Mercaptosuccinic-Acid-Functionalized Gold Nanoparticles for Highly Sensitive Colorimetric Sensing of Fe(III) Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of MSA-Functionalized AuNPs
2.3. Transmission Electron Microscopy
2.4. Dynamic Light Scattering of the AuNPs and Their Complex with Fe(III)
2.5. Fe(III) Ion Detection
2.6. Analysis of Water Samples
3. Results and Discussion
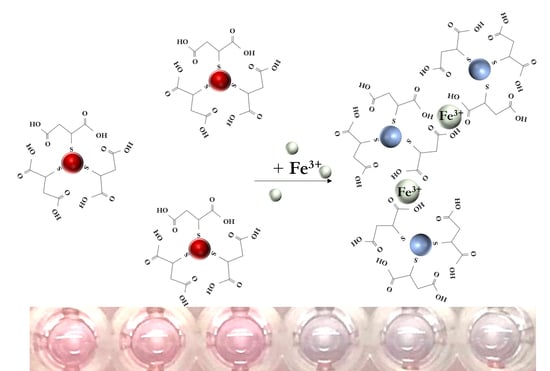
3.1. Sensing Mechanism
3.2. Characterization of MSA-AuNPs
3.3. Optimization of Conditions for Fe3+ Detection
3.4. Colorimetric Determination of Fe(III) Ions and the Analytical Performance
3.5. Selectivity of Fe(III) Ion Detection
3.6. Practical Application of Colorimetric Sensing Probe
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, T.-T.; Yang, J.-X.; Song, J.-M.; Chen, J.-S.; Niu, H.-L.; Mao, C.-J.; Zhang, S.-Y.; Shen, Y.-H. Synthesis of high fluorescence graphene quantum dots and their selective detection for Fe3+ in aqueous solution. Sens. Actuators B Chem. 2017, 243, 863–872. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Iron in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality, WHO/SDE/WSH/03.04/08. In Health Criteria and Other Supporting Information, 2nd ed.; World Health Organization: Geneva, Switzerland, 1996; Volume 2. [Google Scholar]
- Santana-Casiano, J.M.; González-Dávila, M.; Rodríguez, M.J.; Millero, F.J. The effect of organic compounds in the oxidation kinetics of Fe(II). Mar. Chem. 2000, 70, 211–222. [Google Scholar] [CrossRef]
- Millero, F.J.; Sotolongo, S.; Izaguirre, M. The oxidation kinetics of Fe (II) in seawater. Geochim. Cosmochim. Acta 1987, 51, 793–801. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I. Iron toxicity and antioxidant nutrients. Toxicology 2002, 180, 23–32. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Saber-Tehrani, M.; Khoshsafar, H. Preparation and characterization of magnetic nanocomposite of Schiff base/silica/magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry. Talanta 2012, 97, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Boyle, E.A. Determination of iron in seawater by high-resolution isotope dilution inductively coupled plasma mass spectrometry after Mg(OH)2 coprecipitation. Anal. Chim. Acta 1998, 367, 183–191. [Google Scholar] [CrossRef]
- Ariga, T.; Ito, K.; Imura, Y.; Yoshimura, E. High-performance liquid chromatography method for ferric iron chelators using a post-column reaction with Calcein Blue. J. Chromatogr. B Biomed. Appl. 2015, 985, 48–53. [Google Scholar] [CrossRef]
- Didukh-Shadrina, S.L.; Losev, V.N.; Samoilo, A.; Trofimchuk, A.K.; Nesterenko, P.N. Determination of Metals in Natural Waters by Inductively Coupled Plasma Optical Emission Spectroscopy after Preconcentration on Silica Sequentially Coated with Layers of Polyhexamethylene Guanidinium and Sulphonated Nitrosonaphthols. Int. J. Anal. Chem. 2019, 2019, 1467631. [Google Scholar] [CrossRef] [Green Version]
- Fayed, T.A.; El-Nahass, M.N.; El-Daly, H.A.; Shokry, A.A. Development of nanomaterial chemosensors for toxic metal ions sensing. Appl. Organomet. Chem. 2019, 33, e4868. [Google Scholar] [CrossRef]
