Influence of Different Synthesis Methods on the Defect Structure, Morphology, and UV-Assisted Ozone Sensing Properties of Zinc Oxide Nanoplates
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the ZnO Nanostructures
- Precipitation (ZA): The mixture was stirred continuously using a magnetic stirrer for 30 min at room temperature.
- Ultrasonic bath (ZU): The beaker containing the solution was submitted to an ultrasonic bath at 50 mA for 30 min at room temperature.
- Ultrasonic tip (ZP): To perform this synthesis, a protected metallic ultrasonic tip was inserted into the beaker containing the solution, which was then subjected to ultrasonic agitation at 40 mA and 40% amplitude for two sets of 15 min.
- Microwave-assisted hydrothermal (MAH) method (ZM): The mixture was transferred to a Teflon autoclave and placed inside an adapted microwave oven (this setup was described in a previous work [21]). The synthesis was conducted at 130 °C and a heating rate of 10 °C/min for 8 min. The system autogenous pressure reached approximately 300 kPa (~3 atm).
2.2. Characterization of the ZnO Nanostructures
2.3. Preparation of the Sensing Platforms
2.4. Characterization of the Sensing Films
3. Results
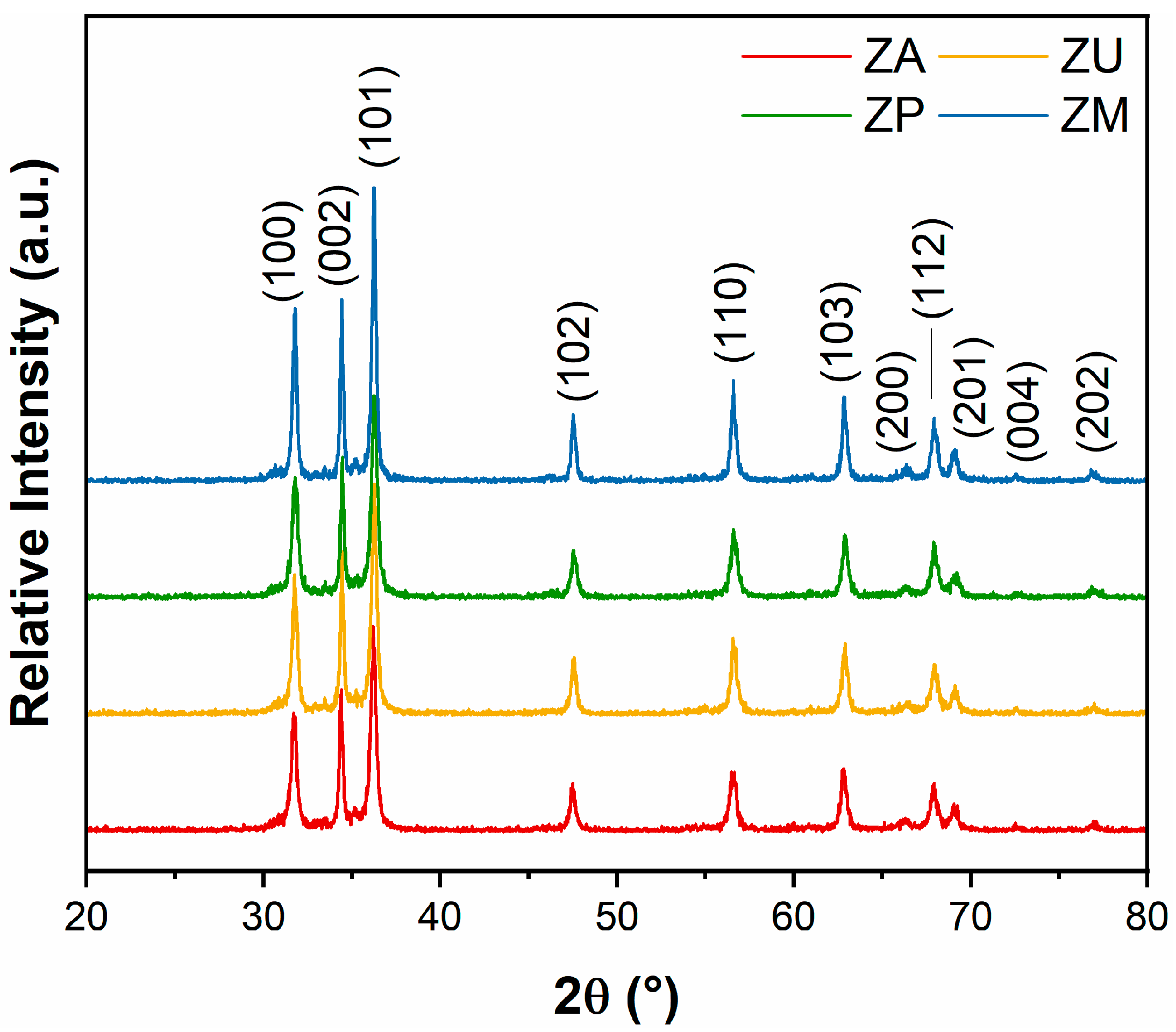
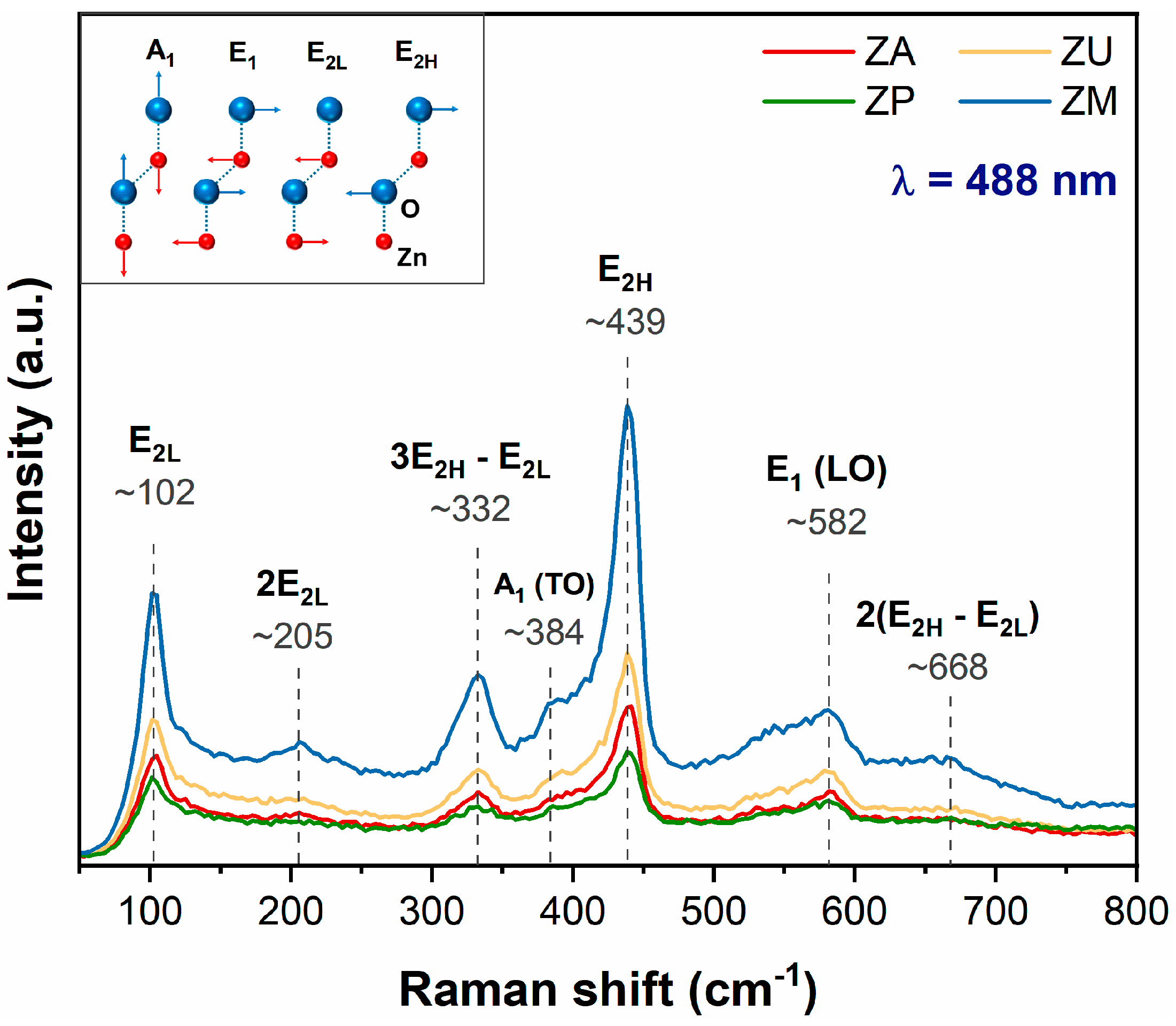
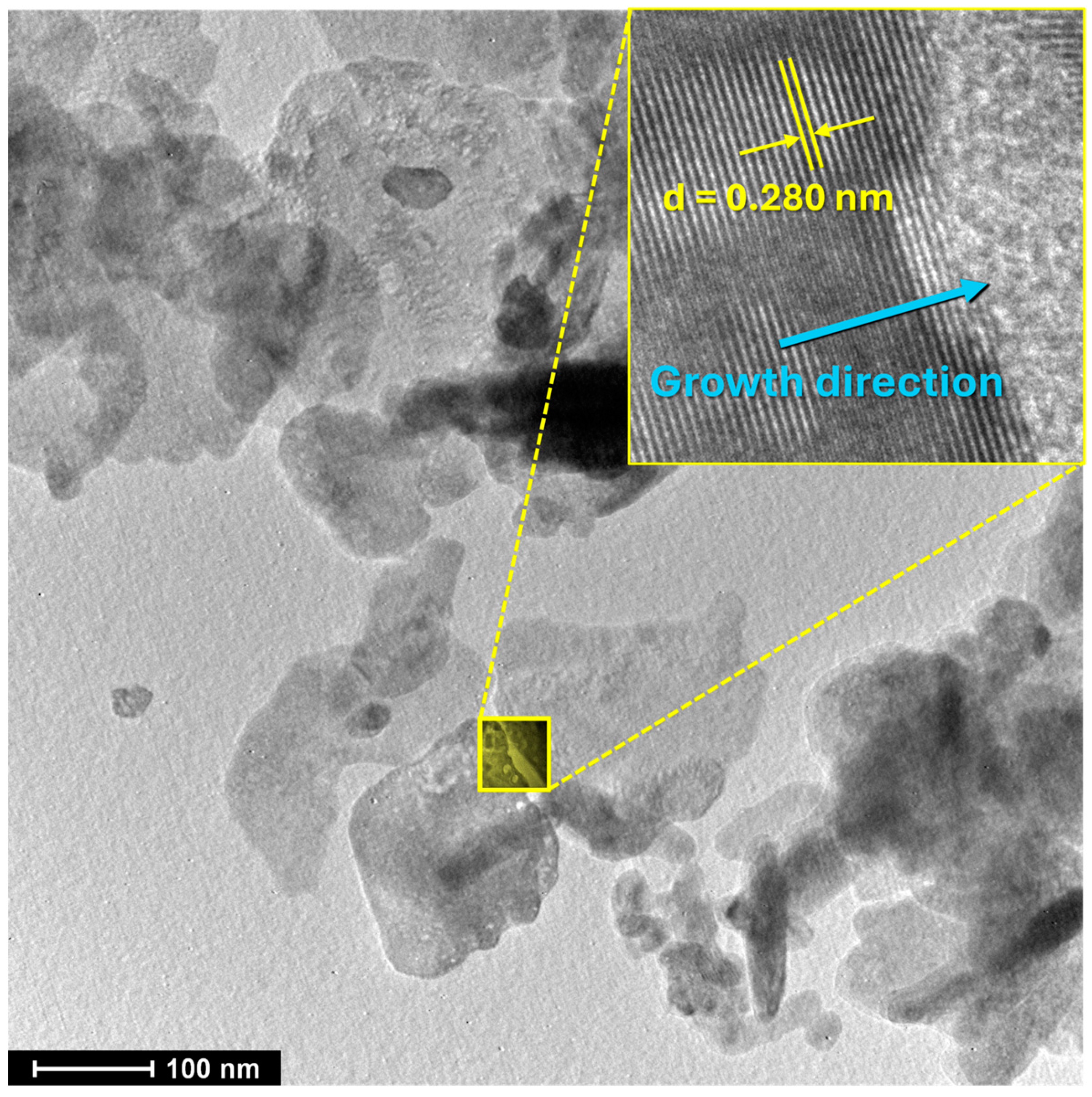
3.1. Characterization of the ZnO Nanostructures
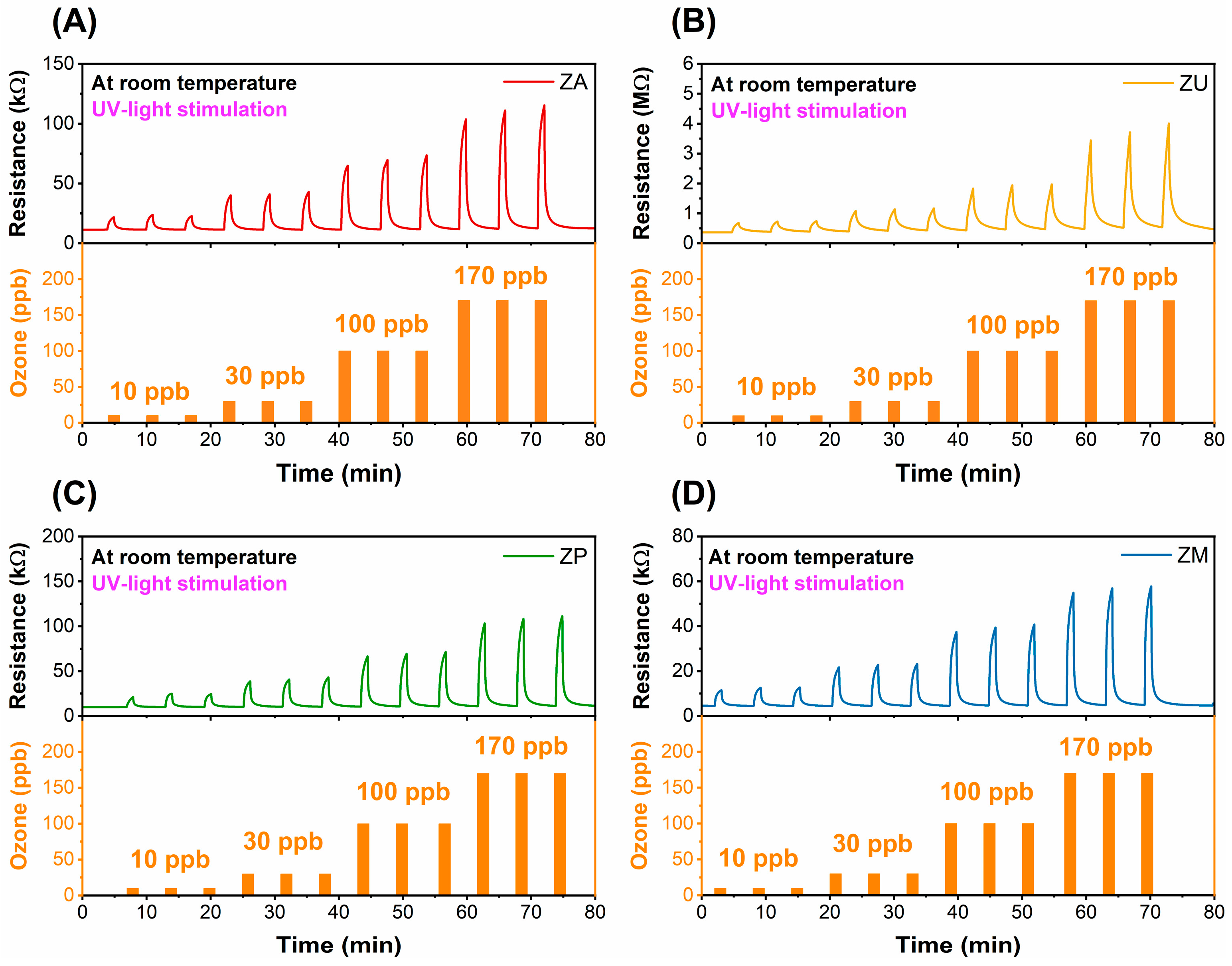
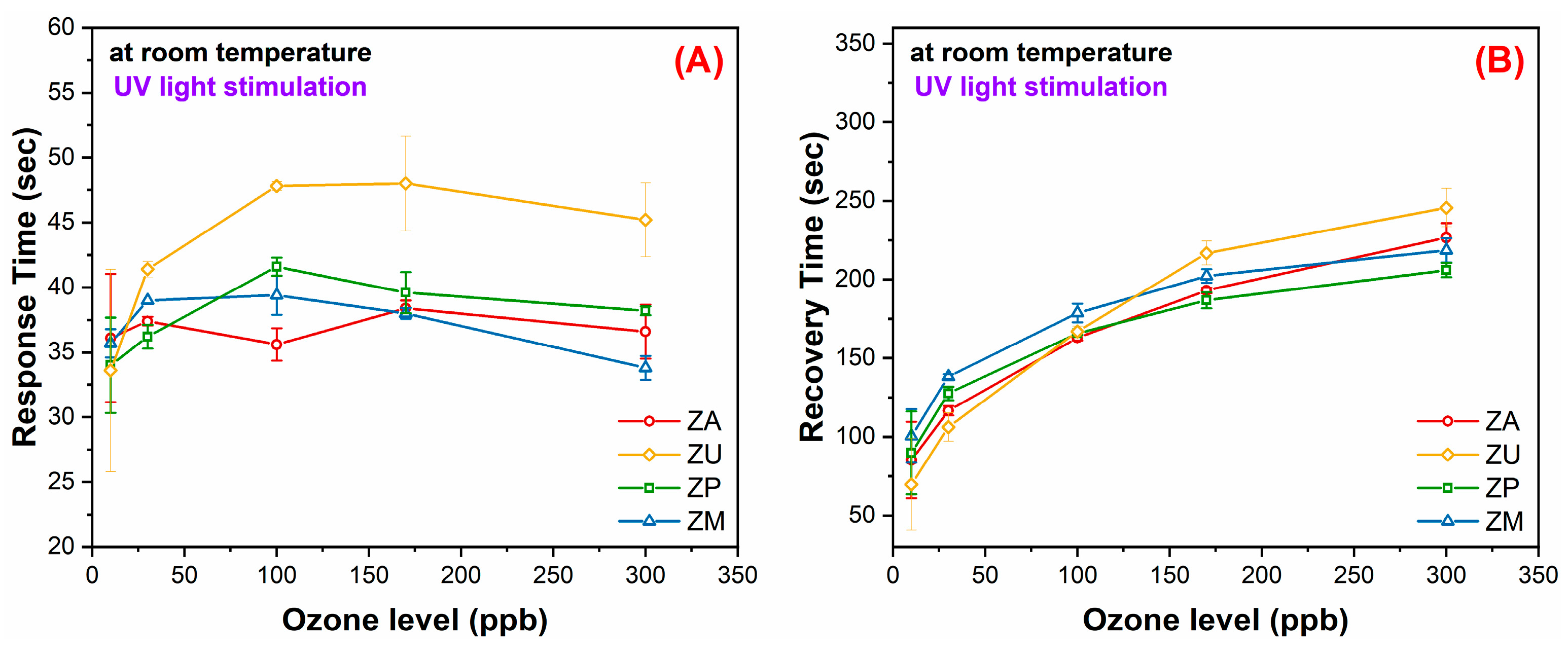
3.2. Characterization of the Sensing Films and Gas-Sensing Measurements
| Sensing Material | Synthesis Method | Operating Temperature | Minimum O3 Level (ppb) | Excitation Wavelengths | Ref. |
|---|---|---|---|---|---|
| ZnO nanorods | Hydrothermal | Room temperature (26 °C) | 100 | UV (351 nm) | [38] |
| Au-modified ZnO nanorods | Hydrothermal | Room temperature (26 °C) | 30 | UV (370 nm) | [71] |
| ZnO thin film | Sputtering | 250 °C | 90 | --------- | [72] |
| SnO2 nanoparticles | Hydrolysis | Room temperature (25 °C) | 12 | UV (315 nm), violet (405 nm) and blue (465 nm) | [73] |
| In2O3 nanostructures | Co-precipitation | 70 °C | 30 | --------- | [74] |
| ZnO nanoplates | Precipitation, ultrasonic bath, ultrasonic tip, and MAH | Room temperature (30 °C) | 10 | UV (375 nm) | This Work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV | Ultraviolet |
| VOC | Volatile organic compounds |
| WHO | World Health Organization |
| MOS | Metal oxide semiconductors |
| MAH | Microwave-assisted hydrothermal |
| ZA | Sample prepared using the precipitation method |
| ZU | Sample prepared using ultrasound-assisted method |
| ZP | Sample prepared using the ultrasonic-tip-assisted method |
| ZM | Sample prepared using the microwave-assisted hydrothermal method |
| BET | Brunauer–Emmett–Teller method |
| FE-SEM | Field-emission scanning electron microscopy |
| DRS | Diffuse reflectance spectroscopy |
| PL | Photoluminescence spectroscopy |
| XRD | X-ray diffraction |
| LO | Longitudinal optical |
| TO | Transverse optical |
| CB | Conduction band |
References
