Characteristics of Peanut Protein-Derived Carbon Dots and Their Application in Cell Imaging and Sensing of Metronidazole
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemicals and Reagents (Materials)
2.2. Instrument
2.3. Quantum Yield (QY) Calculation
2.4. Preparation of Peanut Protein
2.5. Preparation of Peanut Protein Carbon Dots
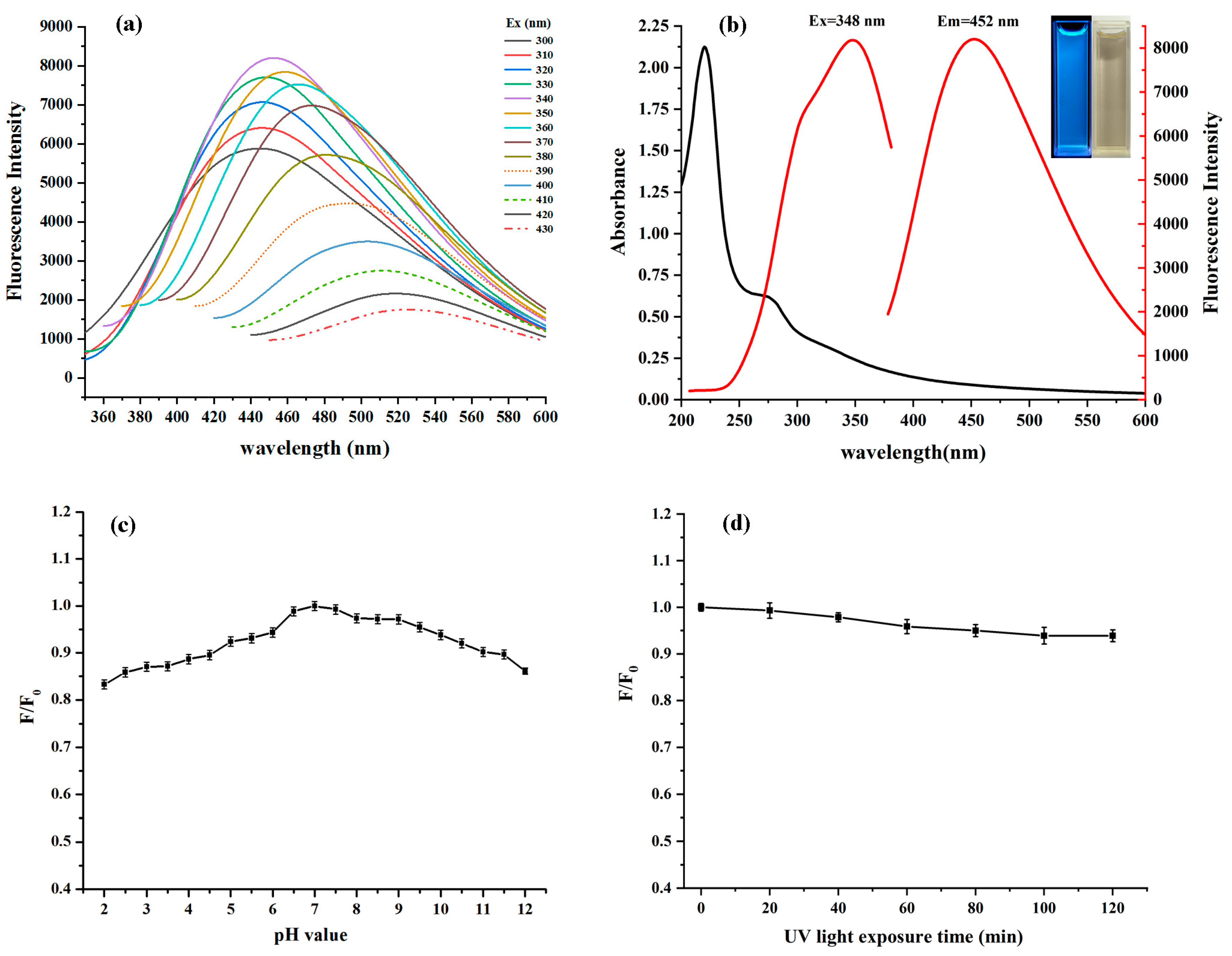
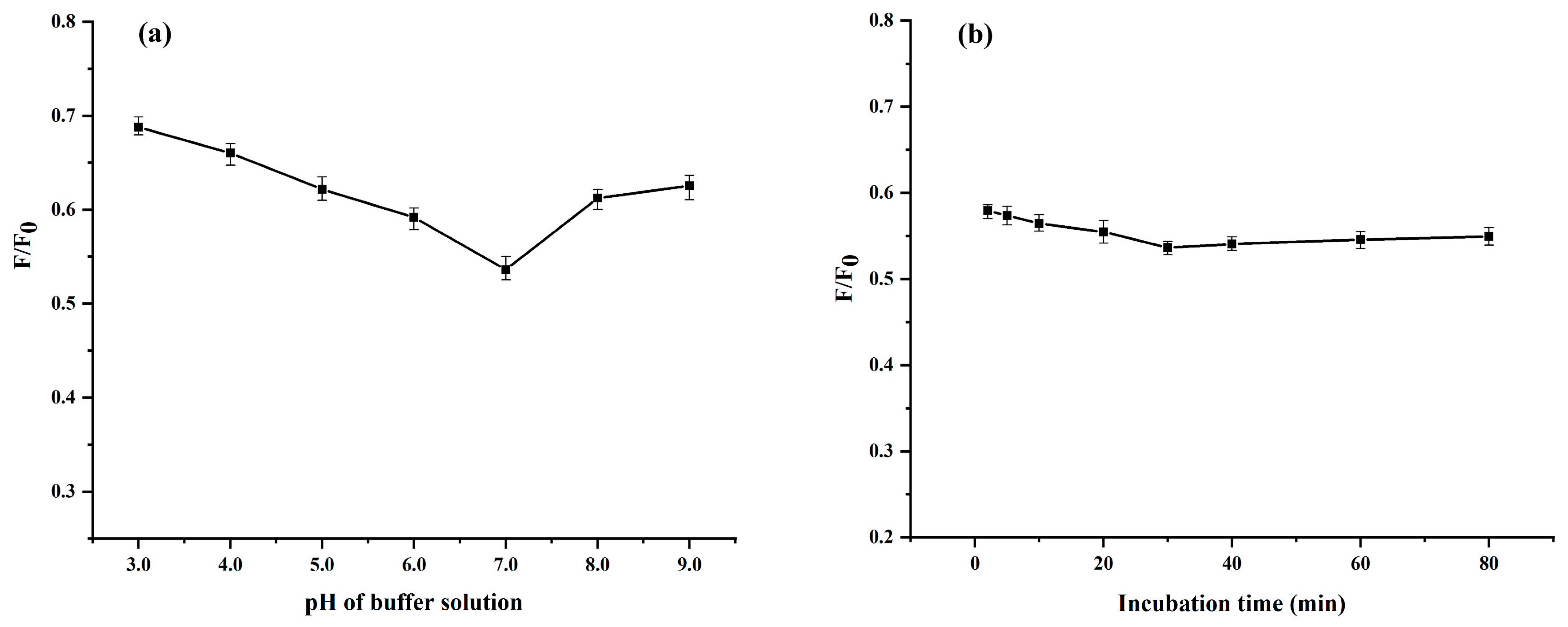
2.6. Effect of pH and Optical Stability of Peanut Protein Carbon Dots
2.7. Cell Counting Kit-8 (CCK-8) Cell Viability Assay and HepG-2 Cells’ Imaging
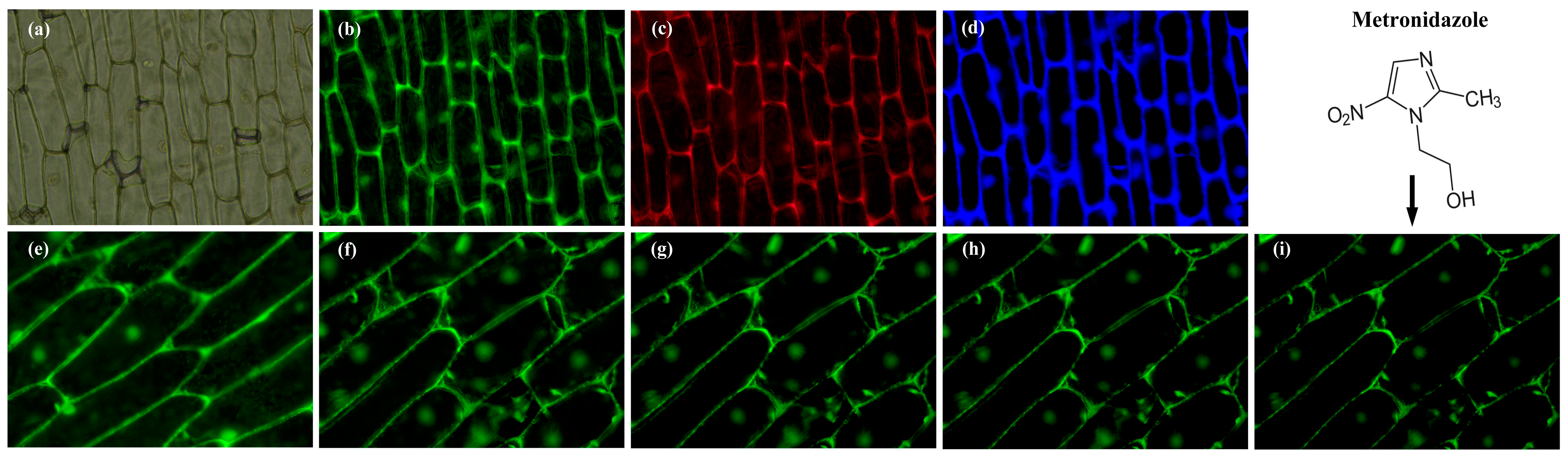
2.8. Imaging of Onion Epidermal Cell
2.9. Quenching Effect of MTZ on the Fluorescence of PP-CDs
2.10. Construction of Calibration Curve for the Determination of MTZ
2.11. Determination of MTZ in Tablet by PP-CDs Fluorescence Sensing
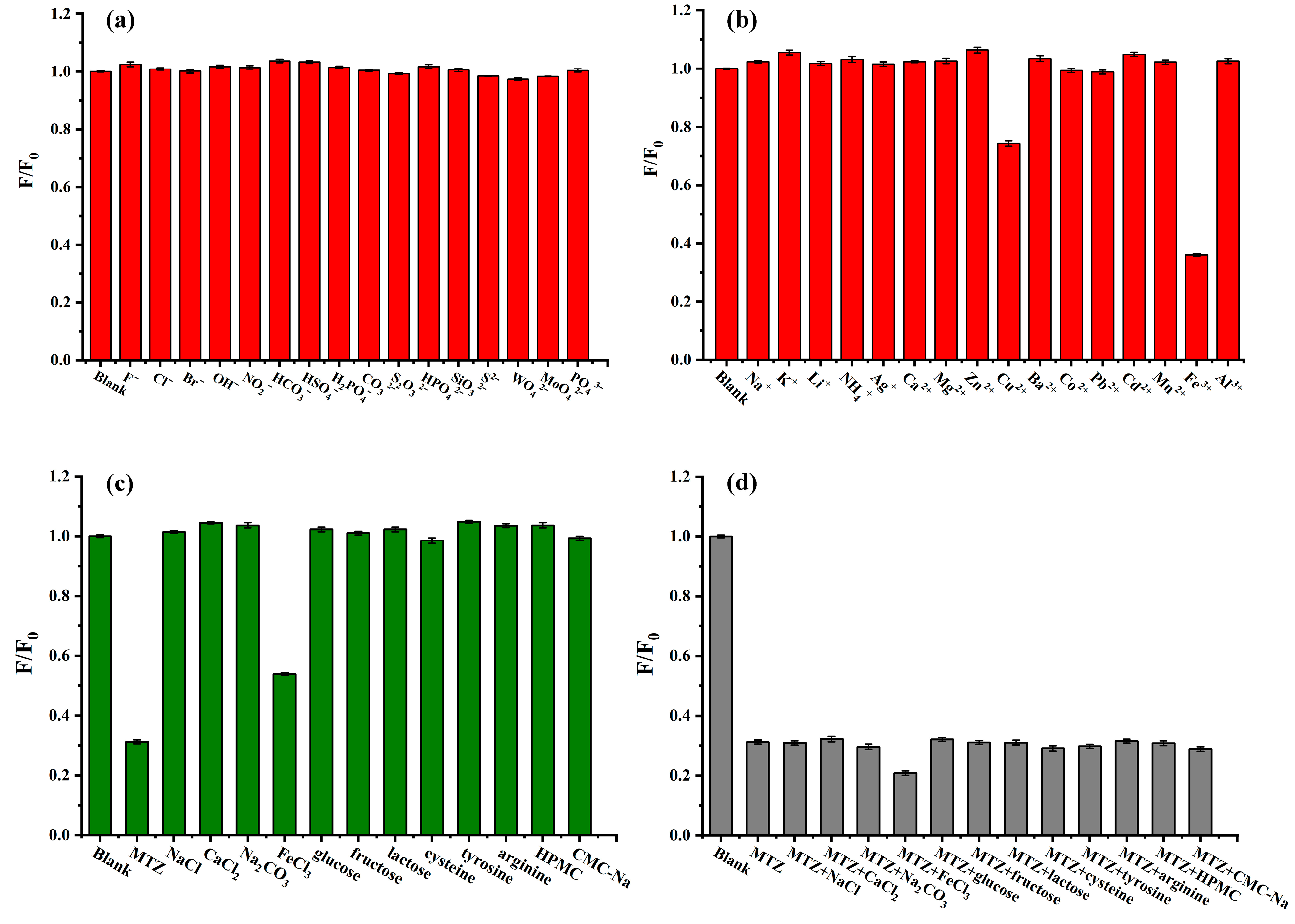
2.12. Evaluation of the Selectivity of Fluorescence Sensing MTZ by PP-CDs
2.13. Statistical Analysis
3. Results
3.1. Comparison of Fluorescence Intensity of Carbon Dots Prepared by Different Peanut Biomass as Carbon Sources
3.2. Optimization of Preparation Conditions for PP CDs via Hydrothermal Reaction
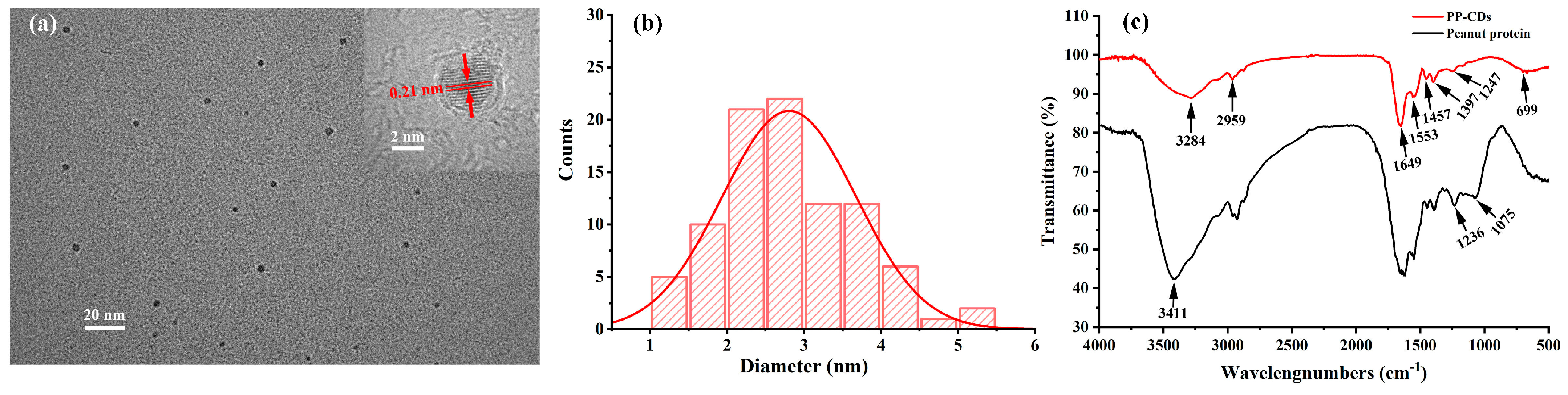
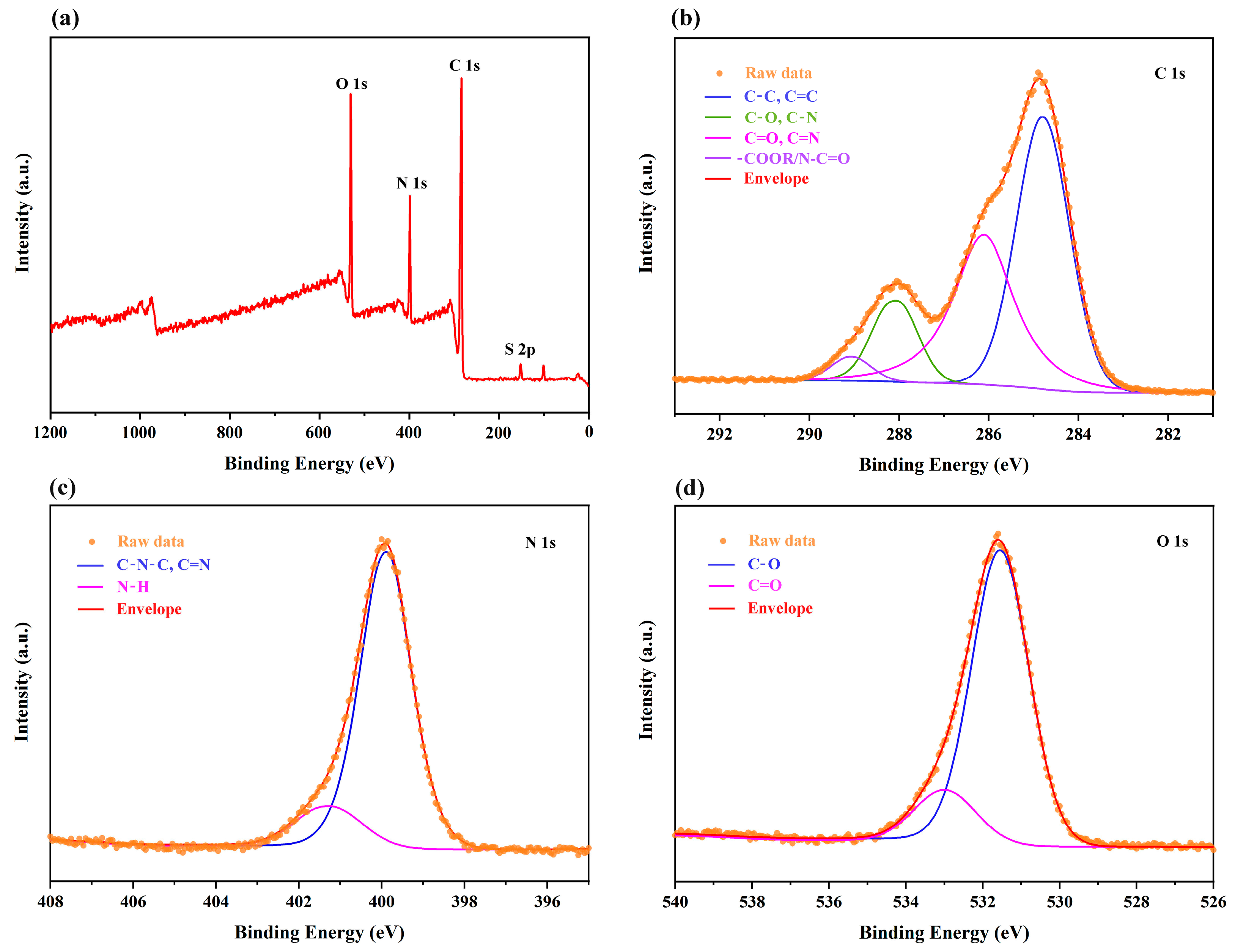
3.3. Characterization of PP-CDs
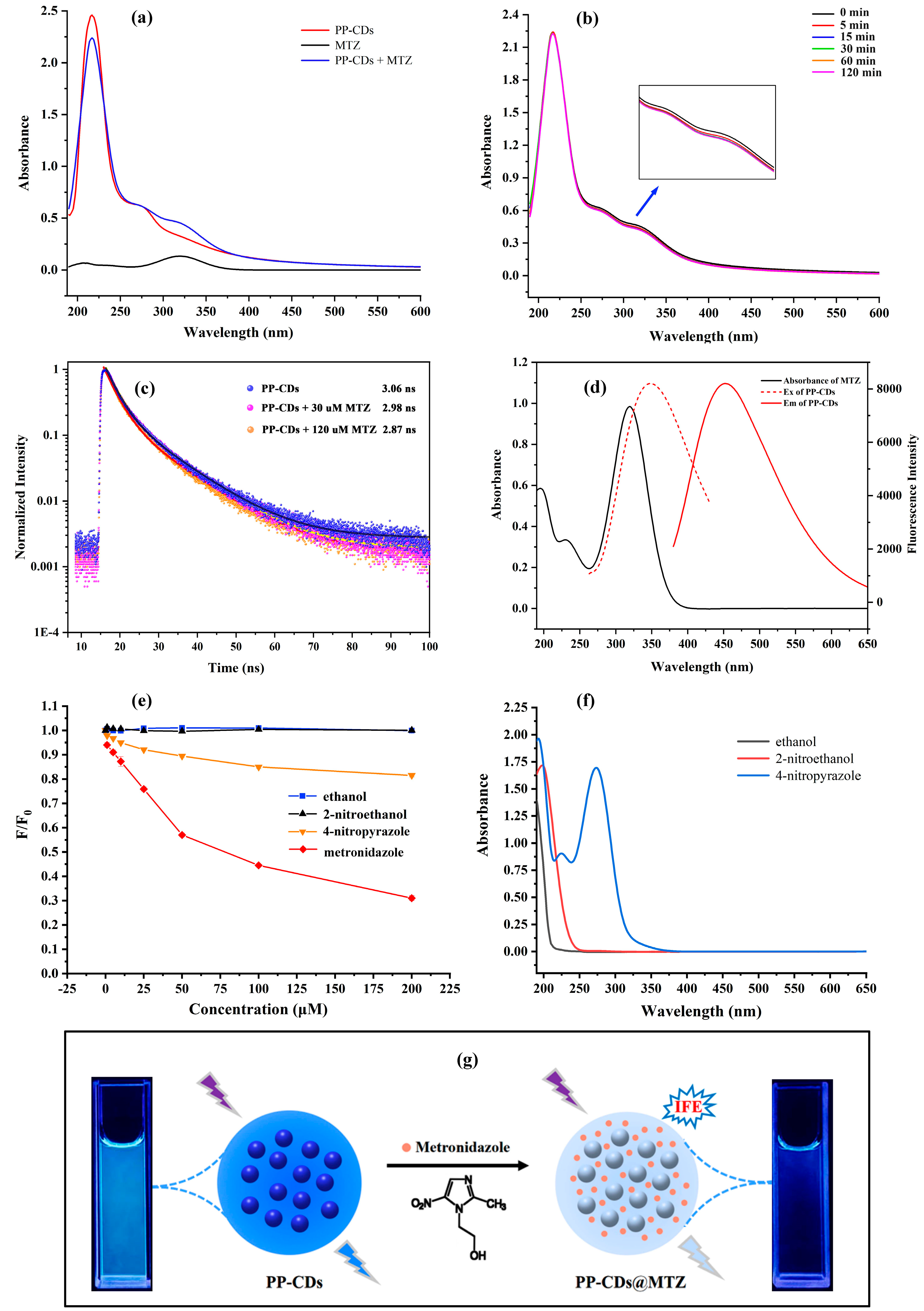
3.4. Fluorescence and UV Spectra of PP-CDs
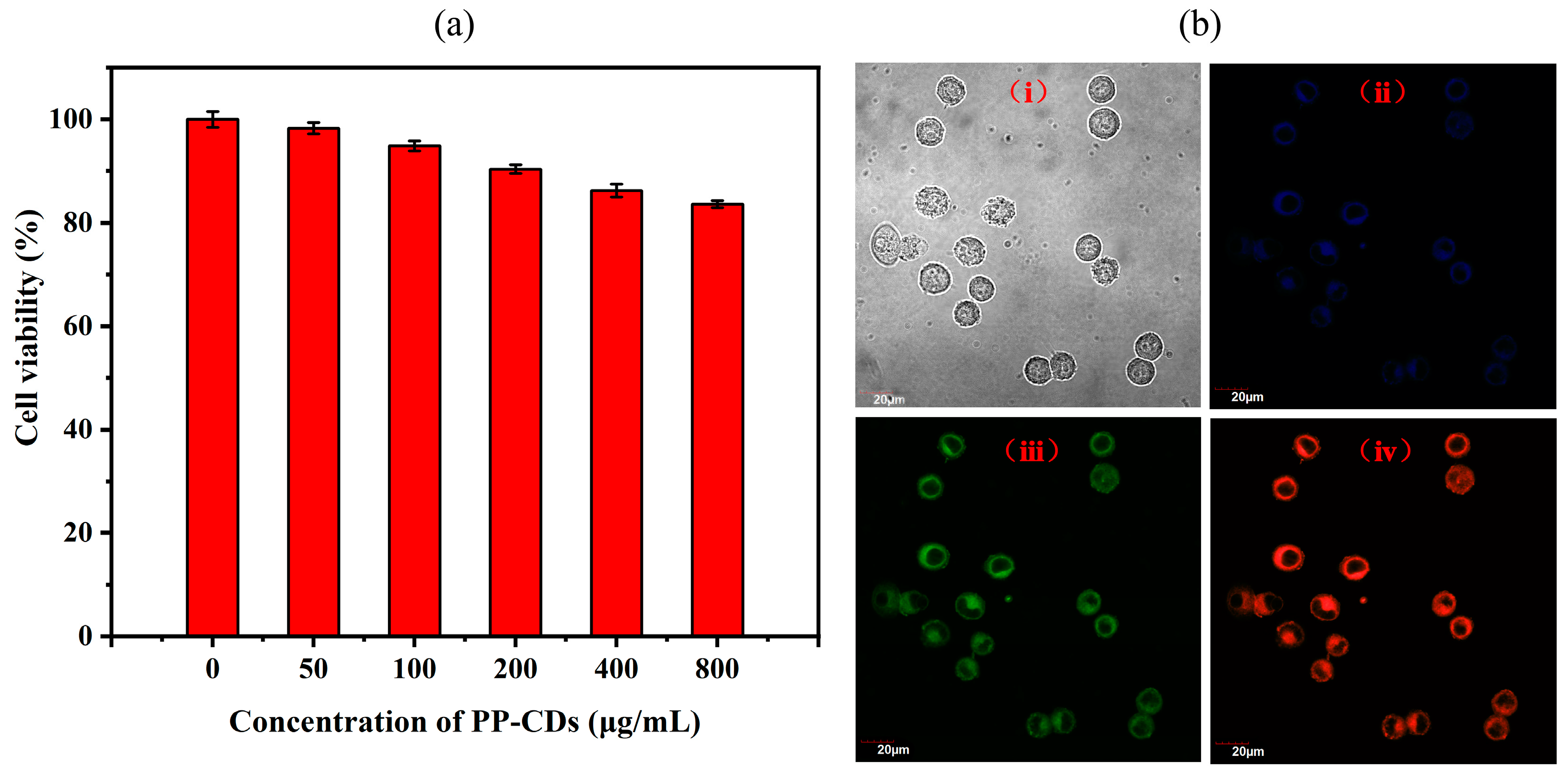
3.5. Cell Counting Kit-8 (CCK-8) Cell Viability Assay
3.6. Imaging of Onion Epidermal Cells with PP-CDs
3.7. Application of PP-CDs in Sensing of MTZ
3.7.1. Optimization of Sensing Conditions
3.7.2. Features of Fluorescence Sensing MTZ by PP-CDs
n = 12; r2 = 0.999; SD = 0.0074; p < 0.0001
3.7.3. Selectivity of Fluorescence Sensing MTZ by PP-CDs
