Electrochemical Sensing and Removal of Cesium from Water Using Prussian Blue Nanoparticle-Modified Screen-Printed Electrodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PB Nanoparticle Ink
Hydrogen Peroxide and Tetrahydrofuran-Mediated Synthesis of PB Nanoparticles
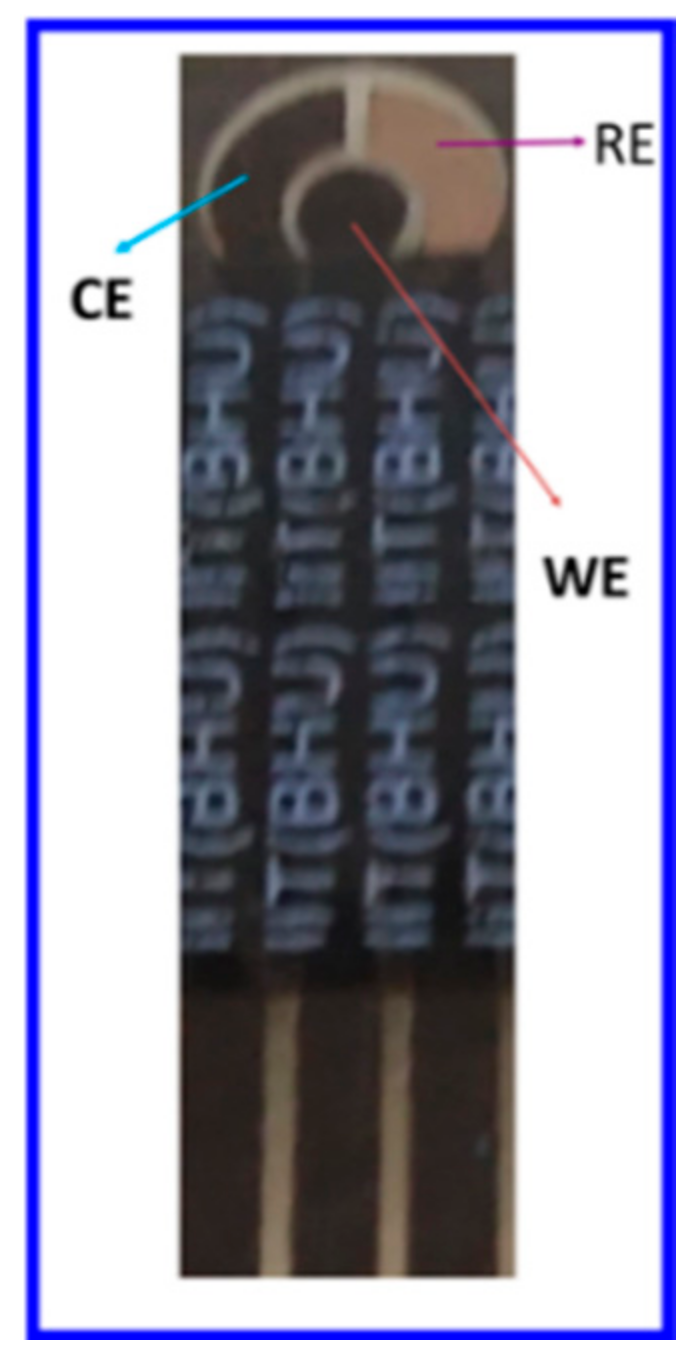
2.3. Fabrication of PB Nanoparticle-Modified Screen-Printed Electrodes
2.4. Electrochemical Measurements
2.5. Assessment of Electrochemical Adsorption
2.6. Fluorometric Method
3. Results and Discussion
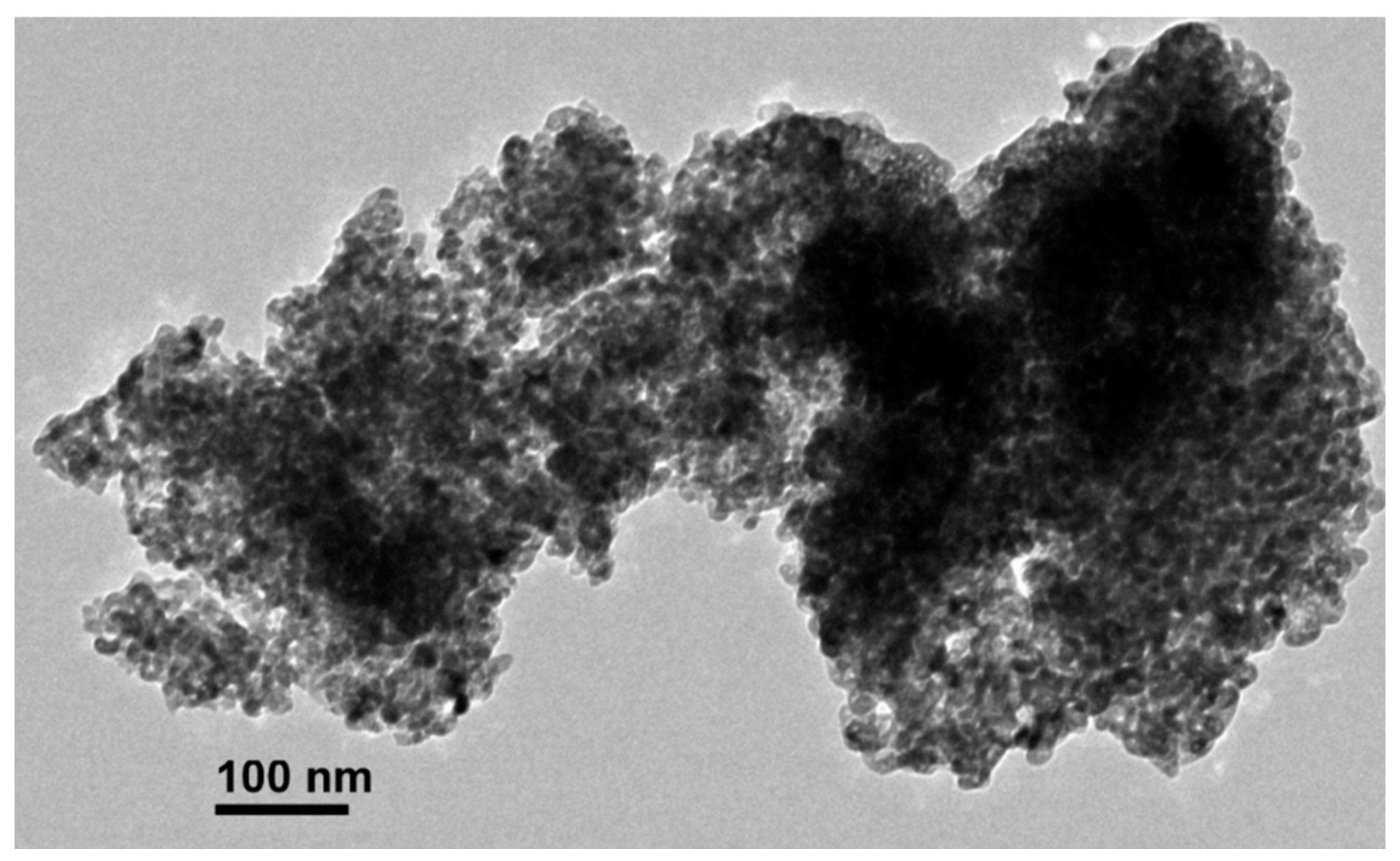
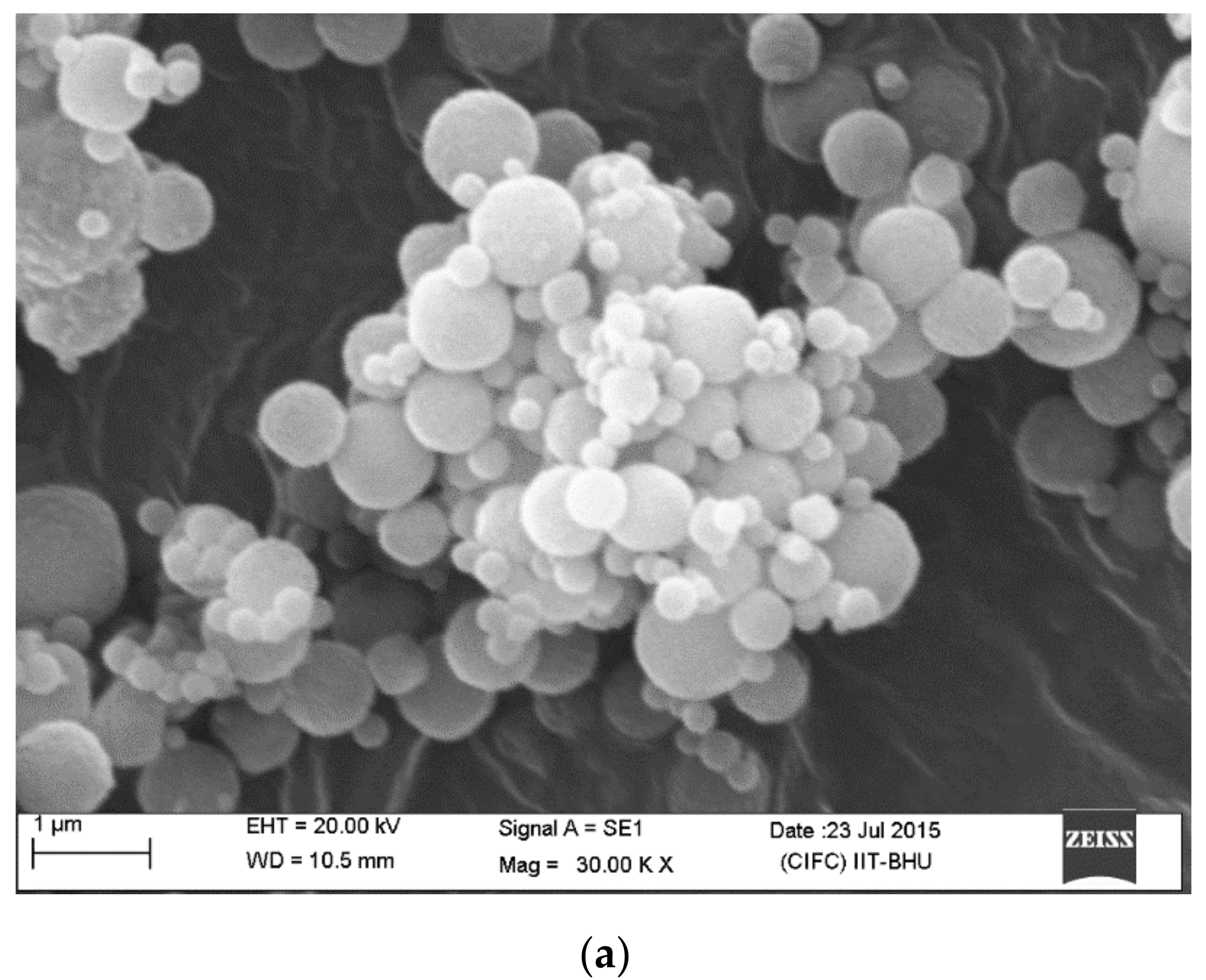
3.1. Tetrahydrofuran and H2O2-Mediated Synthesis of PB Nanoparticle Ink and Electrochemical Performance of PB Nanoparticle-Modified Screen-Printed Electrode
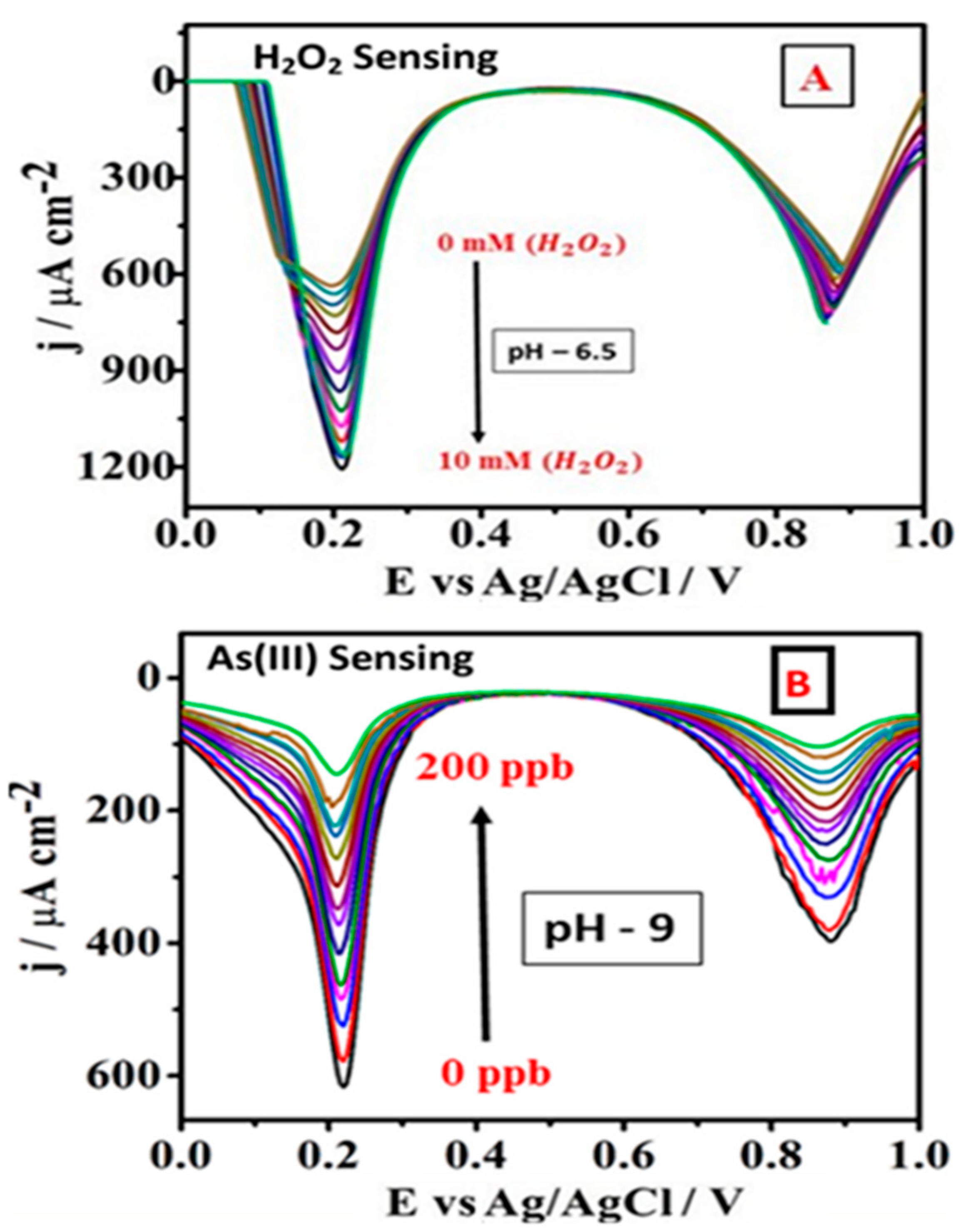
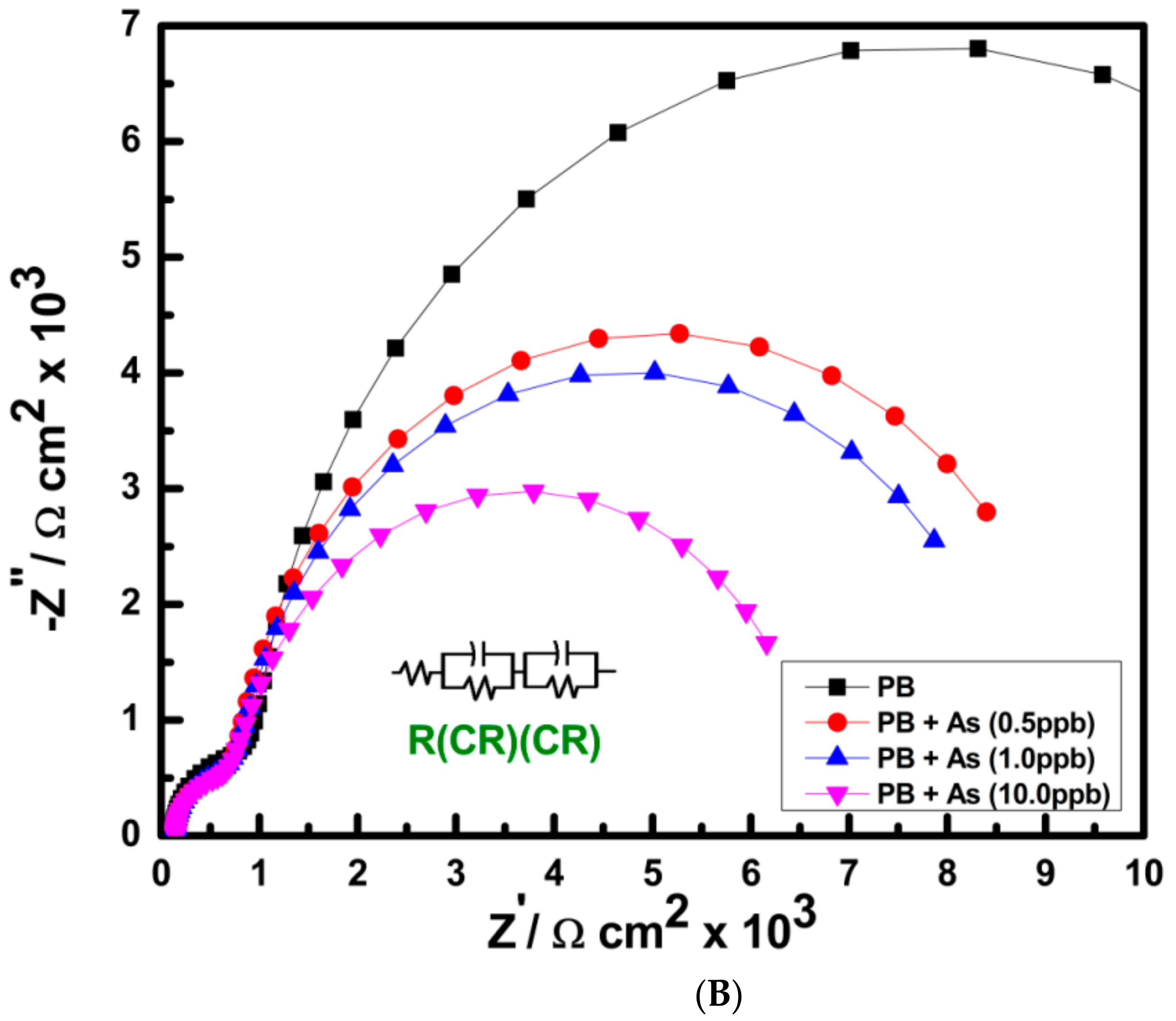
3.2. Electrochemistry of PB Nanoparticle-Modified SPE in the Presence of As(III) and Hydrogen Peroxide
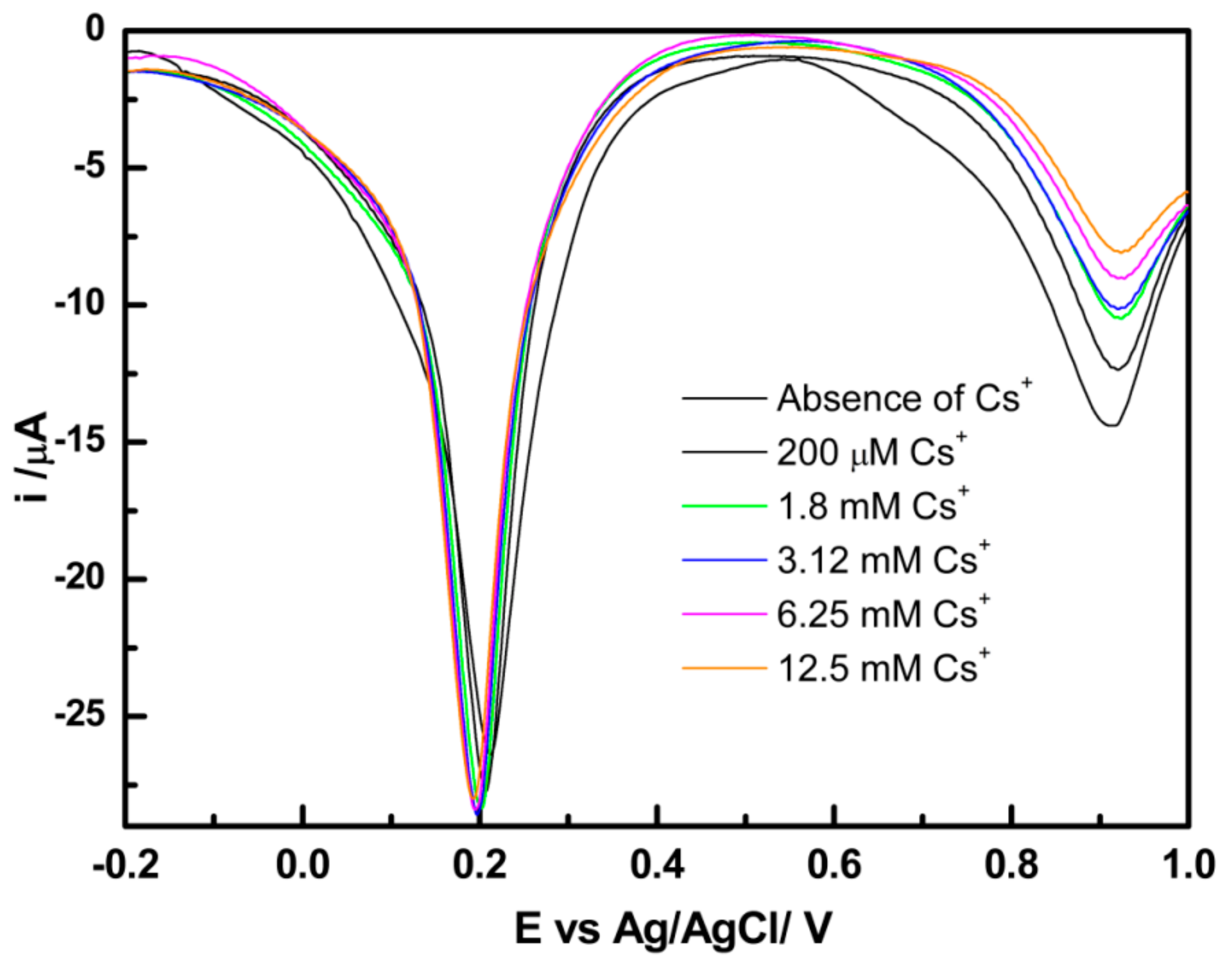
3.3. Electrochemistry of PB Nanoparticle-Modified Screen-Printed Electrodes in the Presence of Cs Ions
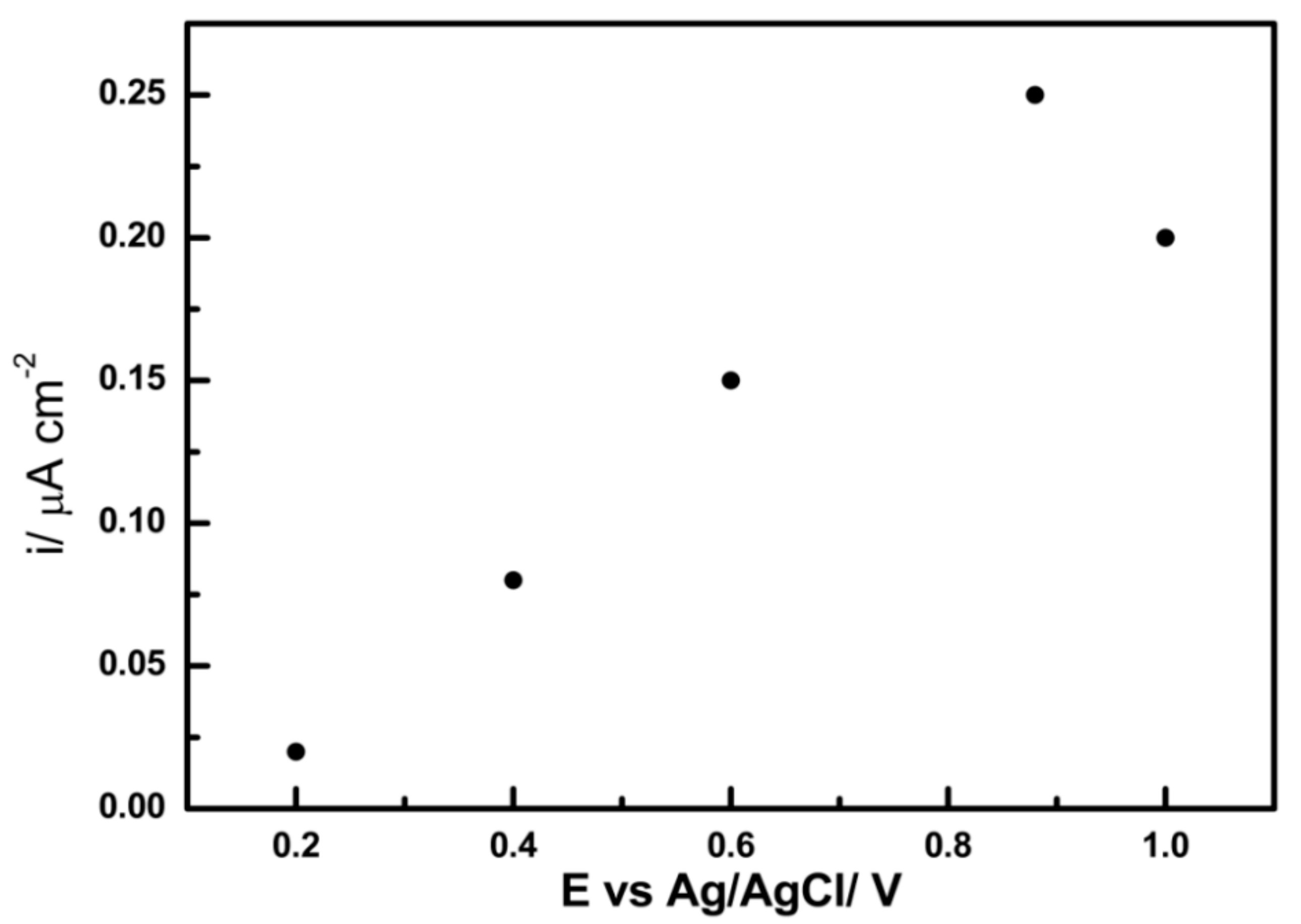
3.4. The Electrochemical Adsorption (EA) Performance of PB Nanoparticle-Modified SPE
3.5. Fluorometric Study