- Hogard, S.; Pearce, R.; Yetka, K.; Gonzalez, R.; Bott, C. Virus Inactivation in Low Ozone Exposure Water Reuse Applications. Water Res. 2024, 256, 121536. [Google Scholar] [CrossRef]
- Dubey, P.; Singh, A.; Yousuf, O. Ozonation: An Evolving Disinfectant Technology for the Food Industry. Food Bioprocess Technol. 2022, 15, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Okolie, J.A.; Mackay, W.; Rateb, M.; Yaseen, M. Ozone Application in Different Industries: A Review of Recent Developments. Chem. Eng. J. 2023, 454, 140188. [Google Scholar] [CrossRef]
- Petani, L.; Koker, L.; Herrmann, J.; Hagenmeyer, V.; Gengenbach, U.; Pylatiuk, C. Recent Developments in Ozone Sensor Technology for Medical Applications. Micromachines 2020, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Komorizono, A.A.; de Lima, B.S.; Mastelaro, V.R. Assessment of the Ozonolysis Effect of RGO-ZnO-Based Ozone Sensors. Sens. Actuators B Chem. 2023, 397, 134621. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Souissi, R.; Bouguila, N.; Bendahan, M.; Aguir, K.; Fiorido, T.; Abderrabba, M.; Halidou, I.; Labidi, A. Ozone Sensing Study of Sprayed β-In2S3 Thin Films. J. Alloys Compd. 2022, 900, 163513. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, S.G. Simultaneous Detection of CO and NH3 Gases at Room Temperature with an Array of ZnS Chemiresistive Sensors and the Superposition Principle. Anal. Chem. 2022, 94, 4602–4609. [Google Scholar] [CrossRef]
- Hambir, S.; Jagtap, S. NO2 Sensing Behavior of Ni-Doped In2O3 Microcubes Based Chemiresistive Gas Sensors. J. Mater. Sci. Mater. Electron. 2023, 34, 1716. [Google Scholar] [CrossRef]
- To, D.T.H.; Park, J.Y.; Yang, B.; Myung, N.V.; Choa, Y.H. Nanocrystalline ZnO Quantum Dot-Based Chemiresistive Gas Sensors: Improving Sensing Performance towards NO2 and H2S by Optimizing Operating Temperature. Sens. Actuators Rep. 2023, 6, 100166. [Google Scholar] [CrossRef]
- Neogi, S.; Ghosh, R. Influence of Dipole Moment on the VOC Sensing Properties of BiFeO3 Microspheres: Addressing the Selectivity towards Acetone. Sens. Actuators B Chem. 2024, 415, 135980. [Google Scholar] [CrossRef]
- Yang, X.Y.; Chen, H.N.; Yue, L.J.; Gong, F.L.; Xie, K.F.; Wei, S.Z.; Zhang, Y.H. Surface Engineering of 1D Na-Doped Pd/WO3 Nanorods for Chemiresistive H2 Sensing. Sens. Actuators B Chem. 2025, 423, 136825. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in Designs and Mechanisms of Semiconducting Metal Oxide Nanostructures for High-Precision Gas Sensors Operated at Room Temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A Review on Chemiresistive ZnO Gas Sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Meng, F.; Huo, S.; Hu, X.; Niu, P.; Wu, E. Strategies and Challenges for Improving the Performance of Two-Dimensional Materials-Based Gas Sensors. Adv. Phys. X 2024, 9, 2288353. [Google Scholar] [CrossRef]
- da Silva, L.F.; M’Peko, J.C.; Catto, A.C.; Bernardini, S.; Mastelaro, V.R.; Aguir, K.; Ribeiro, C.; Longo, E. UV-Enhanced Ozone Gas Sensing Response of ZnO-SnO2 Heterojunctions at Room Temperature. Sens. Actuators B Chem. 2017, 240, 573–579. [Google Scholar] [CrossRef]