- De Acha, N.; Elosúa, C.; Corres, J.M.; Arregui, F.J. Fluorescent Sensors for the Detection of Heavy Metal Ions in Aqueous Media. Sensors 2019, 19, 599. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Q.; Guo, Z.; Ma, H.; Zhang, Y.; Wang, B.; Bin, D.; Wei, Q. Highly selective fluorescent chemosensor for detection of Fe3+ based on Fe3O4@ZnO. Sci. Rep. 2016, 6, 23558. [Google Scholar] [CrossRef] [Green Version]
- Arvapalli, D.M.; Sheardy, A.T.; Alapati, K.C.; Wei, J. High Quantum Yield Fluorescent Carbon Nanodots for detection of Fe (III) Ions and Electrochemical Study of Quenching Mechanism. Talanta 2020, 209, 120538. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, L.; Jia, J.; Chang, D.; Dong, C.; Shuang, S. Fe3+ detection, bioimaging, and patterning based on bright blue-fluorescent N-doped carbon dots. Analyst 2020, 145, 5450–5457. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, Q.; Guo, C.; Sun, Y.; Xie, L.-H.; Li, J.-R. Stable Zr(IV)-Based Metal–Organic Frameworks with Predesigned Functionalized Ligands for Highly Selective Detection of Fe(III) Ions in Water. ACS Appl. Mater. Interfaces 2017, 9, 10286–10295. [Google Scholar] [CrossRef] [PubMed]
- Jayasree, M.; Aparna, R.S.; Anjana, R.R.; Anjali Devi, J.S.; John, N.; Abha, K.; Manikandan, A.; George, S. Fluorescence turn on detection of bilirubin using Fe (III) modulated BSA stabilized copper nanocluster; A mechanistic perception. Anal. Chim. Acta 2018, 1031, 152–160. [Google Scholar] [CrossRef]
- Namgung, H.; Kim, J.; Gwon, Y.; Lee, T.S. Synthesis of poly (p-phenylene) containing a rhodamine 6G derivative for the detection of Fe (III) in organic and aqueous media. RSC Adv. 2017, 7, 39852–39858. [Google Scholar] [CrossRef] [Green Version]
- Harathi, J.; Thenmozhi, K. AIE-active Schiff base compounds as fluorescent probes for the highly sensitive and selective detection of Fe3+ ions. Mater. Chem. Front. 2020, 4, 1471–1482. [Google Scholar] [CrossRef]
- Göde, C.; Yola, M.L.; Yılmaz, A.; Atar, N.; Wang, S. A novel electrochemical sensor based on calixarene functionalized reduced graphene oxide: Application to simultaneous determination of Fe(III), Cd(II) and Pb(II) ions. J. Colloid Interface Sci. 2017, 508, 525–531. [Google Scholar] [CrossRef]
- Xu, N.; Jin, S.; Wang, L. Metal nanoparticles-based nanoplatforms for colorimetric sensing: A review. Rev. Anal. Chem. 2020, 40, 1–11. [Google Scholar] [CrossRef]
- Yang, M.; Chae, J.B.; Kim, C.; Harrison, R.G. A visible chemosensor based on carbohydrazide for Fe(ii), Co(ii) and Cu(ii) in aqueous solution. Photochem. Photobiol. Sci. 2019, 18, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.-Q.; Wang, C.-X.; Yang, J.-X.; Gao, H.-W.; Tian, Y.-P.; Tao, X.-T.; Jiang, M.-H. A highly selective colorimetric chemosensor for detecting the respective amounts of iron(ii) and iron(iii) ions in water. New J. Chem. 2007, 31, 906–910. [Google Scholar] [CrossRef]
- Piriya, V.S.A.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; He, S.; Li, X.; Chen, W. Colorimetric detection of iron ions (III) based on the highly sensitive plasmonic response of the N-acetyl-l-cysteine-stabilized silver nanoparticles. Anal. Chim. Acta 2015, 879, 118–125. [Google Scholar] [CrossRef]