3.7.4. Possible Mechanism of Fluorescence Sensing MTZ by PP-CDs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boukid, F. Peanut protein—An underutilised by-product with great potential: A review. Int. J. Food Sci. Technol. 2022, 57, 5585–5591. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Li, P.F.; Wang, M.M.; Zhang, W.B.; Zhao, W.; Yang, R.J. Fate of phospholipids during aqueous extraction processing of peanut and effect of demulsification treatments on oil-phosphorus-content. Food Chem. 2020, 331, 127367. [Google Scholar] [CrossRef]
- Sousa, A.G.D.; Fernandes, D.C.; Alves, A.M.; de Freitas, J.B.; Naves, M.M.V. Nutritional quality and protein value of exotic almonds and nut from the Brazilian Savanna compared to peanut. Food Res. Int. 2011, 44, 2319–2325. [Google Scholar] [CrossRef]
- Yadav, D.N.; Singh, K.K.; Bhowmik, S.N.; Patil, R.T. Development of peanut milk-based fermented curd. Int. J. Food Sci. Technol. 2010, 45, 2650–2658. [Google Scholar] [CrossRef]
- Isanga, J.; Zhang, G.N. Production and evaluation of some physicochemical parameters of peanut milk yoghurt. LWT—Food Sci. Technol. 2009, 42, 1132–1138. [Google Scholar] [CrossRef]
- Zhang, J.C.; Liu, L.; Jiang, Y.R.; Faisal, S.; Wei, L.L.; Cao, C.J.; Yan, W.H.; Wang, Q. Converting peanut protein biomass waste into “double green” meat substitutes using a high-moisture extrusion process: A multiscale method to explore a process for forming a meat-like fibrous structure. J. Agric. Food Chem. 2019, 67, 10713–10725. [Google Scholar] [CrossRef]
- Zhang, J.C.; Chen, Q.L.; Liu, L.; Zhang, Y.J.; He, N.; Wang, Q. High-moisture extrusion process of transglutaminase-modified peanut protein: Effect of transglutaminase on the mechanics of the process forming a fibrous structure. Food Hydrocoll. 2021, 112, 106346. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Shah, F.; Xu, Y.; Wang, Q. High-moisture extrusion of peanut protein-/carrageenan/sodium alginate/wheat starch mixtures: Effect of different exogenous polysaccharides on the process forming a fibrous structure. Food Hydrocoll. 2020, 99, 105311. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Zhang, M.; Phuhongsung, P. 3D printing of protein-based composite fruit and vegetable gel system. LWT—Food Sci. Technol. 2021, 141, 110978. [Google Scholar] [CrossRef]