- Meng, X.; Kang, S.; Zhao, Z.; Jin, G.; Shao, Z.; Wu, L. Core-Shell Bi2O3/CeO2 Heterojunction for Enhanced Formaldehyde Gas Sensor. Ceram. Int. 2025, 51, 6067–6077. [Google Scholar] [CrossRef]
- Fang, H.; Chen, H.; Tan, R.; Ge, L.; Zhao, T.; Wang, H.; Yuan, S.; Wang, D. A CuO/MoO3-Based SO2 Gas Sensor with Moisture Resistance and Ultra-Fast Response at 90 °C. Sens. Actuators B Chem. 2025, 422, 136627. [Google Scholar] [CrossRef]
- Su, H.; Guan, Y.; Zhou, K.; Ma, C.; Hua, Z.; Wang, X.; Guo, X.; Zeng, D. Ultrasensitive Response and Ultralow Detection Limit of the Acetone Gas Sensor Based on 2D Porous Pd-Doped WO3 Nanosheets. Ceram. Int. 2025, in press. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Q.; Zhu, Y.; Hu, P.; Wu, Z. Highly Selective Room-Temperature Formaldehyde Gas Sensor Based on SnO2 Nanoparticle-Modified In2O3 Microspheres. Appl. Surf. Sci. 2025, 695, 162856. [Google Scholar] [CrossRef]
- Ortega, P.P.; Silva, C.C.; Ramirez, M.A.; Biasotto, G.; Foschini, C.R.; Simões, A.Z. Multifunctional Environmental Applications of ZnO Nanostructures Synthesized by the Microwave-Assisted Hydrothermal Technique. Appl. Surf. Sci. 2021, 542, 148723. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced Sensing Performance of ZnO Nanostructures-Based Gas Sensors: A Review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Butt, M.A.; Piramidowicz, R. Integrated Photonic Sensors for the Detection of Toxic Gasses—A Review. Chemosensors 2024, 12, 143. [Google Scholar] [CrossRef]
- Jian, J.C.; Chang, Y.C.; Chang, S.P.; Chang, S.J. Biotemplate-Assisted Growth of ZnO in Gas Sensors for Ppb-Level NO2 Detection. ACS Omega 2024, 9, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Marani, P.; Morandi, S.; Lettieri, S.; Mazzocchi, M.; Sacerdoti, M.; Carotta, M.C. Growth Mechanisms of ZnO Micro-Nanomorphologies and Their Role in Enhancing Gas Sensing Properties. Sensors 2021, 21, 1331. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Jangir, L.K.; Rathore, K.S.; Kumar, M.; Awasthi, K. Morphology-Dependent Structural and Optical Properties of ZnO Nanostructures. Appl. Phys. A Mater. Sci. Process 2019, 125, 553. [Google Scholar] [CrossRef]
- Li, Y.X.; Guo, Z.; Su, Y.; Jin, X.B.; Tang, X.H.; Huang, J.R.; Huang, X.J.; Li, M.Q.; Liu, J.H. Hierarchical Morphology-Dependent Gas-Sensing Performances of Three-Dimensional SnO2 Nanostructures. ACS Sens. 2017, 2, 102–110. [Google Scholar] [CrossRef]
- Ortega, P.P.; Gherardi, S.; Spagnoli, E.; Fabbri, B.; Astolfi, M.; Zonta, G.; Landini, N.; Malagù, C.; Aldao, C.M.; Ponce, M.A.; et al. Nanostructured Eu-Doped Ceria for Humidity Sensing: A Morphological Perspective. Appl. Surf. Sci. 2024, 661, 160047. [Google Scholar] [CrossRef]
- Srujana, S.; Bhagat, D. Chemical-Based Synthesis of ZnO Nanoparticles and Their Applications in Agriculture. Nanotechnol. Environ. Eng. 2022, 7, 269–275. [Google Scholar] [CrossRef]
- Mahmood, N.B.; Saeed, F.R.; Gbashi, K.R.; Mahmood, U.S. Synthesis and Characterization of Zinc Oxide Nanoparticles via Oxalate Co-Precipitation Method. Mater. Lett. X 2022, 13, 100126. [Google Scholar] [CrossRef]
