Fluoro-Substituted Metal Phthalocyanines for Active Layers of Chemical Sensors
Abstract
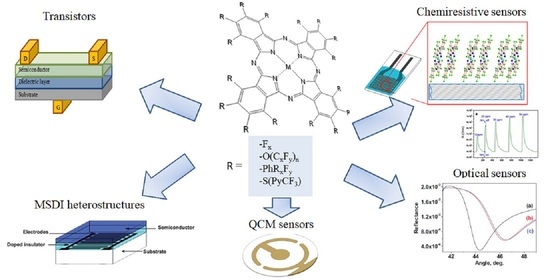
:1. Introduction
2. Brief Overview of Fluorinated Phthalocyanines and Preparation of Their Thin Films
3. Active Layers of the Devices with Electrical Sensor Response
3.1. Sensors Based on MPcFx Films Obtained by PVD
3.2. Solution Processed Films
3.3. Hybrid Materials with Carbon Nanotubes
4. Other Types of Chemical Sensors
4.1. Optical Sensors
4.2. Quartz Crystal Microbalance Sensors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Sagar, R.U.R.; Chen, J.; Liu, J.; Aslam, S.; Nosheen, F.; Anwar, T.; Hussain, N.; Hou, X.; Liang, T. Cobalt phthalocyanine as an efficient catalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 19338–19346. [Google Scholar] [CrossRef]
- Thiruppathiraja, T.; Arokiyanathan, A.L.; Aazaad, B.; Silviya, R.; Lakshmipathi, S. H, OH and COOH functionalized magnesium phthalocyanine as a catalyst for oxygen reduction reaction (ORR)–A DFT study. Int. J. Hydrogen Energy 2020, 45, 8540–8548. [Google Scholar] [CrossRef]
- Farahmand, S.; Ghiaci, M.; Asghari, S. Oxo-vanadium (IV) phthalocyanine implanted onto the modified SBA-15 as a catalyst for direct hydroxylation of benzene to phenol in acetonitrile-water medium: A kinetic study. Chem. Eng. Sci. 2021, 232, 116331. [Google Scholar] [CrossRef]
- Ghadari, R.; Saei, P.S.; Sabri, A.; Ghasemi, Z.; Kong, F. Enhanced phthalocyanine-sensitized solar cell efficiency via cooperation of nitrogen-doped carbon dots. J. Clean. Prod. 2020, 268, 122236. [Google Scholar] [CrossRef]
- Ghadari, R.; Sabri, A.; Saei, P.S.; Kong, F.T.; Marques, H.M. Phthalocyanine-silver nanoparticle structures for plasmon-enhanced dye-sensitized solar cells. Sol. Energy 2020, 198, 283–294. [Google Scholar] [CrossRef]
- Benhaliliba, M. A growth of A–Z phthalocyanine layers onto Si by thermal evaporation process to achieve organic heterojunction diodes. Optik 2020, 217, 164832. [Google Scholar] [CrossRef]
- Raveendra Kiran, M.; Ulla, H.; Satyanarayan, M.N.; Umesh, G. Optoelectronic properties of hybrid diodes based on vanadyl- phthalocyanine and zinc oxide nanorods thin films. Opt. Mater. 2019, 96, 109348. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, P.; Ye, J.; Li, Y.; Li, H.; Zhang, X.; Li, R.; Dong, H.; Hu, W.; Kloc, C. Molecular Crystal Engineering: Tuning Organic Semiconductor from p-type to n-type by Adjusting Their Substitutional Symmetry. Adv. Mater. 2017, 29, 1605053. [Google Scholar] [CrossRef]
- Shao, X.; Wang, S.; Li, X.; Su, Z.; Chen, Y.; Xiao, Y. Single component p-, ambipolar and n-type OTFTs based on fluorinated copper phthalocyanines. Dyes Pigments 2016, 132, 378–386. [Google Scholar] [CrossRef]
- Chia, L.S.; Du, Y.H.; Palale, S.; Lee, P.S. Interaction of Copper Phthalocyanine with Nitrogen Dioxide and Ammonia Investigation Using X-ray Absorption Spectroscopy and Chemiresistive Gas Measurements. ACS Omega 2019, 4, 10388–10395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diab, N.; Morales, D.M.; Andronescu, C.; Masoud, M.; Schuhmann, W. A sensitive and selective graphene/cobalt tetrasulfonated phthalocyanine sensor for detection of dopamine. Sens. Actuators B Chem. 2019, 285, 17–23. [Google Scholar] [CrossRef]
- Chaabene, M.; Gassoumi, B.; Mignon, P.; Ben Chaâbane, R.; Allouche, A.R. New zinc phthalocyanine derivatives for nitrogen dioxide sensors: A theoretical optoelectronic investigation. J. Mol. Graph. Model. 2019, 88, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Chen, J.Y.; Hu, J.Q.; Zhang, L.; Lin, A.L.; Wang, R.; Zheng, B.Y.; Ke, M.R.; Li, X.; Huang, J.D. The substituted zinc(II) phthalocyanines using “sulfur bridge” as the linkages. Synthesis, red-shifted spectroscopic properties and structure-inherent targeted photodynamic activities. Dyes Pigments 2021, 189, 109270. [Google Scholar] [CrossRef]
