Enhanced Capacitive Humidity Sensing Performance at Room Temperature via Hydrogen Bonding of Cyanopyridone-Based Oligothiophene Donor
Abstract
:1. Introduction
2. Materials and Methods
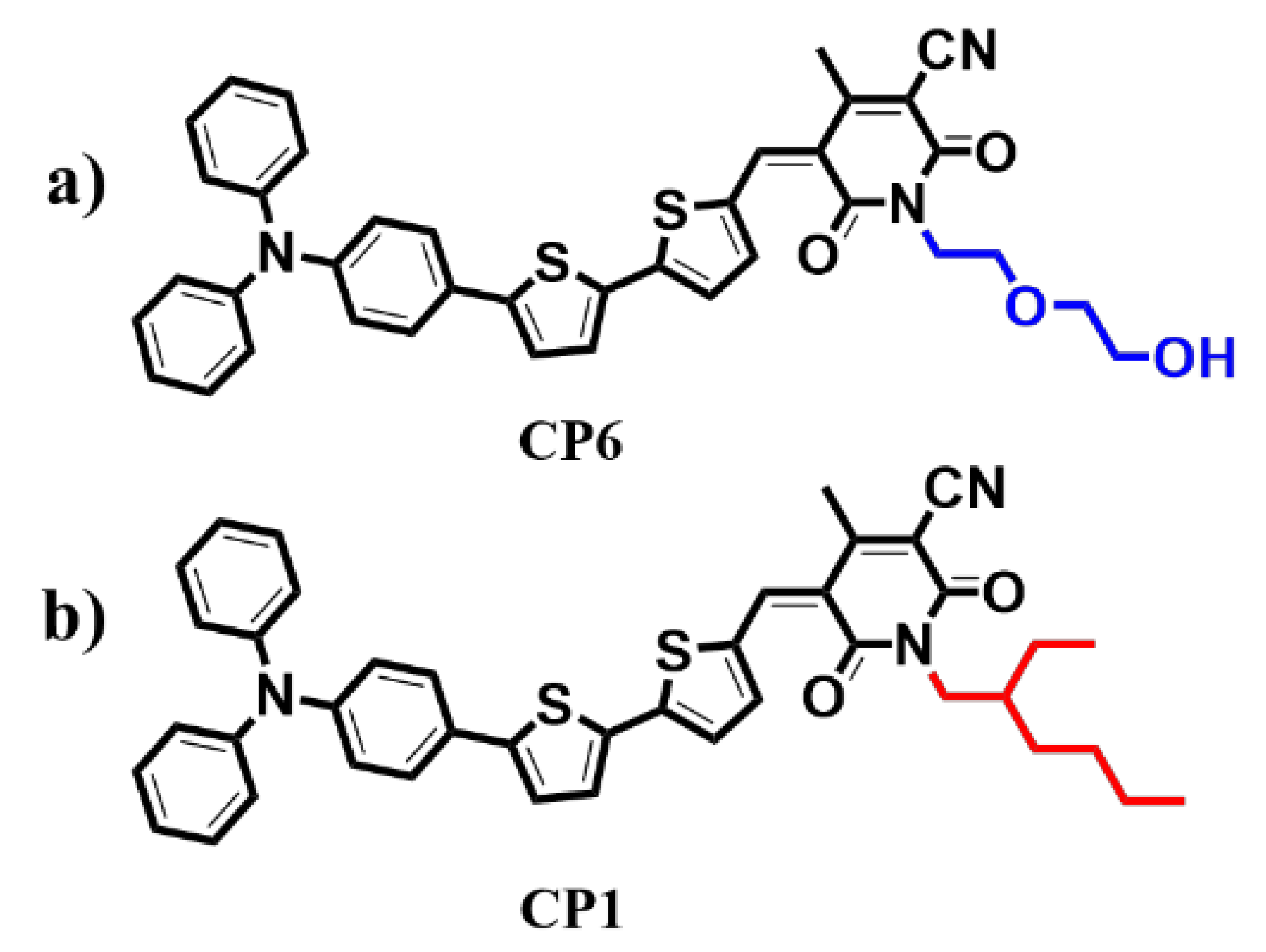
2.1. Synthesis
2.2. Device Fabrication and Characterisation
3. Results and Discussions
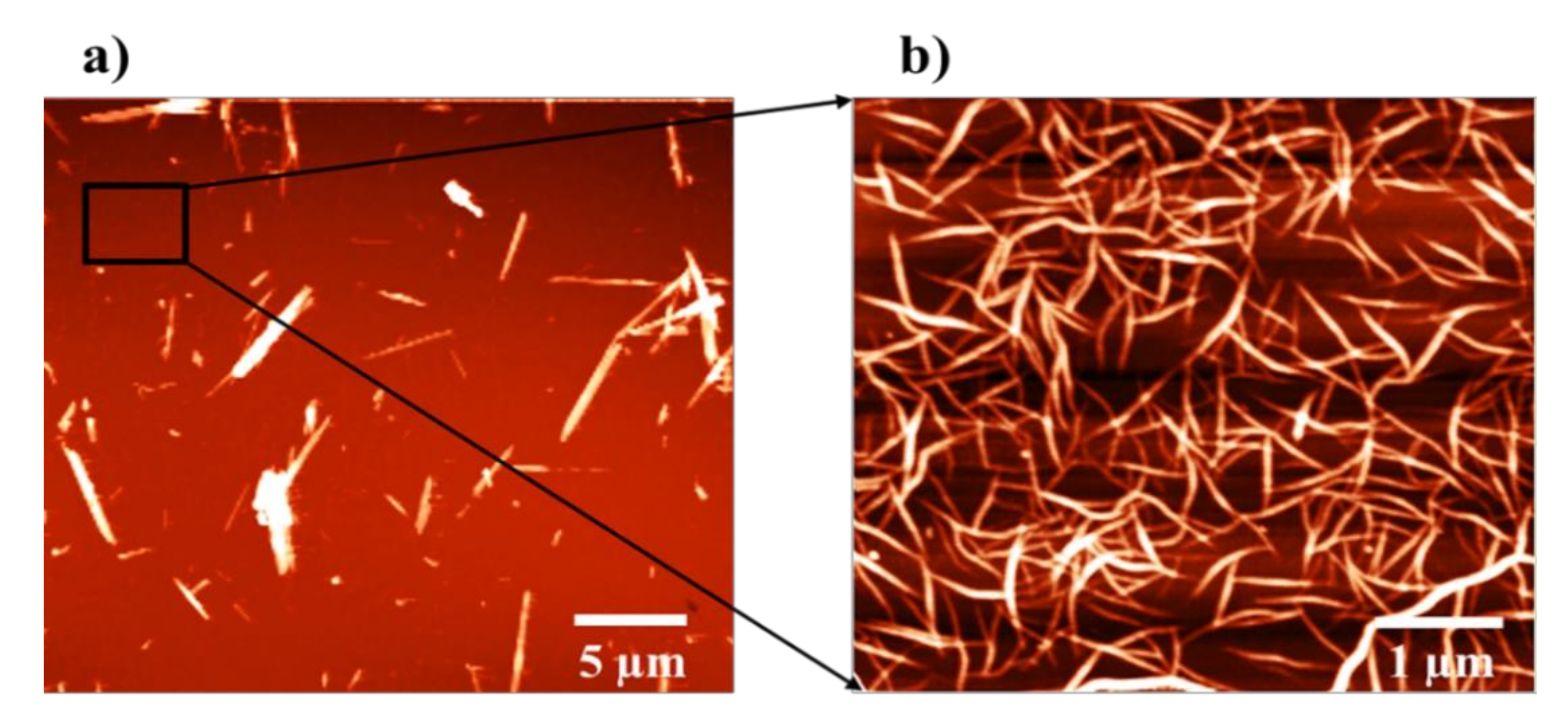
3.1. Surface and Structural Analyses
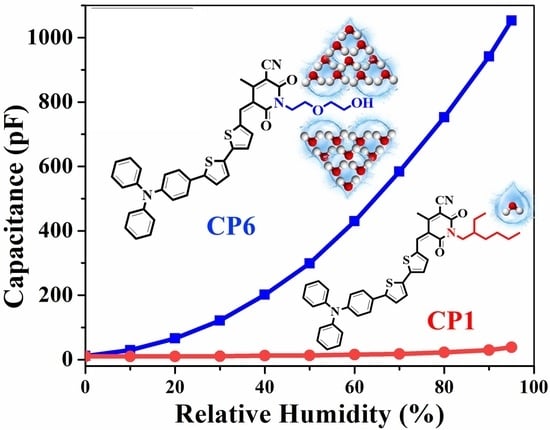
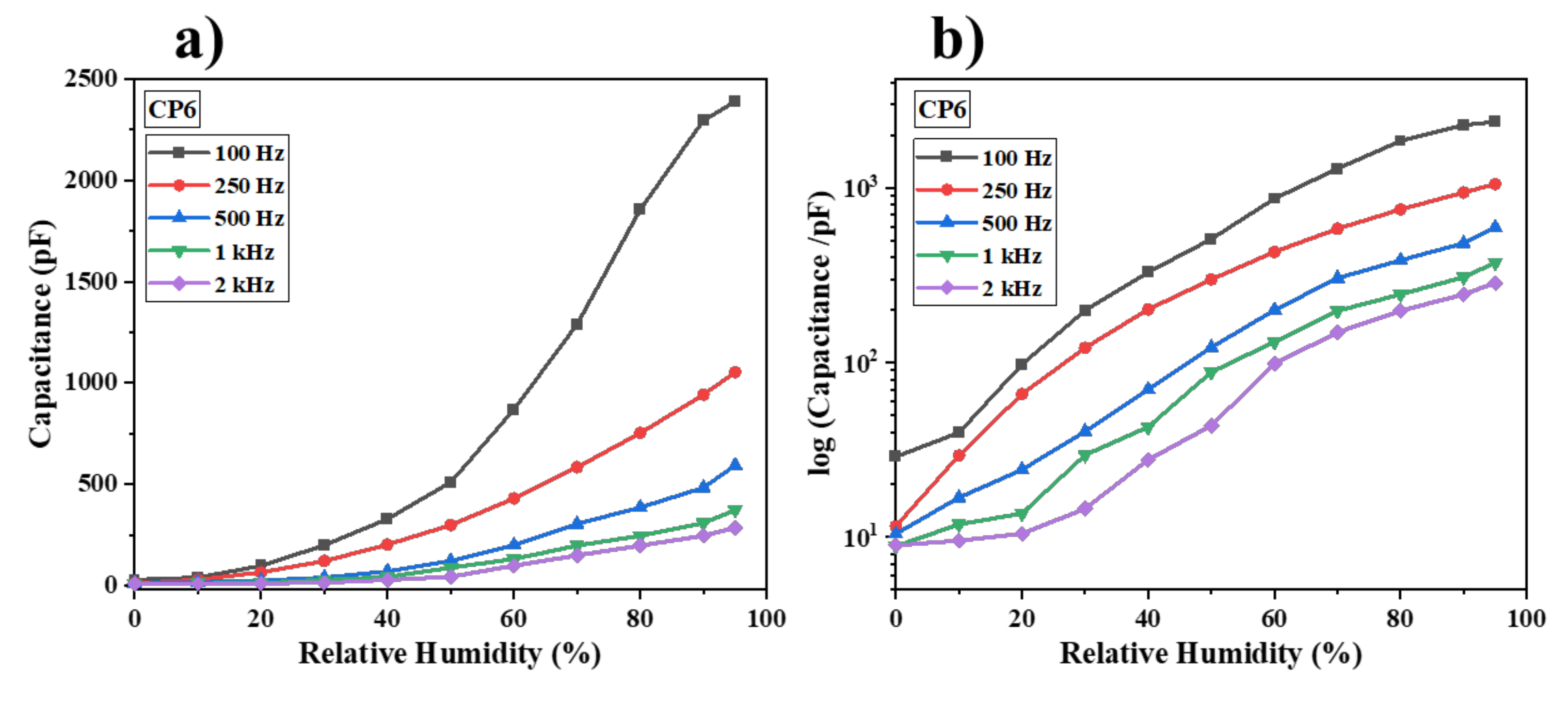
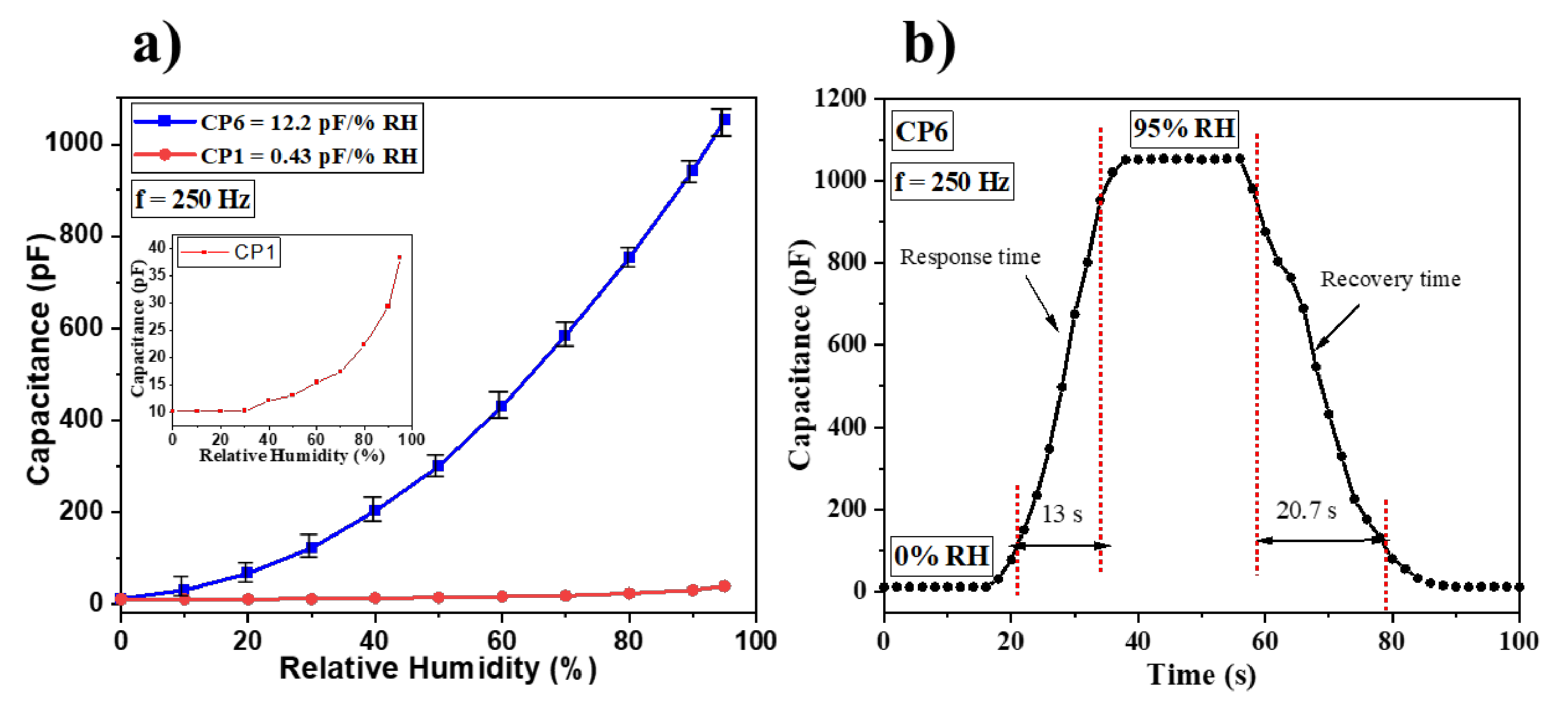
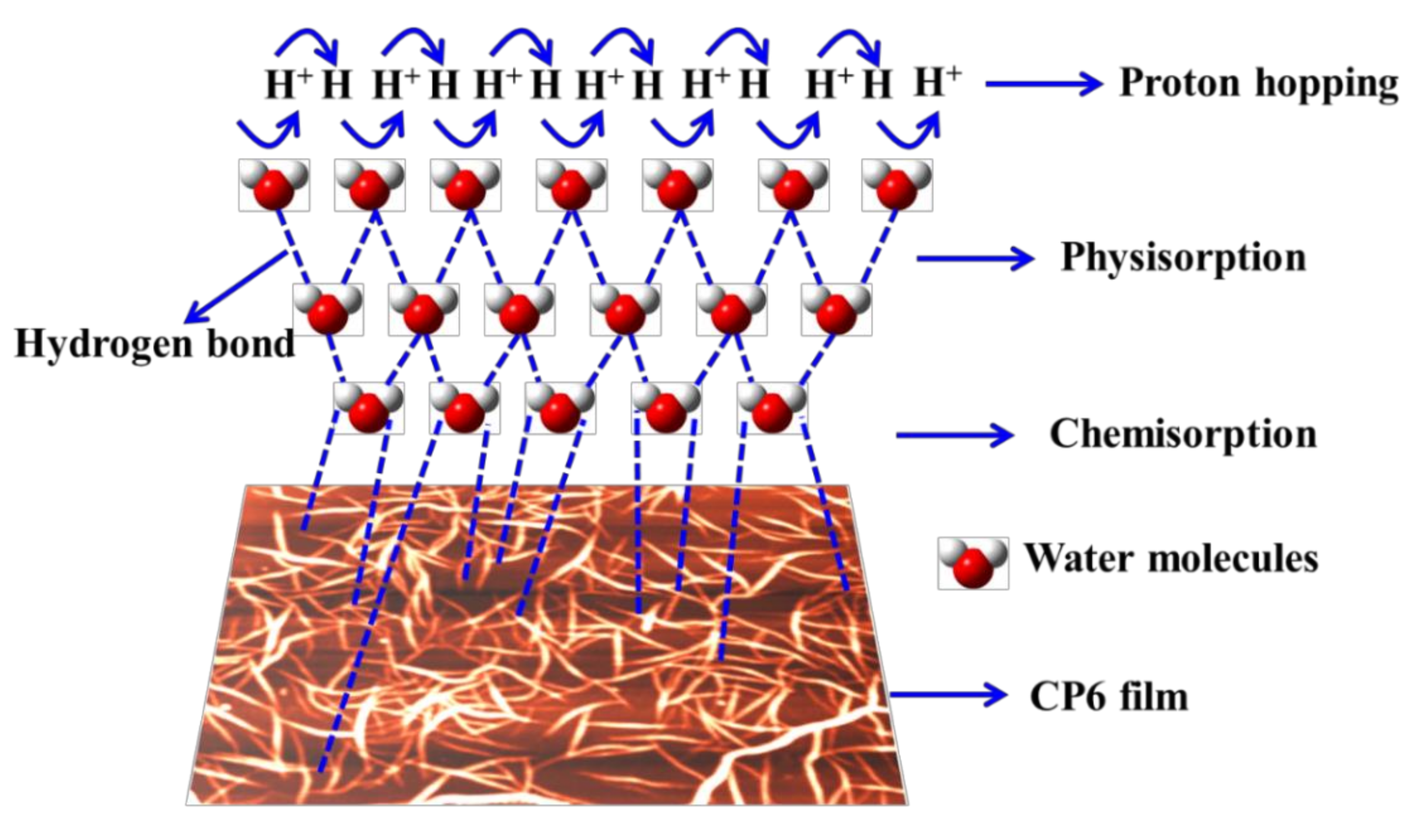
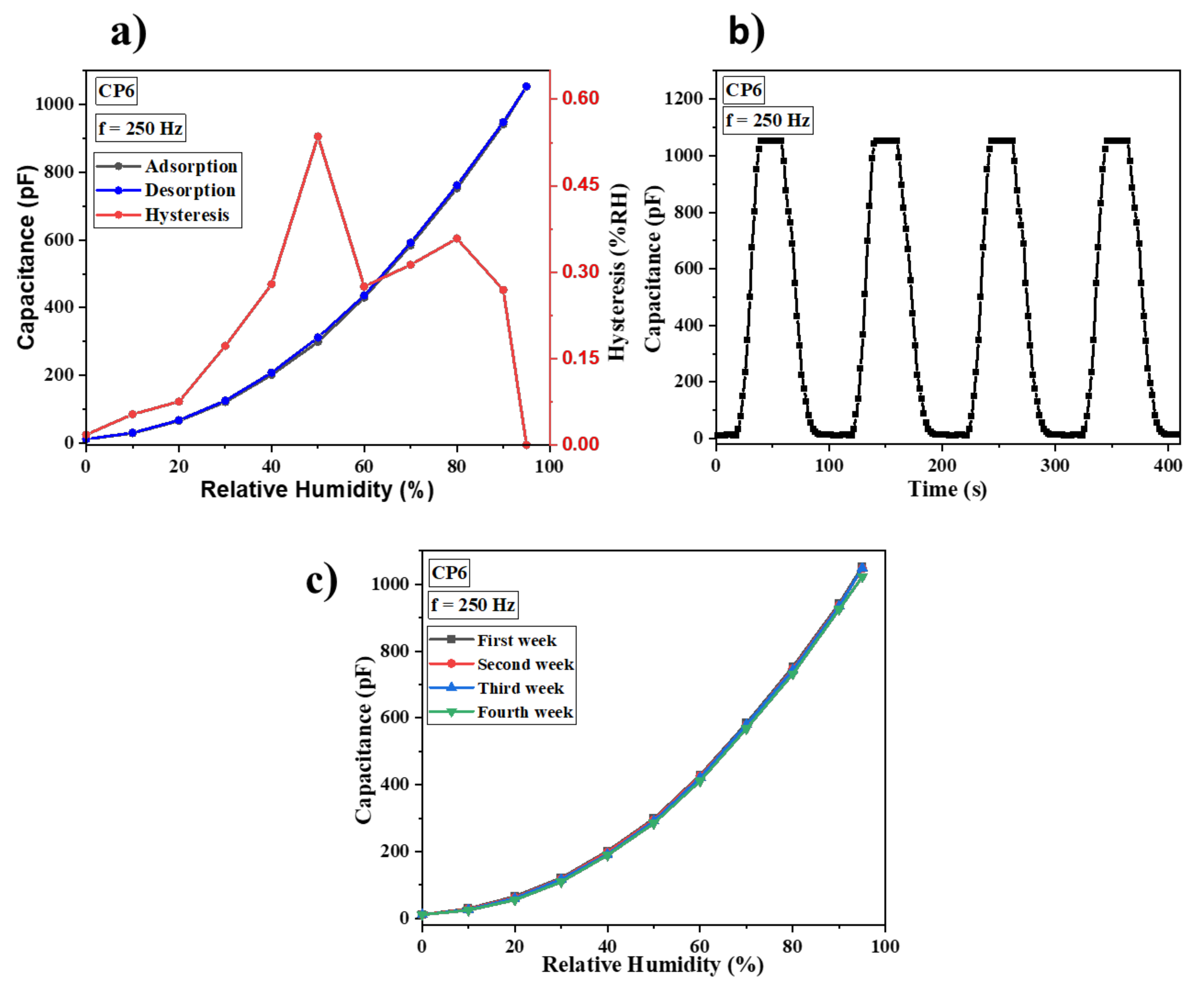
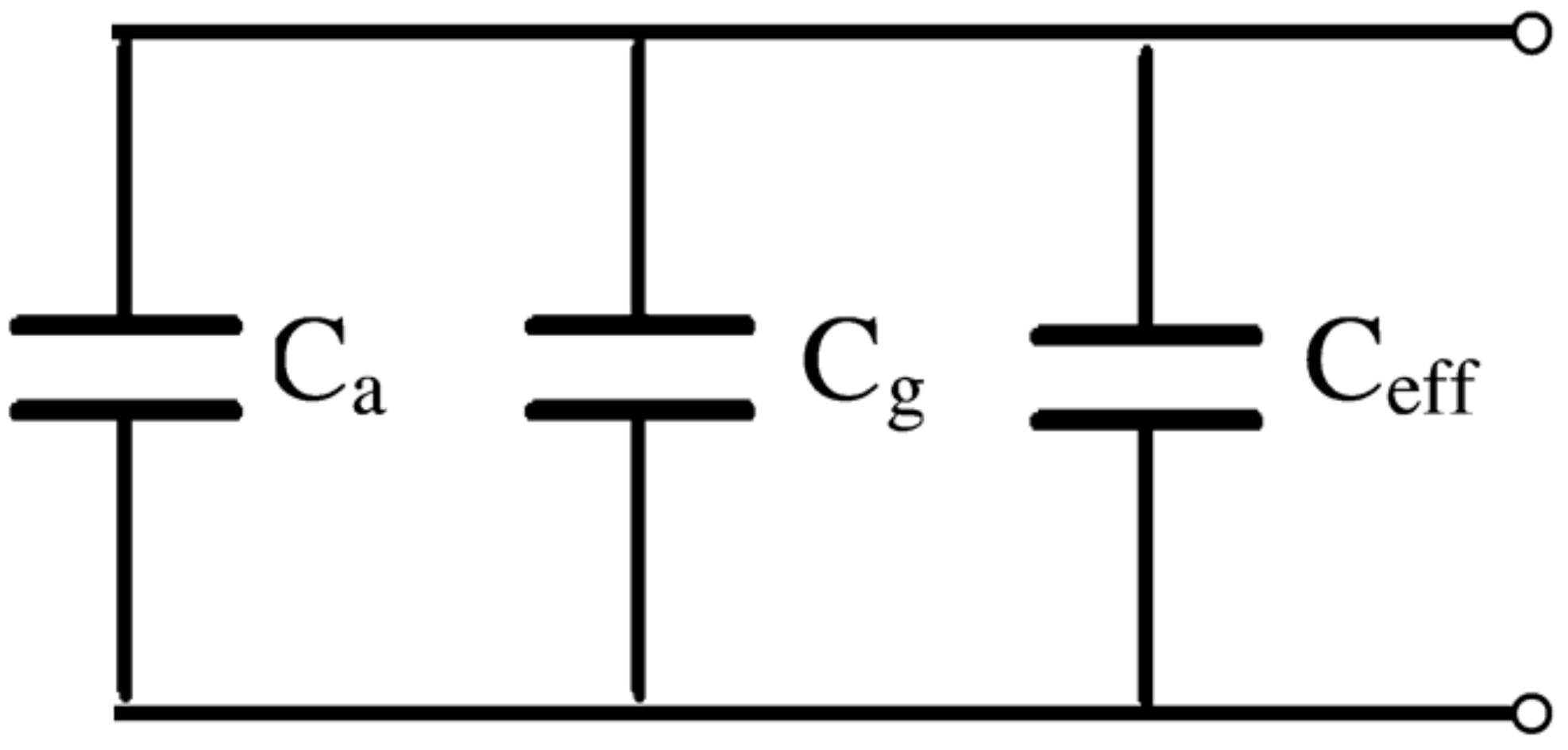
3.2. Humidity Sensing Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, Y.; Zhao, Y.; Chen, M.-Q.; Xia, F. Research advances in microfiber humidity sensors. Small 2018, 14, e1800524. [Google Scholar] [CrossRef]
- Huang, T.; Chou, J.; Sun, T.; Hsiung, S. A device for skin moisture and environment humidity detection. Sens. Actuators B Chem. 2008, 134, 206–212. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Tamagawa, S.; Fujitani, T.; Yamashita, H. Removal of phosphate from aqueous solution using layered double hydroxide prepared from waste iron-making slag. Bull. Chem. Soc. Jpn. 2016, 89, 472–480. [Google Scholar] [CrossRef]
- Antonacci, A.; Arduini, F.; Moscone, D.; Palleschi, G.; Scognamiglio, V. Nanostructured (Bio)sensors for smart agriculture. TrAC Trends Anal. Chem. 2018, 98, 95–103. [Google Scholar] [CrossRef]
- Lang, C.; Hubert, T.; Quaas, H.; Linke, M. Online measurement of humidity in the agri-food chain. Acta Hortic. 2010, 413–417. [Google Scholar] [CrossRef]