Effect of the Addition of Cs+ on the Fluorescent Intensity of Fluorescein
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vipin, A.K.; Fugetsu, B.; Sakata, I.; Isogai, A.; Endo, M.; Li, M.; Dresselhaus, M.S. Cellulose nanofiber backboned Prussian blue nanoparticles as powerful adsorbents for the selective elimination of radioactive cesium. Sci. Rep. 2016, 6, 37009. [Google Scholar] [CrossRef] [Green Version]
- Yasunari, T.J.; Stohl, A.; Hayano, R.S.; Burkhart, J.F.; Eckhardt, S.; Yasunari, T. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc. Natl. Acad. Sci. USA 2011, 108, 19530–19534. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Furukawa, S.; Ohtani, R.; Sukegawa, H.; Nemoto, Y.; Reboul, J.; Kitagawa, S.; Yamauchi, Y. Synthesis of Prussian blue nanoparticles with a hollow interior by controlled chemical etching. Angew. Chem. Int. Ed. 2012, 51, 984–988. [Google Scholar] [CrossRef]
- Köhler, F.H.; Storcheva, O. Paramagnetic Prussian Blue Analogues CsMII [MIII (CN) 6]. The quest for spin on cesium ions by use of 133Cs MAS NMR spectroscopy. Inorg. Chem. 2015, 54, 6801–6806. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Prussian blue: A safe pigment with zeolitic-like activity. Int. J. Mol. Sci. 2021, 22, 780. [Google Scholar] [CrossRef] [PubMed]
