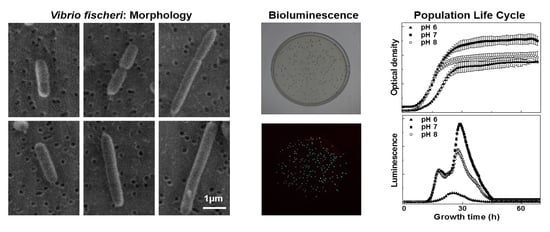
pH-Induced Modulation of Vibrio fischeri Population Life Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organism and Growth Medium
2.2. Aerobic Growth of V. fischeri
2.3. Morphological Analysis
2.4. Viability of V. fischeri Cells
2.5. Characterization of Surface Charge
3. Results
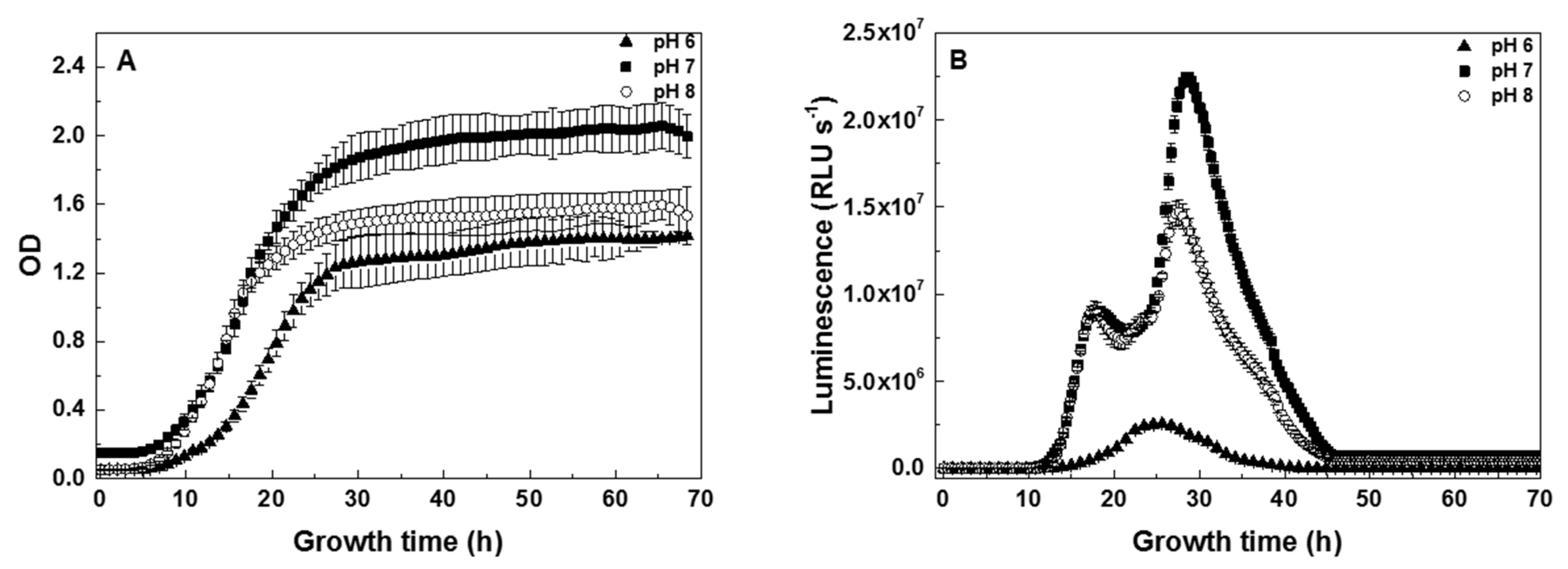
3.1. Dependence of V. fischeri Growth and Bioluminescence on pH
3.2. Surface Charge Analysis
3.3. Viability Assays
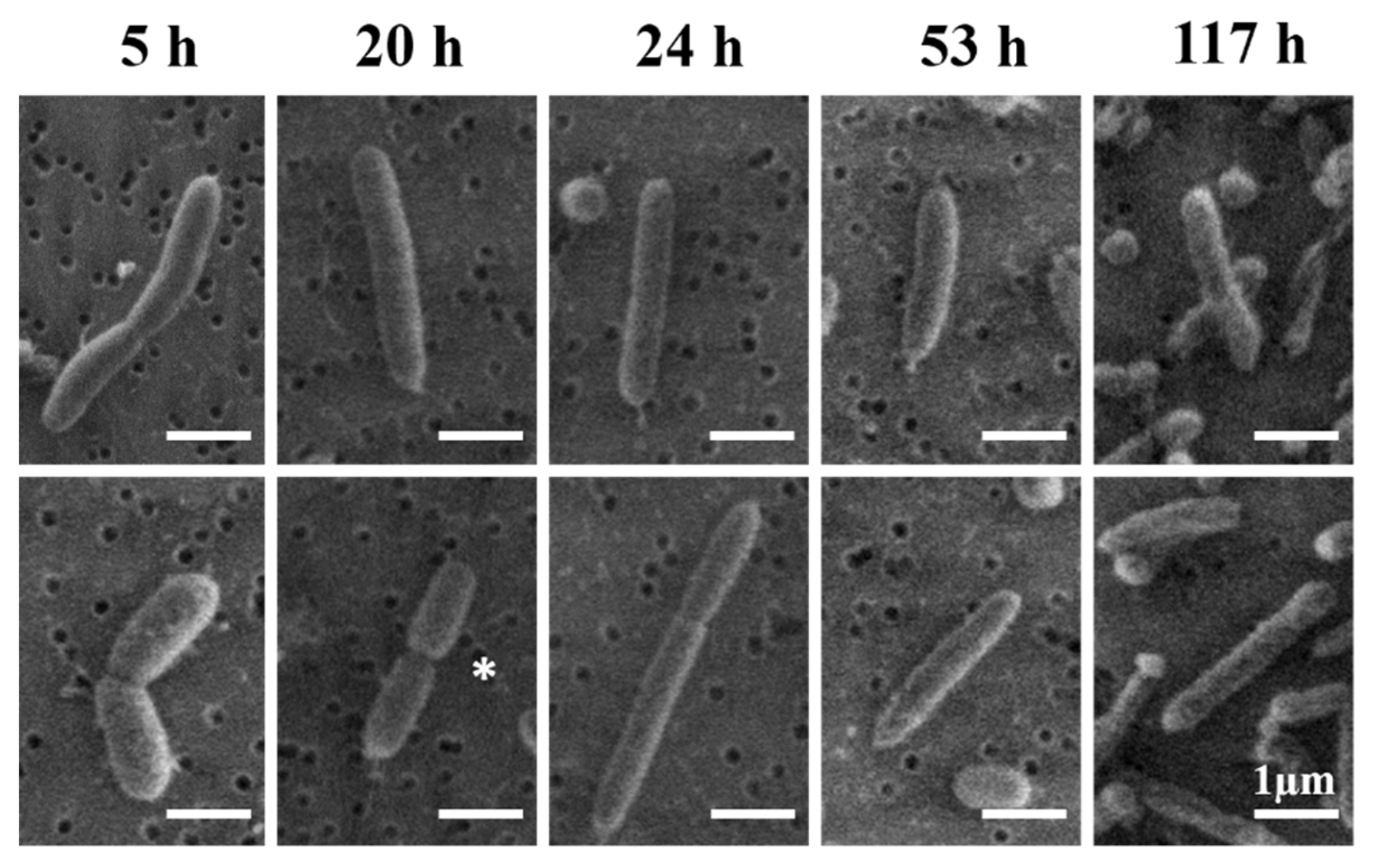
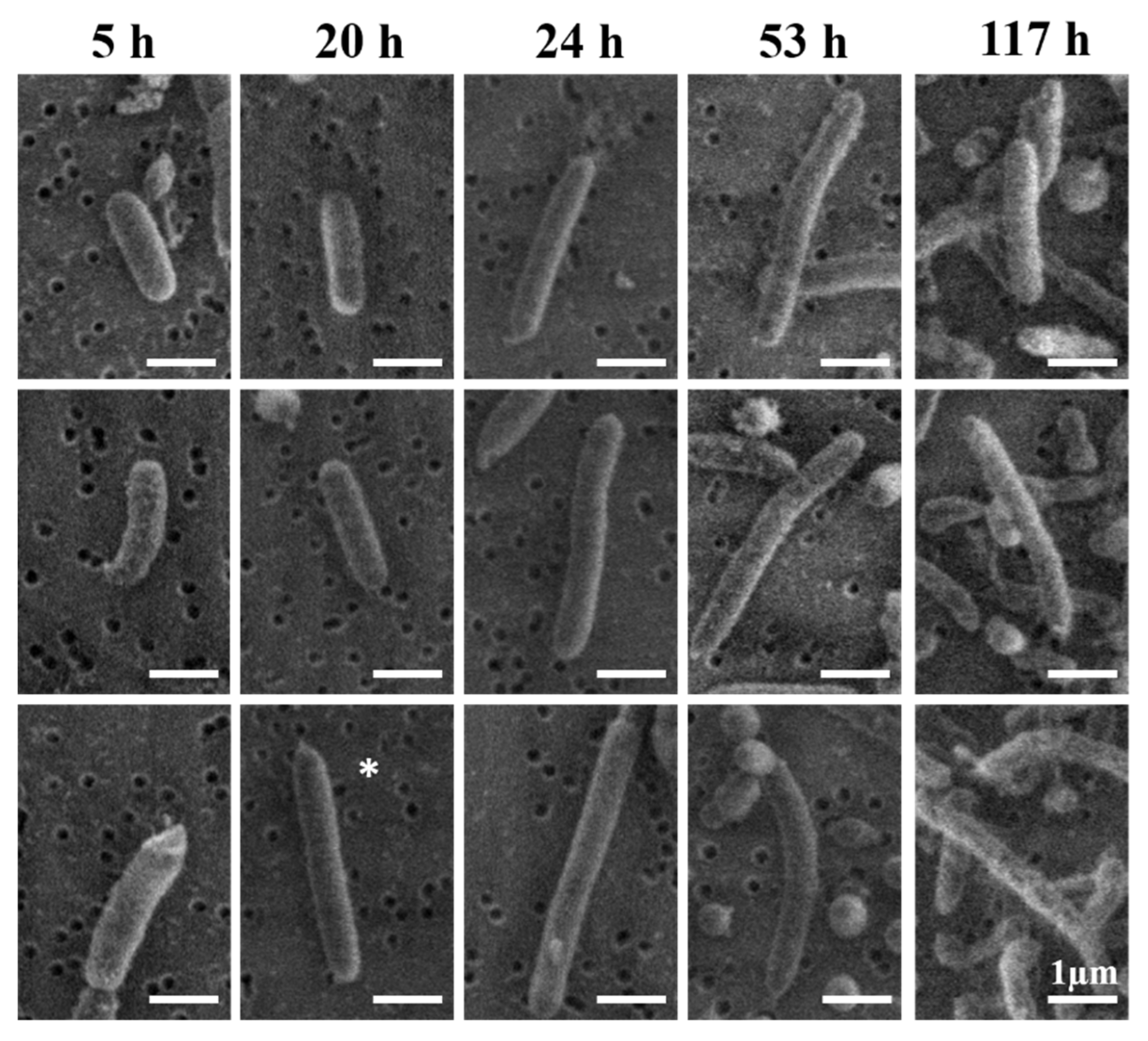
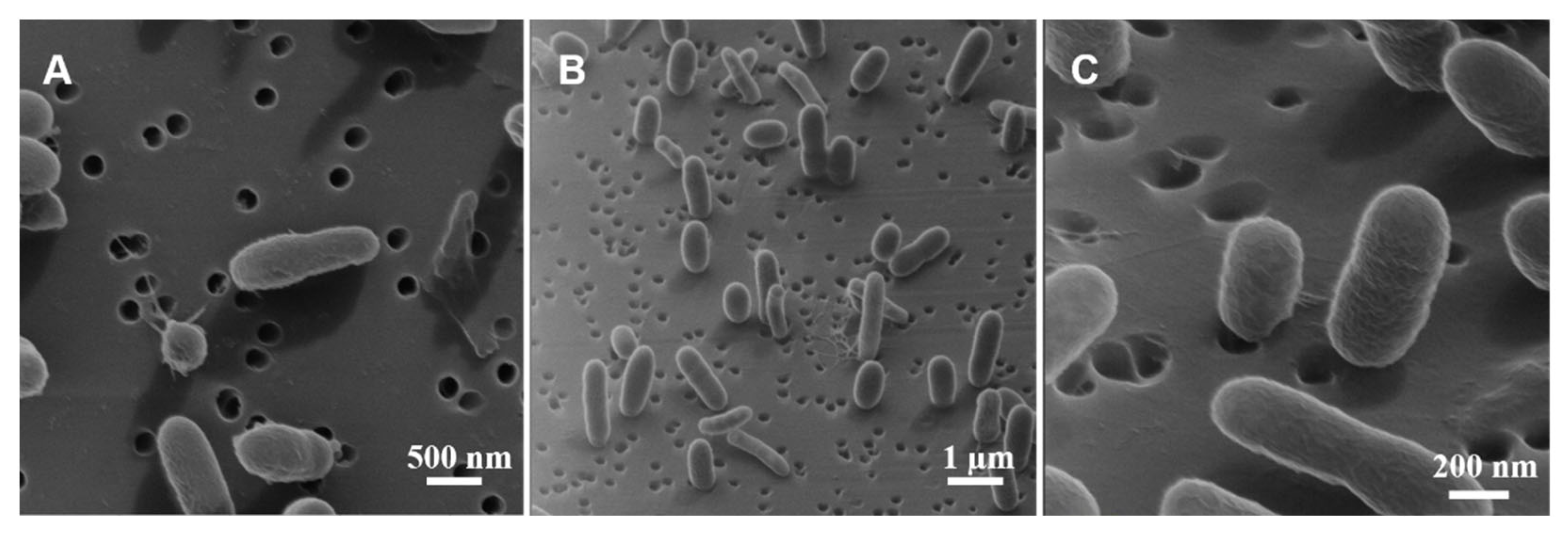
3.4. Morphological Analysis
4. Discussion
4.1. Timing of the V. fischeri Growth Phases
4.2. Surface Charge of V. fischeri Is pH-Independent
4.3. Time-Profiles of V. fischeri Bioluminescence Are pH-Dependent
4.4. Typical Morphology of V. fischeri Cells
4.5. Interpretation of the Apparently Round Cell Morphology
4.6. Mechanism for Producing the Extended-Rod Morphology
4.7. Extended-Rod Morphology as a Metabolic Stress Indicator
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Asif Tahir, M.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Shvets, V.I.; Reshetilov, A.N. A mediator microbial biosensor for assaying general toxicity. Enzym. Microb. Technol. 2020, 132, 109435. [Google Scholar] [CrossRef] [PubMed]
- Mirjani, M.; Soleimani, M.; Salari, V. Toxicity assessment of total petroleum hydrocarbons in aquatic environments using the bioluminescent bacterium Aliivibrio fischeri. Ecotoxicol. Environ. Saf. 2021, 207, 111554. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Venkataraman, C.; Mukherji, S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 2006, 32, 265–268. [Google Scholar] [CrossRef]
- Asano, T.; Wang, P.C.; Iwasaki, A. Spectrophotometric detection of labile zinc (II) released from metallothionein: A simple method to evaluate heavy metal toxicity. J. Biosci. Bioeng. 2010, 109, 638–644. [Google Scholar] [CrossRef]
- Mendonça, E.; Picado, A.; Paixão, S.M.; Silva, L.; Cunha, M.A.; Leitão, S.; Moura, I.; Cortez, C.; Brito, F. Ecotoxicity tests in the environmental analysis of wastewater treatment plants: Case study in Portugal. J. Hazard. Mater. 2009, 163, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Riego Sintes, J.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Grillo, R.; Mattos, B.D.; Antunes, D.R.; Forini, M.M.L.; Monikh, F.A.; Rojas, O.J. Foliage adhesion and interactions with particulate delivery systems for plant nanobionics and intelligent agriculture. Nano Today 2021, 37, 101078. [Google Scholar] [CrossRef]
