Innovative Microfluidic Technologies for Rapid Heavy Metal Ion Detection
Abstract
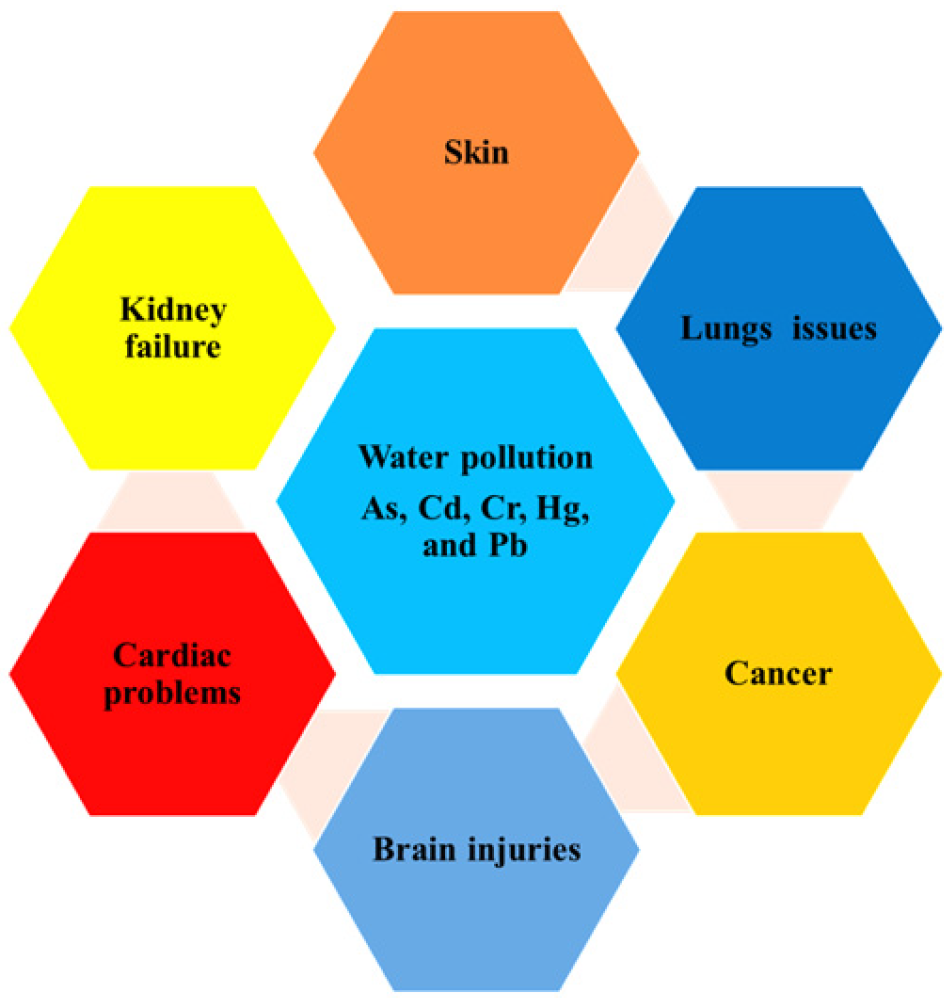
1. Introduction
2. Microfluidic Technologies
2.1. Introduction to Microfluidics
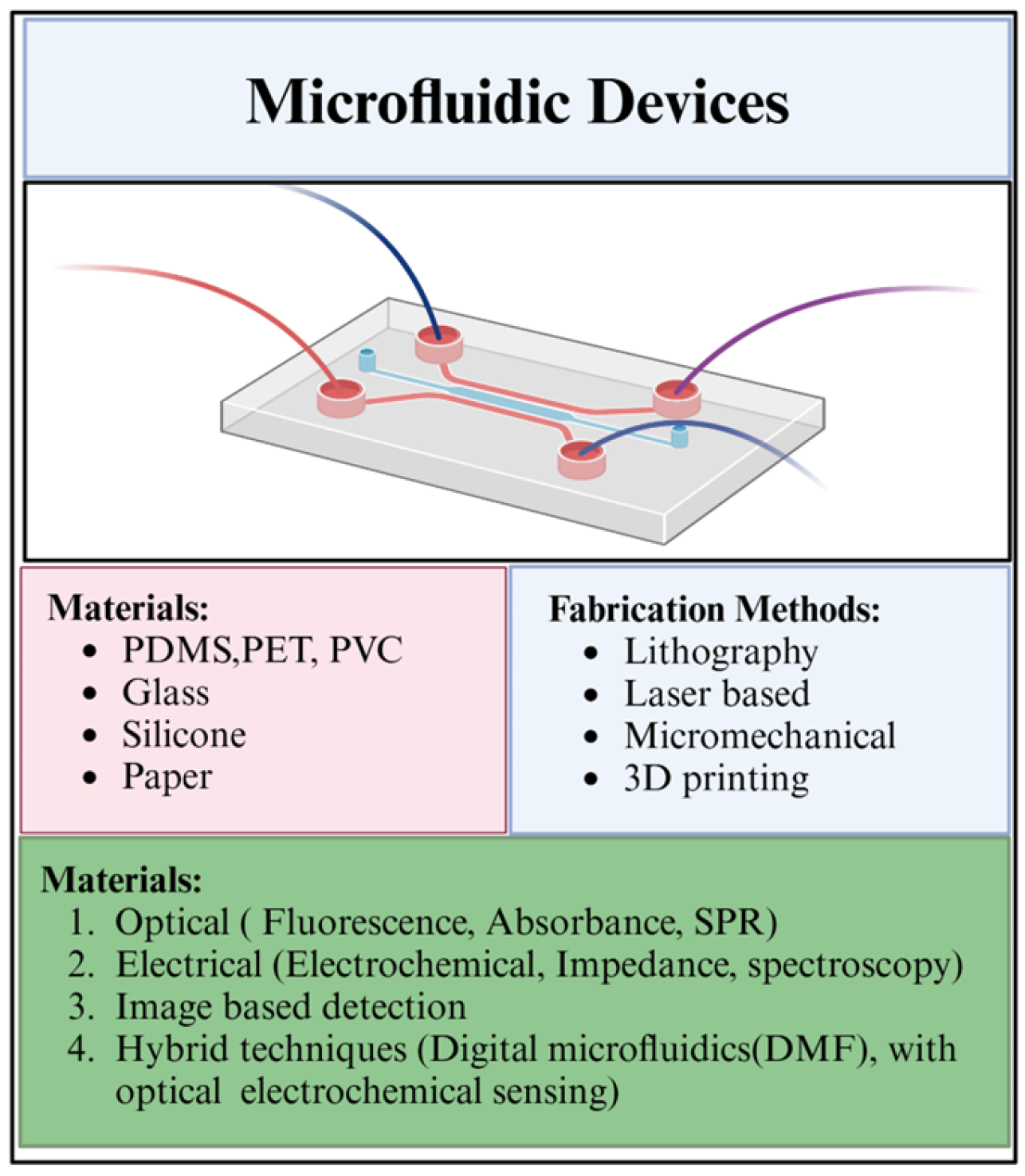
2.2. Materials and Fabrication
2.3. Advancements in Materials and Sensors
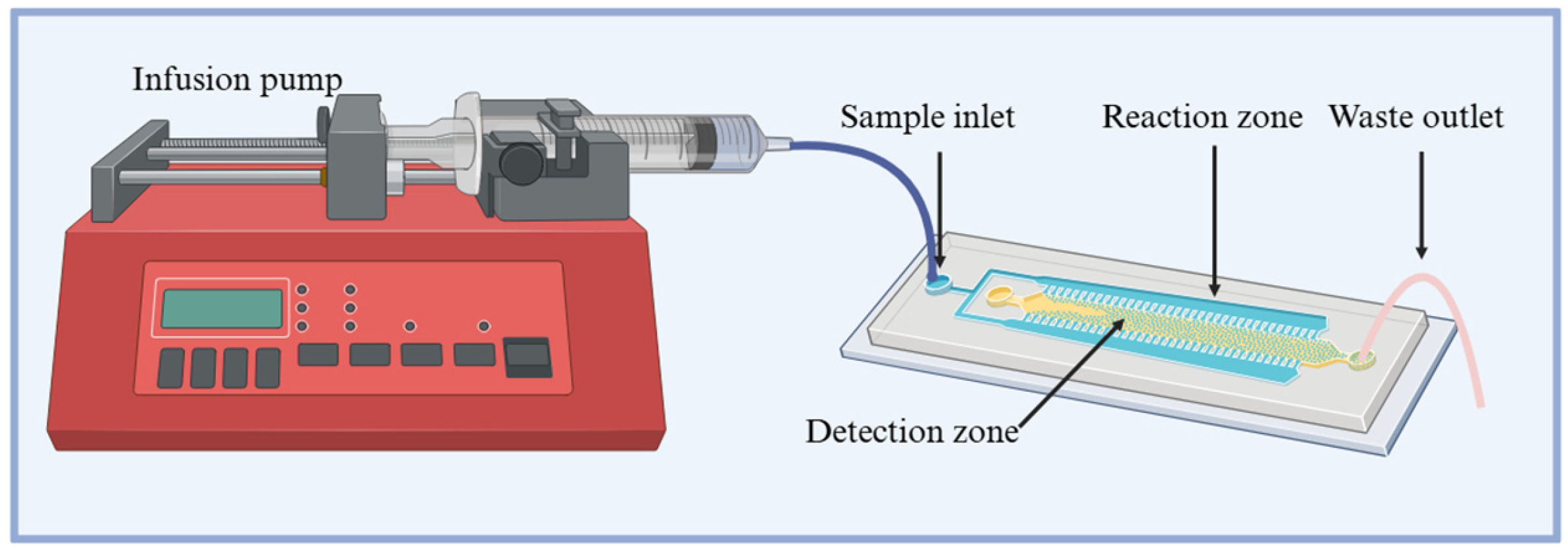
2.4. Detection Methods
2.5. Sample Preparation and Digestion for Non-Labile Metal Detection
2.6. Applications and Future Directions
3. Innovation in Microfluidics Devices for HMI Detection
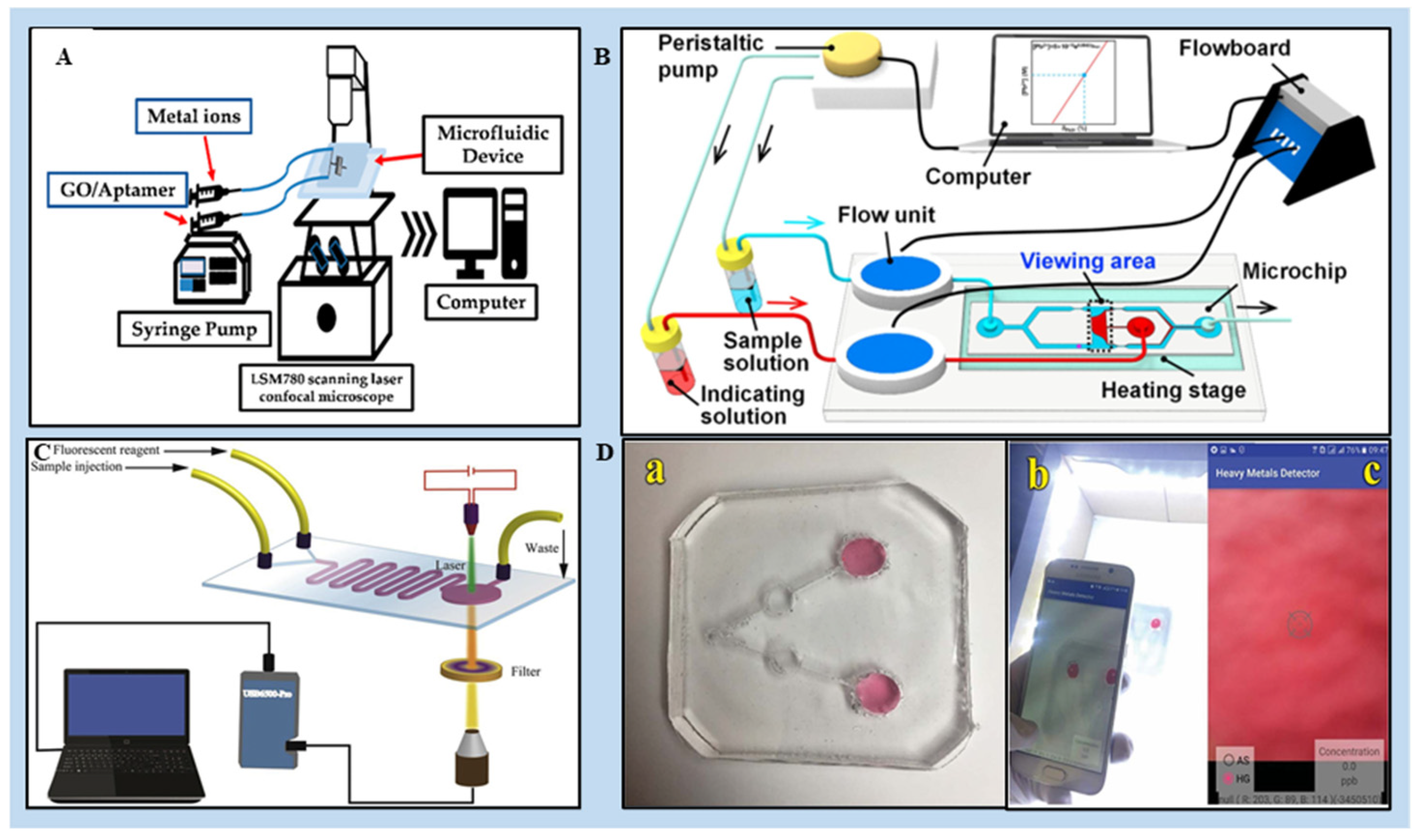
3.1. PDMS-Based LOCs for the Detection of HMIs
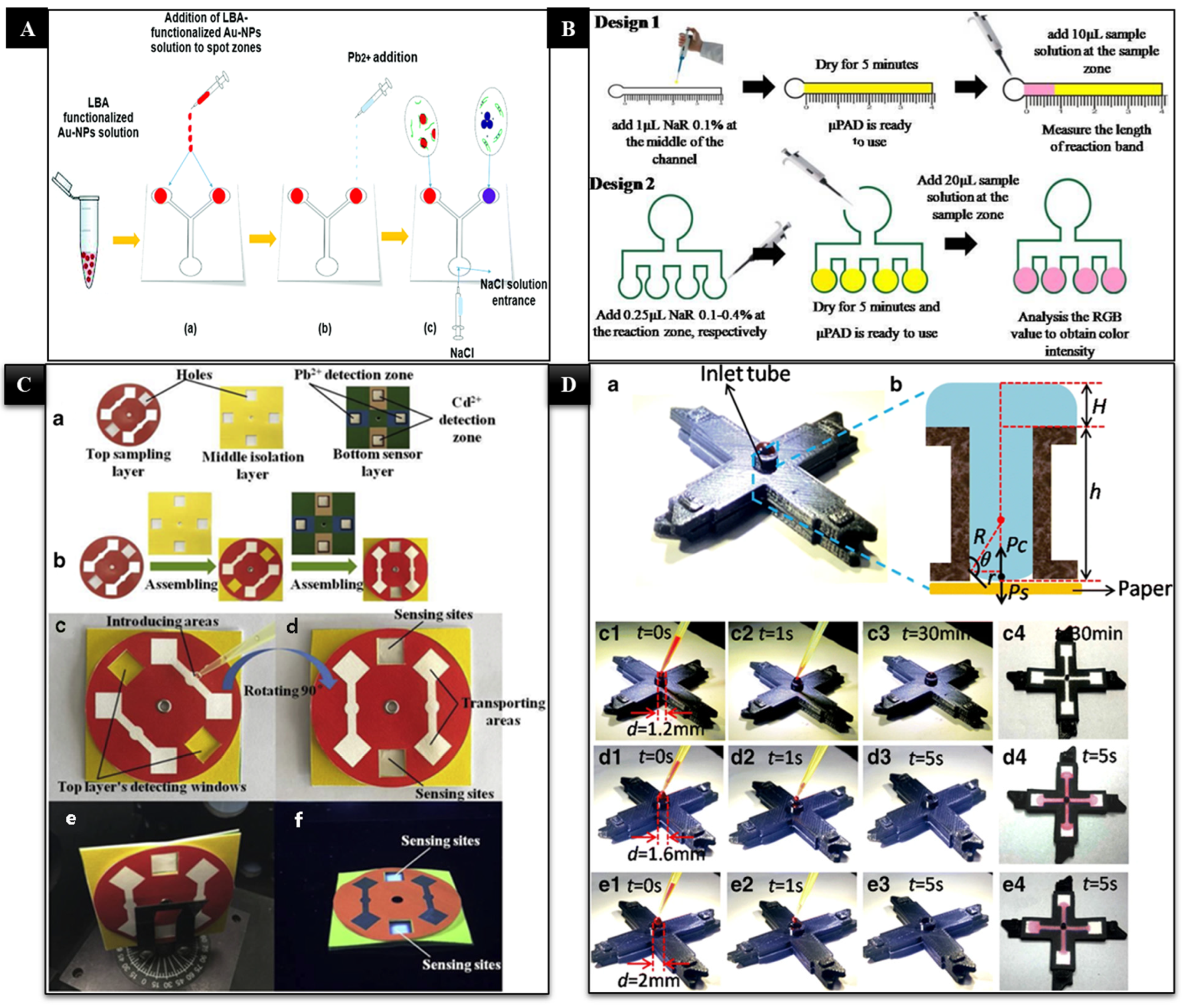
3.2. Paper-Based Microfluidics for the Detection of HMIs
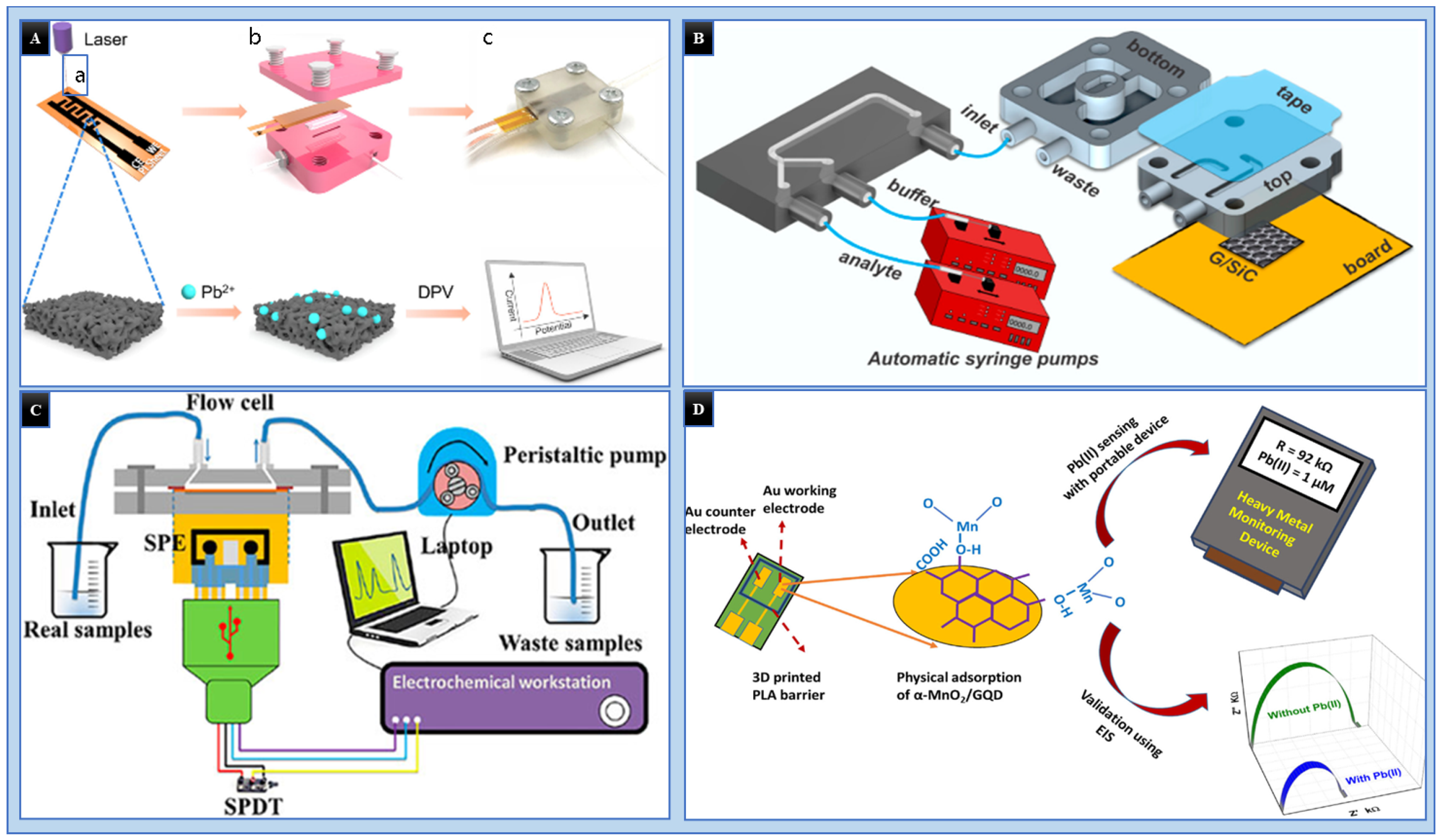
3.3. 3D Printed Microfluidics for the Detection of HMIs
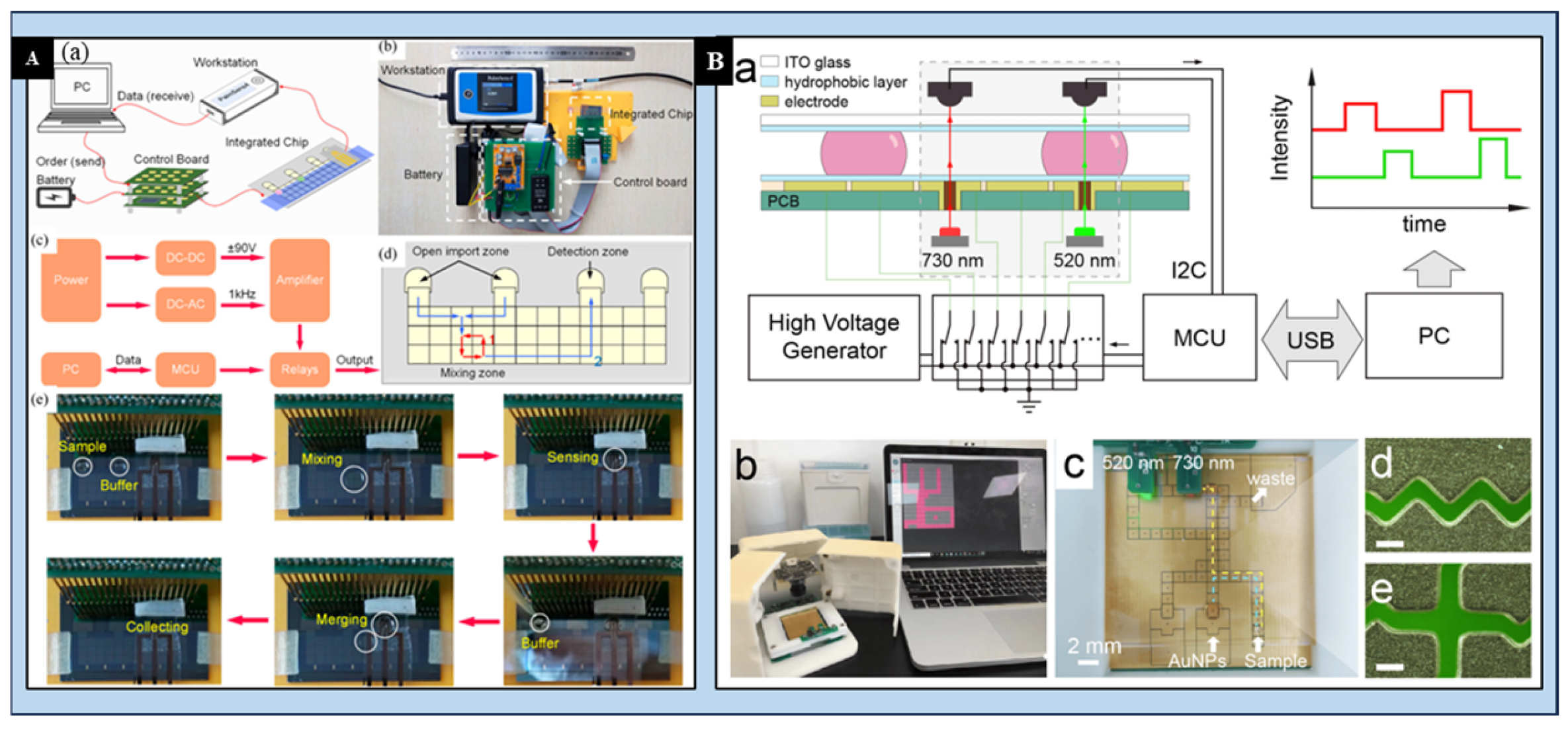
3.4. Digital Microfluidics (DMF) for the Detection of HMIs
4. Discussion on Challenges, Limitations, and Future Perspectives
4.1. Challenges and Limitations
4.2. Future Perspectives
4.2.1. AI Integration for Enhanced Data Analysis and Decision Making
- i.
- Machine Learning Algorithms (MLAs):
- ii.
- Real-Time Sensor Calibration:
- iii.