- Bridgeman, D.; Corral, J.; Quach, A.; Xian, X.; Forzani, E. Colorimetric humidity sensor based on liquid composite materials for the monitoring of food and pharmaceuticals. Langmuir 2014, 30, 10785–10791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, L.; Le, X.; Dong, H.; Xie, J.; Ying, Y.; Ping, J. One-step and large-scale fabrication of flexible and wearable humidity sensor based on laser-induced graphene for real-time tracking of plant transpiration at bio-interface. Biosens. Bioelectron. 2020, 165, 112360. [Google Scholar] [CrossRef]
- Salimifard, P.; Rim, D.; Gomes, C.; Kremer, P.; Freihaut, J.D. Resuspension of biological particles from indoor surfaces: Effects of humidity and air swirl. Sci. Total Environ. 2017, 583, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Berruti, G.; Consales, M.; Giordano, M.; Sansone, L.; Petagna, P.; Buontempo, S.; Breglio, G.; Cusano, A. Radiation hard humidity sensors for high energy physics applications using polyimide-coated fiber Bragg gratings sensors. Sens. Actuators B Chem. 2013, 177, 94–102. [Google Scholar] [CrossRef]
- Png, E.; Srinivasan, S.; Bekiroglu, K.; Chaoyang, J.; Su, R.; Poolla, K. An internet of things upgrade for smart and scalable heating, ventilation and air-conditioning control in commercial buildings. Appl. Energy 2019, 239, 408–424. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic-inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef]
- Najeeb, M.A.; Ahmad, Z.; Shakoor, R.A. Organic thin-film capacitive and resistive humidity sensors: A focus review. adv. mater. interfaces 2018, 5, 1800969. [Google Scholar] [CrossRef]
- Rehman, H.M.U.; Rehman, M.; Saqib, M.; Khan, S.A.; Khan, M.; Yang, Y.; Kim, S.; Rahman, S.; Kim, W.-Y. Highly efficient and wide range humidity response of biocompatible egg white thin film. Nanomaterials 2021, 11, 1815. [Google Scholar] [CrossRef] [PubMed]