- Karimi, M.; Sadeghi, R.; Kokini, J. Human exposure to nanoparticles through trophic transfer and the biosafety concerns that nanoparticle-contaminated foods pose to consumers. Trends Food Sci. Technol. 2018, 75, 129–145. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in cosmetics: Recent updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Trindade, T.; Duarte, A.C.; Pereira, E.; Koopmans, G.F.; Römkens, P.F.A.M. A framework to measure the availability of engineered nanoparticles in soils: Trends in soil tests and analytical tools. TrAC Trends Anal. Chem. 2016, 75, 129–140. [Google Scholar] [CrossRef]
- Németh, I.; Molnár, S.; Vaszita, E.; Molnár, M. The Biolog EcoPlateTM technique for assessing the effect of metal oxide nanoparticles on freshwater microbial communities. Nanomaterials 2021, 11, 1777. [Google Scholar] [CrossRef] [PubMed]
- Fekete-Kertész, I.; Piszmán, D.; Molnár, M. Particle size and concentration dependent ecotoxicity of nano- and microscale TiO2 —Comparative study by different aquatic test organisms of different trophic levels. Water Air Soil Pollut. 2017, 228, 245. [Google Scholar] [CrossRef]
- Trinh, T.X.; Kim, J. Status Quo in data availability and predictive models of nano-mixture toxicity. Nanomaterials 2021, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Ríos, F.; Fernández-Arteaga, A.; Fernández-Serrano, M.; Jurado, E.; Lechuga, M. Silica micro- and nanoparticles reduce the toxicity of surfactant solutions. J. Hazard. Mater. 2018, 353, 436–443. [Google Scholar] [CrossRef]
- Urbanczyk, H.; Ast, J.C.; Higgins, M.J.; Carson, J.; Dunlap, P.V. Reclassification of Vibrio fischeri, Vibrio logei, Vibrio salmonicida and Vibrio wodanis as Aliivibrio fischeri gen. nov., comb. nov., Aliivibrio logei comb. nov., Aliivibrio salmonicida comb. nov. and Aliivibrio wodanis comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2823–2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Tashiro, Y.; Saito, K.; Kawai-Noma, S.; Umeno, D. Directed evolution of Vibrio fischeri LuxR signal sensitivity. J. Biosci. Bioeng. 2016, 122, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Fuqua, W.C.; Winans, C.S.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Madden, D.; Lidesten, B.-M. Bacterial illumination Culturing luminous bacteria. In Bioscience Explained; NCBE, The University of Reading: Reading, UK, 2001; Volume 1, pp. 1–8. [Google Scholar]