- Faruque, H.A.; Choi, E.S.; Kim, J.H.; Kim, S.; Kim, E. In vivo removal of radioactive cesium compound using Prussian blue-deposited iron oxide nanoparticles. Nanomedicine 2019, 14, 3143–3158. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.; Kim, M.; Lim, J.M.; Ryu, J.; Kim, S. Sequential removal of radioactive Cs by electrochemical adsorption and desorption reaction using core-shell structured carbon nanofiber–Prussian blue composites. Chem. Eng. J. 2020, 399, 125817. [Google Scholar] [CrossRef]
- Ohara, E.; Soejima, T.; Ito, S. Removal of low concentration Cs (I) from water using Prussian blue. Inorg. Chim. Acta 2021, 514, 120029. [Google Scholar] [CrossRef]
- Chen, G.R.; Chang, Y.R.; Liu, X.; Kawamoto, T.; Tanaka, H.; Kitajima, A.; Parajuli, D.; Takasaki, M.; Yoshino, K.; Chen, M.L.; et al. Prussian blue (PB) granules for cesium (Cs) removal from drinking water. Sep. Purif. 2015, 143, 146–151. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Lee, W.; Kim, S. Rapid removal of radioactive cesium by polyacrylonitrile nanofibers containing Prussian blue. J. Hazard. Mater. 2018, 347, 106–113. [Google Scholar] [CrossRef]