- Predictive Modeling and Early Warning Systems:
4.2.2. Advancing Field Testability for Robust and User-Friendly Devices
- i.
- Enhanced Robustness and Durability:
- ii.
- Integrated Sample Handling and Preprocessing:
- iii.
- Portable Power Solutions:
4.2.3. Other Promising Directions
- i.
- 3D Printing Advancements:
- ii.
- Paper-Based Microfluidics:
4.2.4. Commercialization Strategies
5. Conclusions
Funding
Conflicts of Interest
References
- Ali, H.; Khan, E. What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’–proposal of a comprehensive Definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Treatment of electroplating industry wastewater: A review on the various techniques. Environ. Sci. Pollut. Res. Int. 2022, 29, 72196–72246. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.M.; Komprdova, K.; Lorinczova, K.; Kuta, J.; Pribylova, P.; Scheringer, M.; Sebejova, L.; Piler, P.; Zvonar, M.; Klanova, J. Human biomonitoring of essential and toxic trace elements (heavy metals and metalloids) in urine of children, teenagers, and young adults from a Central European Cohort in the Czech Republic. J. Expo. Sci. Environ. Epidemiol. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef]
- Sarker, A.; Masud, M.A.A.; Deepo, D.M.; Das, K.; Nandi, R.; Ansary, M.W.R.; Islam, A.; Islam, T. Biological and green remediation of heavy metal contaminated water and soils: A state-of-the-art review. Chemosphere 2023, 332, 138861. [Google Scholar] [CrossRef]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of Heavy Metals and Recent Advances in Their Removal: A Review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Harpprecht, C.; Miranda Xicotencatl, B.; van Nielen, S.; van der Meide, M.; Li, C.; Li, Z.; Tukker, A.; Steubing, B. Future environmental impacts of metals: A systematic review of impact trends, modelling approaches, and challenges. Resour. Conserv. Recycl. 2024, 205, 107572. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Chen, B.; Dong, S. Mercury Contamination in Fish and Its Effects on the Health of Pregnant Women and Their Fetuses, and Guidance for Fish Consumption-A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 15929. [Google Scholar] [CrossRef]
- Kothapalli, C.R. Differential impact of heavy metals on neurotoxicity during development and in aging central nervous system. Curr. Opin. Toxicol. 2021, 26, 33–38. [Google Scholar] [CrossRef]
