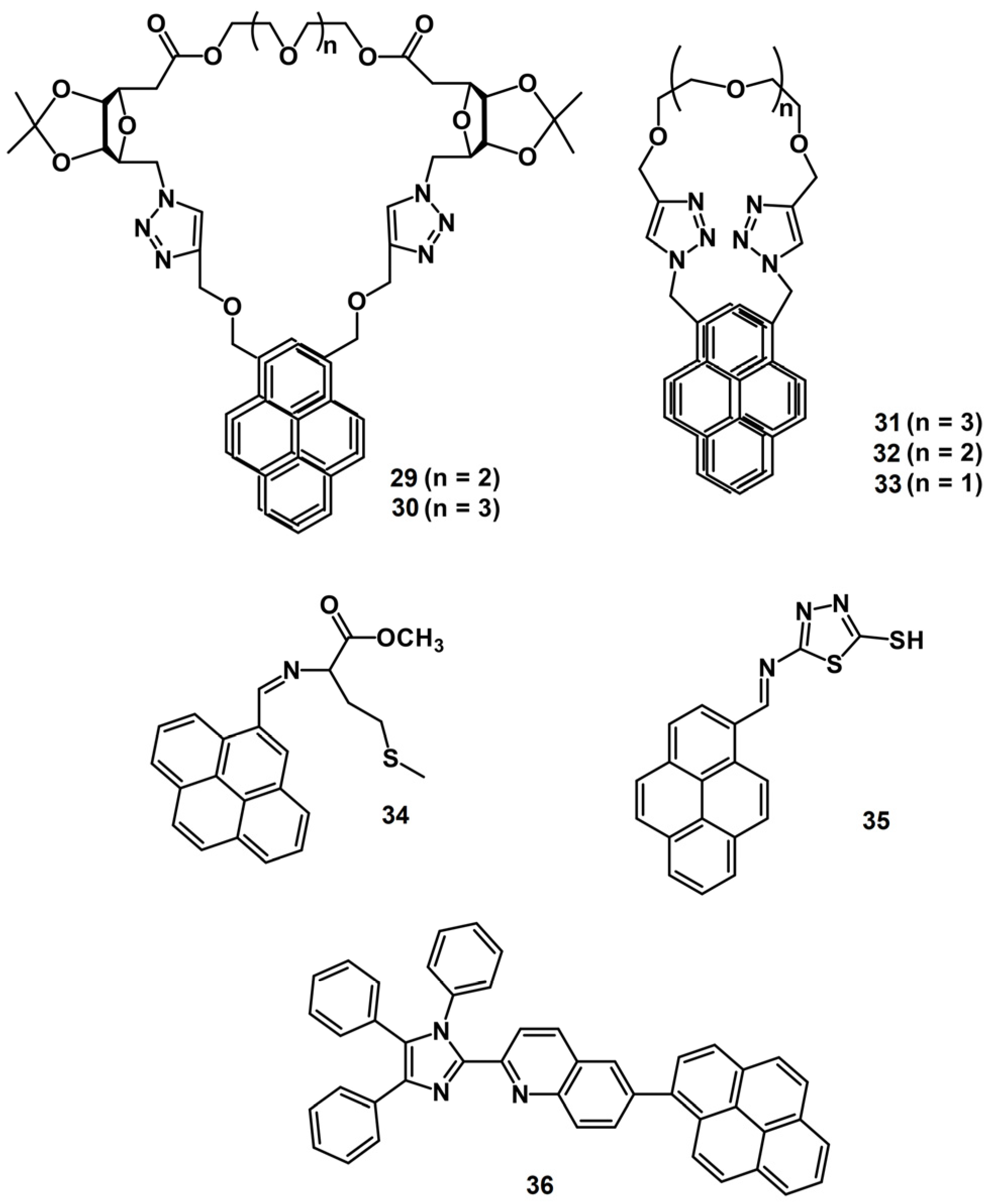
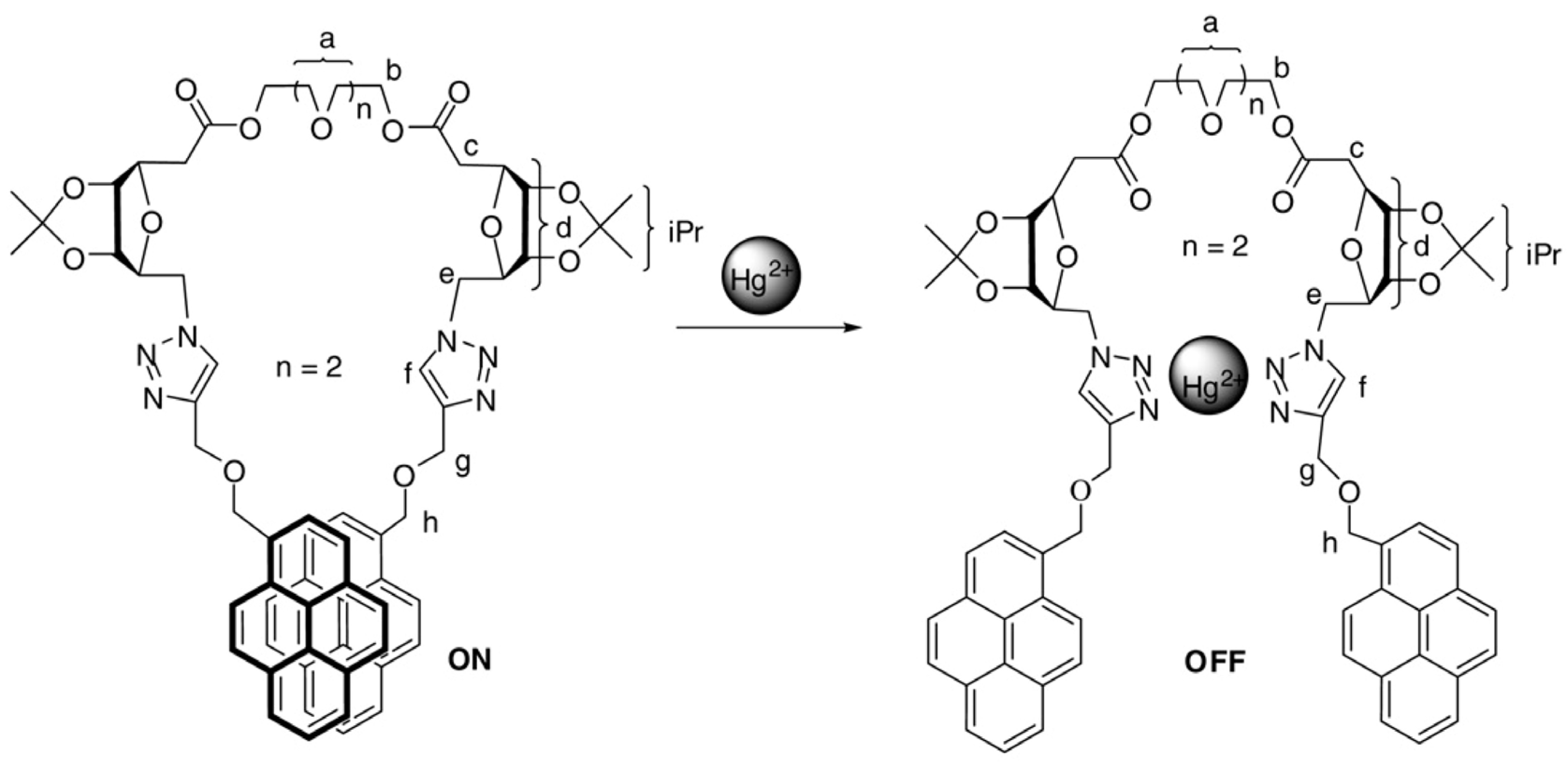
2.1. Excimer Facilitated Hg2+ Detection
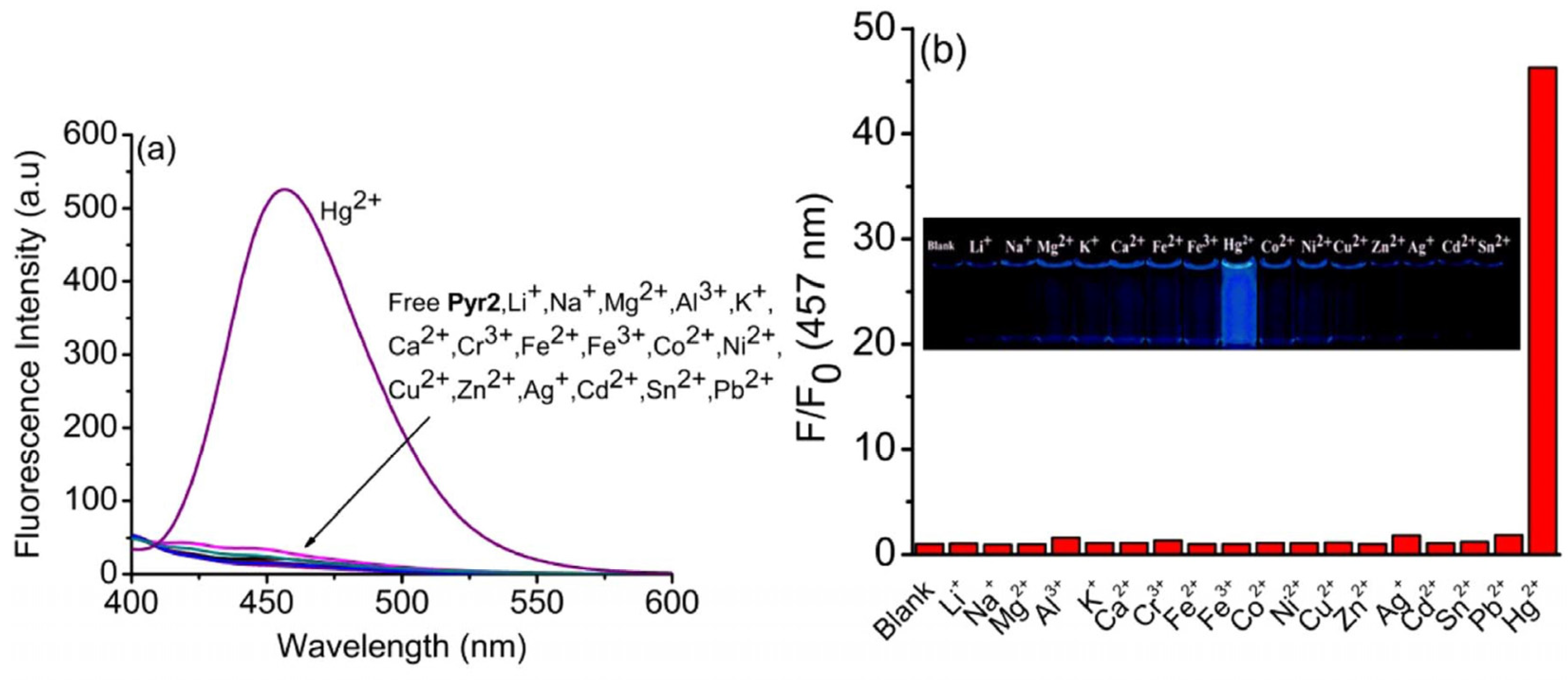
2.2. CHEF-Tuned Hg2+ Detection
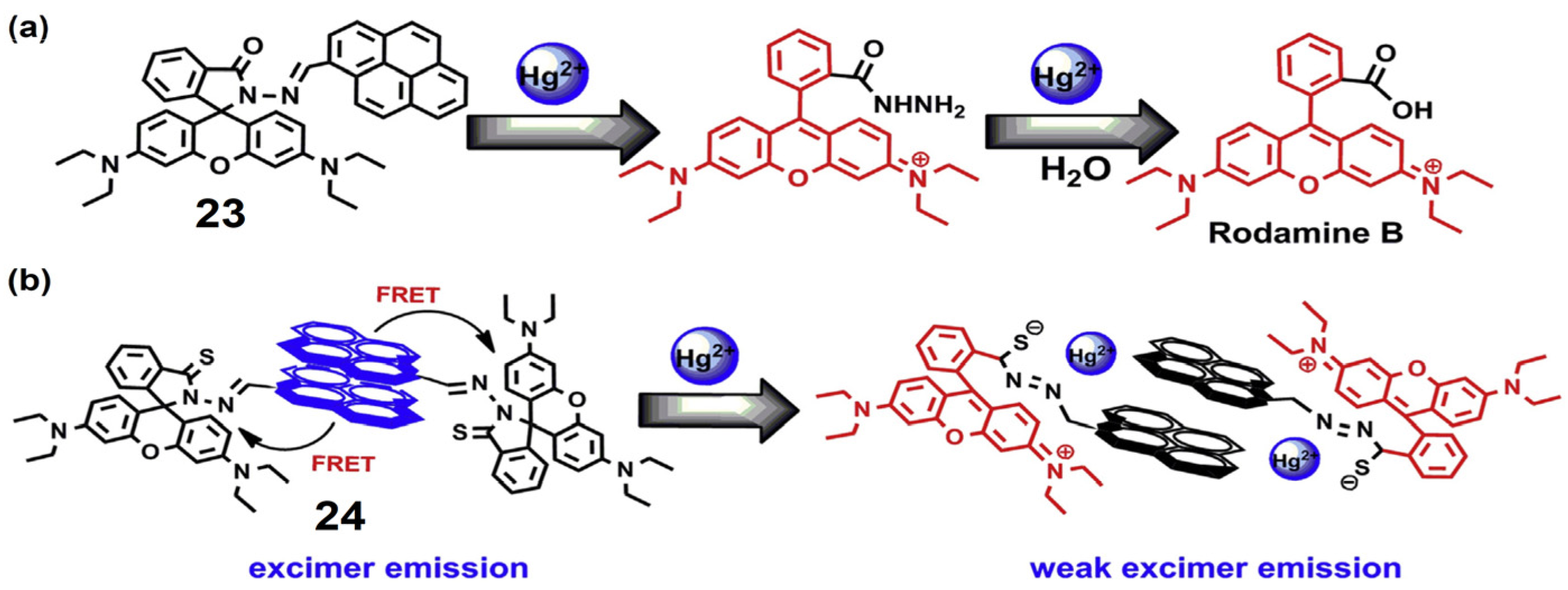
2.3. Pyrene Conjugates for Reaction-Based Detection of Hg2+
2.4. Critical Analysis of PL “Turn-On” Detection of Hg2+
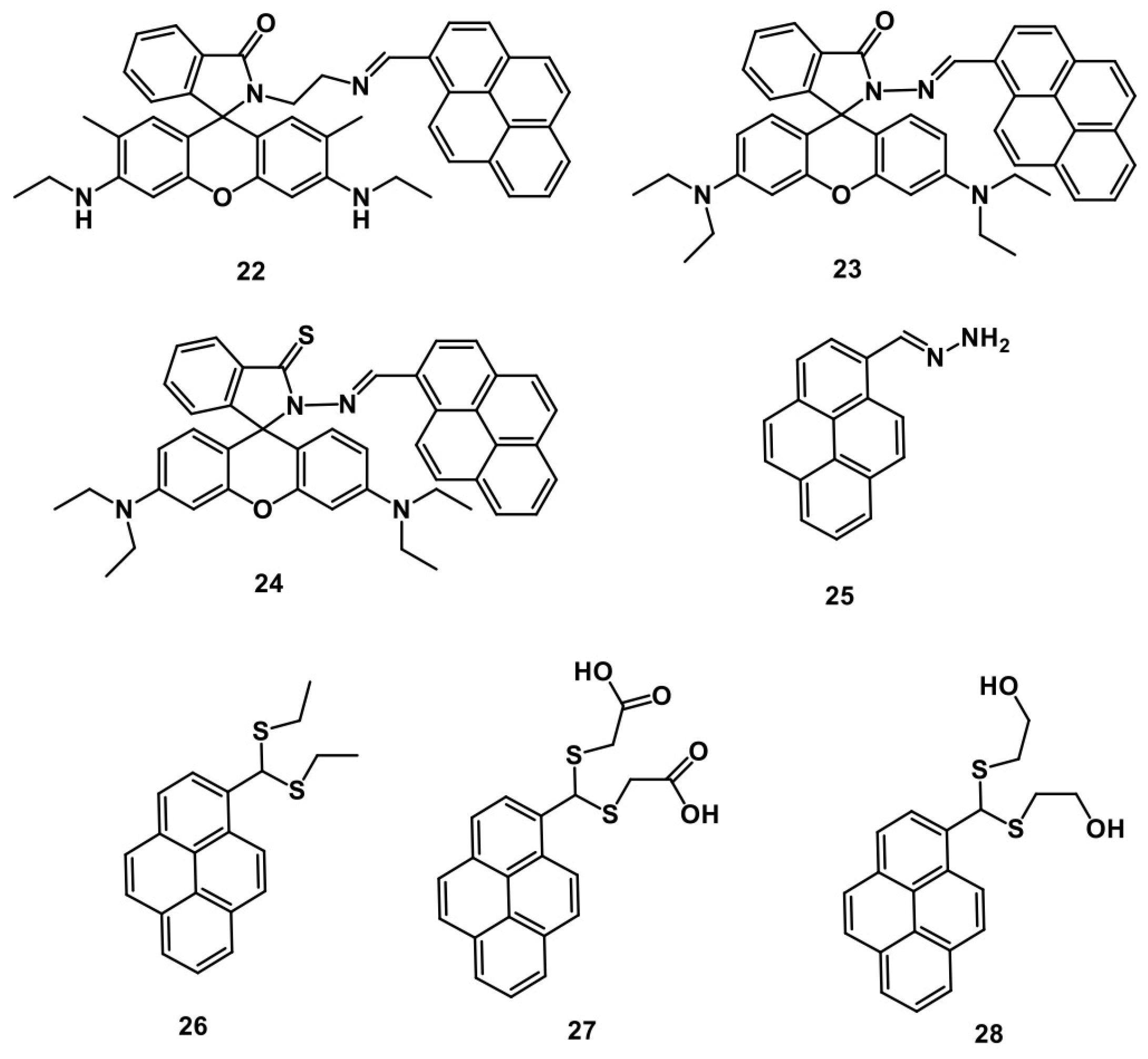
3. Pyrene Derivatives for PL “Turn-Off” Detection of Hg2+
Critical Analysis of PL “Turn-Off” Detection of Hg2+
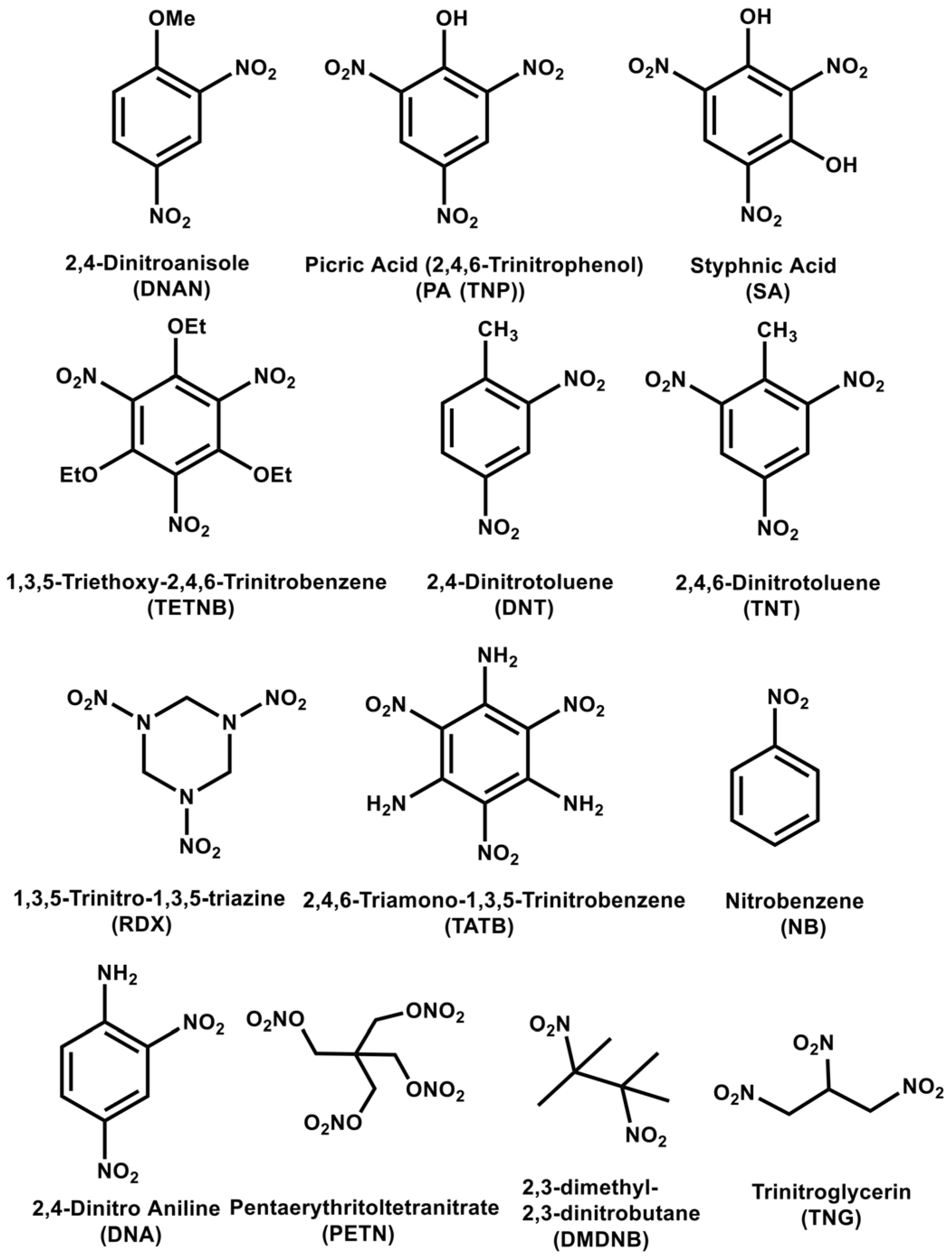
4. Pyrene Derivatives for Explosive Detection
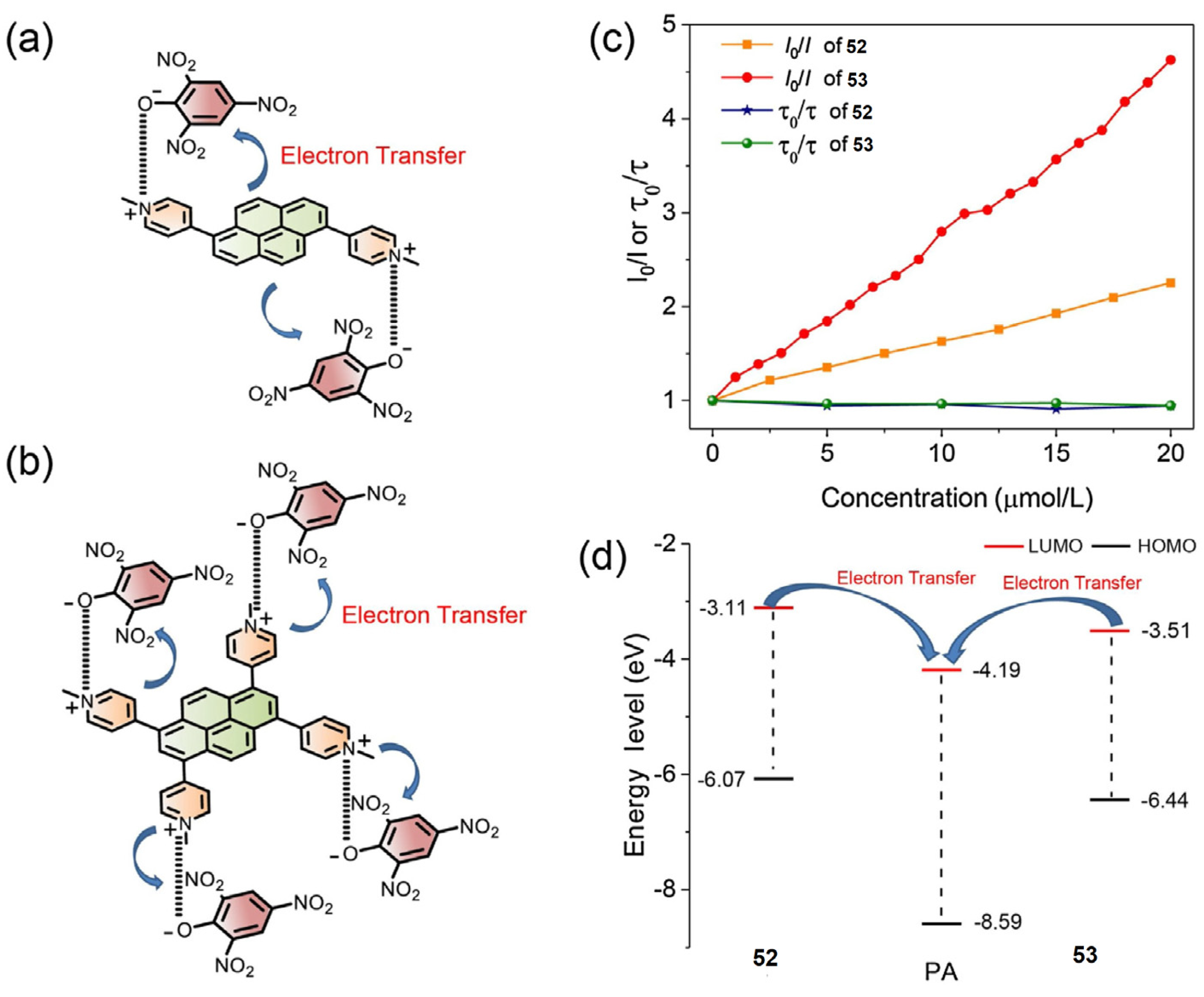
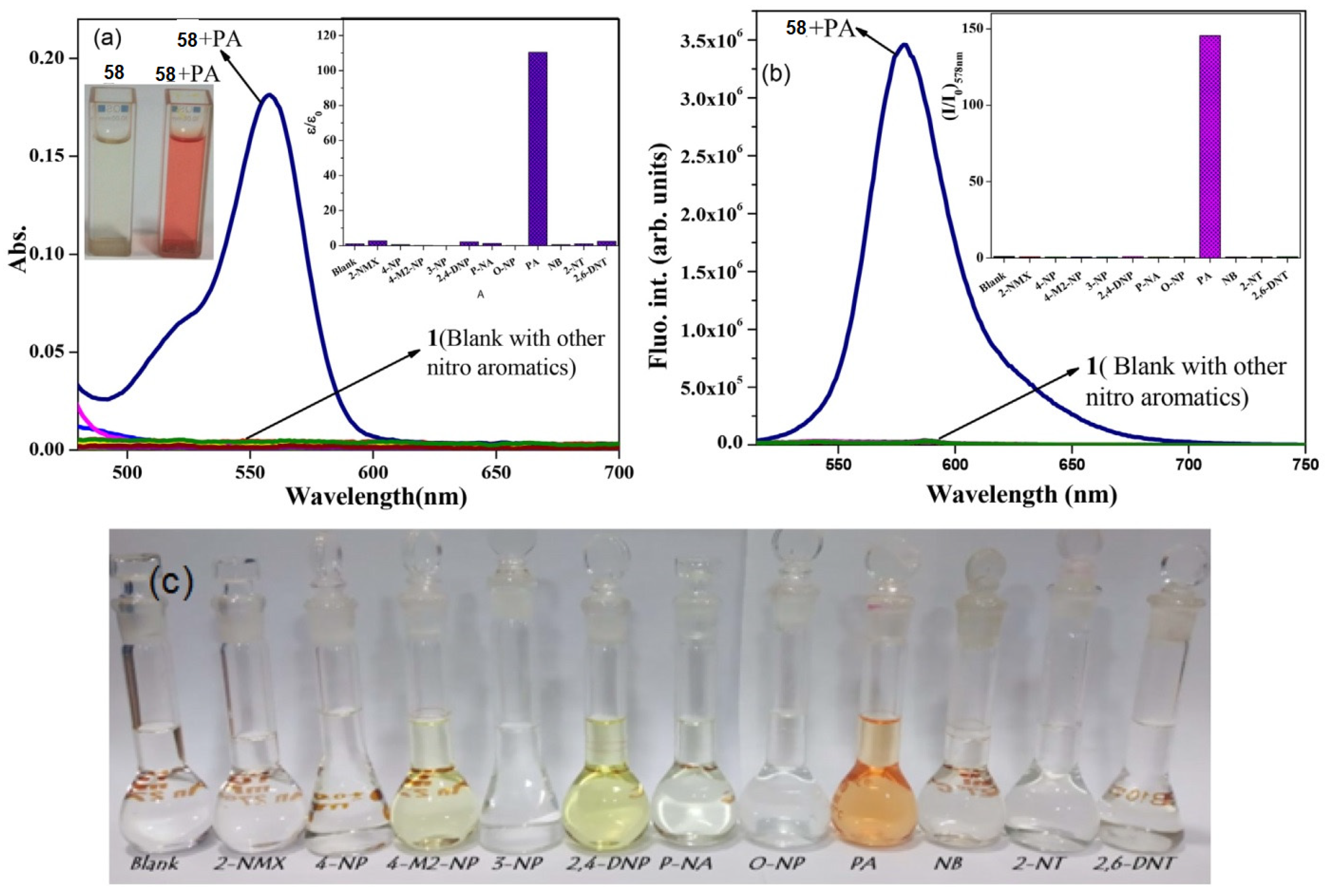
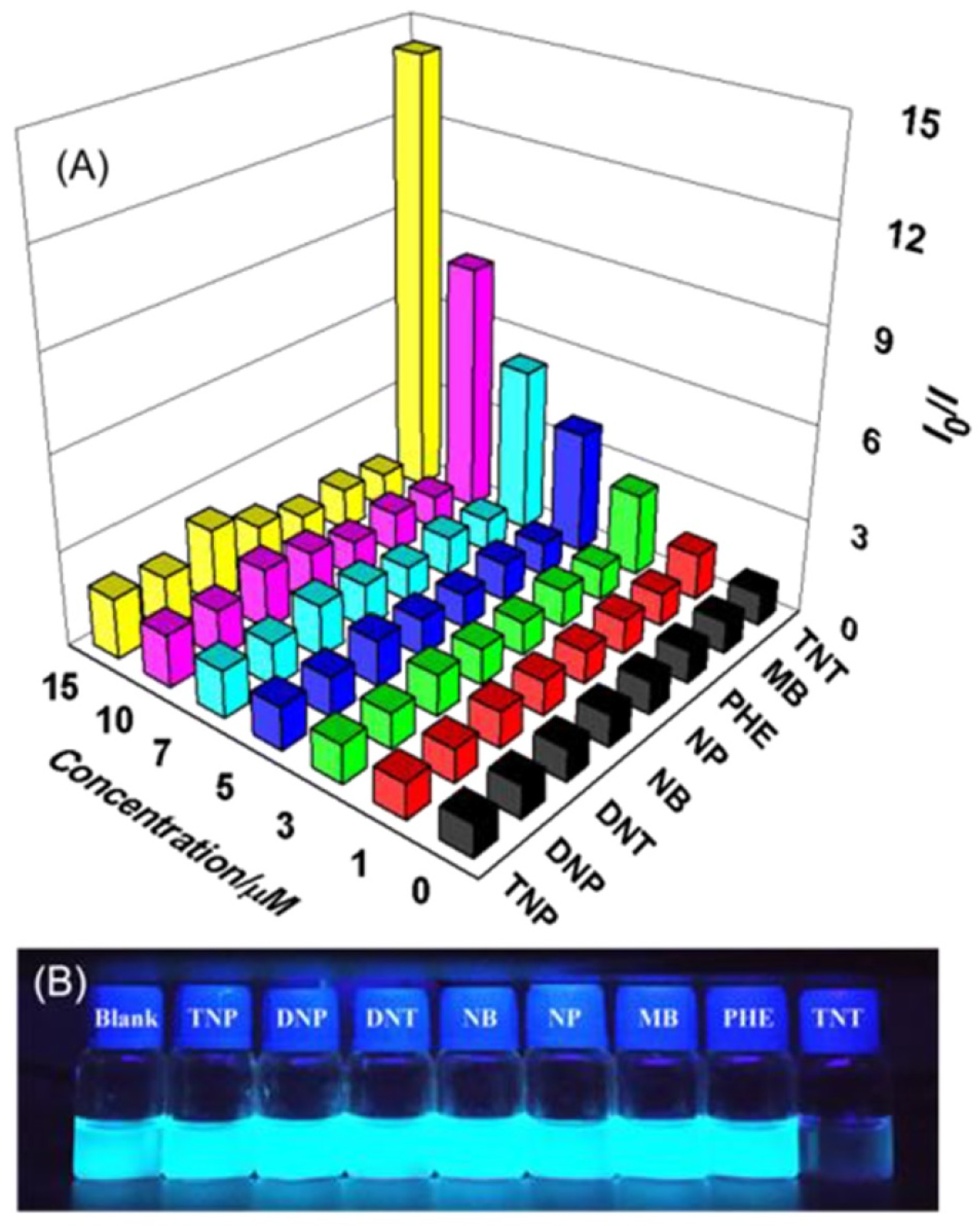
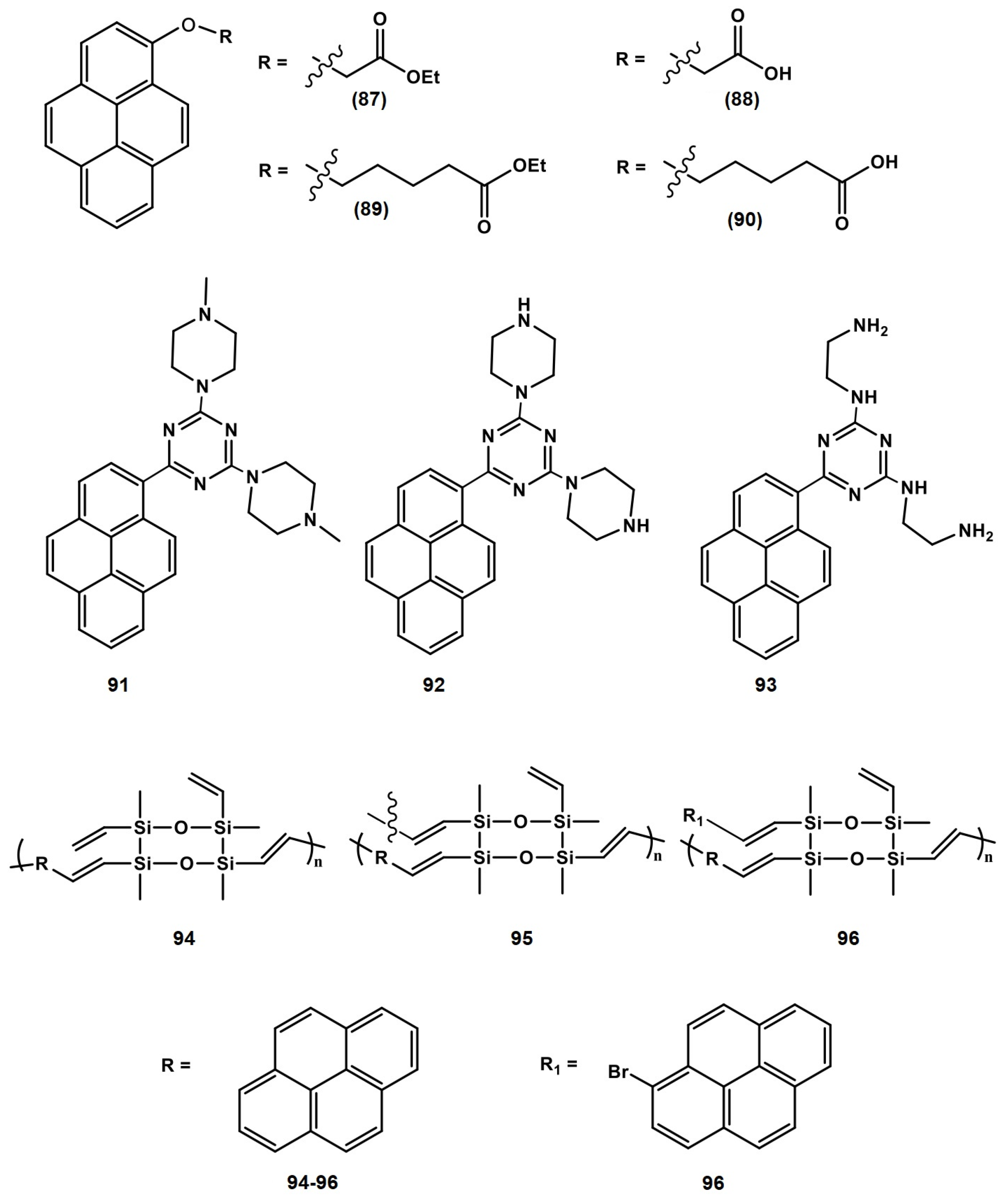
4.1. Pyrene Derivatives for Picric Acid (PA) Detection
Critical Analysis of Detection of PA
4.2. Pyrene Derivatives for Trinitrotoluene (TNT) Detection
Critical Scrutiny of Detection of TNT