- Rauwel, P.; Rauwel, E. Towards the extraction of radioactive cesium-137 from water via graphene/CNT and nanostructured Prussian blue hybrid nanocomposites: A review. Nanomaterials 2019, 9, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, P.C.; Pandey, A.K. Novel synthesis of super peroxidase mimetic polycrystalline mixed metal hexacyanoferrates nanoparticles dispersion. Analyst 2013, 138, 2295–2301. [Google Scholar] [CrossRef]
- Pandey, P.C.; Pandey, A.K. Novel synthesis of Prussian blue nanoparticles and nanocomposite sol: Electro-analytical application in hydrogen peroxide sensing. Electrochim. Acta 2013, 87, 1–8. [Google Scholar] [CrossRef]
- Pandey, P.C.; Pandey, A.K. A Process for the Polyethylenimine and Organic Reducing Agent-Mediated Synthesis of Noble Metal Nanoparticles. Indian Patent 295327, 6 January 2012. [Google Scholar]
- Pandey, P.C.; Shukla, S.; Narayan, R.J. Organotrialkoxysilane-functionalized Prussian Blue nanoparticles-mediated fluorescence sensing of arsenic (III). Nanomaterials 2021, 11, 1145. [Google Scholar] [CrossRef]
- Pandey, P.C.; Panday, D. Tetrahydrofuran and hydrogen peroxide mediated conversion of potassium hexacyanoferrate into Prussian blue nanoparticles: Application to hydrogen peroxide sensing. Electrochim. Acta 2016, 190, 758–765. [Google Scholar] [CrossRef]