- Rittersma, Z. Recent achievements in miniaturised humidity sensors—A review of transduction techniques. Sens. Actuators A Phys. 2002, 96, 196–210. [Google Scholar] [CrossRef]
- Ali, S.; Hassan, A.; Hassan, G.; Bae, J.; Lee, C.H. All-printed humidity sensor based on graphene/methyl-red composite with high sensitivity. Carbon 2016, 105, 23–32. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [Green Version]
- Fatima, N.; Aziz, F.; Ahmad, Z.; Najeeb, M.A.; Azmeer, M.I.; Karimov, K.S.; Ahmed, M.M.; Basheer, S.; Shakoor, R.A.; Sulaiman, K. Compositional engineering of the pi-conjugated small molecular VOPcPhO: Alq3 complex to boost humidity sensing. RSC Adv. 2017, 7, 19780–19786. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Jameel, M.A.; Gupta, A.; Langford, S.J.; Shafiei, M. Capacitive humidity sensing performance of naphthalene diimide derivatives at ambient temperature. Synth. Met. 2021, 275, 116739. [Google Scholar] [CrossRef]
- Fatima, Q.; Haidry, A.A.; Yao, Z.; He, Y.; Li, Z.; Sun, L.; Xie, L. The critical role of hydroxyl groups in water vapor sensing of graphene oxide. Nanoscale Adv. 2019, 1, 1319–1330. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Gupta, A.; Shafiei, M.; Langford, S. Recent advances in perylene diimide-based active materials in electrical mode gas sensing. Chemosensors 2021, 9, 30. [Google Scholar] [CrossRef]
- Wahab, F.; Sayyad, M.H.; Khan, D.N.; Tahir, M.; Aziz, F.; Khan, R.; Karimov, K.S. Sensing properties of cobalt-phthalocyanine-based multipurpose sensor. J. Electron. Mater. 2017, 46, 2045–2052. [Google Scholar] [CrossRef]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Jani, C.H.; Langford, S.J. Chemistry of naphthalene diimides. Chem. Soc. Rev. 2008, 37, 331–342. [Google Scholar] [CrossRef]