4.3. Pyrene Derivatives for RDX and Multi-Explosive Discovery
Critical Views on RDX and Multi-Explosive Encounters
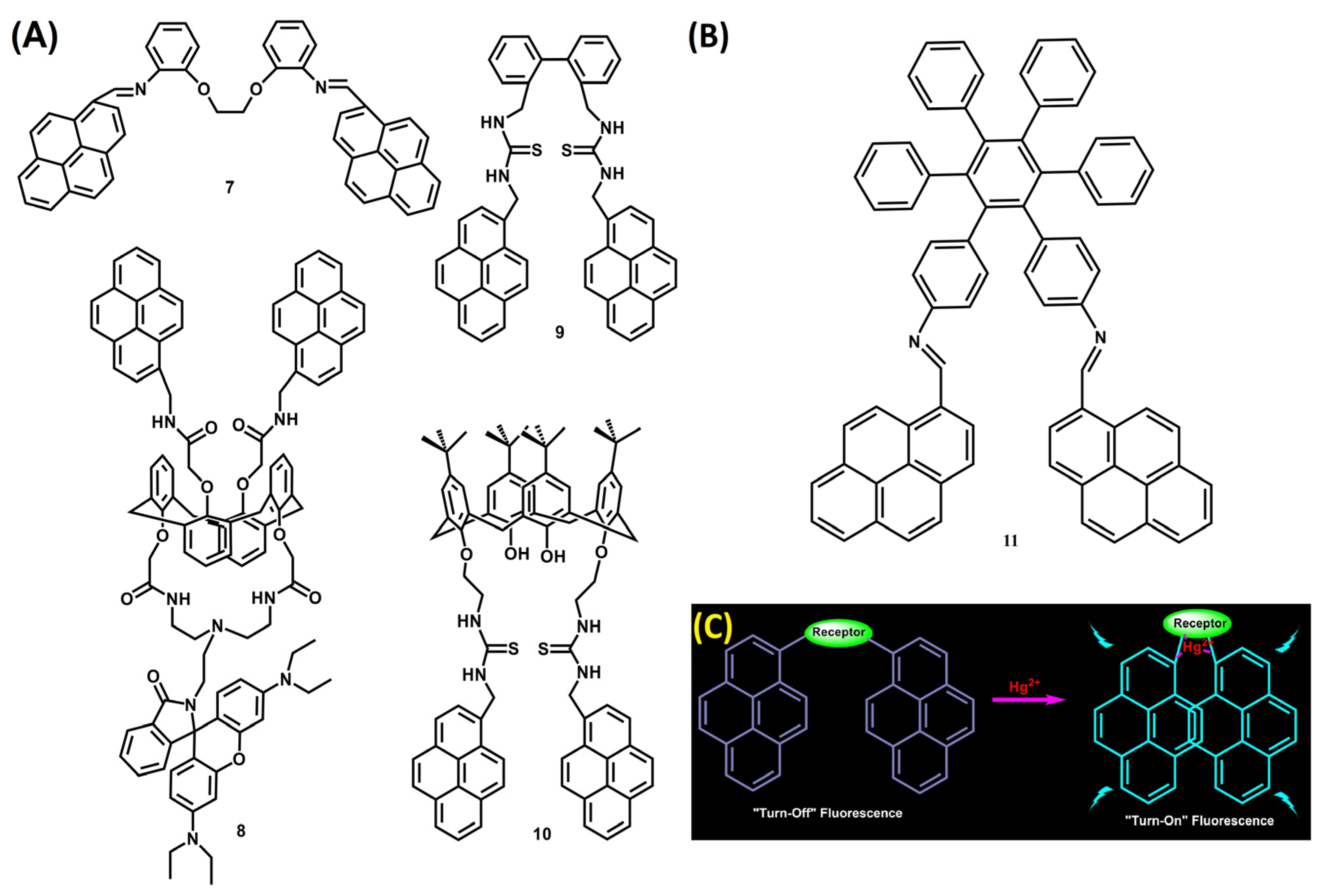
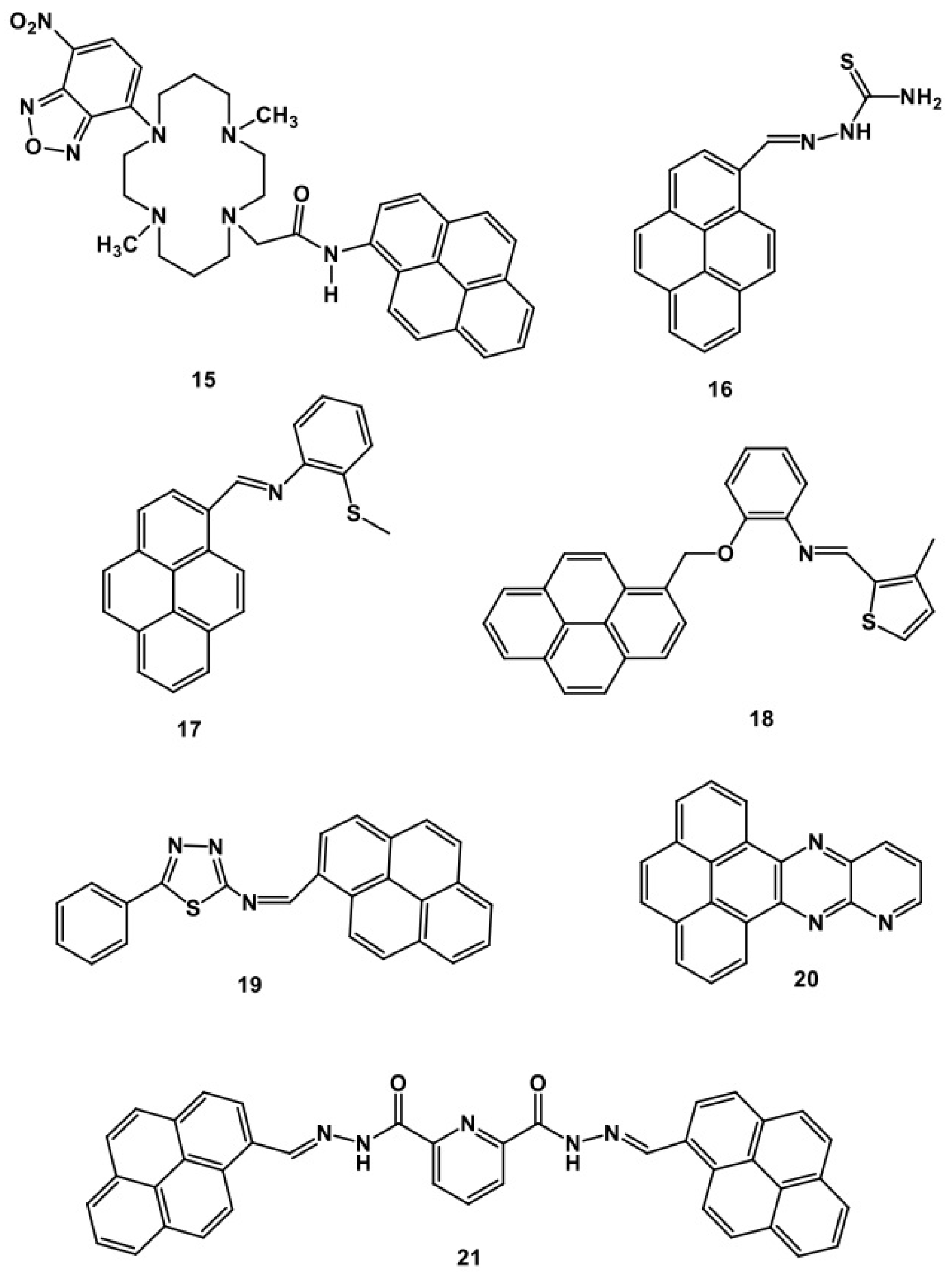
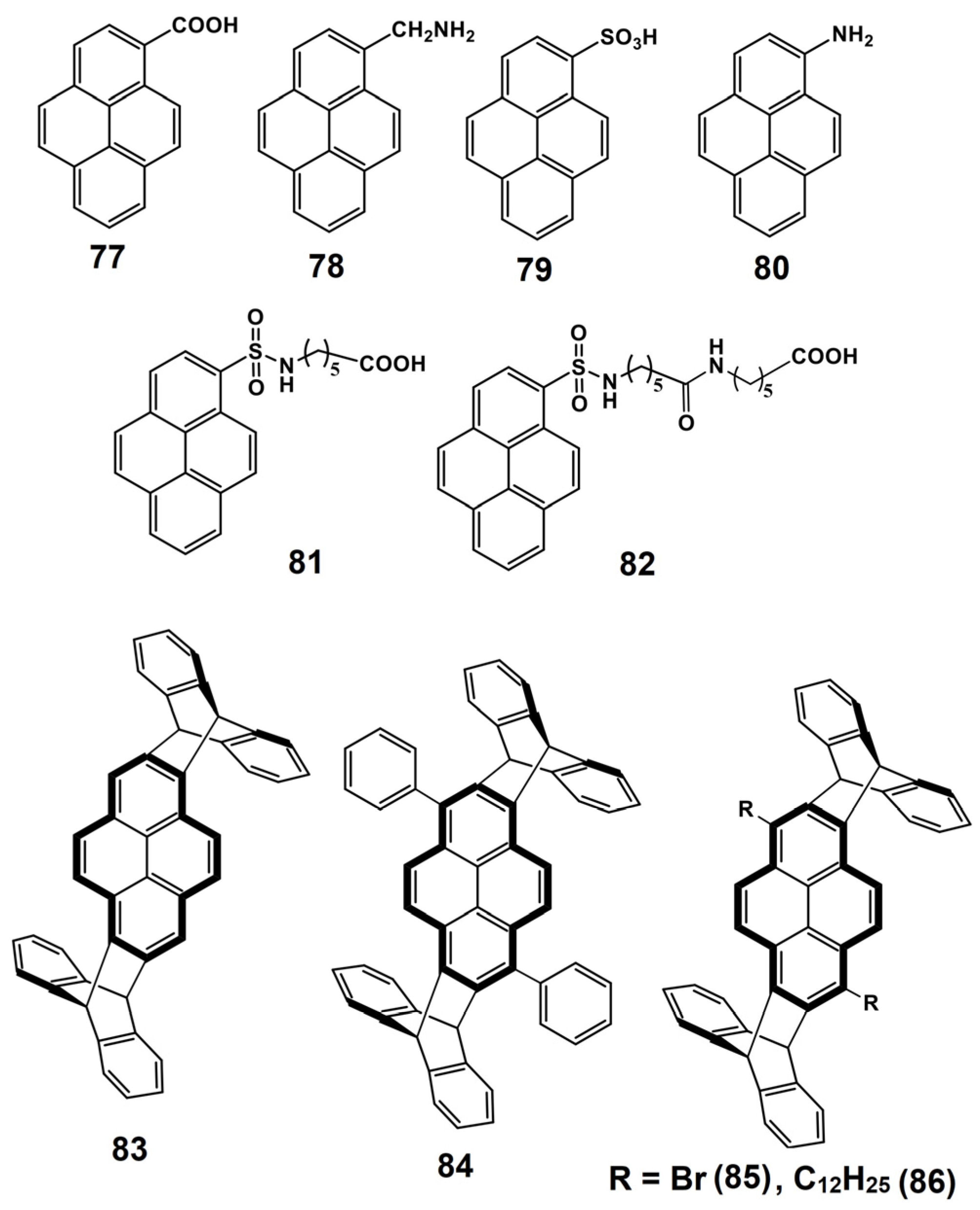
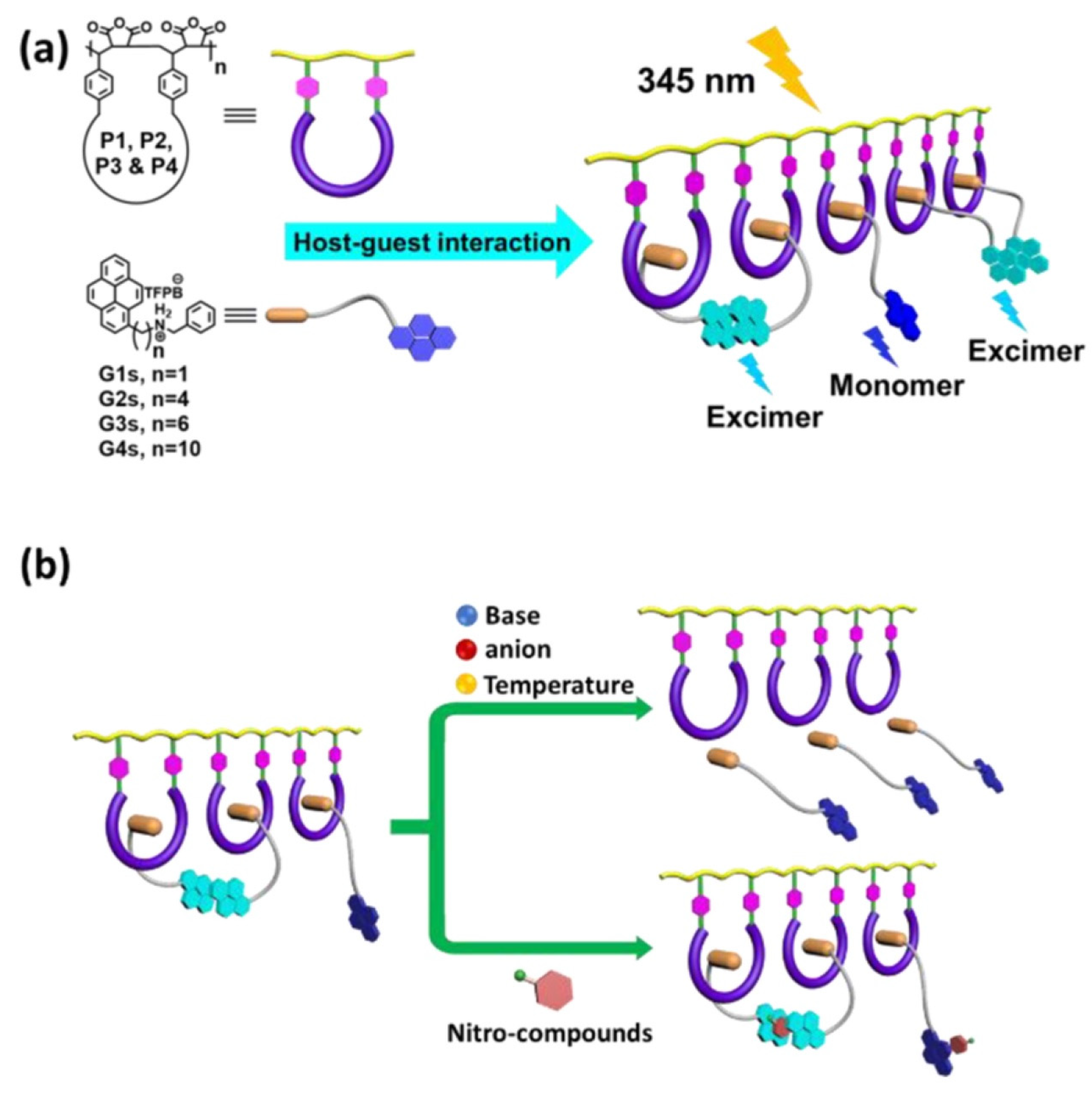
5. Pyrene-Conjugated Hybrid Probes for Hg2+ and Explosive Detection
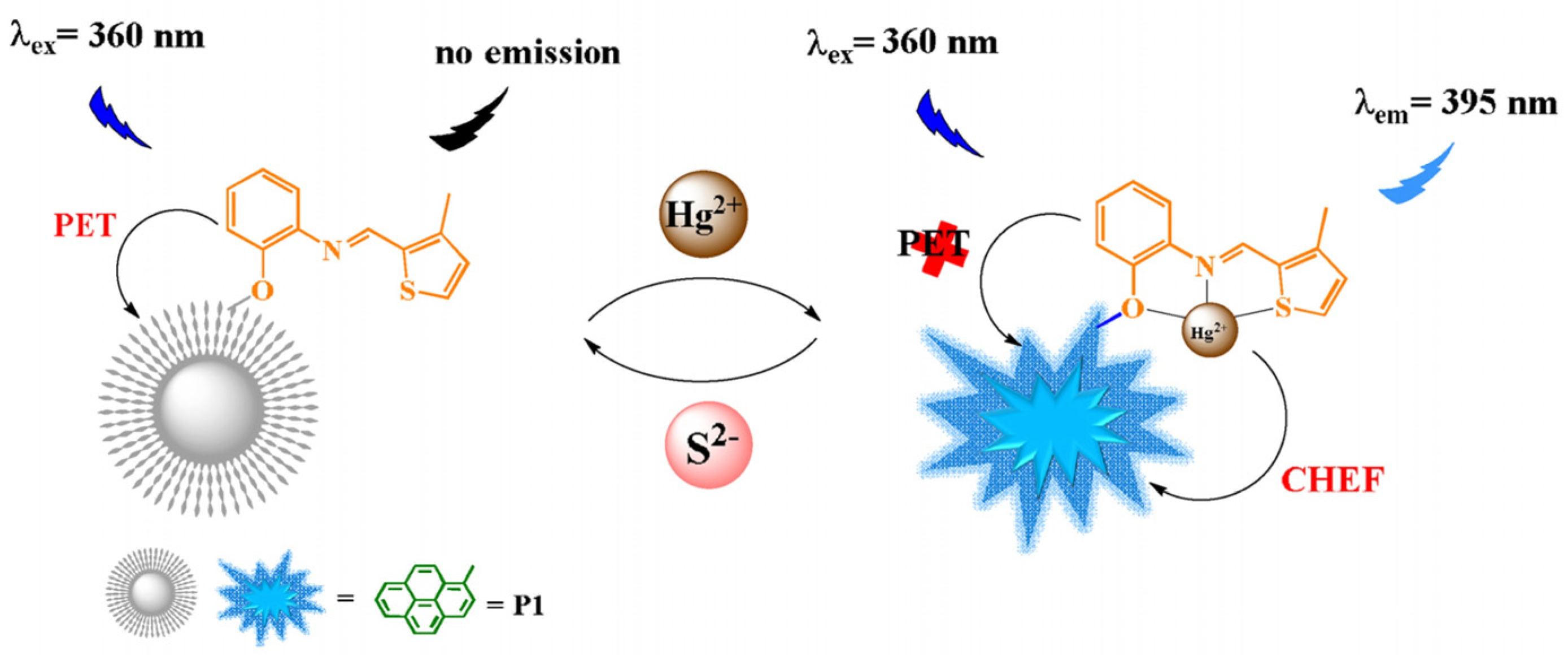
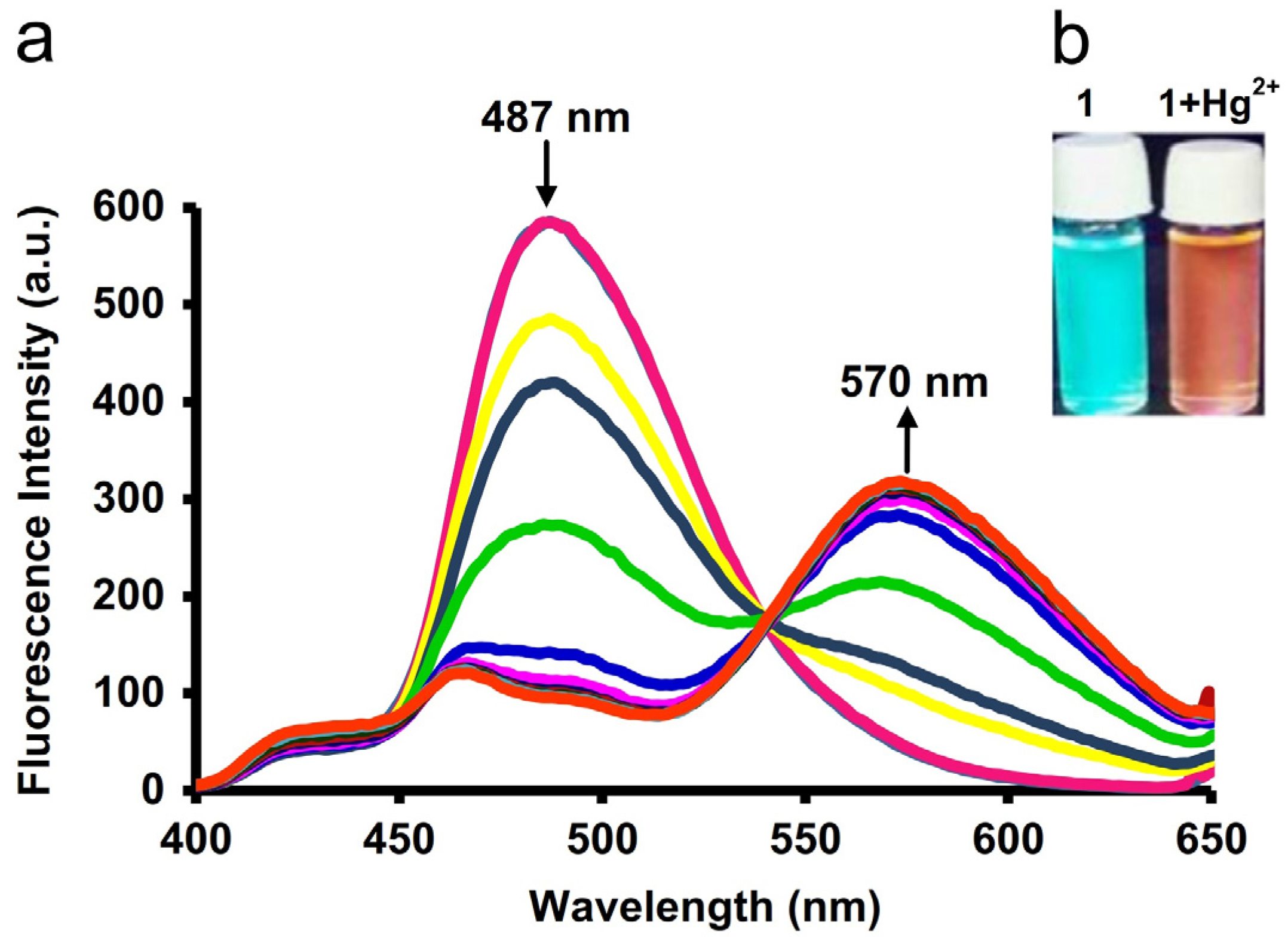
5.1. Hg2+ Sensors
5.2. Explosive Sensors
5.3. Critical Opinions on Hybrid Sensors for Hg2+ and Explosives
6. Design Requirements, Advantages, and Limitations
6.1. Design Requirements
- The probe for Hg2+ must hold hetero atoms or functional groups such as nitrogen/imine (N/−C=N), sulphur/thiols (S/−SH), oxygen/hydroxyl (O/−OH), carboxylic acid (−COOH), etc. This could lead to strong coordination with Hg2+ to deliver either PL enhancement or a quenching response.