- Rykov, A.I.; Wang, J.; Zhang, T.; Nomura, K. Cs sorption by “soluble” and “insoluble” iron hexacyanocobaltates probed by Mössbauer spectroscopy. Hyperfine Interact. 2013, 218, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Qiao, J.; Yuan, J.; Shen, J.; Wang, A.J.; Niu, L. Electrochemical removal of radioactive cesium from nuclear waste using the dendritic copper hexacyanoferrate/carbon nanotube hybrids. Electrochim. Acta 2017, 257, 172–180. [Google Scholar] [CrossRef]
- Murugappan, K.; Lee, J.; Silvester, D.S. Comparative study of screen printed electrodes for ammonia gas sensing in ionic liquids. Electrochem. Commun. 2011, 13, 1435–1438. [Google Scholar] [CrossRef] [Green Version]
- O’Halloran, M.P.; Pravda, M.; Guilbault, G.G. Prussian Blue bulk modified screen-printed electrodes for H2O2 detection and for biosensors. Talanta 2001, 55, 605–611. [Google Scholar] [CrossRef]
- Bonacin, J.A.; Santos, P.L.D.; Katik, V.; Foster, C.W.; Bank, C.E. Use of screen printed electrodes modified by Prussian blue and analogues in sensing of cysteine. Electroanalysis 2018, 30, 170–179. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, E.; Moscone, D.; Palleschi, G.; Killard, A.J. Development of a hydrogen peroxide sensor based on screen-printed electrodes modified with inkjet-printed Prussian blue nanoparticles. Sensors 2014, 14, 14222–14234. [Google Scholar] [CrossRef]
- Chen, R.; Tanaka, H.; Kawamoto, T.; Asai, M.; Fukushima, C.; Na, H.; Kurihara, M.; Watanabe, M.; Arisaka, M.; Nankawa, T. Selective removal of cesium ions from wastewater using copper hexacyanoferrate nanofilms in an electrochemical system. Electrochim. Acta 2013, 87, 119–125. [Google Scholar] [CrossRef]
- Draouil, H.; Alvarez, L.; Causse, J.; Flaud, V.; Zaibi, M.A.; Bantignies, J.L.; Oueslati, M.; Cambedouzou, J. Copper hexacyanoferrate functionalized single-walled carbon nanotubes for selective cesium extraction. New J. Chem. 2017, 41, 7705–7713. [Google Scholar] [CrossRef]
- Vipin, A.K.; Ling, S.; Fugetsu, B. Sodium cobalt hexacyanoferrate encapsulated in alginate vesicle with CNT for both cesium and strontium removal. Carbohydr. Polym. 2014, 111, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, H.; Zhai, J.; Sun, L.; Zhao, Y.; Yu, H. Magnetic Prussian blue/graphene oxide nanocomposites caged in calcium alginate microbeads for elimination of cesium ions from water and soil. Chem. Eng. J. 2014, 246, 10–19. [Google Scholar] [CrossRef]