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-assembly of perylene imide molecules into 1d nanostructures: Methods, morphologies, and applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef]
- Hundal, A.K.; Agarwal, A.; Jameel, M.A.; Ali, S.; Chen, J.-Y.; Kaur, N.; Jones, L.; Li, J.-L.; Langford, S.; Gupta, A. Impact of self-assembly on the photovoltaic properties of a small molecule oligothiophene donor. Sol. Energy 2020, 195, 223–229. [Google Scholar] [CrossRef]
- Hundal, A.K.; Ali, S.; Agarwal, A.; Jameel, M.A.; Jones, L.A.; Li, J.-L.; Evans, R.A.; Langford, S.J.; Gupta, A. Enhanced photovoltaic efficiency via control of self-assembly in cyanopyridone-based oligothiophene donors. J. Phys. Chem. Lett. 2021, 12, 919–924. [Google Scholar] [CrossRef]
- Gupta, A.; Ali, A.; Bilic, A.; Gao, M.; Hegedűs, K.; Singh, B.; Watkins, S.E.; Wilson, G.J.; Bach, U.; Evans, R.A. Absorption enhancement of oligothiophene dyes through the use of a cyanopyridone acceptor group in solution-processed organic solar cells. Chem. Commun. 2012, 48, 1889–1891. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Pramanik, S.; Manna, A.; Bhuyan, S.; Shah, N.F.A.; Radzi, Z.; Abu Osman, N.A. Design and development for capacitive humidity sensor applications of lead-free Ca,Mg,Fe,Ti-oxides-based electro-ceramics with improved sensing properties via physisorption. Sensors 2016, 16, 1135. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Zhang, Y.; Guo, W.; Ruan, S. Characterization and humidity sensing properties of the sensor based on Na2Ti3O7 nanotubes. J. Nanosci. Nanotechnol. 2014, 14, 4303–4307. [Google Scholar] [CrossRef] [PubMed]
- Al-Sehemi, A.G.; Al-Assiri, M.S.; Kalam, A.; Zafar, Q.; Azmer, M.I.; Sulaiman, K.; Ahmad, Z. Sensing performance optimization by tuning surface morphology of organic (D-π-A) dye-based humidity sensor. Sens. Actuators B Chem. 2016, 231, 30–37. [Google Scholar] [CrossRef]