- The pyrene-based probe for Hg2+ must hold exceptional fluorescent ΦF values in two ways: (i) For the PL “Turn-On” sensor, the probe must possess a low ΦF value with weak or no-emission; (ii) For the PL “Turn-Off” sensor, the probe must possess a higher ΦF value with superior emission. This could be achieved by the inclusion of heteroatoms, functional units, Schiff base imine (−C=N) bonds, and extended conjugation, etc. [148,149,150,151], which initiates either PET/FRET On or Off, “CHEF”, and chemodosimetric approaches mechanisms.
- For the chemodosimetric and ratiometric detection of Hg2+, the probe must be designed with highly reactive groups like sulphur/thiols (S/−SH) and a suitable acceptor moiety such as rhodamine, Fluorinated Boron-Dipyrromethene (BODIPY), etc.
- To detect the NACs via the PL “Turn-Off” response, the designed pyrene-based probe must have extended conjugation, “AIE” characteristics, and exceptional π-π stacking. On the other hand, for the PL “Turn-On” response, the probe must be designed with H-bonding and electron/charge transfer units that initiate the emission enhancement.
- To design the pyrene-based hybrid sensor, suitable nanostructured materials must be selected to attach with the proposed pyrene conjugate to afford a weak/highly emissive unique structured material for Hg2+/NACs detection. However, before synthesis, it is important to ensure the hybrid system has a particular affinity for either Hg2+ or NACs.
6.2. Advantages
- Most of the probes that detect Hg2+ ions hold unique luminescent features to afford PL “Turn-On” or “Turn-Off” responses with exceptional changes in ΦF values and can be applied for in vitro/in vivo imaging and real sample analysis. This is advantageous over nanomaterials such as quantum dots (QDs), carbon dots (CDs), nanoparticles, 2D-materials, etc. [152,153,154,155].
- The distinct PL response achieved during the ratiometric Hg2+ sensor may be helpful to quantify the Hg2+ via dual/multi-channel response, which is an advantage over common probes.