- Mahdavi, R.; Talesh, S.S.A. Sol-Gel Synthesis, Structural and Enhanced Photocatalytic Performance of Al Doped ZnO Nanoparticles. Adv. Powder Technol. 2017, 28, 1418–1425. [Google Scholar] [CrossRef]
- Wirunchit, S.; Koetniyom, W. ZnO Nanoparticles Synthesis and Characterization by Hydrothermal Process for Biological Applications. Phys. Status Solidi A 2023, 220, 2200364. [Google Scholar] [CrossRef]
- Apostoluk, A.; Zhu, Y.; Gautier, P.; Valette, A.; Bluet, J.M.; Cornier, T.; Masenelli, B.; Daniele, S. Improved Visible Emission from ZnO Nanoparticles Synthesized via the Co-Precipitation Method. Materials 2023, 16, 5400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Wang, X.; Zhang, A.; Gao, X.; Yagoub, A.E.-G.A.; Ma, H.; Zhou, C. Ultrasound-Assisted Synthesis of Potentially Food-Grade Nano-Zinc Oxide in Ionic Liquids: A Safe, Green, Efficient Approach and Its Acoustics Mechanism. Foods 2022, 11, 1656. [Google Scholar] [CrossRef]
- Gedanken, A.; Perelshtein, I.; Perkas, N. Power Ultrasound for the Production of Nanomaterials. In Power Ultrasonics: Applications of High-Intensity Ultrasound, 2nd ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 431–454. [Google Scholar] [CrossRef]
- Catto, A.C.; Oliveira, M.C.; Ribeiro, R.A.P.; Avansi, W.; da Silva, L.F.; Longo, E. Hematite Rhombuses for Chemiresitive Ozone Sensors: Experimental and Theoretical Approaches. Appl. Surf. Sci. 2021, 563, 150209. [Google Scholar] [CrossRef]
- de Almeida, W.L.; Freisleben, L.C.; Brambilla, B.C.; Isoppo, V.G.; Rodembusch, F.S.; de Sousa, V.C. Influence of Starch Used in the Sol-Gel Synthesis of ZnO Nanopowders. J. Nanoparticle Res. 2023, 25, 1–13. [Google Scholar] [CrossRef]
- Catto, A.C.; Da Silva, L.F.; Ribeiro, C.; Bernardini, S.; Aguir, K.; Longo, E.; Mastelaro, V.R. An Easy Method of Preparing Ozone Gas Sensors Based on ZnO Nanorods. RSC Adv. 2015, 5, 19528–19533. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N. Structural and Vibrational Properties of ZnO Nanoparticles Synthesized by the Chemical Precipitation Method. Phys. E Low Dimens. Syst. Nanostruct. 2014, 58, 130–137. [Google Scholar] [CrossRef]
- Decremps, F.; Pellicer-Porres, J.; Saitta, A.M.; Chervin, J.C.; Polian, A. High-Pressure Raman Spectroscopy Study of Wurtzite ZnO. Phys. Rev. B 2002, 65, 921011–921014. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Zhang, C.; Yang, Y.; Lv, K. Raman Spectra and Microstructure of Zinc Oxide Irradiated with Swift Heavy Ion. Crystals 2019, 9, 395. [Google Scholar] [CrossRef]
- Daiko, Y.; Schmidt, J.; Kawamura, G.; Romeis, S.; Segets, D.; Iwamoto, Y.; Peukert, W. Mechanochemically Induced Sulfur Doping in ZnO via Oxygen Vacancy Formation. Phys. Chem. Chem. Phys. 2017, 19, 13838–13845. [Google Scholar] [CrossRef]
- Kumar Inwati, G.; Kumar, P.; Roos, W.D.; Swart, H.C. Thermally Induced Structural Metamorphosis of ZnO:Rb Nanostructures for Antibacterial Impacts. Colloids Surf. B Biointerfaces 2020, 188, 110821. [Google Scholar] [CrossRef] [PubMed]