- Al-Raqa, S.Y.; Khezami, K.; Kaya, E.N.; Durmuş, M. A novel water soluble axially substituted silicon(IV) phthalocyanine bearing quaternized 4-(4-pyridinyl)phenol groups: Synthesis, characterization, photophysicochemical properties and BSA/DNA binding behavior. Polyhedron 2021, 194, 114937. [Google Scholar] [CrossRef]
- Kuzmina, E.A.; Dubinina, T.V.; Tomilova, L.G. Recent advances in chemistry of phthalocyanines bearing electron-withdrawing halogen, nitro and: N -substituted imide functional groups and prospects for their practical application. New J. Chem. 2019, 43, 9314–9327. [Google Scholar] [CrossRef]
- Kuzmina, E.A.; Dubinina, T.V.; Vasilevsky, P.N.; Saveliev, M.S.; Gerasimenko, A.Y.; Borisova, N.E.; Tomilova, L.G. Novel octabromo-substituted lanthanide(III) phthalocyanines–Prospective compounds for nonlinear optics. Dyes Pigments 2021, 185, 108871. [Google Scholar] [CrossRef]
- Stuzhin, P.A. Fluorinated phthalocyanine and their analogues. In Fluorine in Heterocyclic Chemistry: Volume 1: 5-Membered Heterocycles and Macrocycles; Nenajdenko, V., Ed.; Springer: Cham, Switzerland, 2014; Volume 1, pp. 621–681. ISBN 9783319043463. [Google Scholar]
- Li, Y.; Han, X.; Li, Y.; Zhou, W.; Li, P.; Liu, R.; Lin, X.; Huang, Z.; Feng, X.; Ma, Y. Air-stable, transparent flexible ambipolar organic thin film transistors based on CuPc-F16CuPc bi-channel structure. AIP Adv. 2018, 8, 119903. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Xu, W.; Hu, W.; Zhu, D. Air-stable ambipolar organic field-effect transistor based on a novel bi-channel structure. J. Mater. Chem. 2008, 18, 2420–2422. [Google Scholar] [CrossRef]
- Eguchi, K.M.; Matsushita, M.; Awaga, K. In Situ Real-Time Measurements for Ambipolar Channel Formation Processes in Organic Double-Layer Field-Effect Transistors of CuPc and F16CuPc. J. Phys. Chem. C 2018, 122, 26054–26060. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Gromilov, S.; Krasnov, P.; Basova, T. Fluorinated metal phthalocyanines: Interplay between fluorination degree, films orientation, and ammonia sensing properties. Sensors 2018, 18, 2141. [Google Scholar] [CrossRef] [Green Version]
- Schlettwein, D.; Graaf, H.; Meyer, J.-P.; Oekermann, T.I.; Jaeger, N. Molecular Interactions in Thin Films of Hexadecafluorophthalocyaninatozinc (F16PcZn) as Compared to Islands of N,N′-Dimethylperylene-3,4,9,10-biscarboximide (MePTCDI). J. Phys. Chem. B 1999, 103, 3078–3086. [Google Scholar] [CrossRef]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Valli, L. Phthalocyanine-based Langmuir–Blodgett films as chemical sensors. Adv. Colloid Interface Sci. 2005, 116, 13–44. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, M.; Gaudillat, P.; Suisse, J.M. Phthalocyanine-based hybrid materials for chemosensing. J. Porphyr. Phthalocyanines 2013, 17, 913–919. [Google Scholar] [CrossRef]
- Rodriguez-Méndez, M.L.; Gay, M.; De Saja, J.A. New insights into sensors based on radical bisphthalocyanines. J. Porphyr. Phthalocyanines 2009, 13, 1159–1167. [Google Scholar] [CrossRef]
- Nesakumar, N.; Berchmans, S.; Alwarappan, S. Chemically modified carbon based electrodes for the detection of reduced glutathione. Sens. Actuators B Chem. 2018, 264, 448–466. [Google Scholar] [CrossRef]
- Moraes, F.C.; Cabral, M.F.; Machado, S.A.S.; Mascaro, L.H. Electrocatalytic behavior of glassy carbon electrodes modified with multiwalled carbon nanotubes and cobalt phthalocyanine for selective analysis of dopamine in presence of ascorbic acid. Electroanalysis 2008, 20, 851–857. [Google Scholar] [CrossRef]
- Bhupathiraju, N.V.S.D.K.; Rizvi, W.; Batteas, J.D.; Drain, C.M. Fluorinated porphyrinoids as efficient platforms for new photonic materials, sensors, and therapeutics. Org. Biomol. Chem. 2016, 14, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Casa, S.; Henary, M. Synthesis and Applications of Selected Fluorine-Containing Fluorophores. Molecules 2021, 26, 1160. [Google Scholar] [CrossRef] [PubMed]