- Hybrid pyrene–mesoporous conjugates show specific affinity to Hg2+/NACs by affording a large surface area to deliver greater PL responses. This could be advantageous and reliable compared to other hybrid systems.
6.3. Limitations
- Pyrene is a light-sensitive moiety; hence, the stability and durability of pyrene-based probes are still in question. This limits the long-term use of the pyrene-based probes for Hg2+ and NACs detection.
- Most of the pyrene-based probes for Hg2+ and NACs are operable in organic solvent or semi-aqueous media, and few probes show “AIE” features in an aqueous environment. This could limit the easy operation and real-time performance of sensors.
- Many reports on the use of pyrene derivatives for Hg2+ discrimination lack precise mechanistic evidence and extended applications. This raises doubts about the reliability of the probes and limits further investigations.
- The few reports on pyrene-conjugated hybrid systems for Hg2+ and their multi-NAC sensing ability limit upcoming researchers investigating such a direction. In addition, the test-strip method using probes reported for NAC detection is described without proper protocols. This limits the real applications.
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the Terrestrial Environment: A Review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J.; O’Connor, D. Remediation of Mercury Contaminated Soil, Water, and Air: A Review of Emerging Materials and Innovative Technologies. Environ. Inter. 2020, 134, 105281. [Google Scholar] [CrossRef] [PubMed]
- Gworek, B.; Dmuchowski, W.; Baczewska, A.H.; Brągoszewska, P.; Bemowska-Kałabun, O.; Wrzosek-Jakubowska, J. Air Contamination by Mercury, Emissions and Transformations—A Review. Water Air Soil Pollut. 2017, 228, 123. [Google Scholar] [CrossRef]
- Gworek, B.; Bemowska-Kałabun, O.; Kijeńska, M.; Wrzosek-Jakubowska, J. Mercury in Marine and Oceanic Waters—A Review. Water Air Soil Pollut. 2016, 227, 371. [Google Scholar] [CrossRef]
- Basu, N.; Abass, K.; Dietz, R.; Krümmel, E.; Rautio, A.; Weihe, P. The Impact of Mercury Contamination on Human Health in the Arctic: A State of the Science Review. Sci. Total Environ. 2022, 831, 154793. [Google Scholar] [CrossRef]
- Al-Sulaiti, M.M.; Soubra, L.; Al-Ghouti, M.A. The Causes and Effects of Mercury and Methylmercury Contamination in the Marine Environment: A Review. Curr. Pollut. Rep. 2022, 8, 249–272. [Google Scholar] [CrossRef]
- Mergler, D. Ecosystem Approaches to Mercury and Human Health: A Way Toward the Future. Ambio 2021, 50, 527–531. [Google Scholar] [CrossRef]
- Kollur, S.P.; Shivamallu, C.; Prasad, S.K.; Veerapur, R.; Patil, S.S.; Cull, C.A.; Coetzee, J.F.; Amachawadi, R.G. Recent Advances on the Development of Chemosensors for the Detection of Mercury Toxicity: A Review. Separations 2021, 8, 192. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Fu, X.; Wang, X.; Zhang, H.; Lin, C.-J. Mercury Pollution in China: Implications on the Implementation of the Minamata Convention. Environ. Sci. Process. Impacts 2022, 24, 634–648. [Google Scholar] [CrossRef]
- Santos-Sacramento, L.; Arrifano, G.P.; Lopes-Araújo, A.; Augusto-Oliveira, M.; Albuquerque-Santos, R.; Takeda, P.Y.; Souza-Monteiro, J.R.; Macchi, B.M.; do Nascimento, J.L.M.; Lima, R.R.; et al. Human Neurotoxicity of Mercury in the Amazon: A Scoping Review with Insights and Critical Considerations. Ecotoxicol. Environ. Saf. 2021, 208, 111686. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The Toxicology of Mercury: Current Research and Emerging Trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef]
- Gauthama, B.U.; Narayana, B.; Sarojini, B.K.; Suresh, N.K.; Sangappa, Y.; Kudva, A.K.; Satyanarayana, G.; Raghu, S.V. Colorimetric “off–on” Fluorescent Probe for Selective Detection of Toxic Hg2+ Based on Rhodamine and Its Application for In-Vivo Bioimaging. Microchem. J. 2021, 166, 106233. [Google Scholar] [CrossRef]
- Zhu, N.; Xu, J.; Ma, Q.; Gang, Y.; Li, L.; Liu, S.; Liu, S.; Wang, G. Rhodamine-Based Fluorescent Probe for Highly Selective Determination of Hg2+. ACS Omega 2022, 7, 29236–29245. [Google Scholar] [CrossRef] [PubMed]
- Małuszyński, M.J.; Małuszyńska, I. The Mercury Content in the Upper Layers of Soils in the Selected Area of the Masovian Landscape Park. Sustainability 2022, 14, 405. [Google Scholar] [CrossRef]
- Busairi, N.; Syahir, A. Recent Advances in Mercury Detection: Towards Enabling a Sensitive and Rapid Point-of-Check Measurement. J. Toxicol. Risk Assess. 2018, 4, 1–10. [Google Scholar]
- Shuai, H.; Xiang, C.; Qian, L.; Bin, F.; Xiaohui, L.; Jipeng, D.; Chang, Z.; Jiahui, L.; Wenbin, Z. Fluorescent Sensors for Detection of Mercury: From Small Molecules to Nanoprobes. Dye. Pig. 2021, 187, 109125. [Google Scholar] [CrossRef]
- Sharma, R.; Lee, H.-I. Recent Advances in Polymeric Chemosensors for the Detection and Removal of Mercury Ions in Complex Aqueous Media. J. Macromol. Sci. A 2022, 59, 389–402. [Google Scholar] [CrossRef]
- Zarlaida, F.; Adlim, M. Gold and Silver Nanoparticles and Indicator Dyes as Active Agents in Colorimetric Spot and Strip Tests for Mercury(II) Ions: A Review. Microchim. Acta 2017, 184, 45–58. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, T.-Y.; Woo, M.-A. Trends in Sensor Development Toward Next-Generation Point-of-Care Testing for Mercury. Biosens. Bioelectron. 2021, 183, 113228. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; Wang, K.; Wang, L. A Triarylamine-Based Fluorescent Covalent Organic Framework for Efficient Detection and Removal of Mercury(II) ion. Dye. Pig. 2020, 173, 10788. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.-W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors 2021, 9, 101. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, S.; Li, J.; Chen, L. Nanomaterial-Based Optical Sensors for Mercury Ions. TrAC Trends Anal. Chem. 2016, 82, 175–190. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.; Shi, A.; Bao, L.; Shen, Y.; Shen, R.; Ye, Y. Recent Developments in Spectroscopic Techniques for the Detection of Explosives. Materials 2018, 11, 1364. [Google Scholar] [CrossRef]
- Klapec, D.J.; Czarnopys, G.; Pannuto, J. Interpol Review of Detection and Characterization of Explosives and Explosives Residues 2016–2019. Forensic Sci. Inter. Synergy 2020, 2, 670–700. [Google Scholar]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence Based Explosive Detection: From Mechanisms to Sensory Materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef]
- Ye, M.; Ma, H.; Ni, Q. Research on a Sudden Explosion and its Environmental Impact. IOP Conf. Ser. Mater. Sci. Eng. 2017, 275, 12022. [Google Scholar] [CrossRef]
- Panz, K.; Miksch, K.; Sójka, T. Synergetic Toxic Effect of an Explosive Material Mixture in Soil. Bull. Environ. Contamin. Toxicol. 2013, 91, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Li, J.; Ji, M.; Li, J.; Ma, D.; Liu, A.; Zhao, Y.; Yang, C. Fluorescent Covalent Organic Frameworks: A Promising Material Platform for Explosive Sensing. Front. Chem. 2022, 10, 943813. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent Metal–Organic Frameworks for Chemical Sensing and Explosive Detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.; Wang, L. Nanomaterials for luminescence detection of nitroaromatic explosives. TrAC Trends Anal. Chem. 2015, 65, 13–21. [Google Scholar] [CrossRef]
- Yu, H.A.; DeTata, D.A.; Lewis, S.W.; Silvester, D.S. Recent Developments in the Electrochemical Detection of Explosives: Towards Field-Deployable Devices for Forensic Science. TrAC Trends Anal. Chem. 2017, 97, 374–384. [Google Scholar] [CrossRef]
- Arshad, A.; Wang, H.; Bai, X.; Jiang, R.; Xu, S.; Wang, L. Colorimetric Paper Sensor for Sensitive Detection of Explosive Nitroaromatics Based on Au@Ag Nanoparticles. Spectrochim. Acta A 2019, 206, 16–22. [Google Scholar] [CrossRef]
- Das, P.; Mandal, S.K. Understanding the effect of an amino group on the selective and ultrafast detection of TNP in water using fluorescent organic probes. J. Mater. Chem. C 2018, 6, 3288–3297. [Google Scholar] [CrossRef]
- Verhagen, A.; Kelarakis, A. Carbon Dots for Forensic Applications: A Critical Review. Nanomaterials 2020, 10, 1535. [Google Scholar] [CrossRef]
- Maluleke, R.; Oluwafemi, O.S. Synthetic Approaches, Modification Strategies and the Application of Quantum Dots in the Sensing of Priority Pollutants. Appl. Sci. 2021, 11, 11580. [Google Scholar] [CrossRef]
- Mitri, F.; De Iacovo, A.; De Santis, S.; Giansante, C.; Sotgiu, G.; Colace, L. Chemiresistive Device for the Detection of Nitroaromatic Explosives Based on Colloidal PbS Quantum Dots. ACS Appl. Electron. Mater. 2021, 3, 3234–3239. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W.; Thirumalaivasan, N.; Bhushan, M.; Murugan, A. Sensing Utilities of Cesium Lead Halide Perovskites and Composites: A Comprehensive Review. Sensors 2024, 24, 2504. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Guo, X.; Cui, Q.; Zhang, W.; Dong, W.; Fei, T. Conjugated Polymer Nanoparticles Based on Anthracene and Tetraphenylethene for Nitroaromatics Detection in Aqueous Phase. Chemosensors 2022, 10, 366. [Google Scholar] [CrossRef]
- Tanwar, A.S.; Parui, R.; Garai, R.; Chanu, M.A.; Iyer, P.K. Dual “Static and Dynamic” Fluorescence Quenching Mechanisms Based Detection of TNT via a Cationic Conjugated Polymer. ACS Meas. Sci. Au 2022, 2, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Awasthi, K.; Chandran, S.; Aazaad, B.; Sun, K.W.; Ohta, N.; Wu, S.-P.; Lin, M.-C. Methylammonium Tin Tribromide Quantum Dots for Heavy Metal Ion Detection and Cellular Imaging. ACS Appl. Nano Mater. 2022, 5, 2859–2874. [Google Scholar] [CrossRef]
- Germain, M.E.; Knapp, M.J. Optical Explosives Detection: From Color Changes to Fluorescence Turn-On. Chem. Soc. Rev. 2009, 38, 2543–2555. [Google Scholar] [CrossRef]
- Lee, J.Y.; Root, H.D.; Ali, R.; An, W.; Lynch, V.M.; Bähring, S.; Kim, I.S.; Sessler, J.L.; Park, J.S. Ratiometric Turn-On Fluorophore Displacement Ensembles for Nitroaromatic Explosives Detection. J. Am. Chem. Soc. 2020, 142, 19579–19587. [Google Scholar] [CrossRef]
- Rullyani, C.; Shellaiah, M.; Ramesh, M.; Lin, H.-C.; Chu, C.-W. Pyrene-SH Functionalized OTFT for Detection of Hg2+ Ions in Aquatic Environments. Org. Electron. 2019, 69, 275–280. [Google Scholar] [CrossRef]
- Manandhar, E.; Wallace, K.J. Host–Guest Chemistry of Pyrene-Based Molecular Receptors. Inorg. Chim. Acta 2012, 381, 15–43. [Google Scholar] [CrossRef]
- Gong, Y.; Zhan, X.; Li, Q.; Li, Z. Progress of Pyrene-Based Organic Semiconductor in Organic Field Effect Transistors. Sci. China Chem. 2016, 59, 1623–1631. [Google Scholar] [CrossRef]
- Shellaiah, M.; Wu, Y.-H.; Singh, A.; Ramakrishnam Raju, M.V.; Lin, H.-C. Novel Pyrene- and Anthracene-Based Schiff Base Derivatives as Cu2+ and Fe3+ Fluorescence Turn-on Sensors and for Aggregation Induced Emissions. J. Mater. Chem. A 2013, 1, 1310–1318. [Google Scholar] [CrossRef]