- Raji, R.; Gopchandran, K.G. ZnO Nanostructures with Tunable Visible Luminescence: Effects of Kinetics of Chemical Reduction and Annealing. J. Sci. Adv. Mater. Devices 2017, 2, 51–58. [Google Scholar] [CrossRef]
- da Silva-Neto, M.L.; de Oliveira, M.C.A.; Dominguez, C.T.; Lins, R.E.M.; Rakov, N.; de Araújo, C.B.; Menezes, L.d.S.; de Oliveira, H.P.; Gomes, A.S.L. UV Random Laser Emission from Flexible ZnO-Ag-Enriched Electrospun Cellulose Acetate Fiber Matrix. Sci. Rep. 2019, 9, 11765. [Google Scholar] [CrossRef]
- Rajith Kumar, C.R.; Betageri, V.S.; Nagaraju, G.; Pujar, G.H.; Onkarappa, H.S.; Latha, M.S. Synthesis of Core/Shell (ZnO/Ag) Nanoparticles Using Calotropis Gigantea and Their Applications in Photocatalytic and Antibacterial Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3410–3417. [Google Scholar] [CrossRef]
- Achehboune, M.; Khenfouch, M.; Boukhoubza, I.; Leontie, L.; Doroftei, C.; Carlescu, A.; Bulai, G.; Mothudi, B.; Zorkani, I.; Jorio, A. Microstructural, FTIR and Raman Spectroscopic Study of Rare Earth Doped ZnO Nanostructures. Mater. Today Proc. 2022, 53, 319–323. [Google Scholar] [CrossRef]
- Jung, S.H.; Oh, E.; Lee, K.H.; Yang, Y.; Park, C.G.; Park, W.; Jeong, S.H. Sonochemical Preparation of Shape-Selective ZnO Nanostructures. Cryst. Growth Des. 2008, 8, 265–269. [Google Scholar] [CrossRef]
- Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Gerbreders, A.; Mihailova, I.; Tamanis, E.; Ogurcovs, A. Hydrothermal Synthesis of ZnO Nanostructures with Controllable Morphology Change. CrystEngComm 2020, 22, 1346–1358. [Google Scholar] [CrossRef]
- Resende Leite, R.; Colombo, R.; Eduardo Bimbi Júnior, F.; Roberto de Vasconcelos Lanza, M.; da Silva Barud, H.; Ramos Moreira Afonso, C.; Inês Basso Bernardi, M. Precursor Effect on the Hydrothermal Synthesis of Pure ZnO Nanostructures and Enhanced Photocatalytic Performance for Norfloxacin Degradation. Chem. Eng. J. 2024, 496, 154374. [Google Scholar] [CrossRef]
- Phogat, P.; Shreya; Jha, R.; Singh, S. Harnessing ZnO Morphologies in Energy Application and Sustainable Development. Phys. Scr. 2024, 99, 102004. [Google Scholar] [CrossRef]
- Bagabas, A.; Alshammari, A.; Aboud, M.F.A.; Kosslick, H. Room-Temperature Synthesis of Zinc Oxide Nanoparticles in Different Media and Their Application in Cyanide Photodegradation. Nanoscale Res. Lett. 2013, 8, 516. [Google Scholar] [CrossRef]
- Ortega, P.P.; Aparecido, R.; Amoresi, C.; Daldin, M.; Leonnam, T.; Merízio, G.; Ramirez, M.A.; Aldao, M.; Malaguù, C.; Ponce, M.A.; et al. Insights into the Morphology and Structural Defects of Eu-Doped Ceria Nanostructures for Optoelectronic Applications in Red-Emitting Devices. ACS Appl. Nano Mater. 2024, 7, 12466–12479. [Google Scholar] [CrossRef]