- Yang, H.M.; Hwang, J.R.; Lee, D.Y.; Kim, K.B.; Park, C.W.; Kim, H.R.; Lee, K.W. Eco-friendly one-pot synthesis of Prussian blue-embedded magnetic hydrogel beads for the removal of cesium from water. Sci. Rep. 2018, 8, 11476. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; He, F.; Dai, Y. Prussian blue analog caged in chitosan surface-decorated carbon nanotubes for removal cesium and strontium. J. Radioanal. Nucl. Chem. 2016, 310, 1139–1145. [Google Scholar] [CrossRef]
- Jang, S.C.; Haldorai, Y.; Lee, G.W.; Hwang, S.K.; Han, Y.K.; Roh, C.; Huh, Y.S. Porous three-dimensional graphene foam/Prussian blue composite for efficient removal of radioactive 137Cs. Sci. Rep. 2015, 5, 17510. [Google Scholar] [CrossRef] [Green Version]





| PB-Based Adsorbent | Removal Efficiency | Kd L/g | Initial Conc. mg/L | Isotherm | Kinetic Model | Qmax mg/g | Reference |
|---|---|---|---|---|---|---|---|
| MWCNT–CuHCF | 95 | 568 | 1 | Langmuir Freundich | NA | 310 | [18] |
| SWCNT–CuHCF | NA | NA | NA | NA | NA | 230 | [24] |
| MWCNT–Na–CoHCF–alginate | 53 | 23 | 200 | Langmuir Freundich | PFO PSO | 133 | [25] |
| Fe3O4–FeHCF–GO–alginate beads | 80 | 48.7 | 25–150 | Langmuir Freundich | PSO | 43.5 | [26] |