- Shellaiah, M.; Chen, Y.-T.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Pyrene-Based AIEE Active Nanoprobe for Zn2+ and Tyrosine Detection Demonstrated by DFT, Bioimaging, and Organic Thin-Film Transistor. ACS Appl. Mater. Interfaces 2021, 13, 28610–28626. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.-W. Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications. Biosensors 2022, 12, 550. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Srinivasadesikan, V.; Lin, C.-M.; Sun, K.W.; Ko, F.-H.; Lin, M.-C.; Lin, H.-C. Novel Pyrene Containing Monomeric and Dimeric Supramolecular AIEE Active Nano-Probes Utilized in Selective “Off–On” Trivalent Metal and Highly Acidic pH Sensing with Live Cell Applications. J. Mater. Chem. C 2016, 4, 2056–2071. [Google Scholar] [CrossRef]
- Zhao, Q.; Lai, H.; Chen, H.; Li, H.; He, F. H- and J-Aggregation Inspiring Efficient Solar Conversion. J. Mater. Chem. A 2021, 9, 1119–1126. [Google Scholar] [CrossRef]
- Heo, J.; Murale, D.P.; Yoon, H.Y.; Arun, V.; Choi, S.; Kim, E.; Lee, J.-S.; Kim, S. Recent trends in molecular aggregates: An exploration of biomedicine. Aggregate 2022, 3, e159. [Google Scholar] [CrossRef]
- Fang, H.-P.; Shellaiah, M.; Singh, A.; Raju, M.V.R.; Wu, Y.-H.; Lin, H.-C. Naked Eye and Fluorescent Detections of Hg2+ Ions and Cysteine via J-Aggregation and Deaggregation of a Perylene Bisimide Derivative. Sens. Actuators B 2014, 194, 229–237. [Google Scholar] [CrossRef]
- Shellaiah, M.; Ramakrishna Raju, M.V.; Singh, A.; Lin, H.-C.; Wei, K.-H.; Lin, H.-C. Synthesis of Novel Platinum Complex Core as A Selective Ag+ Sensor and Its H-Bonded Tetrads Self-Assembled with Triarylamine Dendrimers for Electron/Energy Transfers. J. Mater. Chem. A 2014, 2, 17463–17476. [Google Scholar] [CrossRef]
- Shellaiah, M.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. An AIEE Active Anthracene-Based Nanoprobe for Zn2+ and Tyrosine Detection Validated by Bioimaging Studies. Chemosensors 2022, 10, 381. [Google Scholar] [CrossRef]
- Más-Montoya, M.; Janssen, R.A.J. The Effect of H- and J-Aggregation on the Photophysical and Photovoltaic Properties of Small Thiophene–Pyridine–DPP Molecules for Bulk-Heterojunction Solar Cells. Adv. Funct. Mater. 2017, 27, 1605779. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, X.; Xie, Z.; Liu, J.; Han, Y. Optimizing H-/J-Type Aggregation and Vertical Phase Separation to Improve Photovoltaic Efficiency of Small Molecule Solar Cells by Adding a Macromolecule Additive. ACS Appl. Energy Mater. 2018, 1, 6338–6344. [Google Scholar] [CrossRef]
- Yang, M.-H.; Thirupathi, P.; Lee, K.-H. Selective and Sensitive Ratiometric Detection of Hg(II) Ions Using a Simple Amino Acid Based Sensor. Org. Lett. 2011, 13, 5028–5031. [Google Scholar] [CrossRef] [PubMed]
- Areti, S.; Hinge, V.K.; Rao, C.P. Pyrenyl-imino-C2-Glucosyl Conjugate: Synthesis, Characterization, and Ratiometric and Reversible OFF–ON Receptor for Hg2+. Carbohydr. Res. 2014, 399, 64–69. [Google Scholar] [CrossRef]
- Thirupathi, P.; Saritha, P.; Lee, K.-H. Ratiometric Fluorescence Chemosensor Based on Tyrosine Derivatives for Monitoring Mercury Ions in Aqueous Solutions. Org. Biomol. Chem. 2014, 12, 7100–7109. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Rajan, Y.C.; Balu, P.; Murugan, A. A Pyrene-Based Schiff Base Probe for Selective Fluorescence Turn-On Detection of Hg2+ Ions with Live Cell Application. New J. Chem. 2015, 39, 2523–2531. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, X.; Fan, Z. An AIE Active Pyrene-Based Fluorescent Probe for Selective Sensing Hg2+ and Imaging in Live Cells. Spectrochim. Acta A 2019, 223, 117315. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, C.-Y.; Gao, X.-S.; You, X.-Y.; Yao, C. Hg2+-Selective Ratiometric and “Off−On” Chemosensor Based on the Azadiene−Pyrene Derivative. Org. Lett. 2010, 12, 2566–2569. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, M.H.; Zhang, J.F.; Kim, J.S. Pyrene Excimer-Based Calix[4]arene FRET Chemosensor for Mercury(II). J. Org. Chem. 2010, 75, 7159–7165. [Google Scholar] [CrossRef]
- Wang, G.K.; Mi, Q.L.; Zhao, L.Y.; Hu, J.J.; Guo, L.E.; Zou, X.J.; Liu, B.; Xie, X.G.; Zhang, J.F.; Zhao, Q.H.; et al. A Pyrene Derivative for Hg2+-Selective Fluorescent Sensing and Its Application in In Vivo Imaging. Chem. Asian J. 2014, 9, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lavado, J.; Lorente, A.; Flores, E.; Ochoa, A.; Godoy, F.; Jaque, P.; Saitz, C. Elucidating Sensing Mechanisms of A Pyrene Excimer-Based Calix[4]arene for Ratiometric Detection of Hg(ii) and Ag(i) and Cemosensor Behaviour as INHIBITION or IMPLICATION Logic Gates. RSC Adv. 2020, 10, 21963–21973. [Google Scholar] [CrossRef]
- Pramanik, S.; Bhalla, V.; Kumar, M. Mercury Assisted Fluorescent Supramolecular Assembly of Hexaphenylbenzene Derivative for Femtogram Detection of Picric Acid. Anal. Chim. Acta 2013, 793, 99–106. [Google Scholar] [CrossRef]
- Tümay, S.O.; Uslu, A.; Ardıç Alidağı, H.; Kazan, H.H.; Bayraktar, C.; Yolaçan, T.; Durmuş, M.; Yeşilot, S. A Systematic Series of Fluorescence Chemosensors with Multiple Binding sites for Hg(ii) Based on Pyrenyl-Functionalized Cyclotriphosphazenes and Their Application in Live Cell Imaging. New J. Chem. 2018, 42, 14219–14228. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.S.; Park, S.M.; Chang, S.-K. Hg2+-Selective OFF−ON and Cu2+-Selective ON−OFF Type Fluoroionophore Based upon Cyclam. Org. Lett. 2006, 8, 371–374. [Google Scholar] [CrossRef]
- Wang, X.M.; Yan, H.; Feng, X.L.; Chen, Y. 1-Pyrenecarboxaldehyde Thiosemicarbazone: A Novel Fluorescent Molecular Sensor Towards Mercury (II) Ion. Chin. Chem. Lett. 2010, 21, 1124–1128. [Google Scholar] [CrossRef]
- Sivaraman, G.; Anand, T.; Chellappa, D. Development of A Pyrene Based “Turn On” Fluorescent Chemosensor for Hg2+. RSC Adv. 2012, 2, 10605–10609. [Google Scholar] [CrossRef]
- Tümay, S.O.; Yeşilot, S. Highly Selective “Turn-On” Fluorescence Determination of Mercury Ion in Food and Environmental Samples Through Novel Anthracene and Pyrene Appended Schiff Bases. J. Photochem. Photobiol. A 2021, 407, 113093. [Google Scholar] [CrossRef]
- Bai, C.-B.; Xu, P.; Zhang, J.; Qiao, R.; Chen, M.-Y.; Mei, M.-Y.; Wei, B.; Wang, C.; Zhang, L.; Chen, S.-S. Long-Wavelength Fluorescent Chemosensors for Hg2+ based on Pyrene. ACS Omega 2019, 4, 14621–14625. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Kaur, P.; Singh, K. A Fluorescent Probe for the Detection of Hg2+: Shift From “On-State A” to “On-State B”. Talanta 2014, 130, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, V.; KulathuIyer, S. Twisted Pyrene with Perfect Hetero Atomic Cavity Optical Sensor for Hg22+ and Pb2+. Inorg. Chem. Commun. 2020, 121, 108187. [Google Scholar] [CrossRef]
- Ahamed, B.N.; Ghosh, P. An Integrated System of Pyrene and Rhodamine-6G for Selective Colorimetric and Fluorometric Sensing of Mercury(II). Inorg. Chim. Acta 2011, 372, 100–107. [Google Scholar] [CrossRef]
- Chu, K.-H.; Zhou, Y.; Fang, Y.; Wang, L.-H.; Li, J.-Y.; Yao, C. Rhodamine–Pyrene Conjugated Chemosensors for Ratiometric Detection of Hg2+ Ions: Different Sensing Behavior Between a Spirolactone and a Sirothiolactone. Dye. Pig. 2013, 98, 339–346. [Google Scholar] [CrossRef]
- Bist, A.; Cho, S.J.; Ahmed, N. A Simple Pyrene-Based Highly Sensitive Turn-On Fluorescent Chemodosimeter for Hg2+. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 75–81. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Naha, S.; Thirumalaivasan, N.; Velmathi, S.; Wu, S.-P. A Novel Nanomolar Highly Selective Fluorescent Probe for Imaging Mercury (II) in Living cells and Zebrafish. Sens. Actuators B 2018, 277, 673–678. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, T.; Ou, Z.; Cai, W.; Yang, G.; Li, Y.; Xu, M.; Li, Q. Highly Sensitive and Selective Turn-On Fluorescent Chemosensors for Hg2+ Based on Thioacetal Modified Pyrene. Talanta 2018, 178, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Lu, C.-Y.; Cheng, H.-J.; Chen, S.-J.; Hu, C.-H.; Wu, A.-T. A Pyrenyl-Appended Triazole-Based Ribose as A Fluorescent Sensor for Hg2+ Ion. Carbohyd. Res. 2010, 345, 2557–2561. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Wu, S.-P. Highly Selective Fluorescent Sensors for Mercury(II) Ions and Their Applications in Living Cell Imaging. Tetrahedron 2013, 69, 1965–1969. [Google Scholar] [CrossRef]
- Banerjee, A.; Karak, D.; Sahana, A.; Guha, S.; Lohar, S.; Das, D. Methionine–Pyrene Hybrid-Based Fluorescent Probe for Trace Level Detection and Estimation of Hg(II) in Aqueous Environmental Samples: Experimental and Computational Studies. J. Hazard. Mater. 2011, 186, 738–744. [Google Scholar] [CrossRef]
- Rani, B.K.; John, S.A. Fluorogenic Mercury Ion Sensor Based on Pyrene-Amino Mercapto Thiadiazole Unit. J. Hazard. Mater. 2018, 343, 98–106. [Google Scholar] [CrossRef]
- Harsha, K.G.; Appalanaidu, E.; Chereddy, N.R.; Baggi, T.R.; Rao, V.J. Pyrene Tethered Imidazole Derivative for the Qualitative and Quantitative Detection of Mercury Present in Various Matrices. Sens. Actuators B 2018, 256, 528–534. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Fu, Y.; Zhu, C.; Cheng, Y. Novel Fluorescent Sensor for Ag+ and Hg2+ Based on the BINOL-Pyrene Derivative via Click Reaction. Tetrahedron 2011, 67, 3181–3186. [Google Scholar] [CrossRef]
- Puangsamlee, T.; Tachapermpon, Y.; Kammalun, P.; Sukrat, K.; Wainiphithapong, C.; Sirirak, J.; Wanichacheva, N. Solvent Control Bifunctional Fluorescence Probe for Selective Detection of Cu2+ and Hg2+ Via the Excimer of Pyrenylacetamide Subunits. J. Lumin. 2018, 196, 227–235. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.Y.; Lee, S.H. Self-assembly of Pyrene Boronic Acid-Based Chemodosimeters for Highly Efficient Mercury(II) Ion Detection. Tetrahed. Lett. 2019, 60, 151048. [Google Scholar] [CrossRef]
- Merz, V.; Merz, J.; Kirchner, M.; Lenhart, J.; Marder, T.B.; Krueger, A. Pyrene-Based “Turn-Off” Probe with Broad Detection Range for Cu2+, Pb2+ and Hg2+ Ions. Chem. Eur. J. 2021, 27, 8118–8126. [Google Scholar] [CrossRef] [PubMed]
- Shaligram, S.; Wadgaonkar, P.P.; Kharul, U.K. Fluorescent Polymeric Ionic Liquids for the Detection of Nitroaromatic Explosives. J. Mater. Chem. A 2014, 2, 13983–13989. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Y.; Shaw, G.; Carrier, A.; Dey, S.; Zhao, J.; Lei, Y. Fundamental Study of Electrospun Pyrene–Polyethersulfone Nanofibers Using Mixed Solvents for Sensitive and Selective Explosives Detection in Aqueous Solution. ACS Appl. Mater. Interfaces 2015, 7, 13189–13197. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yao, Z.; Ge, W.; Qiao, Y.; Zhang, L.; Cao, Z.; Wu, H.-C. Selective and Sensitive Detection of Picric Acid Based on A Water-Soluble Fluorescent Probe. RSC Adv. 2016, 6, 38328–38331. [Google Scholar] [CrossRef]