- Hendrix, Y.; Rauwel, E.; Nagpal, K.; Haddad, R.; Estephan, E.; Boissière, C.; Rauwel, P. Revealing the Dependency of Dye Adsorption and Photocatalytic Activity of ZnO Nanoparticles on Their Morphology and Defect States. Nanomaterials 2023, 13, 1998. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.W.; Jung, S.H.; Lee, B.R.; Oh, E.; Lee, K.H. Precursor Effects of Citric Acid and Citrates on ZnO Crystal Formation. Langmuir 2009, 25, 3825–3831. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, A.F.; Lemos, S.C.S.; Leite, E.R.; Longo, E.; Andrés, J. Back to the Basics: Probing the Role of Surfaces in the Experimentally Observed Morphological Evolution of ZnO. Nanomaterials 2023, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.P.; Semancik, S. Controlling the Morphology of Zinc Oxide Nanorods Crystallized from Aqueous Solutions: The Effect of Crystal Growth Modifiers on Aspect Ratio. Chem. Mater. 2007, 19, 4016–4022. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, Y.; Tian, X.; Sun, Y. Asymmetric ZnO Nanostructures: Morphology Controlled Synthesis, Intrinsic Electric Fields Induced Growth Mechanism and n-Butanol Gas-Sensing Performance. J. Cryst. Growth 2021, 566–567, 126158. [Google Scholar] [CrossRef]
- Ma, Q.; Fang, Y.; Liu, Y.; Song, J.; Fu, X.; Li, H.; Chu, S.; Chen, Y. Facile Synthesis of ZnO Morphological Evolution with Tunable Growth Habits: Achieving Superior Gas-Sensing Properties of Hemispherical ZnO/Au Heterostructures for Triethylamine. Phys. E Low. Dimens. Syst. Nanostruct. 2019, 106, 180–186. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Morais, M.; Nunes, D.; Oliveira, M.J.; Rovisco, A.; Pimentel, A.; Águas, H.; Fortunato, E.; Martins, R. High UV and Sunlight Photocatalytic Performance of Porous ZnO Nanostructures Synthesized by a Facile and Fast Microwave Hydrothermal Method. Materials 2021, 14, 2385. [Google Scholar] [CrossRef]
- Choudhary, S.; Sahu, K.; Bisht, A.; Satpati, B.; Mohapatra, S. Rapid Synthesis of ZnO Nanowires and Nanoplates with Highly Enhanced Photocatalytic Performance. Appl. Surf. Sci. 2021, 541, 148484. [Google Scholar] [CrossRef]
- Arunkumar, G.; Deviga, G.; Mariappan, M.; Pannipara, M.; Al-Sehemi, A.G.; Soliman, U.A.; Anthony, S.P. Carbon Encapsulated ZnO Nanoplates for Efficient Removal of Organic Dyes from Aqueous Medium by Adsorption: Role of Organic Ligand and Calcination Temperature. J. Mol. Liq. 2024, 403, 124852. [Google Scholar] [CrossRef]
- Cui, J.; Shi, L.; Xie, T.; Wang, D.; Lin, Y. UV-Light Illumination Room Temperature HCHO Gas-Sensing Mechanism of ZnO with Different Nanostructures. Sens. Actuators B Chem. 2016, 227, 220–226. [Google Scholar] [CrossRef]
- Juabrum, S.; Chankhanittha, T.; Nanan, S. Hydrothermally Grown SDS-Capped ZnO Photocatalyst for Degradation of RR141 Azo Dye. Mater. Lett. 2019, 245, 1–5. [Google Scholar] [CrossRef]
- Alvi, N.H.; ul Hasan, K.; Nur, O.; Willander, M. The Origin of the Red Emission in N-Zno Nanotubes/p-Gan White Light Emitting Diodes. Nanoscale Res. Lett. 2011, 6, 130. [Google Scholar] [CrossRef]
- Carotta, M.C.; Cervi, A.; Fioravanti, A.; Gherardi, S.; Giberti, A.; Vendemiati, B.; Vincenzi, D.; Sacerdoti, M. A Novel Ozone Detection at Room Temperature through UV-LED-Assisted ZnO Thick Film Sensors. Thin Solid Film. 2011, 520, 939–946. [Google Scholar] [CrossRef]