- Yao, F.; Gao, Y.H.; Chen, F.S.; Xia, Y.M. Effects of electrospinning parameters on peanut protein isolate nanofibers diameter. CyTA—J. Food. 2021, 19, 729–738. [Google Scholar] [CrossRef]
- Yao, F.; Zheng, Y.; Gao, Y.; Du, Y.; Chen, F. Electrospinning of peanut protein isolate/poly-L-lactic acid nanofibers containing tetracycline hydrochloride for wound healing. Ind. Crops Prod. 2023, 194, 116262. [Google Scholar] [CrossRef]
- Justino, H.D.M.; dos Santos, I.F.; de Soua, R.C.N.; Sanches, E.A.; Bezerra, J.D.; Lamarao, C.V.; Pires, A.C.D.; Campelo, P.H. Exploring ultrasound-assisted technique for enhancing techno-functional properties of plant proteins: A comprehensive review. Int. J. Food Sci. Technol. 2024, 59, 498–511. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci. Technol. 2013, 34, 109–123. [Google Scholar] [CrossRef]
- Ning, F.J.; Ge, Z.Z.; Qiu, L.; Wang, X.Q.; Luo, L.P.; Xiong, H.; Huang, Q.R. Double-induced se-enriched peanut protein nanoparticles preparation, characterization and stabilized food-grade pickering emulsions. Food Hydrocoll. 2020, 99, 105308. [Google Scholar] [CrossRef]
- John, B.K.; Mathew, J.; Sreekanth, K.; Radhakrishnan, E.K.; Mathew, B. Biomass derived carbon quantum dots as a versatile platform for fluorescent sensing, catalytic reduction, fluorescent ink and anticancer agents. Mater. Today Sustain. 2024, 26, 100715. [Google Scholar] [CrossRef]
- Vishnukumar, P.; Sankaranarayanan, S.; Hariram, M. Carbon dots from renewable resources: A review on precursor choices and potential applications. In Green Nanomaterials, 1st ed.; Springer: Singapore, 2020; Volume 126, pp. 159–208. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.; Chakradhar, D.; Perumal, S.; Shim, J.J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Venugopalan, P.; Vidya, N. Bilimbi (Averrhoa bilimbi) fruit derived carbon dots for dual sensing of Cu (II) and quinalphos. Int. J. Environ. Anal. Chem. 2022, 104, 6613–6626. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 15, 303–311. [Google Scholar] [CrossRef]