- Deshmukh, S.C.; Rana, S.; Shinde, S.V.; Dhara, B.; Ballav, N.; Talukdar, P. Selective Sensing of Metal Ions and Nitro Explosives by Efficient Switching of Excimer-to-Monomer Emission of an Amphiphilic Pyrene Derivative. ACS Omega 2016, 1, 371–377. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Baranova, A.A.; Lugovik, K.I.; Khokhlov, K.O.; Chuvashov, R.D.; Dinastiya, E.M.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Linear and V-Shaped Push–Pull Systems on A Base of Pyrimidine Scaffold with A Pyrene-Donative Fragment for Detection of Nitroaromatic Compounds. J. Iran. Chem. Soc. 2018, 15, 787–797. [Google Scholar] [CrossRef]
- Sodkhomkhum, R.; Masik, M.; Watchasit, S.; Suksai, C.; Boonmak, J.; Youngme, S.; Wanichacheva, N.; Ervithayasuporn, V. Imidazolylmethylpyrene Sensor for Dual Optical Detection of Explosive Chemical: 2,4,6-Trinitrophenol. Sens. Actuators B 2017, 245, 665–673. [Google Scholar] [CrossRef]
- Islam, A.S.M.; Sasmal, M.; Maiti, D.; Dutta, A.; Show, B.; Ali, M. Design of a Pyrene Scaffold Multifunctional Material: Real-Time Turn-On Chemosensor for Nitric Oxide, AIEE Behavior, and Detection of TNP Explosive. ACS Omega 2018, 3, 10306–10316. [Google Scholar] [CrossRef]
- Hu, Y.; Joung, J.F.; Jeong, J.-E.; Jeong, Y.; Woo, H.Y.; She, Y.; Park, S.; Yoon, J. 2-(Benzothiazol-2-yl)pyren-1-ol, A New Excited State Intramolecular Proton Transfer-Based Fluorescent Sensor for Nitroaromatic Compounds. Sens. Actuators B 2019, 280, 298–305. [Google Scholar] [CrossRef]
- Kathiravan, A.; Gowri, A.; Khamrang, T.; Kumar, M.D.; Dhenadhayalan, N.; Lin, K.-C.; Velusamy, M.; Jaccob, M. Pyrene-Based Chemosensor for Picric Acid—Fundamentals to Smartphone Device Design. Anal. Chem. 2019, 91, 13244–13250. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Song, J.; Wang, F.; Kan, L.; Song, J.; Wang, W.; Ma, W.; Zhang, W.; He, G. Pyrenoviologen-Based Fluorescent Sensor for Detection of Picric Acid in Aqueous Solution. Chin. Chem. Lett. 2019, 30, 1984–1988. [Google Scholar] [CrossRef]
- Panigrahi, A.; Sahu, B.P.; Mandani, S.; Nayak, D.; Giri, S.; Sarma, T.K. AIE Active Fluorescent Oorganic Nanoaggregates for Selective Detection of Phenolic-Nitroaromatic Explosives and Cell Imaging. J. Photochem. Photobiol. A 2019, 374, 194–205. [Google Scholar] [CrossRef]
- Kundu, B.K.; Pragti; Reena; Mobin, S.M.; Mukhopadhyay, S. Mechanistic and Thermodynamic Aspects of A Pyrene-Based Fluorescent Probe to Detect Picric Acid. New J. Chem. 2019, 43, 11483–11492. [Google Scholar] [CrossRef]
- Pherkkhuntod, C.; Ervithayasuporn, V.; Chanmungkalakul, S.; Wang, C.; Liu, X.; Harding, D.J.; Kiatkamjornwong, S. Water-Soluble Polyaromatic-Based Imidazolium for Detecting Picric Acid: Pyrene vs. Anthracene. Sens. Actuators B 2021, 330, 129287. [Google Scholar] [CrossRef]
- Jindal, G.; Kaur, N. Fluorimetric Quantification of Picric Acid in Aqueous Medium Via Smartphone and Invisible Ink Applications Using Pyrene Based Sensor. Inorg. Chem. Commun. 2022, 140, 109481. [Google Scholar] [CrossRef]
- Charan Behera, K.; Mallick, D.; Narayan Patra, B.; Bag, B. A Pyrene-Rhodamine FRET Couple as A Chemosensor for Selective Detection of Picric Acid. Spectrochim. Acta A 2022, 271, 120934. [Google Scholar] [CrossRef]
- Beyazkilic, P.; Yildirim, A.; Bayindir, M. Formation of Pyrene Excimers in Mesoporous Ormosil Thin Films for Visual Detection of Nitro-explosives. ACS Appl. Mater. Interfaces 2014, 6, 4997–5004. [Google Scholar] [CrossRef]
- Venkatramaiah, N.; Firmino, A.D.G.; Almeida Paz, F.A.; Tomé, J.P.C. Fast Detection of Nitroaromatics Using Phosphonate Pyrene Motifs as Dual Chemosensors. Chem. Commun. 2014, 50, 9683–9686. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, P.; Li, Z.; Liu, J.; Yang, Y.; Han, K. New Insights Into the Sensing Mechanism of A Phosphonate Pyrene Chemosensor for TNT. Phys. Chem. Chem. Phys. 2018, 20, 19539–19545. [Google Scholar] [CrossRef]
- Hong, J.-H.; Choi, J.-H.; Cho, D.-G. Simple Pyrene Derivatives as Fluorescence Sensors for TNT and RDX in Micelles. Bull. Korean Chem. Soc. 2014, 35, 3158–3162. [Google Scholar] [CrossRef]
- Sadieva, L.K.; Taniya, O.S.; Kovalev, I.S.; Kopchuk, D.S.; Zyryanov, G.V.; Rusinov, V.L.; Chupakhin, O.N. Pyrene-1-Carboxylic Acid Polyethylene Glycol Esters: Synthesis and Photophysical Studies. Russ. Chem. Bull. 2021, 70, 1174–1179. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Yu, C.; Zhang, L.; Song, G.; Yao, Z. Self-Assembly of Nanoscale Induced Excimers of 12-Pyren-1-yldodecanoic Acid for TNT Detection. ACS Appl. Nano Mater. 2019, 2, 3453–3458. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Sadieva, L.K.; Taniya, O.S.; Yurk, V.M.; Minin, A.S.; Kopchuk, D.S.; Zyryanov, G.V.; Charushin, V.N.; Chupakhin, O.N. Bispyrenylalkane Chemosensor for the Naked-eye Detection of Nitro-explosives. Chim. Techno Acta 2021, 8, 20218209. [Google Scholar] [CrossRef]
- Sriyab, S.; Jorn-Iat, K.; Prompinit, P.; Wolschann, P.; Hannongbua, S.; Suramitr, S. Photophysical Properties of 1-Pyrene-based Derivatives for Nitroaromatic Explosives Detection: Experimental and Theoretical Studies. J. Lumin. 2018, 203, 492–499. [Google Scholar] [CrossRef]
- Hao, H.; Ye, Z.; Dai, H.; Liu, C.; Yi, A.; Xu, B.; Shi, G.; Su, S.; Azad, F.; Chi, Z. Pyrenyl-Based Aggregation-Induced Emission Luminogen for Highly Sensitive and Selective Detection of 2,4,6-Trinitrotoluene in Water. ChemistrySelect 2021, 6, 12182–12187. [Google Scholar] [CrossRef]
- Alidağı, H.A.; Tümay, S.O.; Şenocak, A.; Yeşilot, S. Pyrene Functionalized Cyclotriphosphazene-based Dyes: Synthesis, Intramolecular Excimer Formation, and Fluorescence Receptor for the Detection of Nitro-aromatic Compounds. Dye. Pig. 2018, 153, 172–181. [Google Scholar] [CrossRef]
- Ardic Alidagi, H.; Tümay, S.O.; Şenocak, A.; Çiftbudak, Ö.F.; Çoşut, B.; Yeşilot, S. Constitutional Isomers of Dendrimer-like Pyrene Substituted Cyclotriphosphazenes: Synthesis, Theoretical Calculations, and Use as Fluorescence Receptors for the Detection of Explosive Nitroaromatics. New J. Chem. 2019, 43, 16738–16747. [Google Scholar] [CrossRef]
- Öztürk, B.Ö.; Şehitoğlu, S.K. Pyrene Substituted Amphiphilic ROMP Polymers as Nano-Sized Fluorescence Sensors for Detection of TNT in Water. Polymer 2019, 183, 121868. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Celebioglu, A.; Bayir, S.; Gorur, M.; Doganci, E.; Yilmaz, F.; Uyar, T. Highly Fluorescent Pyrene-Functional Polystyrene Copolymer Nanofibers for Enhanced Sensing Performance of TNT. ACS Appl. Mater. Interfaces 2015, 7, 21038–21046. [Google Scholar] [CrossRef]
- Singla, P.; Kaur, P.; Singh, K. Discrimination in Excimer Emission Quenching of Pyrene by Nitroaromatics. Tetrahed. Lett. 2015, 56, 2311–2314. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Taniya, O.S.; Slovesnova, N.V.; Kim, G.A.; Santra, S.; Zyryanov, G.V.; Kopchuk, D.S.; Majee, A.; Charushin, V.N.; Chupakhin, O.N. Fluorescent Detection of 2,4-DNT and 2,4,6-TNT in Aqueous Media by Using Simple Water-Soluble Pyrene Derivatives. Chem. Asian J. 2016, 11, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Khasanov, A.F.; Kopchuk, D.S.; Kovalev, I.S.; Taniya, O.S.; Giri, K.; Slepukhin, P.A.; Santra, S.; Rahman, M.; Majee, A.; Charushin, V.N.; et al. Extended Cavity Pyrene-based Iptycenes for the Turn-Off Fluorescence Detection of RDX and Common Nitroaromatic Explosives. New J. Chem. 2017, 41, 2309–2320. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Taniya, O.S.; Kopchuk, D.S.; Giri, K.; Mukherjee, A.; Santra, S.; Majee, A.; Rahman, M.; Zyryanov, G.V.; Bakulev, V.A.; et al. 1-Hydroxypyrene-based Micelle-Forming Sensors for the Visual Detection of RDX/TNG/PETN-based Bomb Plots in Water. New J. Chem. 2018, 42, 19864–19871. [Google Scholar] [CrossRef]
- Mosca, L.; Karimi Behzad, S.; Anzenbacher, P., Jr. Small-Molecule Turn-On Fluorescent Probes for RDX. J. Am. Chem. Soc. 2015, 137, 7967–7969. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, P.; Ma, Y.; Tang, Z.; Yang, Y.; Han, K. Reconsideration of the Detection and Fluorescence Mechanism of a Pyrene-Based Chemosensor for TNT. J. Phys. Chem. A 2018, 122, 1400–1405. [Google Scholar] [CrossRef]
- Gou, Z.; Wang, A.; Tian, M.; Zuo, Y. Pyrene-Based Monomer-Excimer Dual Response Organosilicon Polymer for the Selective Detection of 2,4,6-Trinitrotoluene (TNT) and 2,4,6-Trinitrophenol (TNP). Mater. Chem. Front. 2022, 6, 607–612. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Shi, X.; Liu, L.; Chang, W.; Li, J. Multiple Stimuli-Responsiveness Fluorescent Probe Derived from Cyclopolymers and Pyrene-Ended Ammonium Salts. ACS Appl. Polym. Mater. 2020, 2, 2246–2251. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, B.; Guo, Y.; Du, J.; Xiao, D.; Bo, L. A Highly Sensitive and Selective Turn-On Fluorogenic and Colorimetric Sensor Based on Pyrene-Functionalized Magnetic Nanoparticles for Hg2+ Detection and Cell Imaging. Talanta 2013, 117, 338–344. [Google Scholar] [CrossRef]
- Razi, S.S.; Ali, R.; Srivastava, P.; Misra, A. Smart Excimer Fluorescence Probe for Visual Detection, Cell Imaging and Extraction of Hg2+. RSC Adv. 2015, 5, 79538–79547. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Panneerselvam, P.; Ravikumar, A.; Morad, N. Magnetic Core-Shell Fibrous Silica Functionalized with Pyrene Derivative for Highly Sensitive and Selective Detection of Hg (II) Ion. J. Dispers. Sci. Technol. 2019, 40, 1368–1377. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, H.; Hong, W.; Shi, G. Fluorescence Detection of Mercury Ions in Aqueous Media with the Complex of A Cationic Oligopyrene Derivative and Oligothymine. Analyst 2009, 134, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Z.M.; Alimli, D.; Tonta, M.M.; Kose, M.E.; Yilmaz, F. Highly Sensitive and Reusable Mercury (II) Sensor Based on Fluorescence Quenching of Pyrene Moiety in Polyacrylamide-Based Cryogel. Sens. Actuators B 2017, 242, 362–368. [Google Scholar] [CrossRef]
- Ali, S.; Mansha, M.; Baig, N.; Khan, S.A. Cost-Effective and Selective Fluorescent Chemosensor (Pyr-NH@SiO2 NPs) for Mercury Detection in Seawater. Nanomaterials 2022, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, Z.; Qiao, M.; Peng, J.; Ding, L. Pyrene-Functionalized Mesoporous Silica as A Fluorescent Nanosensor for Selective Detection of Hg2+ in Aqueous Solution. Colloids Surf. A 2022, 637, 128269. [Google Scholar] [CrossRef]
- Chen, W.; Zuckerman, N.B.; Konopelski, J.P.; Chen, S. Pyrene-Functionalized Ruthenium Nanoparticles as Effective Chemosensors for Nitroaromatic Derivatives. Anal. Chem. 2010, 82, 461–465. [Google Scholar] [CrossRef]
- Du, H.; He, G.; Liu, T.; Ding, L.; Fang, Y. Preparation of Pyrene-Functionalized Fluorescent Film with A Benzene Ring in Spacer and Sensitive Detection to Picric Acid in Aqueous Phase. J. Photochem. Photobiol. A 2011, 217, 356–362. [Google Scholar] [CrossRef]