- Azmer, M.I.; Ahmad, Z.; Sulaiman, K.; Al-Sehemi, A.G. Humidity dependent electrical properties of an organic material DMBHPET. Measurement 2015, 61, 180–184. [Google Scholar] [CrossRef]
- Li, B.; Tian, Q.; Su, H.; Wang, X.; Wang, T.; Zhang, D. High sensitivity portable capacitive humidity sensor based on In2O3 nanocubes-decorated GO nanosheets and its wearable application in respiration detection. Sens. Actuators B Chem. 2019, 299, 126973. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Ahmad, Z.; Mallick, S.; Touati, F.; Al-Thani, N. Capacitive type humidity sensor based on PANI decorated Cu–ZnS porous microspheres. Talanta 2020, 219, 121361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Tomer, V.K.; Jena, N.; Bala, I.; Sharma, N.; Nepak, D.; Pal, S.K. Truxene based porous, crystalline covalent organic frameworks and it’s applications in humidity sensing. Molecules 2017, 49, 50. [Google Scholar]
- Niu, H.; Yue, W.; Li, Y.; Yin, F.; Gao, S.; Zhang, C.; Kan, H.; Yao, Z.; Jiang, C.; Wang, C. Ultrafast-response/recovery capacitive humidity sensor based on arc-shaped hollow structure with nanocone arrays for human physiological signals monitoring. Sens. Actuators B Chem. 2021, 334, 129637. [Google Scholar] [CrossRef]
- Khadse, V.; Thakur, S.; Patil, K.; Patil, P. Humidity-sensing studies of cerium oxide nanoparticles synthesized by non-isothermal precipitation. Sens. Actuators B Chem. 2014, 203, 229–238. [Google Scholar] [CrossRef]
- Yao, W.; Chen, X.; Zhang, J. A capacitive humidity sensor based on gold–PVA core–shell nanocomposites. Sens. Actuators B Chem. 2010, 145, 327–333. [Google Scholar] [CrossRef]