- Nealson, K.H.; Platt, T.; Hastings, J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970, 104, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, K.L.E. Correlations of Vibrio fischeri bacteria test data with bioassay data for other organisms. Environ. Health Perspect. 1998, 106, 583–591. [Google Scholar]
- Dunn, A.K. Vibrio fischeri metabolism: Symbiosis and beyond. Adv. Microb. Physiol. 2012, 61, 37–68. [Google Scholar] [CrossRef]
- Bose, J.L.; Kim, U.; Bartkowski, W.; Gunsalus, R.P.; Overley, A.M.; Lyell, N.L.; Visick, K.L.; Stabb, E.V. Bioluminescence in Vibrio fischeri is controlled by the redox-responsive regulator ArcA. Mol. Microbiol. 2007, 65, 538–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-H.; Tian, X. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef] [PubMed]
- Lupp, C.; Ruby, E.G. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J. Bacteriol. 2005, 187, 3620–3629. [Google Scholar] [CrossRef] [Green Version]
- Manefield, M.; Chong, G. Quorum Sensing signal synthesis may represent a selective advantage independent of its role in regulation of bioluminescence in Vibrio fischeri. PLoS ONE 2013, 8, e6744. [Google Scholar] [CrossRef]
- Pérez, P.D.; Hagen, S.J. Heterogeneous response to a quorum-sensing signal in the luminescence of individual Vibrio fischeri. PLoS ONE 2010, 5, e15473. [Google Scholar] [CrossRef] [Green Version]
- Scheerer, S.; Gomez, F.; Lloyd, D. Bioluminescence of Vibrio fischeri in continuous culture: Optimal conditions for stability and intensity of photoemission. J. Microbiol. Methods 2006, 67, 321–329. [Google Scholar] [CrossRef]
- Rizzo, L. Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res. 2011, 45, 4311–4340. [Google Scholar] [CrossRef] [PubMed]
- Marugán, J.; Bru, D.; Pablos, C.; Catalá, M. Comparative evaluation of acute toxicity by Vibrio fischeri and fern spore based bioassays in the follow-up of toxic chemicals degradation by photocatalysis. J. Hazard. Mater. 2012, 213–214, 117–122. [Google Scholar] [CrossRef]