- Baxter, J.B.; Schmuttenmaer, C.A. Conductivity of ZnO Nanowires, Nanoparticles, and Thin Films Using Time-Resolved Terahertz Spectroscopy. J. Phys. Chem. B 2006, 110, 25229–25239. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.H.; Sawyer, S.M. Oxygen Adsorption and Photoconduction Models for Metal Oxide Semiconductors: A Review. IEEE Sens. J. 2021, 21, 16409–16427. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Ponce, M.A.; Aldao, C.M.; Castro, M.S. Influence of Particle Size on the Conductance of SnO2 Thick Films. J. Eur. Ceram. Soc. 2003, 23, 2105–2111. [Google Scholar] [CrossRef]
- Joshi, N.; da Silva, L.F.; Shimizu, F.M.; Mastelaro, V.R.; M’Peko, J.C.; Lin, L.; Oliveira, O.N. UV-Assisted Chemiresistors Made with Gold-Modified ZnO Nanorods to Detect Ozone Gas at Room Temperature. Microchim. Acta 2019, 186, 418. [Google Scholar] [CrossRef]
- Güell, F.; Galdámez-Martínez, A.; Martínez-Alanis, P.R.; Catto, A.C.; da Silva, L.F.; Mastelaro, V.R.; Santana, G.; Dutt, A. ZnO-Based Nanomaterials Approach for Photocatalytic and Sensing Applications: Recent Progress and Trends. Mater. Adv. 2023, 4, 3685–3707. [Google Scholar] [CrossRef]
- De Palma, J.V.N.; Catto, A.C.; de Oliveira, M.C.; Ribeiro, R.A.P.; Teodoro, M.D.; da Silva, L.F. Light-Assisted Ozone Gas-Sensing Performance of SnO2 Nanoparticles: Experimental and Theoretical Insights. Sens. Actuators Rep. 2022, 4, 100081. [Google Scholar] [CrossRef]
- Sui, N.; Zhang, P.; Zhou, T.; Zhang, T. Selective Ppb-Level Ozone Gas Sensor Based on Hierarchical Branch-like In2O3 Nanostructure. Sens. Actuators B Chem. 2021, 336, 129612. [Google Scholar] [CrossRef]
- Jafarova, V.N.; Orudzhev, G.S. Structural and Electronic Properties of ZnO: A First-Principles Density-Functional Theory Study within LDA(GGA) and LDA(GGA)+U Methods. Solid. State Commun. 2021, 325, 114166. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A Review on ZnO: Fundamental Properties and Applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Samavati, A.; Awang, A.; Samavati, Z.; Fauzi Ismail, A.; Othman, M.H.D.; Velashjerdi, M.; Eisaabadi, B.G.; Rostami, A. Influence of ZnO Nanostructure Configuration on Tailoring the Optical Bandgap: Theory and Experiment. Mater. Sci. Eng. B 2021, 263, 114811. [Google Scholar] [CrossRef]
- Wahyuono, R.A.; Hermann-Westendorf, F.; Dellith, A.; Schmidt, C.; Dellith, J.; Plentz, J.; Schulz, M.; Presselt, M.; Seyring, M.; Rettenmeyer, M.; et al. Effect of Annealing on the Sub-Bandgap, Defects and Trapping States of ZnO Nanostructures. Chem. Phys. 2017, 483–484, 112–121. [Google Scholar] [CrossRef]
- Nandi, A.; Majumder, R.; Nag, P.; Datta, S.K.; Saha, H.; Majumdar, S. Precursor Dependent Tailoring of Morphology and Bandgap of Zinc Oxide Nanostructures. J. Mater. Sci. Mater. Electron. 2017, 28, 10885–10892. [Google Scholar] [CrossRef]
- Desimone, P.M.; Zonta, G.; Giulietti, G.; Ortega, P.P.; Aldao, C.M.; Simões, A.Z.; Moura, F.; Ponce, M.A.; Foschini, C.R. Towards Carbon Monoxide Detection Based on ZnO Nanostructures. Mater. Sci. Eng. B 2024, 299, 117003. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and Shape Effects on the Band Gap of Semiconductor Compound Nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef]