- Ali, S.; Jameel, M.A.; Harrison, C.J.; Gupta, A.; Shafiei, M.; Langford, S.J. Nanoporous naphthalene diimide surface enhances humidity and ammonia sensing at room temperature. Sens. Actuators B Chem. 2021, 351, 130972. [Google Scholar] [CrossRef]
- Safian, N.A.M.; Anuar, A.; Omar, A.-Z.; Bawazeer, T.M.; Alsenany, N.; Alsoufi, M.S.; Supangat, A.; Roslan, N.A. Enhanced sensitivity of zinc phthalocyanine-based microporous humidity sensors by varying size of electrode gaps. Sens. Actuators B Chem. 2021, 343, 130158. [Google Scholar] [CrossRef]
- Mondal, P.P.; Mahapatra, P.L.; Das, S.; Saha, D. Study on the novel capacitive moisture sensing behaviour of nickel chromite nanoparticle based thick film. Measurement 2020, 163, 107992. [Google Scholar] [CrossRef]
- Roslan, N.A.; Abu Bakar, A.; Bawazeer, T.M.; Alsoufi, M.S.; Alsenany, N.; Majid, W.H.A.; Supangat, A. Enhancing the performance of vanadyl phthalocyanine-based humidity sensor by varying the thickness. Sens. Actuators B Chem. 2019, 279, 148–156. [Google Scholar] [CrossRef]
- Ali, S.; Tahir, M.; Mehboob, N.; Wahab, F.; Langford, S.J.; Said, S.M.; Sarker, M.R.; Julai, S.; Ali, S.H.M. Amino anthraquinone: Synthesis, characterization, and its application as an active material in environmental sensors. Material 2020, 13, 960. [Google Scholar] [CrossRef] [Green Version]
- Raza, E.; Asif, S.; Aziz, F.; Azmer, M.I.; Malik, H.A.; Teh, C.-H.; Najeeb, M.A.; Zafar, Q.; Ahmad, Z.; Wahab, F.; et al. Influence of thermal annealing on a capacitive humidity sensor based on newly synthesized macroporous PBObzT. Sens. Actuators B Chem. 2016, 235, 146–153. [Google Scholar] [CrossRef]
- Tahir, M.; Sayyad, M.H.; Clark, J.; Wahab, F.; Aziz, F.; Shahid, M.; Chaudry, J.A. Humidity, light and temperature dependent characteristics of Au/N-BuHHPDI/Au surface type multifunctional sensor. Sens. Actuators B 2014, 192, 565–571. [Google Scholar] [CrossRef]