- Miyashiro, T.; Ruby, E.G. Shedding light on bioluminescence regulation in Vibrio fischeri. Mol. Microbiol. 2013, 84, 795–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.R.; Martins, P.M.; Lanceros-Mendez, S.; Teixeira, S.; Carabineiro, S.A.C.; Kuehn, K.; Cuniberti, G.; Alves, M.M.; Pereira, L. Ciprofloxacin wastewater treated by UVA photocatalysis: Contribution of irradiated TiO2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio fischeri. RSC Adv. 2016, 6, 95494–95503. [Google Scholar] [CrossRef]
- Silva, A.R.; Soares, O.S.G.P.; Pereira, M.F.R.; Alves, M.M.; Pereira, L. Tailoring carbon nanotubes to enhance their efficiency as electron shuttle on the biological removal of acid orange 10 under anaerobic conditions. Nanomaterials 2020, 10, 2496. [Google Scholar] [CrossRef]
- Silva, A.R.; Cavaleiro, A.J.; Soares, O.S.G.P.; Braga, C.S.N.; Salvador, A.F.; Pereira, M.F.R.; Alves, M.M.; Pereira, L. Detoxification of ciprofloxacin in an anaerobic bioprocess supplemented with magnetic carbon nanotubes: Contribution of adsorption and biodegradation mechanisms. Int. J. Mol. Sci. 2021, 22, 2932. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, P.; Liang, P.; Hao, W.; Li, M.; Huang, X. Dual-signal-biosensor based on luminescent bacteria biofilm for real-time online alert of Cu (II) shock. Biosens. Bioelectron. 2019, 142, 111500. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Venkataraman, C.; Mukherji, S. Toxicity assessment of organic pollutants: Reliability of bioluminescence inhibition assay and univariate QSAR models using freshly prepared Vibrio fischeri. Toxicol. In Vitro 2008, 22, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.J.; Jennings, V.L.K.; Rayner-Brandes, M.H. Assessing chemical toxicity with the bioluminescent Photobacterium (Vibrio fischeri): A comparison of three commercial systems. Water Res. 2001, 35, 3448–3456. [Google Scholar]
- Bayo, J.; Angosto, J.M.; Gómez-López, M.D. Ecotoxicological screening of reclaimed disinfected wastewater by Vibrio fischeri bioassay after a chlorination-dechlorination process. J. Hazard. Mater. 2009, 172, 166–171. [Google Scholar] [CrossRef]
- Minetto, D.; Libralato, G.; Ghirardini, A.V. Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: An overview. Environ. Int. 2014, 66, 18–27. [Google Scholar] [CrossRef]