- Bhat, S.V.; Deepak, F.L. Tuning the Bandgap of ZnO by Substitution with Mn2+, Co2+ and Ni2+. Solid State Commun. 2005, 135, 345–347. [Google Scholar] [CrossRef]
- Joseph, D.P.; Venkateswaran, C. Bandgap Engineering in ZnO By Doping with 3d Transition Metal Ions. J. At. Mol. Phys. 2011, 2011, 270540. [Google Scholar] [CrossRef]
- Umavathi, S.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Virik, P.; Al-Mulhm, N.; Subash, M.; Gopinath, K.; Kavitha, C. Green Synthesis of ZnO Nanoparticles for Antimicrobial and Vegetative Growth Applications: A Novel Approach for Advancing Efficient High Quality Health Care to Human Wellbeing. Saudi J. Biol. Sci. 2021, 28, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Habibi-Yangjeh, A.; Pirhashemi, M.; Ghosh, S. ZnO/ZnBi2O4 Nanocomposites with p-n Heterojunction as Durable Visible-Light-Activated Photocatalysts for Efficient Removal of Organic Pollutants. J. Alloys Compd. 2020, 826, 154229. [Google Scholar] [CrossRef]
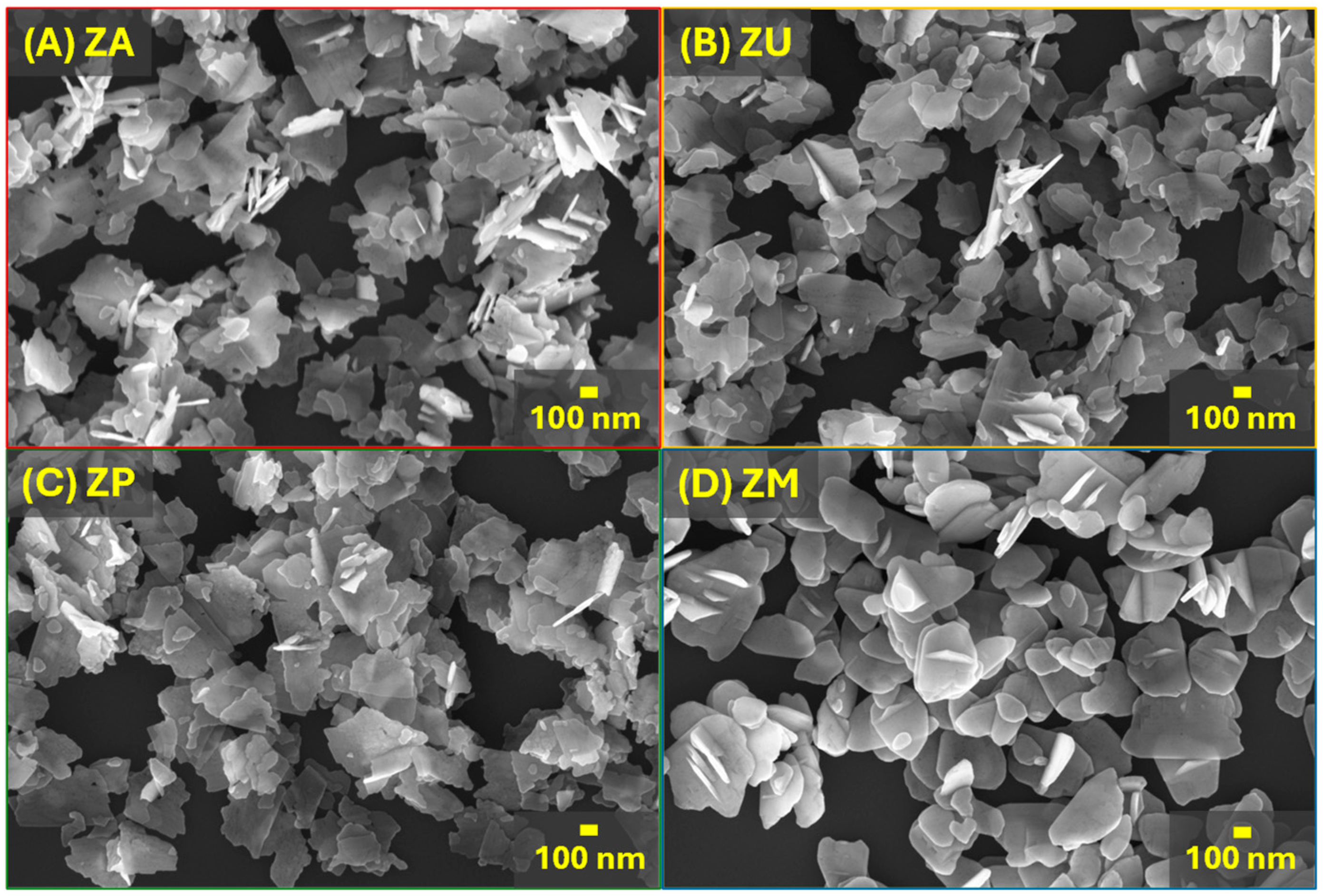
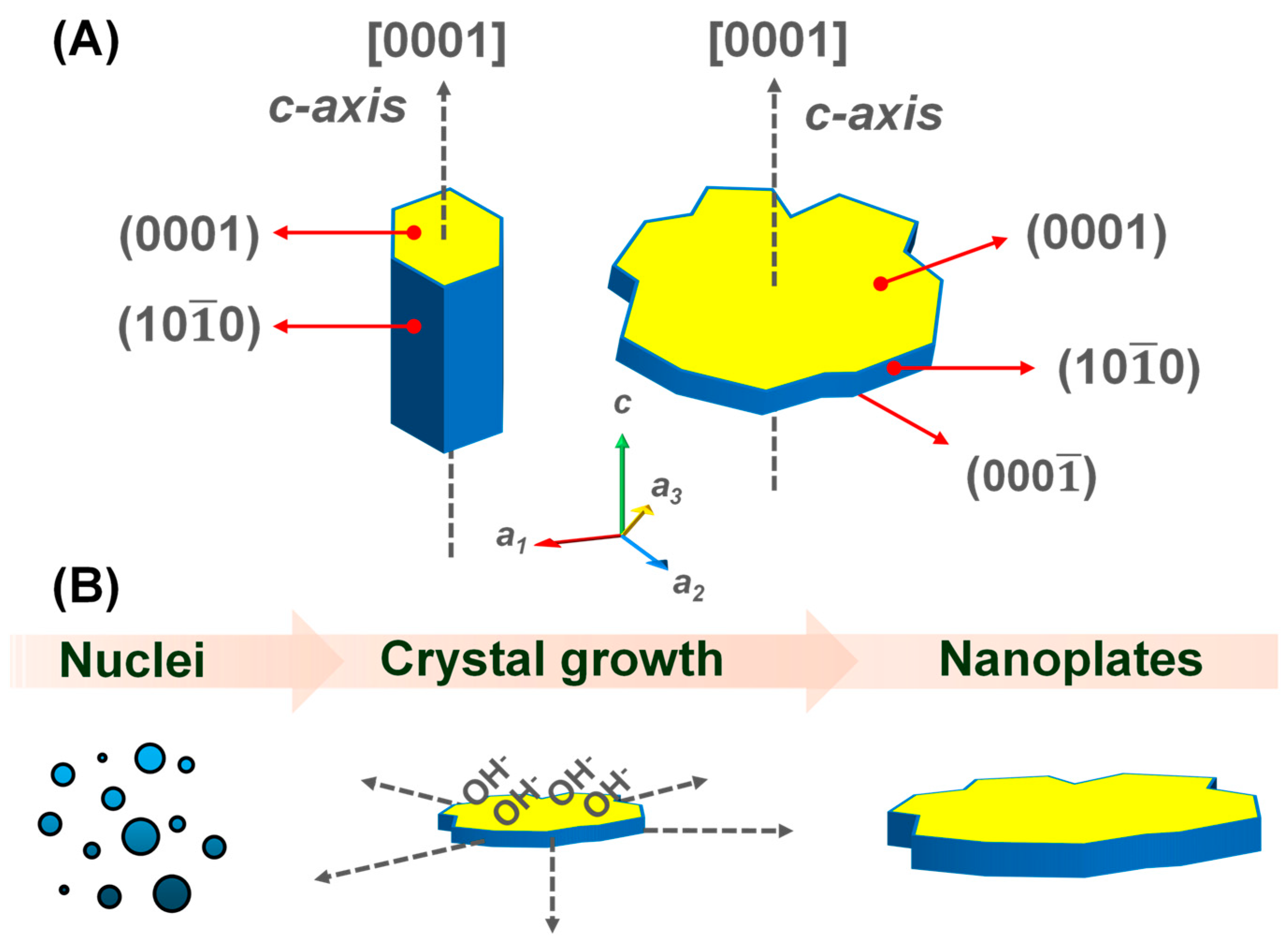
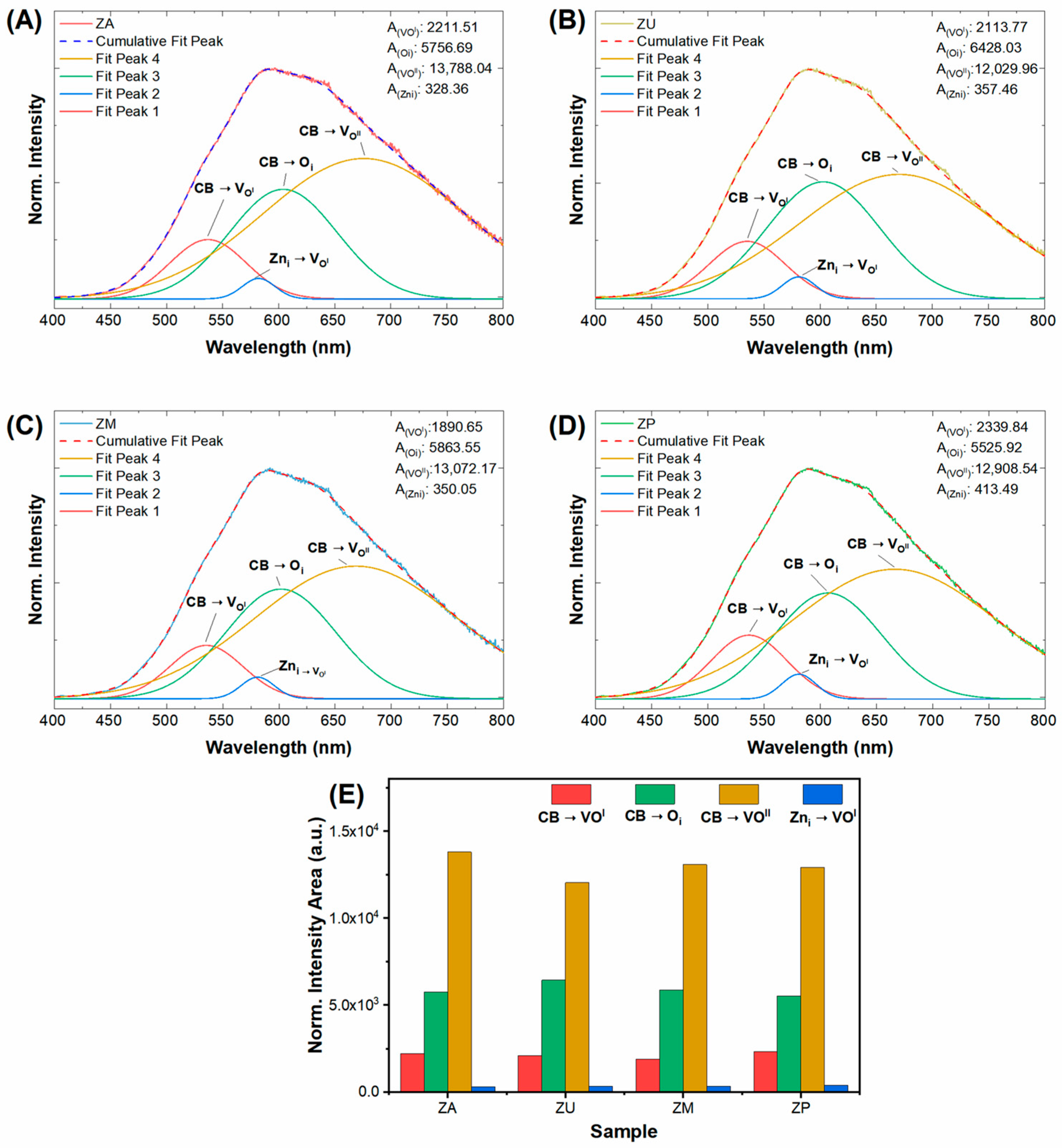
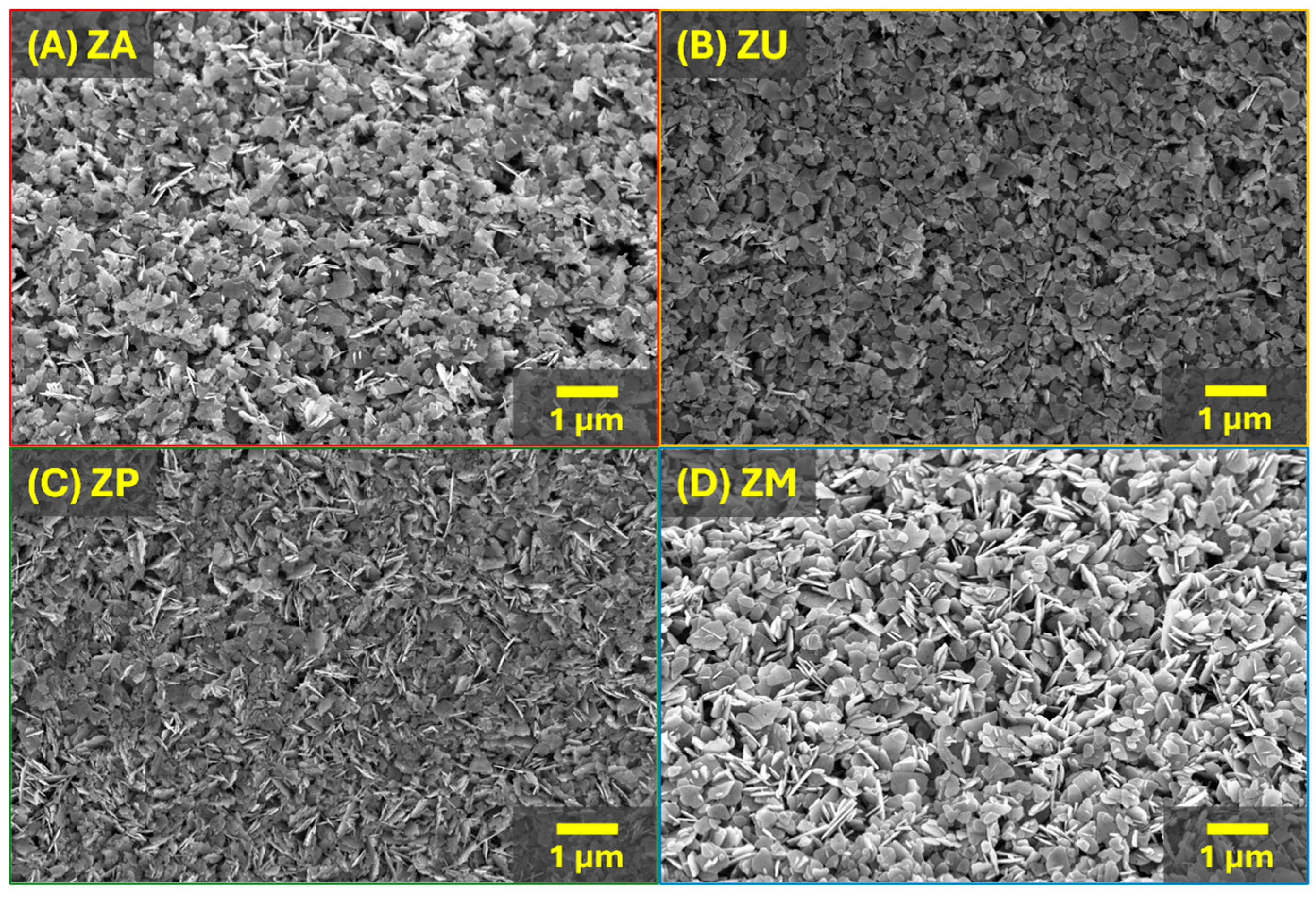
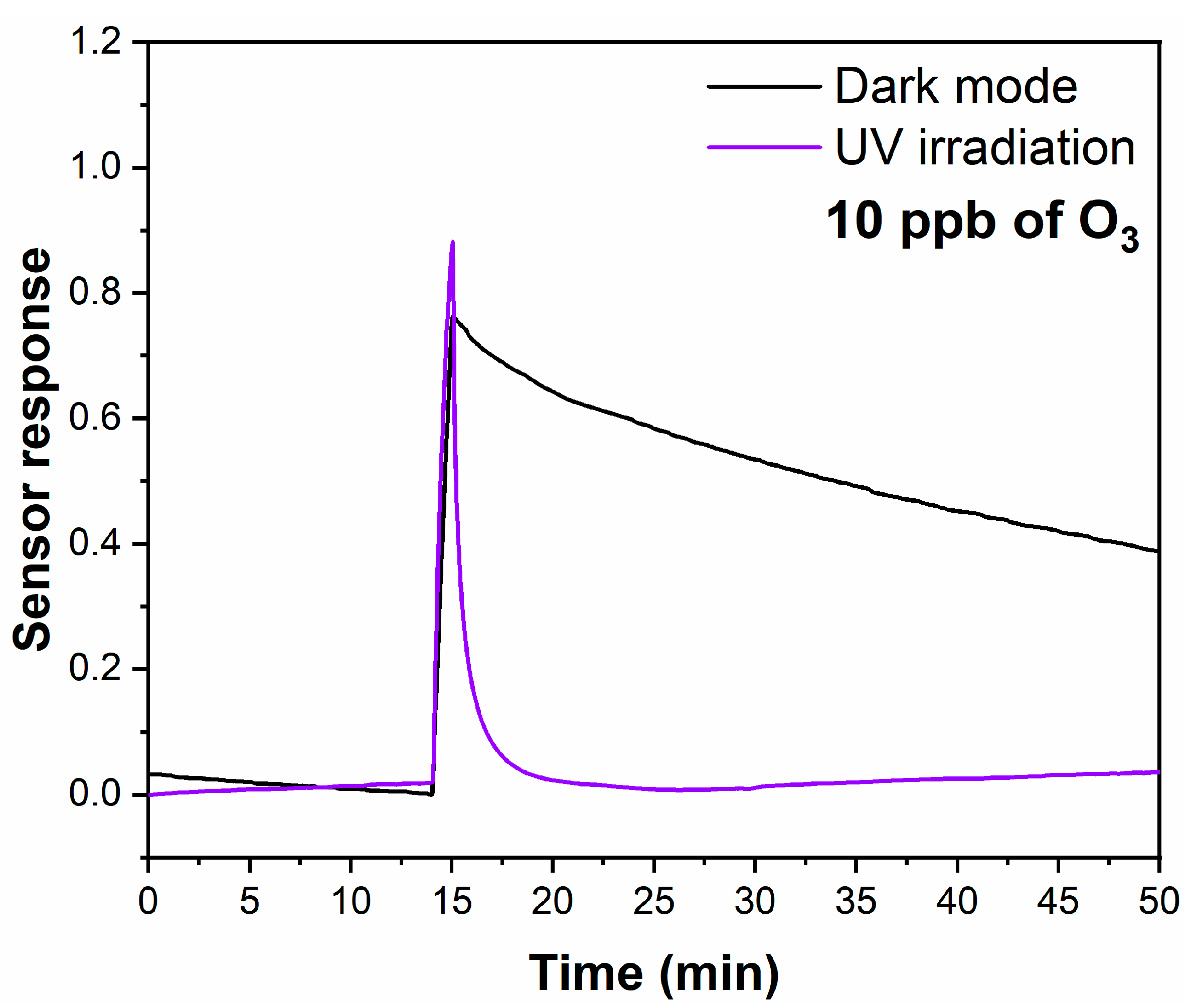


| Sample | Top Area (µm2) | Thickness (nm) | Specific Surface Area (m2/g) |
|---|---|---|---|
| ZA | 0.0142 ± 0.0017 | 17.1 ± 0.4 | 15.86 ± 0.05 |
| ZU | 0.0122 ± 0.0013 | 22.8 ± 0.6 | 19.37 ± 0.08 |
| ZP | 0.0122 ± 0.0014 | 17.7 ± 0.6 | 24.75 ± 0.03 |
| ZM | 0.0332 ± 0.0033 | 26.0 ± 0.6 | 15.32 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, P.P.; Palma, J.V.N.; Doimo, A.L.; Líbero, L.; Yamakawa, G.F.; Merízio, L.G.; Aguiar, E.C.; Silva, L.F.; Longo, E. Influence of Different Synthesis Methods on the Defect Structure, Morphology, and UV-Assisted Ozone Sensing Properties of Zinc Oxide Nanoplates. Chemosensors 2025, 13, 152. https://doi.org/10.3390/chemosensors13040152
Ortega PP, Palma JVN, Doimo AL, Líbero L, Yamakawa GF, Merízio LG, Aguiar EC, Silva LF, Longo E. Influence of Different Synthesis Methods on the Defect Structure, Morphology, and UV-Assisted Ozone Sensing Properties of Zinc Oxide Nanoplates. Chemosensors. 2025; 13(4):152. https://doi.org/10.3390/chemosensors13040152
Chicago/Turabian StyleOrtega, Pedro P., João V. N. Palma, Ana L. Doimo, Laura Líbero, Gabriel F. Yamakawa, Leonnam G. Merízio, Ederson C. Aguiar, Luís F. Silva, and Elson Longo. 2025. "Influence of Different Synthesis Methods on the Defect Structure, Morphology, and UV-Assisted Ozone Sensing Properties of Zinc Oxide Nanoplates" Chemosensors 13, no. 4: 152. https://doi.org/10.3390/chemosensors13040152
APA StyleOrtega, P. P., Palma, J. V. N., Doimo, A. L., Líbero, L., Yamakawa, G. F., Merízio, L. G., Aguiar, E. C., Silva, L. F., & Longo, E. (2025). Influence of Different Synthesis Methods on the Defect Structure, Morphology, and UV-Assisted Ozone Sensing Properties of Zinc Oxide Nanoplates. Chemosensors, 13(4), 152. https://doi.org/10.3390/chemosensors13040152