- Khan, A.; Riahi, Z.; Kim, J.T.; Rhim, J.W. Carboxymethyl cellulose/gelatin film incorporated with eggplant peel waste-derived carbon dots for active fruit packaging applications. Int. J. Biol. Macromol. 2024, 271, 132715. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Muthuchamy, N.; Lee, Y.R. Hydrophilic nitrogen-doped carbon dots from biowaste using dwarf banana peel for environmental and biological applications. Fuel 2020, 275, 117821. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhou, Y.; Yan, S.H.; Qian, S.N.; Wang, Y.H.; Ju, E.G.; Zhang, C.B. Herbal medicine-inspired carbon quantum dots with antibiosis and hemostasis effects for promoting wound healing. ACS Appl. Mater. Interfaces. 2024, 16, 8527–8537. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Zhang, R.; Zhang, T.; Liu, X.M.; Zhao, Y.F.; Yang, M.Q.; Cheng, H.Y.; Zhang, Q.M.; Zhang, Y.J.; Wu, X.P.; et al. Synthesis, applications and biosafety evaluation of carbon dots derived from herbal medicine. Biomed. Mater. 2023, 18, 042004. [Google Scholar] [CrossRef]
- Koutamehr, M.E.; Moradi, M.; Tajik, H.; Molaei, R.; Heshmati, M.K.; Alizadeh, A. Sour whey-derived carbon dots; synthesis, characterization, antioxidant activity and antimicrobial performance on foodborne pathogens. LWT—Food Sci. Technol. 2023, 184, 114978. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Zhou, Y.; Wu, Y.; Sun, Q.; Le, T. Preparation of phosphorus-doped cow milk-derived carbon quantum dots and detection of Au3+. J. Food Process Eng. 2023, 46, e14349. [Google Scholar] [CrossRef]
- Kurian, M.; Ganapathy, D.; Jain, A.R. Recent advances of metronidazole—A review. Drug Invent. Today 2018, 10, 3536–3541. [Google Scholar]
- Mahugo-Santana, C.; Sosa-Ferrera, Z.; Ma Esther, T.P.; José, J.S.R. Analytical methodologies for the determination of nitroimidazole residues in biological and environmental liquid samples: A review. Anal. Chim. Acta. 2010, 665, 113–122. [Google Scholar] [CrossRef]