- Bystrzejewska-Piotrowska, G.; Golimowski, J.; Urban, P.L. Nanoparticles: Their potential toxicity, waste and environmental management. Waste Manag. 2009, 29, 2587–2595. [Google Scholar] [CrossRef]
- Hussain, S.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Murdock, R.C.; Yu, K.O.; Mattie, D.M.; Schlager, J.J. Toxicity evaluation for safe use of nanomaterials: Recent achievements and technical challenges. Adv. Mater. 2009, 21, 1549–1559. [Google Scholar] [CrossRef]
- Neal, A.L. What can be inferred from bacterium—nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology 2008, 17, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Tartanson, M.-A.; Soussan, L.; Rivallin, M.; Pecastaings, S.; Chis, C.V.; Penaranda, D.; Roques, C.; Faur, C. Dynamic mechanisms of the bactericidal action of an Al2O3-TiO2-Ag granular material on an Escherichia coli strain. Appl. Environ. Microbiol. 2015, 81, 7135–7142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Hughes, J.; Chen, Y. Impacts of hematite nanoparticle exposure on biomechanical, adhesive, and surface electrical properties of Escherichia coli cells. Appl. Environ. Microbiol. 2012, 78, 3905–3915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.-L.; Shao, M.-F.; Xu, Y.-S.; Luo, Y.; Zhang, K.; Ouyang, F.; Li, J. Non-selective separation of bacterial cells with magnetic nanoparticles facilitated by varying surface charge. Front. Microbiol. 2016, 7, 1891. [Google Scholar] [CrossRef] [PubMed]
- Westmeier, D.; Hahlbrock, A.; Reinhardt, C.; Fröhlich-Nowoisky, J.; Wessler, S.; Vallet, C.; Pöschl, U.; Knauer, S.K.; Stauber, R.H. Nanomaterial–microbe cross-talk: Physicochemical principles and (patho) biological consequences. Chem. Soc. Rev. 2018, 47, 5312–5337. [Google Scholar] [CrossRef] [PubMed]
- Kurvet, I.; Juganson, K.; Vija, H.; Sihtmäe, M.; Blinova, I.; Syvertsen-Wiig, G.; Kahru, A. Toxicity of nine (doped) rare earth metal oxides and respective individual metals to aquatic microorganisms Vibrio fischeri and Tetrahymena thermophila. Materials 2017, 10, 754. [Google Scholar] [CrossRef] [Green Version]