- Wu, B.; Hou, S.; Peng, D.; Wang, Y.; Wang, C.; Xu, F.; Xu, H. Response of soil micro-ecology to different levels of cadmium in alkaline soil. Ecotoxicol. Environ. Saf. 2018, 166, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Verzelloni, P.; Urbano, T.; Wise, L.A.; Vinceti, M.; Filippini, T. Cadmium exposure and cardiovascular disease risk: A systematic review and dose-response meta-analysis. Environ. Pollut. 2024, 345, 123462. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zheng, W. Cadmium Exposure: Mechanisms and Pathways of Toxicity and Implications for Human Health. Toxics 2024, 12, 388. [Google Scholar] [CrossRef]
- Monteiro De Oliveira, E.C.; Caixeta, E.S.; Santos, V.S.V.; Pereira, B.B. Arsenic exposure from groundwater: Environmental contamination, human health effects, and sustainable solutions. J. Toxicol. Environ. Health B Crit. Rev. 2021, 24, 119–135. [Google Scholar] [CrossRef]
- Wadgaonkar, P.; Wang, Z.; Chen, F. Endoplasmic reticulum stress responses and epigenetic alterations in arsenic carcinogenesis. Environ. Pollut. 2024, 347, 123565. [Google Scholar] [CrossRef]
- EU 20202184; European Union Standards for Drinking Water Quality Are Outlined in Directive EU 20202184. European Union: Brussels, Belgium, 2020.