- Rao, Y.S.; Rao, N.S.M.; Murthy, B.S. Direct spectrophotometric determination of metronidazole and ornidazole. J. Indian Chem. Soc. 2012, 89, 981–984. [Google Scholar] [CrossRef]
- Mahrouse, M.A.; Elkady, E.F. Validated spectrophotometric methods for the simultaneous determination of ciprofloxacin hydrochloride and metronidazole in tablets. Chem. Pharm. Bull. 2011, 59, 1485–1493. [Google Scholar] [CrossRef]
- El-Ghobashy, M.R.; Abo-Talib, N.F. Spectrophotometric methods for the simultaneous determination of binary mixture of metronidazole and diloxanide furoate without prior separation. J. Adv. Res. 2010, 1, 323–329. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Ghoneim, E.; El-Hefnawy, G.; El-Hallag, I. Voltammetry and determination of metronidazole at a carbon fiber microdisk electrode. Talanta 2005, 66, 869–874. [Google Scholar] [CrossRef]
- Lu, S.; Wu, K.; Dang, X.; Hu, S. Electrochemical reduction and voltammetric determination of metronidazole at a nanomaterial thin film coated glassy carbon electrode. Talanta 2004, 63, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.A.; Mossa, H.A.; Barsoum, B.N. Application of nuclear magnetic resonance spectroscopy for quantitative analysis of miconazole, metronidazole and sulfamethoxazole in pharmaceutical and urine samples. J. Pharm. Biomed. Anal. 2006, 41, 654–661. [Google Scholar] [CrossRef]
- Ho, C.; Sin, D.W.M.; Wong, K.M.; Tang, H.P.O. Determination of dimetridazole and metronidazole in poultry and porcine tissues by gas chromatography–electron capture negative ionization mass spectrometry. Anal. Chim. Acta. 2005, 530, 23–31. [Google Scholar] [CrossRef]
- King’ori, L.D.; Walker, R.B. HPLC method for simultaneous analysis of ranitidine and metronidazole in dosage forms. Asian J. Chem. 2014, 26, 426–430. [Google Scholar] [CrossRef]