- Turhan, H.; Tukenmez, E.; Karagoz, B.; Bicak, N. Highly Fluorescent Sensing of Nitroaromatic Explosives in Aqueous Media Using Pyrene-Linked PBEMA Microspheres. Talanta 2018, 179, 107–114. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, L.; Lü, F.; Liu, T.; Fang, Y. Fluorescent Film Sensors Based on SAMs of Pyrene Derivatives for Detecting Nitroaromatics in Aqueous Solutions. Spectrochim. Acta A 2012, 97, 31–37. [Google Scholar] [CrossRef]
- Liu, T.; Ding, L.; Zhao, K.; Wang, W.; Fang, Y. Single-Layer Assembly of Pyrene End-Capped Terthiophene and Its Sensing Performances to Nitroaromatic Explosives. J. Mater. Chem. 2012, 22, 1069–1077. [Google Scholar] [CrossRef]
- Sarkar, K.; Salinas, Y.; Campos, I.; Martínez-Máñez, R.; Marcos, M.D.; Sancenón, F.; Amorós, P. Organic–Inorganic Hybrid Mesoporous Materials as Regenerable Sensing Systems for the Recognition of Nitroaromatic Explosives. ChemPlusChem 2013, 78, 684–694. [Google Scholar] [CrossRef]
- Reddy, K.L.; Kumar, A.M.; Dhir, A.; Krishnan, V. Selective and Sensitive Fluorescent Detection of Picric Acid by New Pyrene and Anthracene Based Copper Complexes. J. Fluoresc. 2016, 26, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, D.; Fu, Y.; He, Q.; Cao, H.; Li, W.; Cheng, J. Enhanced Fluorescence of Functionalized Silica Microsphere Based on Whispering Gallery Mode for Nitrate Ester Explosives and Hexogen Vapour Detection. J. Mater. Chem. C 2017, 5, 2114–2122. [Google Scholar] [CrossRef]
- Salinas, Y.; Agostini, A.; Pérez-Esteve, É.; Martínez-Máñez, R.; Sancenón, F.; Dolores Marcos, M.; Soto, J.; Costero, A.M.; Gil, S.; Parra, M.; et al. Fluorogenic Detection of Tetryl and TNT Explosives Using Nanoscopic-Capped Mesoporous Hybrid Materials. J. Mater. Chem. A 2013, 1, 3561–3564. [Google Scholar] [CrossRef]
- Thakarda, J.; Agrawal, B.; Anil, D.; Jana, A.; Maity, P. Detection of Trace-Level Nitroaromatic Explosives by 1-Pyreneiodide-Ligated Luminescent Gold Nanostructures and Their Forensic Applications. Langmuir 2020, 36, 15442–15449. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, H.; Liang, S.; Xu, Q. Rapid and Sensitive Fluorescence Sensing Detection of Nitroaromatic Compounds in Water Samples Based on Pyrene Functionalized Nanofibers Mat Prepared via Green Approach. Microchem. J. 2021, 165, 106175. [Google Scholar] [CrossRef]
- Beyazkilic, P.; Yildirim, A.; Bayindir, M. Nanoconfinement of Pyrene in Mesostructured Silica Nanoparticles for Trace Detection of TNT in the Aqueous Phase. Nanoscale 2014, 6, 15203–15209. [Google Scholar] [CrossRef]
- Chethanakumar; Budri, M.; Gudasi, K.B.; Vadavi, R.S.; Bhat, S.S. Luminescent Pyrene-based Schiff base Receptor for Hazardous Mercury(II) Detection Demonstrated by Cell Imaging and Test Strip. J. Fluoresc. 2023, 33, 539–551. [Google Scholar] [CrossRef]
- Yang, X.-L.; Hu, D.-Y.; Chen, Q.; Li, L.; Li, P.-X.; Ren, S.-B.; Bertuzzo, M.; Chen, K.; Han, D.-M.; Zhou, X.-H.; et al. A Pyrene-Cored Conjugated Microporous Polycarbazole for Sensitive and Selective Detection of Hazardous Explosives. Inorg. Chem. Commun. 2019, 107, 107453. [Google Scholar] [CrossRef]
- Xue, Z.-M.; Zhang, X.-T.; Zhao, W.-X.; Yang, G.; Wang, C.-Z.; Chen, S.-H.; Lin, H.-T.; Elsegood, M.R.J.; Yamato, T. Extended π-Conjugated Pyrene-Based Chemosensors for Highly Sensitive and Selective Detection of Nitro-Contaminants. Microchem. J. 2025, 209, 112699. [Google Scholar] [CrossRef]
- Gawas, P.P.; Selvaraj, K.; Pamanji, R.; Selvin, J.; Nutalapati, V. Highly Sensitive Fluorescence Turn-OFF and Reversible Chemical Sensor for Hg2+ Ion Based on Pyrene Appended 2-Thiohydantoin. Chemosphere 2024, 352, 141470. [Google Scholar] [CrossRef]
- Shellaiah, M.; Manikandan, E.; Sun, K.W.; Venkatachalam, V. Luminescent Probes/Conjugates Derived from Quantum Dots, Nanoparticles, and Nanoclusters for Cyanide (CN−) Detection. Nano Exp. 2024, 5, 42001. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Carbon Dot-Based Fluorescent Detection of Biothiols. Biosensors 2023, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.-W. Review on Anti-Aggregation-Enabled Colorimetric Sensing Applications of Gold and Silver Nanoparticles. Chemosensors 2022, 10, 536. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Chowdhury, A.D.; Doong, R.-A. Highly Sensitive and Selective Detection of Mercury Ions using N, S-Codoped Graphene Quantum Dots and Its Paper Strip Based Sensing Application in Wastewater. Sens. Actuators B 2017, 252, 1169–1178. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Diamond-Based Electrodes for Detection of Metal Ions and Anions. Nanomaterials 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chen, Y.; Guo, X.; Liu, W.; Zhang, L.; Lv, C.; Xu, Y.; Jin, Y.; Li, B. Aggregation-Induced Chemiluminescence System for Sensitive Detection of Mercury Ions. Anal. Bioanal. Chem. 2021, 413, 625–633. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Cheng, Y.; Wang, S.; Zhou, Z.; Zhang, P.; Zhu, X.; Wang, B.; Zhang, H.; Xie, S.; et al. Fluorogenic Detection of Mercury Ion in Aqueous Environment Using Hydrogel-Based AIE Sensing Films. Aggregate 2023, 4, e287. [Google Scholar] [CrossRef]
- To, K.C.; Ben-Jaber, S.; Parkin, I.P. Recent Developments in the Field of Explosive Trace Detection. ACS Nano 2020, 14, 10804–10833. [Google Scholar] [CrossRef]
- Ni, J.-S.; Lu, G.-H. Natural Protoberberine Alkaloid–Montmorillonite Nanocomposite Powders with AIE Features for Visualizing High-Resolution Latent Fingerprints. Spectrochim. Acta A 2023, 300, 122908. [Google Scholar] [CrossRef]
- Syiemlieh, C.; Narayanan, M.; Velusamy, M.; Kathiravan, A. Pyrene Based AIE-Active Probe for Selective Detection of Picric Acid Through the Inner Filter Effect Channel in Aqueous Medium. J. Mol. Liq. 2024, 407, 125125. [Google Scholar] [CrossRef]
- Cui, F.; Xie, Z.; Yang, R.; Zhang, Y.; Liu, Y.; Zheng, H.; Han, X. Aggregation-Induced Emission Enhancement (AIEE) Active Bispyrene-Based Fluorescent Probe: “Turn-Off” Fluorescence for the Detection of Nitroaromatics. Spectrochim. Acta A 2024, 314, 124222. [Google Scholar] [CrossRef] [PubMed]
- Jamuna, K.; Govindharaj, P.; Krishnan, A.; Savitha Devi, N.; Sebastian, A.T.; Selvapalam, N.; Mukherjee, M.; Data, P.; Gayathri, S.; Sivakumar, S.; et al. Development of Pyrene Embedded Luminophore via π-linker: Room Temperature Phosphorescence (RTP) and Sensing towards Nitroaromatics (NACs). ChemPhotoChem 2024, 8, e202400046. [Google Scholar] [CrossRef]
- Shepeleva, I.I.; Birin, K.P.; Polivanovskaia, D.A.; Martynov, A.G.; Shokurov, A.V.; Tsivadze, A.Y.; Selektor, S.L.; Gorbunova, Y.G. Unusual ‘Turn-on’ Ratiometric Response of Fluorescent Porphyrin-Pyrene Dyads to the Nitroaromatic Compounds. Chemosensors 2023, 11, 43. [Google Scholar] [CrossRef]
- Liu, L.-N.; Tao, H.; Chen, G.; Chen, Y.; Cao, Q.-Y. An Amphiphilic Pyrene-Based Probe for Multiple Channel Sensing of Mercury Ions. J. Luminesc. 2018, 203, 189–194. [Google Scholar] [CrossRef]






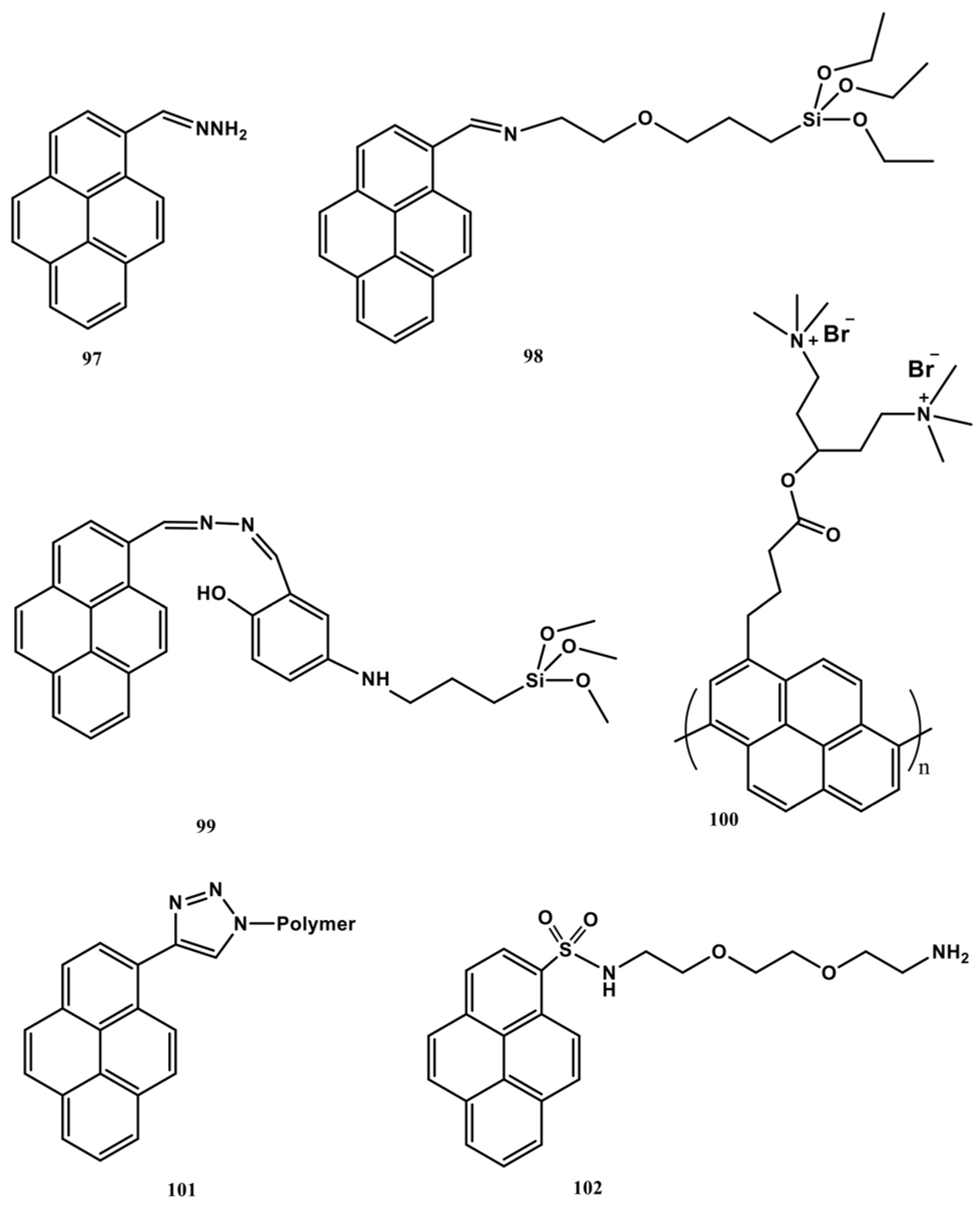

| Probe Number | Detection Conditions (Solvent; pH) | Linear Regression | Detection Limit (LOD) | Assigned Mechanism | Applications | Ref |
|---|---|---|---|---|---|---|
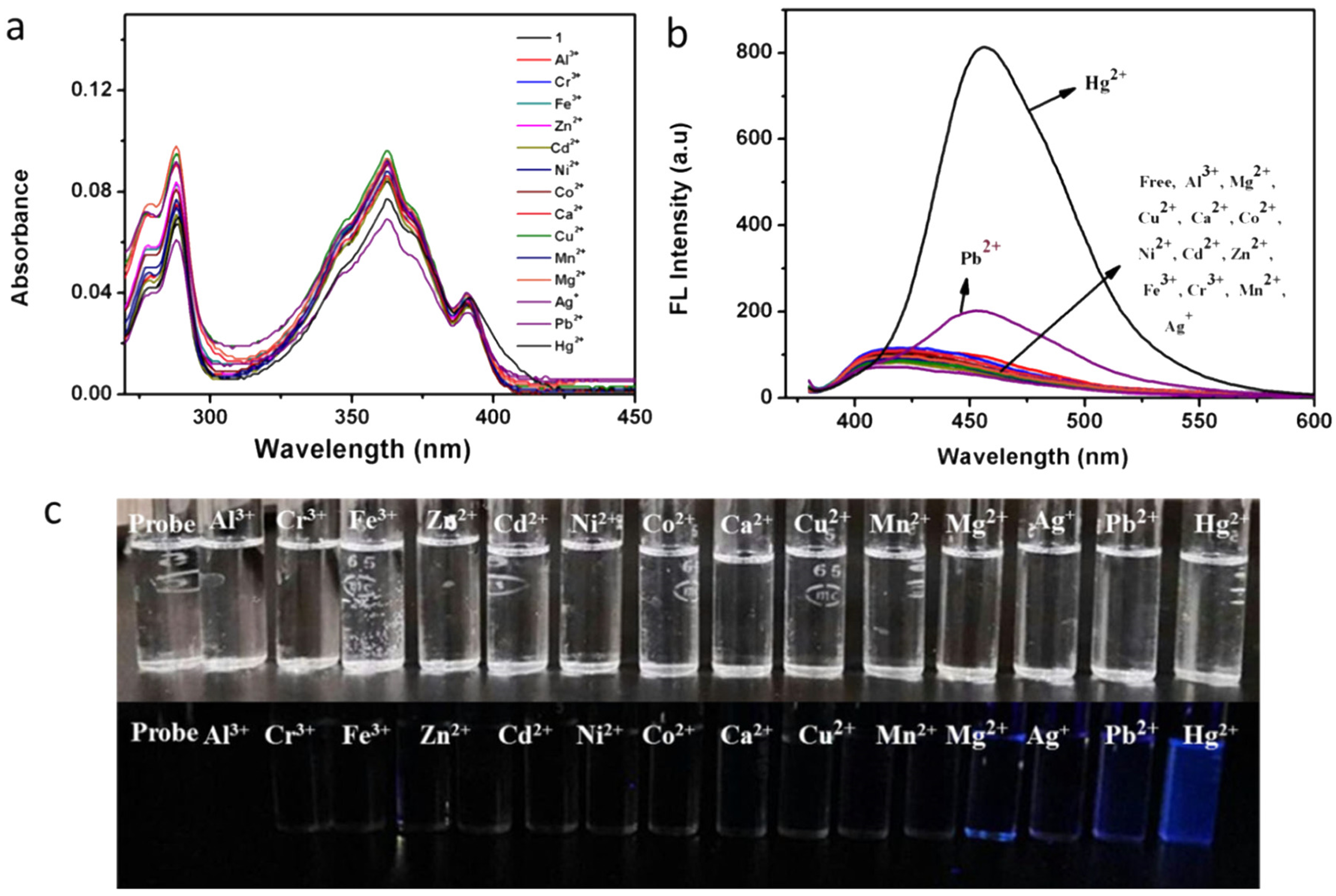
| 1 | H2O/DMF (98:2, v/v, 10 mM HEPES; pH 7.4) | 0–750 nM | 57.2 nM | 2:1 (1-Hg2+) excimer formation | NA | [61] |
| 2 | Ethanol; NA | NA | 45 ± 5 nM | 2:1 (2-Hg2+) excimer formation | NA | [62] |
| 3 and 4 | H2O–DMSO, 95:5, v/v, 10 mM HEPES; pH 7.4 | 0–500 nM (for both) | 22.2 nM (3) and 44 nM (4) | 2:1 (3-Hg2+ and 4-Hg2+) excimer formation | NA | [63] |
| 5 | DMSO/H2O (v/v = 7/3); pH 7.0 | 0–60 µM | 2.82 µM | 2:1 (5-Hg2+) excimer formation and PET | Cellular Imaging | [64] |
| 6 | H2O/DMF (v/v = 2:3, PBS buffer); pH 7.0 | 0–20 µM | 0.42 µM | 2:1 (6-Hg2+) excimer formation and PET | Real water and cellular Imaging | [65] |
| 7 | HEPES-CH3CN (80:20, v/v); pH 7.2 | NA | 0.2 µM | 1:1 (7-Hg2+) excimer formation | NA | [66] |
| 8 | CHCl3/CH3CN (1:1, v/v); NA | NA | 5 µM | FRET “On” | NA | [67] |
| 9 | DMSO/HEPES buffer (20Mm; 9:1 v/v); pH 7.4 | 0–1 µM | 0.74 µM | 1:1 (9-Hg2+) excimer formation | Cellular, C-elegans, and Zebra imaging | [68] |
| 10 | CH3CN/DMSO (99:1); NA | 0–1 µM | 8.11 nM | 1:1 (10-Hg2+) excimer formation | Logic gate applications | [69] |
| 11 | H2O/EtOH (1:1, v/v) buffered with HEPES; pH = 7.05 | 0–100 µM | 4.5 nM | 1:1 (11-Hg2+) excimer formation | Test Strip Method | [70] |
| 12, 13, and 14 | CH3CN; NA | 0–50 µM, 0–25 µM, and 0–20 µM, respectively | 0.223 µM, 0.114 µM, and 0.050 µM, respectively | 1:1, 1:2, and 1:3 (12-Hg2+, 13-Hg2+, and 14-Hg2+) excimer formation and “CHEF” | Cellular Imaging | [71] |
| 15 | H2O-CH3CN (10:90, v/v; pH 4.8 | NA | 7.9 µM | “CHEF” | NA | [72] |
| 16 | CH3CN; NA | NA | 3.98 µM | “CHEF” | NA | [73] |
| 17 | H2O–CH3CN (30:70, v/v); NA | 0.1–10 µM | 22 nM | “CHEF” | Cellular imaging | [74] |
| 18 | H2O:DMSO (1:2 v/v); pH:8.0 | 0.008–38 μM | 8.32 nM | “CHEF” | Test paper, food, and environmental samples | [75] |
| 19 | HEPES buffer (10 mM)/CH3CN (30:70, v/v); pH = 7.4 | 0–21 µM | 36 nM | “CHEF” | Test strips and silica gel plate | [76] |
| 20 | THF:H2O (99:1, v/v); NA | NA | 1.31 µM | Excimer, “CHEF”, and ICT | Test strips | [77] |
| 21 | THF; NA | NA | 12 µM | “CHEF” | NA | [78] |
| 22 | CH3CN; NA | NA | NA | FRET and Chemodosimeter | NA | [79] |
| 23 and 24 | Ethanol/H2O (1:1, v/v); pH 7.0 | 0–300 µM and 0 –4 µM | 19.1 µM and 0.43 µM | FRET and Chemodosimeter | Water samples | [80] |
| 25 | CH3CN–CH2Cl2 (1:1, v/v); NA | NA | NA | Chemodosimeter | NA | [81] |
| 26 | H2O-CH3CN (v/v = 50/50); NA | 0–30 µM | 55 nM | Chemodosimeter | Cellular and Zebra imaging | [82] |
| 27 and 28 | Ethanol/PBS buffer (2:1, v/v); pH 7.4 | 0–3.5 µM and 0–12 µM | 1.49 nM and 1.03 nM | Chemodosimeter and ICT | Cellular imaging and real water samples | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shellaiah, M.; Sun, K.-W.; Anandan, K.; Murugan, A.; Venkatachalam, V.; Bhushan, M.; Sivakumar, M.; Manikandan, E.; Kaliaperumal, K.; Li, W.-T. Luminescent Pyrene-Derivatives for Hg2+ and Explosive Detection. Chemosensors 2025, 13, 145. https://doi.org/10.3390/chemosensors13040145
Shellaiah M, Sun K-W, Anandan K, Murugan A, Venkatachalam V, Bhushan M, Sivakumar M, Manikandan E, Kaliaperumal K, Li W-T. Luminescent Pyrene-Derivatives for Hg2+ and Explosive Detection. Chemosensors. 2025; 13(4):145. https://doi.org/10.3390/chemosensors13040145
Chicago/Turabian StyleShellaiah, Muthaiah, Kien-Wen Sun, K. Anandan, Arumugam Murugan, Vijayaraj Venkatachalam, Mayank Bhushan, Mani Sivakumar, E. Manikandan, Kumaravel Kaliaperumal, and Wen-Tai Li. 2025. "Luminescent Pyrene-Derivatives for Hg2+ and Explosive Detection" Chemosensors 13, no. 4: 145. https://doi.org/10.3390/chemosensors13040145
APA StyleShellaiah, M., Sun, K.-W., Anandan, K., Murugan, A., Venkatachalam, V., Bhushan, M., Sivakumar, M., Manikandan, E., Kaliaperumal, K., & Li, W.-T. (2025). Luminescent Pyrene-Derivatives for Hg2+ and Explosive Detection. Chemosensors, 13(4), 145. https://doi.org/10.3390/chemosensors13040145