- GB-5749-2006; National Standard of the People’s Republic of China, GB-5749-2006-Standards-for-Drinking-Water-Quality. National Health Commission of the People’s Republic of China: Beijing, China, 2006.
- EPA–822-R-02–047; National Recommended Water Quality Criteria. Office of Water, Office of Science and Technology, US EPA: Washington, DC, USA, 2002.
- WHO. Guidelines for Drinking Water Quality, 4th ed.; WHO Press: Geneva, Switzerland, 2011. [Google Scholar]
- Arjomandi, M.; Shirkhanloo, H. A review: Analytical methods for heavy metals determination in environment and human samples. Anal. Methods Environ. Chem. J. 2019, 2, 97–126. [Google Scholar] [CrossRef]
- Sulthana, S.F.; Iqbal, U.M.; Suseela, S.B.; Anbazhagan, R.; Chinthaginjala, R.; Chitathuru, D.; Ahmad, I.; Kim, T.H. Electrochemical Sensors for Heavy Metal Ion Detection in Aqueous Medium: A Systematic Review. ACS Omega 2024, 9, 25493–25512. [Google Scholar] [CrossRef]
- William, T.; Jerry Winberry, J. Compendium of Methods for Determination of Inorganic Compounds in Ambient Air; Center for Environmental Research Information Office of Research and Development U.S. Environmental Protection Agency Cincinnati: Cincinnati, OH, USA, 1999; pp. 3–31. [Google Scholar]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Gong, T.; Liu, J.; Liu, X.; Liu, J.; Xiang, J.; Wu, Y. A sensitive and selective sensing platform based on CdTe QDs in the presence of l-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chem. 2016, 213, 306–312. [Google Scholar] [CrossRef]
- Lebedev, A.; Sinikova, N.; Nikolaeva, S.; Poliakova, O.; Khrushcheva, M.; Pozdnyakov, S. Metals and organic pollutants in snow surrounding an iron factory. Environ. Chem. Lett. 2003, 1, 107–112. [Google Scholar] [CrossRef]
- Xia, L.; Zhu, D.; Wei, Y. Affirmation of the Method that Determination of Water Heavy Metals by Flame Atomic Absorption Spectrophotometry. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 052086. [Google Scholar] [CrossRef]
- Wang, M.; Lai, J.; Ni, Y.; Zhou, W. A microwave digestion method for determination of heavy metals in shallow lake sediments by graphite furnace atomic absorption spectrometry. Chin. J. Anal. Lab. 2012, 31, 51–54. [Google Scholar]
- Sitko, R.; Janik, P.; Zawisza, B.; Talik, E.; Margui, E.; Queralt, I. Green approach for ultratrace determination of divalent metal ions and arsenic species using total-reflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent. Anal. Chem. 2015, 87, 3535–3542. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Sun, Y.-Z.; Zhang, C.; Yan, J.; Zhao, H.-L.-B. Simultaneous determination of arsenic, lead and cadmium in cosmetics by ICP-OES. China Surfactant Deterg. Cosmet. 2017, 47, 598–602. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Chen, B.; He, M.; Hu, B. Chip-based array magnetic solid phase microextraction on-line coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in cells. Analyst 2015, 140, 5619–5626. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, S.K.; Choi, I.; Lee, J.; Yi, J. Molecular Level Detection of Heavy Metal Ions Using Atomic Force Microscope. Clean. Technol. 2005, 11, 69–74. [Google Scholar]
- Evans, E.H.; Pisonero, J.; Smith, C.M.M.; Taylor, R.N. Atomic spectrometry update: Review of advances in atomic spectrometry and related techniques. J. Anal. At. Spectrom. 2021, 36, 868–891. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Walgama, C.; Fernand Narcisse, V.E.; Grant, C. Electrochemical and Colorimetric Nanosensors for Detection of Heavy Metal Ions: A Review. Sensors 2023, 23, 9080. [Google Scholar] [CrossRef]
- Hutton, L.A.; O’Neil, G.D.; Read, T.L.; Ayres, Z.J.; Newton, M.E.; Macpherson, J.V. Electrochemical X-ray fluorescence spectroscopy for trace heavy metal analysis: Enhancing X-ray fluorescence detection capabilities by four orders of magnitude. Anal. Chem. 2014, 86, 4566–4572. [Google Scholar] [CrossRef]
- Shirsat, M.D.; Hianik, T. Electrochemical Detection of Heavy Metal Ions Based on Nanocomposite Materials. J. Compos. Sci. 2023, 7, 473. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Huang, N.M.; Zainal, Z.; Ahmad, S.A.A. Significance of nanomaterials in electrochemical sensors for nitrate detection: A review. Trends Environ. Anal. Chem. 2021, 31, e00135. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, H.; Huang, Y.; Zhao, R. Recent Advances in AIEgens for Metal Ion Biosensing and Bioimaging. Molecules 2019, 24, 4593. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Farooq, N.; Singh, N.; Hashmi, A.A. Recent developments in Schiff base centered optical and chemical sensors for metal ion recognition. J. Mol. Liq. 2024, 401, 124678. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Li, X.; Gao, H.; Yang, L.; Jiang, C. Design of microfluidic fluorescent sensor arrays for real-time and synchronously visualized detection of multi-component heavy metal ions. Chem. Eng. J. 2024, 493, 152636. [Google Scholar] [CrossRef]
- Wang, G.; Chu, L.T.; Hartanto, H.; Utomo, W.B.; Pravasta, R.A.; Chen, T.H. Microfluidic Particle Dam for Visual and Quantitative Detection of Lead Ions. ACS Sens. 2020, 5, 19–23. [Google Scholar] [CrossRef]
- Zeng, S.; Zhu, H.; Sohan, A.; Liu, J.; Wan, X.; Lin, X.; Yin, B. A remote-controlled portable workstation for highly sensitive and real-time chemiluminescent detection of cadmium. Food Chem. 2024, 452, 139549. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Cui, W.; Ren, Z.; Song, Y.; Ren, C.L. Development and potential for point-of-care heavy metal sensing using microfluidic systems: A brief review. Sens. Actuators A Phys. 2022, 344, 113733. [Google Scholar] [CrossRef]
- Sekhwama, M.; Mpofu, K.; Sudesh, S.; Mthunzi-Kufa, P. Integration of microfluidic chips with biosensors. Discov. Appl. Sci. 2024, 6, 458. [Google Scholar] [CrossRef]
- Cheng, Z.; Gu, Y.; Li, S.; Wang, Y.; Chen, H.; Cheng, J.; Liu, P. Enclosed casting of epoxy resin for rapid fabrication of rigid microfluidic chips. Sens. Actuators B Chem. 2017, 252, 785–793. [Google Scholar] [CrossRef]