- Tousi, M.M.; Zhang, Y.; Wan, S.; Yu, L.; Hou, C.; Yan, N.; Fink, Y.; Wang, A.; Jia, X. Scalable fabrication of highly flexible porous polymer-based capacitive humidity sensor using convergence fiber drawing. Polymers 2019, 11, 1985. [Google Scholar] [CrossRef] [Green Version]
- Saqib, M.; Khan, S.A.; Mutee Ur Rehman, H.M.; Yang, Y.; Kim, S.; Rehman, M.M.; Young Kim, W. High-performance humidity sensor based on the graphene flower/zinc oxide composite. Nanomaterials 2021, 11, 242. [Google Scholar] [CrossRef]
- Khan, S.A.; Saqib, M.; Rehman, M.M.; Mutee Ur Rehman, H.M.; Rahman, S.A.; Yang, Y.; Kim, W.Y. A full-range flexible and printed humidity sensor based on a solution-processed p (vdf-trfe)/graphene-flower composite. Nanomaterials 2021, 11, 1915. [Google Scholar] [CrossRef]
- Andrés, M.A.; Vijjapu, M.T.; Surya, S.G.; Shekhah, O.; Salama, K.N.; Serre, C.; Eddaoudi, M.; Roubeau, O.; Gascón, I. Methanol and humidity capacitive sensors based on thin films of MOF nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4155–4162. [Google Scholar] [CrossRef]
| Materials | Bandwidth (%RH) | Operating Frequency | Hysteresis (%) | TRes/TRec (s) | Sensitivity (pF/%RH) | Refs. |
|---|---|---|---|---|---|---|
| PANI/Cu–ZnS | 30–90 | - | 1.5 | 42/24 | 12 | [33] |
| AU/PVA | 11–93 | - | - | 145/94 | 0.05 | [38] |
| NDI-1 | 10–95 | 250 Hz | 0.72 | 20.1/6.28 | 10.13 | [39] |
| ZnPc | 20–100 | 1 kHz | 9.13 | 15/10 | 1.03 | [40] |
| CoCr2O4 | 05–95 | 100 Hz | - | 100/150 | 3 | [41] |
| VTP | 30–95 | 500 Hz | 1.26 | 15/10 | 0.09 | [42] |
| AAQ | 40–88 | 120 Hz | - | 20/26 | 0.4 | [43] |
| PBObzT2 | 50–95 | 100 Hz | 2.76 | 10/4 | 5 | [44] |
| TMBHPET | 37–99 | 100 Hz | 2.4 | 15/15 | 0.064 | [30] |