- ISO 11348-1 INTERNATIONAL STANDARD. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part1: Method Using Freshly Prepared Bacteria; ISO—International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- ISO 11348-3 INTERNATIONAL STANDARD. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part3: Method Using Freeze-Dried Bacteria; ISO—International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- Sousa, C.; Petrovykh, D.Y. Characterization of Bio-nanosystems. In Advances in Processing Technologies for Bio-Based Nanosystems in Food; Ramos, O., Pereira, R., Cerqueira, M., Martins, J., Teixeira, J., Malcata, F., Vicente, A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 271–306. ISBN 9780128115169. [Google Scholar]
- Waters, P.; Lloyd, D. Salt, pH and temperature dependencies of growth and bioluminescence of three species of luminous bacteria analysed on gradient plates. Microbiology 1985, 131, 2865–2869. [Google Scholar] [CrossRef] [Green Version]
- Fulladosa, E.; Murat, J.C.; Villaescusa, I. Effect of cadmium (II), chromium (VI), and arsenic (V) on long-term viability- and growth-inhibition assays using Vibrio fischeri marine bacteria. Arch. Environ. Contam. Toxicol. 2005, 49, 299–306. [Google Scholar] [CrossRef]
- Schmitz, R.P.H.; Eisentrager, A.; Dott, W. Miniaturized kinetic growth inhibition assays with Vibrio fischeri and Pseudomonas putida (application, validation and comparison). J. Microbiol. Methods 1998, 31, 159–166. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, Y.; He, G.-X.; Katagori, N.; Chen, H. A comparison of conventional methods for the quantification of bacterial cells after exposure to metal oxide nanoparticles. BMC Microbiol. 2014, 14, 222. [Google Scholar] [CrossRef] [Green Version]
- Hannig, C.; Hannig, M.; Rehmer, O.; Braun, G.; Hellwig, E.; Al-Ahmad, A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch. Oral Biol. 2007, 52, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Sequeira, D.; Kolen’ko, Y.V.; Pinto, I.M.; Petrovykh, D.Y. Analytical protocols for separation and electron microscopy of nanoparticles interacting with bacterial cells. Anal. Chem. 2015, 87, 4641–4648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.S.; Khodadad, C.L.M.; Ahrendt, S.R.; Parrish, M.L. Impact of simulated microgravity on the normal developmental time line of an animal-bacteria symbiosis. Sci. Rep. 2013, 3, 1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, A.K.; Stabb, E.V. Genetic analysis of trimethylamine N- oxide reductases in the light organ symbiont Vibrio fischeri ES114. J. Bacteriol. 2008, 190, 5814–5823. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.P.; Mitik-Dineva, N.; Mocanasu, R.C.; Murphy, S.; Wang, J.; van Riessen, G.; Crawford, R.J. Vibrio fischeri and Escherichia coli adhesion tendencies towards photolithographically modified nanosmooth poly (tert-butyl methacrylate) polymer surfaces. Nanotechnol. Sci. Appl. 2008, 1, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Barbarossa, M.V.; Kuttler, C.; Fekete, A.; Rothballer, M. A delay model for quorum sensing of Pseudomonas putida. BioSystems 2010, 102, 148–156. [Google Scholar] [CrossRef]
- Gusnard, D.; Kirschner, R.H. Cell and organelle shrinkage during preparation for scanning electron microscopy: Effects of fixation, dehydration and critical point drying. J. Microsc. 1977, 110, 51–57. [Google Scholar] [CrossRef]
- Millikan, D.S.; Ruby, E.G. Vibrio fischeri flagellin A is essential for normal motility and for symbiotic competence during initial squid light organ colonization. J. Bacteriol. 2004, 186, 4315–4325. [Google Scholar] [CrossRef] [Green Version]
- Falcioni, T.; Papa, S.; Campana, R.; Manti, A.; Battistelli, M.; Baffone, W.; Bo, C. State transitions of Vibrio parahaemolyticus VBNC cells evaluated by flow cytometry. Cytom. Part B Clin. Cytom. 2008, 74, 272–281. [Google Scholar] [CrossRef]
- Oliver, J.D.; Nilsson, L.; Kjelleberg, S. Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl. Environ. Microbiol. 1991, 57, 2640–2644. [Google Scholar] [CrossRef] [Green Version]
- Oliver, J.D. The viable but non-culturable state in the human pathogen Vibrio vulnificus. FEMS Microbiol. Lett. 1995, 133, 203–208. [Google Scholar] [CrossRef]
- Joens, M.S.; Huynh, C.; Kasuboski, J.M.; Ferranti, D.; Sigal, Y.J.; Zeitvogel, F.; Obst, M.; Burkhardt, C.J.; Curran, K.P.; Chalasani, S.H.; et al. Helium Ion Microscopy (HIM) for the imaging of biological samples at sub-nanometer resolution. Sci. Rep. 2013, 3, 3514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, R.; Notte, J.; Ward, B. The ALIS He ion source and its application to high resolution microscopy. Phys. Procedia 2008, 1, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Ward, B.W.; Notte, J.A.; Economou, N.P. Helium ion microscope: A new tool for nanoscale microscopy and metrology. J. Vac. Sci. Technol. B 2006, 24, 2871–2874. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.; Huang, K.C. How and why cells grow as rods. BMC Biol. 2014, 12, 54. [Google Scholar] [CrossRef] [Green Version]