- Akay, C.; Özkan, S.A.; Sentürk, Z.; Cevheroglu, S. Simultaneous determination of metronidazole and miconazole in pharmaceutical dosage forms by RP-HPLC. Farmaco 2002, 57, 953–957. [Google Scholar] [CrossRef]
- Elkady, E.F.; Mahrouse, M.A. Reversed-phase ion-pair HPLC and TLC-densitometric methods for the simultaneous determination of ciprofloxacin hydrochloride and metronidazole in tablets. Chromatographia 2011, 73, 297–305. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Shang, X.; Chao, D.; Zhou, L.; Zhang, H. Highly sensitive and selective detection of Fe3+ by utilizing carbon quantum dots as fluorescent probes. Chem. Phys. Lett. 2018, 705, 1–6. [Google Scholar] [CrossRef]
- Chen, D.; Song, J.; Yang, H.; Xiong, S.; Liu, Y.; Liu, R. Effects of acid and alkali treatment on the properties of proteins recovered from whole Gutted Grass Carp (Ctenopharyngodon idellus) using isoelectric solubilization/precipitation. J. Food Qual. 2016, 39, 567–857. [Google Scholar] [CrossRef]
- Baig, M.M.F.; Chen, Y.C. Bright carbon dots as fluorescence sensing agents for bacteria and curcumin. J. Colloid Interface Sci. 2017, 501, 341–349. [Google Scholar] [CrossRef]
- Liang, Y. A silicon-cored tetraphenyl benzene derivative with aggregation-induced emission enhancement as a fluorescent probe for nitroaromatic compounds detection. Spectrochim. Acta Part A 2019, 216, 395–403. [Google Scholar] [CrossRef]
- Vissers, Y.M.; Iwan, M.; Adel-Patient, K.; Stahl Skov, P.; Rigby, N.M.; Johnson, P.E.; Mandrup Müller, P.; Przybylski-Nicaise, L.; Schaap, M.; Ruinemans-Koerts, J.; et al. Effect of roasting on the allergenicity of major peanut allergens Ara h 1 and Ara h 2/6: The necessity of degranulation assays. Clin. Exp. Allergy. 2011, 41, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Maleki, S.J.; Rodriguez, J.; Burbano, C.; Muzquiz, M.; Jimenez, M.A.; Pedrosa, M.M.; Cuadrado, C.; Crespo, J.F. Heat and pressure treatments effects on peanut allergenicity. Food Chem. 2012, 132, 360–366. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.L.; Feng, X.T.; Zhang, F.; Yang, Y.Z.; Liu, X.G. Effect of reaction temperature on structure and fluorescence properties of nitrogen-doped carbon dots. Appl. Surf. Sci. 2016, 387, 1236–1246. [Google Scholar] [CrossRef]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of nuts and their potential health benefits-an overview. Foods. 2023, 12, 942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.X.; Gao, H.; Li, X.L. Synthesis and optical properties of nitrogen and sulfur co-doped graphene quantum dots. New J. Chem. 2014, 38, 4615–4621. [Google Scholar] [CrossRef]
- Cen, Q.; Xu, H.; Chen, J.; Zhao, S.; Li, W.; Zheng, M.; Xiang, J.; Luo, L.; Chen, F.; Zhang, H.; et al. Functionalized biomass carbon dots for improved growth of lettuce and Exocarpium Citri Grandis saplings. ACS Appl. Nano Mater. 2023, 6, 16604–16614. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Yan, Z.; Chen, J. Simultaneous determination of temperature and erlotinib by novel carbon-based sensitive nanoparticles. Sen. Actuators B Chem. 2018, 255, 986–994. [Google Scholar] [CrossRef]
- Li, H.T.; He, X.D.; Kang, Z.H.; Huang, H.; Liu, Y.; Liu, J.L.; Lian, S.Y.; Tsang, C.H.A.; Yang, X.B.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, H.J.; Zhou, X.Y.; Gong, X.J.; Xiao, L.X.; Liu, L.Z.; Yang, Z.Q. Bright-yellow-emissive nitrogen-doped carbon nanodots as a fluorescent nanoprobe for the straightforward detection of glutathione in food samples. Food Chem. 2020, 325, 126946. [Google Scholar] [CrossRef]
- Zhang, S.-R.; Cai, S.-K.; Wang, G.-Q.; Cui, J.-Z.; Gao, C.-Z. One-step synthesis of N, P-doped carbon quantum dots for selective and sensitive detection of Fe2+ and Fe3+ and scale inhibition. J. Mol. Struct. 2021, 1246, 131173. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Liu, J.; Yang, Q.; Zhang, B.; Gao, Y.; Wang, Y.; Lu, G. Dual functional N- and S-co-doped carbon dots as the sensor for temperature and Fe3+ ions. Sensors Actuators B Chem. 2017, 242, 1272–1280. [Google Scholar] [CrossRef]