| DMBHPET | 45–95 | 1 kHz | - | 10/15 | 0.012 | [31] |
| PDI derivative | 60–90 | 120 Hz | - | 60/70 | 41 | [45] |
| NDI derivative | 10–95 | 250 Hz | 2.06 | 374/607 | 475 | [18] |
| Porous polymer | 15–80 | - | 9.08 | - | 0.4 | [46] |
| GrF | 15–86 | 100 Hz | - | 0.4/4 | 7.77 | [47] |
| P(VDF-TrFE) | 08–98 | 100 Hz | - | 0.8/2.5 | 0.05 | [48] |
| MOF NPs | 04–88 | - | - | 420/510 | 0.35 | [49] |
| CP6 | 10–95 | 250 Hz | 0.53 | 13/20.7 | 12.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Jameel, M.A.; Harrison, C.J.; Gupta, A.; Evans, R.A.; Shafiei, M.; Langford, S.J. Enhanced Capacitive Humidity Sensing Performance at Room Temperature via Hydrogen Bonding of Cyanopyridone-Based Oligothiophene Donor. Chemosensors 2021, 9, 320. https://doi.org/10.3390/chemosensors9110320
Ali S, Jameel MA, Harrison CJ, Gupta A, Evans RA, Shafiei M, Langford SJ. Enhanced Capacitive Humidity Sensing Performance at Room Temperature via Hydrogen Bonding of Cyanopyridone-Based Oligothiophene Donor. Chemosensors. 2021; 9(11):320. https://doi.org/10.3390/chemosensors9110320
Chicago/Turabian StyleAli, Salman, Mohammed A. Jameel, Christopher J. Harrison, Akhil Gupta, Richard A. Evans, Mahnaz Shafiei, and Steven J. Langford. 2021. "Enhanced Capacitive Humidity Sensing Performance at Room Temperature via Hydrogen Bonding of Cyanopyridone-Based Oligothiophene Donor" Chemosensors 9, no. 11: 320. https://doi.org/10.3390/chemosensors9110320
APA StyleAli, S., Jameel, M. A., Harrison, C. J., Gupta, A., Evans, R. A., Shafiei, M., & Langford, S. J. (2021). Enhanced Capacitive Humidity Sensing Performance at Room Temperature via Hydrogen Bonding of Cyanopyridone-Based Oligothiophene Donor. Chemosensors, 9(11), 320. https://doi.org/10.3390/chemosensors9110320