- Mitrogiannopoulou, A.M.; Tselepi, V.; Ellinas, K. Polymeric and Paper-Based Lab-on-a-Chip Devices in Food Safety: A Review. Micromachines 2023, 14, 986. [Google Scholar] [CrossRef] [PubMed]
- Raffy, S.; Palleau, E.; Calvignac, B.; Brotons, G.; Lefebvre, G.; Rolley, N.; Teychene, S.; Viguier, B.; Cerezo, S.C.; Truan, G.; et al. “All in One” Epoxy-Based Microfluidic Chips at Your Fingertips. ACS Appl. Polym. Mater. 2021, 3, 801–810. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef]
- Filippidou, M.K.; Chatzandroulis, S. Microfluidic Devices for Heavy Metal Ions Detection: A Review. Micromachines 2023, 14, 1520. [Google Scholar] [CrossRef]
- Amini, Y.; Hassanvand, A.; Ghazanfari, V.; Shadman, M.M.; Heydari, M.; Alborzi, Z.S. Optimization of liquid-liquid extraction of calcium with a serpentine microfluidic device. Int. Commun. Heat. Mass. Transf. 2023, 140, 106551. [Google Scholar] [CrossRef]
- Marsousi, S.; Karimi-Sabet, J.; Moosavian, M.A.; Amini, Y. Liquid-liquid extraction of calcium using ionic liquids in spiral microfluidics. Chem. Eng. J. 2019, 356, 492–505. [Google Scholar] [CrossRef]
- Ding, R.; Cheong, Y.H.; Ahamed, A.; Lisak, G. Heavy Metals Detection with Paper-Based Electrochemical Sensors. Anal. Chem. 2021, 93, 1880–1888. [Google Scholar] [CrossRef]
- Xing, G.; Lin, J.M. Microfluidics for foodborne bacteria analysis: Moving toward multiple technologies integration. Biomicrofluidics 2024, 18, 061301. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Li, X.; Li, M.; Wu, Q.; Song, Y.; Zhu, Z.; Yang, C. Integrated microfluidic devices for in vitro diagnostics at point of care. Aggregate 2022, 3, e184. [Google Scholar] [CrossRef]
- Nigam, A.; Sharma, N.; Tripathy, S.; Kumar, M. Development of semiconductor based heavy metal ion sensors for water analysis: A review. Sens. Actuators A Phys. 2021, 330, 112879. [Google Scholar] [CrossRef]
- Buledi, J.A.; Amin, S.; Haider, S.I.; Bhanger, M.I.; Solangi, A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ. Sci. Pollut. Res. Int. 2021, 28, 58994–59002. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zeinu, K.M.; Gao, S.; Liu, B.; Yang, J.; Hu, J. Recent Advances and Perspective on Design and Synthesis of Electrode Materials for Electrochemical Sensing of Heavy Metals. Energy Environ. Mater. 2018, 1, 113–131. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Numan, A.; Gill, A.A.S.; Rafique, S.; Guduri, M.; Zhan, Y.; Maddiboyina, B.; Li, L.; Singh, S.; Nguyen Dang, N. Rationally engineered nanosensors: A novel strategy for the detection of heavy metal ions in the environment. J. Hazard. Mater. 2021, 409, 124493. [Google Scholar] [CrossRef]
- Chauhan, S.; Dahiya, D.; Sharma, V.; Khan, N.; Chaurasia, D.; Nadda, A.K.; Varjani, S.; Pandey, A.; Bhargava, P.C. Advances from conventional to real time detection of heavy metal(loid)s for water monitoring: An overview of biosensing applications. Chemosphere 2022, 307, 136124. [Google Scholar] [CrossRef]
- Tseng, H.Y.; Lizama, J.H.; Alvarado, N.A.S.; Hou, H.H. Lab-on-PCB: One step away from the accomplishment of muTAS? Biomicrofluidics 2022, 16, 031302. [Google Scholar] [CrossRef]
- Lace, A.; Cleary, J. A Review of Microfluidic Detection Strategies for Heavy Metals in Water. Chemosensors 2021, 9, 60. [Google Scholar] [CrossRef]
- Rai, P.K.; Islam, M.; Gupta, A. Microfluidic devices for the detection of contamination in water samples: A review. Sens. Actuators A Phys. 2022, 347, 113926. [Google Scholar] [CrossRef]
- Alhalaili, B.; Popescu, I.N.; Rusanescu, C.O.; Vidu, R. Microfluidic Devices and Microfluidics-Integrated Electrochemical and Optical (Bio)Sensors for Pollution Analysis: A Review. Sustainability 2022, 14, 12844. [Google Scholar] [CrossRef]
- Eddaif, L.; Shaban, A.; Telegdi, J. Sensitive detection of heavy metals ions based on the calixarene derivatives-modified piezoelectric resonators: A review. Int. J. Environ. Anal. Chem. 2019, 99, 824–853. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Wang, S.; Liu, Q.; Feng, H.; Ma, X.; Guo, J. Electrochemical microfluidics techniques for heavy metal ion detection. Analyst 2018, 143, 4230–4246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Han, X. Screen printed electrode containing bismuth for the detection of cadmium ion. J. Electroanal. Chem. 2023, 933, 117291. [Google Scholar] [CrossRef]
- Choudhari, U.; Jagtap, S.; Ramgir, N.; Debnath, A.K.; Muthe, K.P. Screen-printed electrochemical sensors for environmental monitoring of heavy metal ion detection. Rev. Chem. Eng. 2023, 39, 1227–1268. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Teresa Gutierrez-Wing, M.; Choi, J.-W. Review—Recent Progress in Portable Fluorescence Sensors. J. Electrochem. Soc. 2021, 168, 017502. [Google Scholar] [CrossRef]
- Lin, Y.; Gritsenko, D.; Feng, S.; Teh, Y.C.; Lu, X.; Xu, J. Detection of heavy metal by paper-based microfluidics. Biosens. Bioelectron. 2016, 83, 256–266. [Google Scholar] [CrossRef]
- Xu, G.; Song, P.; Xia, L. Examples in the detection of heavy metal ions based on surface-enhanced Raman scattering spectroscopy. Nanophotonics 2021, 10, 4419–4445. [Google Scholar] [CrossRef]
- Liu, T.; Li, S.; Deng, J.; Wang, G.; Liu, Y. Design and study of microfluidic differential phase surface plasmon resonance sensor. J. Phys. Conf. Ser. 2022, 2206, 012026. [Google Scholar] [CrossRef]
- Zhong, Y.; Ji, M.; Hu, Y.; Li, G.; Xiao, X. Progress of environmental sample preparation for elemental analysis. J. Chromatogr. A 2022, 1681, 463458. [Google Scholar] [CrossRef]
- Nadumane, S.S.; Biswas, R.; Mazumder, N. Integrated microfluidic platforms for heavy metal sensing: A comprehensive review. Anal. Methods 2024, 16, 2810–2823. [Google Scholar] [CrossRef]
- Kwon, S.-Y.; Kim, Y.-I.; Kim, Y.-K.; Lee, Y.-B.; Mok, J.H. Microwave-assisted sample preparation for screening of heavy metal elements in food additives by ICP-MS. Lwt 2024, 208, 116708. [Google Scholar] [CrossRef]