- Ye, Q.; Yan, F.; Luo, Y.; Wang, Y.; Zhou, X.; Chen, L. Formation of N, S-codoped fluorescent carbon dots from biomass and their application for the selective detection of mercury and iron ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta. 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods. 2020, 12, 1266. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]



| Sample | MTZ Solution Added (μL) | Concentration of MTZ Solution Added (μM) | Measured MTZ Concentration (μM) | Recovery (%) c | RSD (%) d |
|---|---|---|---|---|---|
| Sample 1 a | none | none | 1.562 ± 0.047 | - | 3.01 |
| 50 | 0.5 | 1.996 ± 0.054 | 96.80 | 2.71 | |
| 100 | 1.0 | 2.492 ± 0.071 | 97.27 | 2.85 | |
| 200 | 2.0 | 3.410 ± 0.118 | 95.73 | 3.46 | |
| Sample 2 b | none | none | 10.683 ± 0.036 | - | 3.51 |
| 50 | 2.5 | 12.726 ± 0.443 | 96.53 | 3.48 | |
| 100 | 5.0 | 14.973 ± 0.428 | 95.47 | 2.86 | |
| 200 | 10.0 | 19.818 ± 0.639 | 95.82 | 3.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Hu, Z.; Zhang, W.; Zhang, Y.; Xiao, J.; Lei, S. Characteristics of Peanut Protein-Derived Carbon Dots and Their Application in Cell Imaging and Sensing of Metronidazole. Chemosensors 2025, 13, 151. https://doi.org/10.3390/chemosensors13040151
Liao J, Hu Z, Zhang W, Zhang Y, Xiao J, Lei S. Characteristics of Peanut Protein-Derived Carbon Dots and Their Application in Cell Imaging and Sensing of Metronidazole. Chemosensors. 2025; 13(4):151. https://doi.org/10.3390/chemosensors13040151
Chicago/Turabian StyleLiao, Junyan, Zhixiong Hu, Weinong Zhang, Yanpeng Zhang, Jiangrong Xiao, and Shenglan Lei. 2025. "Characteristics of Peanut Protein-Derived Carbon Dots and Their Application in Cell Imaging and Sensing of Metronidazole" Chemosensors 13, no. 4: 151. https://doi.org/10.3390/chemosensors13040151
APA StyleLiao, J., Hu, Z., Zhang, W., Zhang, Y., Xiao, J., & Lei, S. (2025). Characteristics of Peanut Protein-Derived Carbon Dots and Their Application in Cell Imaging and Sensing of Metronidazole. Chemosensors, 13(4), 151. https://doi.org/10.3390/chemosensors13040151