- Uzunoğlu, D.; Ergüt, M.; Kodaman, C.G.; Özer, A. Biosynthesized Silver Nanoparticles for Colorimetric Detection of Fe3+ Ions. Arab. J. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Liu, G.; Lu, M.; Huang, X.; Li, T.; Xu, D. Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening. Sensors 2018, 18, 4166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlina, A.N.; Sharma, A.K.; Zherdev, A.V.; Gaur, M.S.; Dzantiev, B.B. Colorimetric determination of lead using gold nanoparticles. Anal. Lett. 2015, 48, 766–782. [Google Scholar] [CrossRef]
- Berlina, A.N.; Komova, N.S.; Zherdev, A.V.; Gaur, M.S.; Dzantiev, B.B. Colorimetric Technique for Antimony Detection Based on the Use of Gold Nanoparticles Conjugated with Poly-A Oligonucleotide. Appl. Sci. 2019, 9, 4782. [Google Scholar] [CrossRef] [Green Version]
- Berlina, A.N.; Zherdev, A.V.; Pridvorova, S.M.; Gaur, M.S.; Dzantiev, B.B. Rapid Visual Detection of Lead and Mercury via Enhanced Crosslinking Aggregation of Aptamer-Labeled Gold Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 5489–5495. [Google Scholar] [CrossRef]
- Frost, M.S.; Dempsey, M.J.; Whitehead, D.E. The response of citrate functionalised gold and silver nanoparticles to the addition of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 15–24. [Google Scholar] [CrossRef]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications-A Review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef]
- Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Progress in rapid optical assays for heavy metal ions based on the use of nanoparticles and receptor molecules. Microchim. Acta 2019, 186, 172. [Google Scholar] [CrossRef]
- Wu, S.-P.; Chen, Y.-P.; Sung, Y.-M. Colorimetric detection of Fe3+ ions using pyrophosphate functionalized gold nanoparticles. Analyst 2011, 136, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Wang, X.-F.; Huo, D.-Q.; Hou, C.-J.; Fa, H.-B.; Yang, M.; Zhang, L. Colorimetric measurement of Fe3+ using a functional paper-based sensor based on catalytic oxidation of gold nanoparticles. Sens. Actuators B Chem. 2017, 242, 1265–1271. [Google Scholar] [CrossRef]
- Buduru, P.; BC, S.R.R. Oxamic acid and p-aminobenzoic acid functionalized gold nanoparticles as a probe for colorimetric detection of Fe3+ ion. Sens. Actuators B Chem. 2016, 237, 935–943. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Shinde, S.; Saratale, R.; Syed, A.; Ameen, F.; Ghodake, G. Spectrophotometric determination of Fe(III) by using casein-functionalized gold nanoparticles. Microchim. Acta 2017, 184, 4695–4704. [Google Scholar] [CrossRef]
- Thatai, S.; Khurana, P.; Prasad, S.; Kumar, D. A new way in nanosensors: Gold nanorods for sensing of Fe(III) ions in aqueous media. Microchem. J. 2014, 113, 77–82. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Woo, J.Y.; Han, C.-S. Colorimetric detection of Fe(III) ions using label-free gold nanoparticles and acidic thiourea mixture. Sens. Actuators B Chem. 2013, 181, 114–118. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Lakshmipriya, T.; Awazu, K. Colorimetric detection of controlled assembly and disassembly of aptamers on unmodified gold nanoparticles. Biosens. Bioelectron. 2014, 51, 115–123. [Google Scholar] [CrossRef]