- Ursell, T.S.; Nguyen, J.; Monds, R.D.; Colavin, A.; Billings, G.; Ouzounov, N.; Gitai, Z.; Shaevitz, J.W.; Huang, K.C. Rod-like bacterial shape is maintained by feedback between cell curvature and cytoskeletal localization. Proc. Natl. Acad. Sci. USA 2014, 111, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Kruse, T.; Bork-Jensen, J.; Gerdes, K. The morphogenetic MreBCD proteins of Escherichia coli form an essential membrane-bound complex. Mol. Microbiol. 2005, 55, 78–89. [Google Scholar] [CrossRef]
- Postma, J.F.; de Valk, S.; Dubbeldam, M.; Maas, J.L.; Tonkes, M.; Schipper, C.A.; Kater, B.J. Confounding factors in bioassays with freshwater and marine organisms. Ecotoxicol. Environ. Saf. 2002, 53, 226–237. [Google Scholar] [CrossRef]
| pH | Growth at Specified pH 1 (ζ-Potential, mV) | Exposed to Specified pH 2 (ζ-Potential, mV) |
|---|---|---|
| 6 | −40.8 ± 5.9 | −18.0 ± 1.2 |
| 6.5 | - | −37.9 ± 0.8 |
| 7 | −44.5 ± 3.9 | −38.9 ± 0.7 |
| 7.5 | - | −37.5 ± 0.6 |
| 8 | −45.8 ± 7.4 | −38.4 ± 0.9 |
| Growth Time (h) | Length (µm) | Diameter (µm) |
|---|---|---|
| 5 | 3.2 ± 1.9 | 0.59 ± 0.1 |
| 20 | 2.2 ± 1.0 | 0.63 ± 0.1 |
| 24 | 1.9 ± 0.6 | 0.59 ± 0.1 |
| 53 | 2.1 ± 0.9 | 0.59 ± 0.1 |
| 117 | 2.1 ± 0.8 | 0.53 ± 0.1 |
| Average | 2.2 ± 1 | 0.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.R.; Sousa, C.; Exner, D.; Schwaiger, R.; Alves, M.M.; Petrovykh, D.Y.; Pereira, L. pH-Induced Modulation of Vibrio fischeri Population Life Cycle. Chemosensors 2021, 9, 283. https://doi.org/10.3390/chemosensors9100283
Silva AR, Sousa C, Exner D, Schwaiger R, Alves MM, Petrovykh DY, Pereira L. pH-Induced Modulation of Vibrio fischeri Population Life Cycle. Chemosensors. 2021; 9(10):283. https://doi.org/10.3390/chemosensors9100283
Chicago/Turabian StyleSilva, Ana Rita, Cláudia Sousa, Daniela Exner, Ruth Schwaiger, Maria Madalena Alves, Dmitri Y. Petrovykh, and Luciana Pereira. 2021. "pH-Induced Modulation of Vibrio fischeri Population Life Cycle" Chemosensors 9, no. 10: 283. https://doi.org/10.3390/chemosensors9100283
APA StyleSilva, A. R., Sousa, C., Exner, D., Schwaiger, R., Alves, M. M., Petrovykh, D. Y., & Pereira, L. (2021). pH-Induced Modulation of Vibrio fischeri Population Life Cycle. Chemosensors, 9(10), 283. https://doi.org/10.3390/chemosensors9100283