- Huang, W.H.; Mai, V.P.; Wu, R.Y.; Yeh, K.L.; Yang, R.J. A Microfluidic Aptamer-Based Sensor for Detection of Mercury(II) and Lead(II) Ions in Water. Micromachines 2021, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhao, N.; Lin, S.; Wang, W.; Zhang, M.-J.; Su, Y.-Y.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Smart microfluidic analogue of Wheatstone-bridge for real-time continuous detection with ultrasensitivity and wide dynamic range. Chem. Eng. J. 2021, 407, 127138. [Google Scholar] [CrossRef]
- Peng, G.; Chen, Y.; Deng, R.; He, Q.; Liu, D.; Lu, Y.; Lin, J.M. Highly sensitive and selective determination of Hg(II) based on microfluidic chip with on-line fluorescent derivatization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 1–6. [Google Scholar] [CrossRef]
- Motalebizadeh, A.; Bagheri, H.; Asiaei, S.; Fekrat, N.; Afkhami, A. New portable smartphone-based PDMS microfluidic kit for the simultaneous colorimetric detection of arsenic and mercury. RSC Adv. 2018, 8, 27091–27100. [Google Scholar] [CrossRef]
- Mishra, N.; Dhwaj, A.; Verma, D.; Prabhakar, A. Cost-effective microabsorbance detection based nanoparticle immobilized microfluidic system for potential investigation of diverse chemical contaminants present in drinking water. Anal. Chim. Acta 2022, 1205, 339734. [Google Scholar] [CrossRef]
- Peng, G.; He, Q.; Lu, Y.; Huang, J.; Lin, J.-M. Flow injection microfluidic device with on-line fluorescent derivatization for the determination of Cr(III) and Cr(VI) in water samples after solid phase extraction. Anal. Chim. Acta 2017, 955, 58–66. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, G.; Bi, J.; Bao, K.; Wang, P. In-situ and ultrasensitive detection of mercury (II) ions (Hg2+) using the localized surface plasmon resonance (LSPR) nanosensor and the microfluidic chip. Sens. Actuators A Phys. 2023, 349, 114074. [Google Scholar] [CrossRef]
- Mohan, J.M.; Dudala, S.; Amreen, K.; Javed, A.; Dubey, S.K.; Goel, S. Microfluidic Device Integrated With PDMS Microchannel and Unmodified ITO Glass Electrodes for Highly Sensitive, Specific, and Point-of-Care Detection of Copper and Mercury. IEEE Trans. Nanobiosci. 2023, 22, 881–888. [Google Scholar] [CrossRef]
- Fakhri, N.; Hosseini, M.; Tavakoli, O. Aptamer-based colorimetric determination of Pb2+ using a paper-based microfluidic platform. Anal. Methods 2018, 10, 4438–4444. [Google Scholar] [CrossRef]
- Wisang, Y.F. Microfluidic Paper-based Analytical Devices (µPADs) For Analysis Lead using Naked Eye and Colorimetric Detections. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 032033. [Google Scholar] [CrossRef]
- Zhou, J.; Li, B.; Qi, A.; Shi, Y.; Qi, J.; Xu, H.; Chen, L. ZnSe quantum dot based ion imprinting technology for fluorescence detecting cadmium and lead ions on a three-dimensional rotary paper-based microfluidic chip. Sens. Actuators B Chem. 2020, 305, 127462. [Google Scholar] [CrossRef]
- Wang, M.; Song, Z.; Jiang, Y.; Zhang, X.; Wang, L.; Zhao, H.; Cui, Y.; Gu, F.; Wang, Y.; Zheng, G. A three-dimensional pinwheel-shaped paper-based microfluidic analytical device for fluorescence detection of multiple heavy metals in coastal waters by rational device design. Anal. Bioanal. Chem. 2021, 413, 3299–3313. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, B.; Wang, J.; Qi, J.; Li, J.; Ma, J.; Chen, L. A rotary multi-positioned cloth/paper hybrid microfluidic device for simultaneous fluorescence sensing of mercury and lead ions by using ion imprinted technologies. J. Hazard. Mater. 2022, 428, 128165. [Google Scholar] [CrossRef]
- Dindorkar, G.; Rathee, V.; Balpande, S.; Kalambe, J. Detection of Mercury in Water using Filter Paper Based Channel and Colorimetric-Android Readout. Int. J. Eng. Adv. Technol. 2019, 9, 434–438. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Hasanzadeh, M.; Pashazadeh-Panahi, P.; Seidi, F. Application of Cys A@AuNPs supported amino acids towards rapid and selective identification of Hg(II) and Cu(II) ions in aqueous solution: An innovative microfluidic paper-based (μPADs) colorimetric sensing platform. J. Mol. Liq. 2021, 338, 117020. [Google Scholar] [CrossRef]
- Jin, B.; Li, Z.; Zhao, G.; Ji, J.; Chen, J.; Yang, Y.; Xu, R. Upconversion fluorescence-based paper disc for multiplex point-of-care testing in water quality monitoring. Anal. Chim. Acta 2022, 1192, 339388. [Google Scholar] [CrossRef]
- Sun, X.; Li, B.; Qi, A.; Tian, C.; Han, J.; Shi, Y.; Lin, B.; Chen, L. Improved assessment of accuracy and performance using a rotational paper-based device for multiplexed detection of heavy metals. Talanta 2018, 178, 426–431. [Google Scholar] [CrossRef]
- Shang, Q.; Zhang, P.; Li, H.; Liu, R.; Zhang, C. A flow chemiluminescence paper-based microfluidic device for detection of chromium (III) in water. J. Innov. Opt. Health Sci. 2019, 12, 1950016. [Google Scholar] [CrossRef]
- Li, F.; Hu, Y.; Li, Z.; Liu, J.; Guo, L.; He, J. Three-dimensional microfluidic paper-based device for multiplexed colorimetric detection of six metal ions combined with use of a smartphone. Anal. Bioanal. Chem. 2019, 411, 6497–6508. [Google Scholar] [CrossRef]
- Alahmad, W.; Tungkijanansin, N.; Kaneta, T.; Varanusupakul, P. A colorimetric paper-based analytical device coupled with hollow fiber membrane liquid phase microextraction (HF-LPME) for highly sensitive detection of hexavalent chromium in water samples. Talanta 2018, 190, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, A.; Hussen, A.; Kaneta, T. Speciation of chromium in water samples using microfluidic paper-based analytical devices with online oxidation of trivalent chromium. Anal. Bioanal. Chem. 2021, 413, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Zhang, Q.; Yang, C.; Yang, W.; Liu, J.; Li, C.; Tao, S. Laser-Induced Carbon Electrodes in a Three-Dimensionally Printed Flow Reactor for Detecting Lead Ions. ACS Omega 2021, 6, 12470–12479. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, M.F.; Shtepliuk, I.; Filippini, D.; Puglisi, D.; Vagin, M.; Yakimova, R.; Eriksson, J. Epitaxial Graphene Sensors Combined with 3D-Printed Microfluidic Chip for Heavy Metals Detection. Sensors 2019, 19, 2393. [Google Scholar] [CrossRef]