| Fe3O4–FeHCF–hydrogel | 99.5 | 0.4 | 100–500 | Langmuir | PSO | 41.5 | [27] |
| Chitosan–FeHCF–CNT | NA | 42.5 | 1–100 | Langmuir Freundich | PFO PSO | 219 | [28] |
| Redlich Peterson | Webb Morris Elovich | ||||||
| rGO–FeHCF | 99.5 | 6.46 | 0.2 | Langmuir Freundich | NA | 18.67 | [29] |
| FeHCF modified screen-printed electrode | 95 | 580 | 1 | Langmuir Freundich | NA | 325 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, P.C.; Yadav, H.P.; Shukla, S.; Narayan, R.J. Electrochemical Sensing and Removal of Cesium from Water Using Prussian Blue Nanoparticle-Modified Screen-Printed Electrodes. Chemosensors 2021, 9, 253. https://doi.org/10.3390/chemosensors9090253
Pandey PC, Yadav HP, Shukla S, Narayan RJ. Electrochemical Sensing and Removal of Cesium from Water Using Prussian Blue Nanoparticle-Modified Screen-Printed Electrodes. Chemosensors. 2021; 9(9):253. https://doi.org/10.3390/chemosensors9090253
Chicago/Turabian StylePandey, Prem. C., Hari Prakash Yadav, Shubhangi Shukla, and Roger J. Narayan. 2021. "Electrochemical Sensing and Removal of Cesium from Water Using Prussian Blue Nanoparticle-Modified Screen-Printed Electrodes" Chemosensors 9, no. 9: 253. https://doi.org/10.3390/chemosensors9090253