- Iarossi, M.; Schiattarella, C.; Rea, I.; De Stefano, L.; Fittipaldi, R.; Vecchione, A.; Velotta, R.; Ventura, B.D. Colorimetric Immunosensor by Aggregation of Photochemically Functionalized Gold Nanoparticles. ACS Omega 2018, 3, 3805–3812. [Google Scholar] [CrossRef]
- Alizadeh, S.; Nazari, Z. A Review on Gold Nanoparticles Aggregation and Its Applications. J. Chem. Rev. 2020, 2, 228–242. [Google Scholar]
- Cheney, G.E.; Fernando, Q.; Freiser, H. Some Metal Chelates of Mercaptosuccinic Acid. J. Phys. Chem. A 1959, 63, 2055–2057. [Google Scholar] [CrossRef]
- Larkworthy, L.F.; Sattari, D. Some complexes of thiomalate with bivalent transition metal ions and gold (I). J. Inorg. Nucl. Chem. 1980, 42, 551–559. [Google Scholar] [CrossRef]
- Pawelec, M.; Stochel, G.; van Eldik, R. Mechanistic information on the copper-catalysed autoxidation of mercaptosuccinic acid in aqueous solution. Dalton Trans. 2004, 2004, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Zhu, T.; Glasser, G.; Knoll, W.; Kreiter, M. Preparation of gold nanoparticles in an aqueous medium using 2-mercaptosuccinic acid as both reduction and capping agent. J. Nanosci. Nanotechnol. 2008, 8, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar]
- Shervedani, R.K.; Hatefi-Mehrjardi, A.; Asadi-Farsani, A. Sensitive determination of iron(III) by gold electrode modified with 2-mercaptosuccinic acid self-assembled monolayer. Anal. Chim. Acta 2007, 601, 164–171. [Google Scholar] [CrossRef]
- Millero, F. Speciation of metals in natural waters. Geochem. Trans. 2001, 2, 57. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Mashhadizadeh, M.H.; Talemi, R.P. Used gold nano-particles as an on/off switch for response of a potentiometric sensor to Al(III) or Cu(II) metal ions. Anal. Chim. Acta 2011, 692, 109–115. [Google Scholar] [CrossRef]
- See, W.P.; Heng, L.Y.; Nathan, S. Highly Sensitive Aluminium(III) Ion Sensor Based on a Self-assembled Monolayer on a Gold Nanoparticles Modified Screen-printed Carbon Electrode. Anal. Sci. 2015, 31, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Koç, Ö.K.; Üzer, A.; Apak, R. A colorimetric probe based on 4-mercaptophenol and thioglycolic acid-functionalized gold nanoparticles for determination of phytic acid and Fe(III) ions. Microchim. Acta 2020, 187, 586. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Green Synthesized Gold Nanoparticles as a Probe for the Detection of Fe3+ Ions in Water. J. Clust. Sci. 2014, 25, 969–978. [Google Scholar] [CrossRef]
- Ho, T.T.-T.; Dang, C.-H.; Huynh, T.K.-C.; Hoang, T.K.-D.; Nguyen, T.-D. In situ synthesis of gold nanoparticles on novel nanocomposite lactose/alginate: Recyclable catalysis and colorimetric detection of Fe(III). Carbohydr. Polym. 2021, 251, 116998. [Google Scholar] [CrossRef] [PubMed]
- Andreani, A.S.; Kunarti, E.S.; Hashimoto, T.; Hayashita, T.; Santosa, S.J. Fast and selective colorimetric detection of Fe3+ based on gold nanoparticles capped with ortho-hydroxybenzoic acid. J. Environ. Chem. Eng. 2021, 9, 105962. [Google Scholar] [CrossRef]





| Particles | Concentration of Fe(III) (ng/mL) | Average Particle Size (nm) | Surface Zeta Potential (mV) |
|---|---|---|---|
| MSA-AuNP | 0 | 27.4 | −27.9 |
| 35 | 132.3 | −20.6 | |
| 100 | 689.3 | +14.9 |
| Element | Result (p = 0.95) (µg/mL) | MRL (µg/mL) |
|---|---|---|
| Al | 0.04 ± 0.011 | 0.5 |