- Zhao, G.; Tran, T.T.; Modha, S.; Sedki, M.; Myung, N.V.; Jassby, D.; Mulchandani, A. Multiplexed Anodic Stripping Voltammetry Detection of Heavy Metals in Water Using Nanocomposites Modified Screen-Printed Electrodes Integrated with a 3D-Printed Flow Cell. Front. Chem. 2022, 10, 815805. [Google Scholar] [CrossRef]
- Gupta, A.K.; Khanna, M.; Roy, S.; Pankaj; Nagabooshanam, S.; Kumar, R.; Wadhwa, S.; Mathur, A. Design and development of a portable resistive sensor based on α-MnO2/GQD nanocomposites for trace quantification of Pb(II) in water. IET Nanobiotechnol. 2021, 15, 505–511. [Google Scholar] [CrossRef]
- Katseli, V.; Thomaidis, N.; Economou, A.; Kokkinos, C. Miniature 3D-printed integrated electrochemical cell for trace voltammetric Hg2+ determination. Sens. Actuators B. Chem. 2020, 308, 127715. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Pournara, A.; Manos, M.; Spanopoulos, I.; Kanatzidis, M.; Tziotzi, T.; Petkov, V.; Margariti, A.; Oikonomopoulos, P.; et al. 3D-printed lab-in-a-syringe voltammetric cell based on a working electrode modified with a highly efficient Ca-MOF sorbent for the determination of Hg(II). Sens. Actuators B Chem. 2020, 321, 128508. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, W.; Zhang, Q.; Zhang, K.; Liang, C.; Wang, D.; Liu, X.; Zhan, X. A portable microfluidic electrochemical sensing platform for rapid detection of hazardous metal Pb2+ based on thermocapillary convection using 3D Ag-rGO-f-Ni(OH)2/NF as a signal amplifying element. J. Hazard. Mater. 2023, 448, 130923. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Niu, P.; Shen, T.; Yuan, Y.; Bai, Y.; Wang, Z. On-site low-power sensing nodes for distributed monitoring of heavy metal ions in water. Nanotechnol. Precis. Eng. 2021, 4, 013005. [Google Scholar] [CrossRef]
- Gu, Z.; Luo, J.J.; Ding, L.W.; Yan, B.Y.; Zhou, J.L.; Wang, J.G.; Wang, H.F.; Kong, C. Colorimetric Sensing with Gold Nanoparticles on Electrowetting-Based Digital Microfluidics. Micromachines 2021, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Kumar, N.; Audibert, J.-F.; Ghasemi, R.; Lefevre, J.-P.; Ha-Thi, M.-H.; Mongin, C.; Leray, I. Water-soluble aluminium fluorescent sensor based on aggregation-induced emission enhancement. N. J. Chem. 2019, 43, 15302–15310. [Google Scholar] [CrossRef]
- Han, S.; Liu, X.; Wang, L.; Wang, Y.; Zheng, G. An efficient protocol to use feedback-controlling digital microfluidic fluorimetric sensor for detection of mercury (II) in coastal seawaters. MethodsX 2019, 6, 1443–1453. [Google Scholar] [CrossRef] [PubMed]

| Metals | EPA μg/L | EU μg/L | China μg/L | WHO μg/L | Refs. |
|---|---|---|---|---|---|
| Arsenic (As) | 10 | 10 | 10 | 10 | |
| Cadmium (Cd) | 5 | 5 | 5 | 3 | |
| Chromium (Cr) | 100 | 50 | 50 | 50 | [17,18,19,20] |
| Lead (Pb) | 15 | 10 | 10 | 10 | |
| Mercury (Hg) | 2 | 1 | 1 | 1 |
| HMI | LOD | Device Material | Detection Method | Ref. |
|---|---|---|---|---|
| Hg2+, Pb2+ | 0.70 ppb, 0.53 ppb | PDMS-GO | Fluorescence | [77] |
| Pb2+ | 10−14 M | PDMS | Electrochemical | [78] |
| Hg2+ | 0.031 μM | PDMS-Glass | Fluorescence | [79] |
| As3+, Hg2+ | 710–1278 μg L−1, 10.77–53.86 μg L−1 | PDMS | Colorimetry | [80] |
| Pb2+, Cr3+, Hg2+ | 0.5 ppb | PDMS-Glass | Absorbance | [81] |
| Cr3+ | 0.094 nM | PDMS | Fluorescence (LIF) | [82] |
| Hg2+ | 2.7 pM | PDMS | Absorption (LSPR) | [83] |
| Hg2+ | 3.19 μM | PDMS-ITO | Electrochemical | [84] |
| Pb2+ | 1.2 nM | Paper | Colorimetry | [85] |
| Pb2+ | 0.756 mgL−1 | Paper | Colorimetry | [86] |
| Cd2+, Pb2+ | 0.245 µg L−1, 0.335 µg L−1 | Paper | Fluorescence | [87] |
| Cd2+, Pb2+, Hg2+ | 0.007–0.015 µg L−1 | Paper | Fluorescence | [88] |
| Hg2+, Pb2+ | 0.18 µg L−1, 0.07 µg L−1 | Paper | Fluorescence | [89] |
| Hg2+ | 0.1 gL−1–0.001 mg L−1 | Paper | Colorimetry | [90] |
| Hg2+ | 0.001 ppm | Paper | Colorimetry | [91] |
| Hg2+, Pb2+ | 20 nM, 4 nM | Paper | Fluorescence | [92] |
| Cr6+ | 0.18 mg L−1 | Paper | Fluorescence | [93] |
| Cr3+ | 0.0245 mg L−1 | Paper | Chemiluminescence | [94] |
| Cr6+ | 0.1 mg L−1 | Paper | Colorimetry | [95] |
| Cr6+ | 3 µg L−1 | Paper | Colorimetry | [96] |
| Cr3+, Cr6+ | 0.008 mg L−1, 0.07 mg L−1 | Paper | Colorimetry | [97] |
| Pb2+ | 0.0330 mg L−1 | 3D Material | Electrochemical | [98] |
| Pb2+ | 95 nM | 3D Material | Electrochemical | [99] |
| As3+, Pb2+, Cd2+ | 2.4 µg L−1, 1.2 µg L−1, 0.8 µg L−1 | 3D Material | Electrochemical | [100] |
| Pb2+ | 0.81 nM | 3D Material | Electrochemical | [101] |
| Hg2+ | 0.52 µg L−1 | 3D Material | Electrochemical | [102] |
| Hg2+ | 0.6 μg L−1 | 3D Material | Electrochemical | [103] |
| Pb2+ | 0.00498 µg L−1 | 3D Material | Electrochemical | [104] |
| Pb2+ | 1 ppb | DMF | Electrochemical | [105] |
| Hg2+ | 2 ppb | DMF | Colorimetry | [106] |
| Al3+ | 4.1 ppb | DMF | Fluorescence | [107] |
| Hg2+ | 0.5 ppb | DMF | Fluorescence | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauf, M.F.; Lin, Z.; Rauf, M.K.; Lin, J.-M. Innovative Microfluidic Technologies for Rapid Heavy Metal Ion Detection. Chemosensors 2025, 13, 149. https://doi.org/10.3390/chemosensors13040149
Rauf MF, Lin Z, Rauf MK, Lin J-M. Innovative Microfluidic Technologies for Rapid Heavy Metal Ion Detection. Chemosensors. 2025; 13(4):149. https://doi.org/10.3390/chemosensors13040149
Chicago/Turabian StyleRauf, Muhammad Furqan, Zhenda Lin, Muhammad Kamran Rauf, and Jin-Ming Lin. 2025. "Innovative Microfluidic Technologies for Rapid Heavy Metal Ion Detection" Chemosensors 13, no. 4: 149. https://doi.org/10.3390/chemosensors13040149
APA StyleRauf, M. F., Lin, Z., Rauf, M. K., & Lin, J.-M. (2025). Innovative Microfluidic Technologies for Rapid Heavy Metal Ion Detection. Chemosensors, 13(4), 149. https://doi.org/10.3390/chemosensors13040149