| As | 0.0007 ± 0.00027 | 0.05 |
| B | 0.17 ± 0.033 | 0.5 |
| Ca | 40.22 ± 6.03 | - |
| Cd | <0.000024 | 0.001 |
| Co | 0.00008 ± 0.000032 | 0.1 |
| Cr | 0.02 ± 0.004 | 0.05 |
| Cu | 0.002 ± 0.0006 | 1 |
| Fe | 0.17 ± 0.034 | 0.3 |
| Hg | <0.00018 | 0.0005 |
| I | 0.003 ± 0.001 | - |
| K | 5.59 ± 0.84 | - |
| Li | 0.006 ± 0.0017 | 0.03 |
| Mg | 9.88 ± 1.48 | - |
| Mn | 0.01 ± 0.003 | 0.1 |
| Na | 8.28 ± 1.24 | 200 |
| Ni | 0.01 ± 0.003 | 0.1 |
| P | 0.37 ± 0.074 | - |
| Pb | 0.0005 ± 0.00019 | 0.03 |
| Se | 0.0007 ± 0.00028 | 0.01 |
| Si | 1.49 ± 0.22 | 10 |
| Sn | 0.0002 ± 0.00008 | - |
| Sr | 0.15 ± 0.03 | 7 |
| V | 0.0009 ± 0.00036 | 0.1 |
| Zn | 0.03 ± 0.008 | 5 |
| Sample | Initial Found (ng/mL) | Added (ng/mL) | Total Found (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| Drinking water | 10.6 ± 0.2 | 15 | 26.4 ± 0.09 | 105 |
| 20 | 29.7 ± 0.43 | 95.5 | ||
| Tap water | 18.5 ± 0.4 | 30 | 49.8 ± 0.6 | 104 |
| 25 | 40.9 ± 0.8 | 89.6 | ||
| Spring water | 27.8 ± 0.2 | 30 | 61.05 ± 0.01 | 110 |
| 25 | 54.09 ± 0.7 | 105 | ||
| 20 | 52.95 ± 0.02 | 126 |
| Label | Capping Reagent | Samples | Time of Analysis | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Rapid homogeneous assays | |||||
| AuNPs | MSA | Water samples | <1 min | 23 ng/mL | This work |
| Au NPs | Casein | Human urine and water samples | <1 min | 25 ng/mL | [36] |
| Ag NPs | N-acetyl-l-cysteine | - | - | 4.4 ng/mL | [24] |
| AuNPs | 4-mercaptophenol and thioglycolic acid | - | 1 min | 55.85 ng/mL | [52] |
| More time-consuming, less sensitive homogeneous assays | |||||
| Au NPs | Ascorbic acid, some proteins and flavonoids from Hibiscus cannabinus as a reducing agent | - | 2 min | 8100 ng/mL | [53] |
| Au NPs | Oxamic acid, p-aminobenzoic acid | Water, urine, and plasma samples | 15 min | 330 ng/mL | [35] |
| Au NPs | Pyrophosphate | Lake water samples | 30 min | 312 ng/mL | [33] |
| AuNPs | Lactose/alginate | - | 20 min | 44.7 ng/mL | [54] |
| AuNPs | acidic thiourea | - | 30 min + 15 min | 50.2 ng/mL | [38] |
| AuNPs | Ortho-hydroxybenzoic acid | Rice field water, river water, and seawater | - | 513 ng/mL | [55] |
| AuNPs | 4-mercaptophenol and thioglycolic acid | - | 1 min | 55.85 ng/mL | [52] |
| AgNPs | B. variegata leaf extract | - | ~5 min | 116.2 ng/mL | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komova, N.S.; Serebrennikova, K.V.; Berlina, A.N.; Pridvorova, S.M.; Zherdev, A.V.; Dzantiev, B.B. Mercaptosuccinic-Acid-Functionalized Gold Nanoparticles for Highly Sensitive Colorimetric Sensing of Fe(III) Ions. Chemosensors 2021, 9, 290. https://doi.org/10.3390/chemosensors9100290
Komova NS, Serebrennikova KV, Berlina AN, Pridvorova SM, Zherdev AV, Dzantiev BB. Mercaptosuccinic-Acid-Functionalized Gold Nanoparticles for Highly Sensitive Colorimetric Sensing of Fe(III) Ions. Chemosensors. 2021; 9(10):290. https://doi.org/10.3390/chemosensors9100290
Chicago/Turabian StyleKomova, Nadezhda S., Kseniya V. Serebrennikova, Anna N. Berlina, Svetlana M. Pridvorova, Anatoly V. Zherdev, and Boris B. Dzantiev. 2021. "Mercaptosuccinic-Acid-Functionalized Gold Nanoparticles for Highly Sensitive Colorimetric Sensing of Fe(III) Ions" Chemosensors 9, no. 10: 290. https://doi.org/10.3390/chemosensors9100290