- Carrión, E.N.; Loas, A.; Patel, H.H.; Pelmuş, M.; Ramji, K.; Gorun, S.M. Fluoroalkyl phthalocyanines: Bioinspired catalytic materials. J. Porphyr. Phthalocyanines 2018, 22, 371–397. [Google Scholar] [CrossRef] [Green Version]
- Klyamer, D.D.; Sukhikh, A.S.; Trubin, S.V.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V.; Hassan, A.K. Tetrafluorosubstituted Metal Phthalocyanines: Interplay between Saturated Vapor Pressure and Crystal Structure. Cryst. Growth Des. 2020, 20, 1016–1024. [Google Scholar] [CrossRef]
- Harbeck, M.; Taşaltın, C.; Gürol, I.; Musluoğlu, E.; Ahsen, V.; Öztürk, Z.Z. Preferential sorption of polar compounds by fluoroalkyloxy substituted phthalocyanines for the use in sorption based gas sensors. Sens. Actuators B Chem. 2010, 150, 616–624. [Google Scholar] [CrossRef]
- Jiang, H.; Ye, J.; Hu, P.; Wei, F.; Du, K.; Wang, N.; Ba, T.; Feng, S.; Kloc, C. Fluorination of metal phthalocyanines: Single-crystal growth, efficient N-channel organic field-effect transistors, and structure-property relationships. Sci. Rep. 2014, 4, 7573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.M.; Mutyala, A.K.; Cha, M.J.; Seo, J.H.; Kang, Y.A.; Park, J.S. Preparation of Alkylated and Perfluorinated ZnPc-modified Carbon Nanotubes and their Application as Conductive Fillers for Poly(vinylidene fluoride) Composite Dielectrics. Bull. Korean Chem. Soc. 2017, 38, 1190–1195. [Google Scholar] [CrossRef]
- Skonieczny, R.; Popielarski, P.; Bała, W.; Fabisiak, K.; Paprocki, K.; Jancelewicz, M.; Kowalska, M.; Szybowicz, M. Effect of annealing temperature on optical and electrical properties of metallophthalocyanine thin films deposited on silicon substrate. Mater. Sci. Pol. 2016, 34, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Schuster, B.E.; Basova, T.V.; Plyashkevich, V.A.; Peisert, H.; Chassé, T. Effects of temperature on structural and morphological features of CoPc and CoPcF16 thin films. Thin Solid Films 2010, 518, 7161–7166. [Google Scholar] [CrossRef]
- Cook, M.J.; Chambrier, I. Phthalocyanine Thin Films: Deposition and Structural Studies. In The Porphyrin Handbook; Academic Press: Cambridge, MA, USA, 2003; pp. 37–127. ISBN 9780080923918. [Google Scholar]
- Basova, T.V.; Kiselev, V.G.; Dubkov, I.S.; Latteyer, F.; Gromilov, S.A.; Peisert, H.; Chassè, T. Optical spectroscopy and XRD study of molecular orientation, polymorphism, and phase transitions in fluorinated vanadyl phthalocyanine thin films. J. Phys. Chem. C 2013, 117, 7097–7106. [Google Scholar] [CrossRef]
- Wright, J. Gas adsorption on phthalocyanines and its effects on electrical properties. Prog. Surf. Sci. 1989, 31, 1–60. [Google Scholar] [CrossRef]
- Schön, J.H.; Bao, Z. Influence of disorder on the electron transport properties in fluorinated copper-phthalocyanine thin films. J. Appl. Phys. 2001, 89, 3526–3528. [Google Scholar] [CrossRef]
- Ye, R.; Baba, M.; Suzuki, K.; Mori, K. Improved performance of fluorinated copper phthalocyanine thin film transistors using an organic p-n junction: Effect of copper phthalocyanine film thickness. Thin Solid Films 2009, 517, 3001–3004. [Google Scholar] [CrossRef]
- Ye, R.; Baba, M.; Ohishi, Y.; Mori, K.; Suzuki, K. On the correlation between morphology and electronic properties of fluorinated copper phthalocyanine (F16CuPc) thin films. Mol. Cryst. Liq. Cryst. 2006, 444, 203–210. [Google Scholar] [CrossRef]
- Ballirano, P.; Caminiti, R.; Ercolani, C.; Maras, A.; Orrù, M.A. X-ray powder diffraction structure reinvestigation of the α and β forms of cobalt phthalocyanine and kinetics of the α→β phase transition. J. Am. Chem. Soc. 1998, 120, 12798–12807. [Google Scholar] [CrossRef]
- Vergnat, C.; Landais, V.; Legrand, J.F.; Brinkmann, M. Orienting semiconducting nanocrystals on nanostructured polycarbonate substrates: Impact of substrate temperature on polymorphism and in-plane orientation. Macromolecules 2011, 44, 3817–3827. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Gromilov, S.A.; Kruchinin, V.N.; Spesivtsev, E.V.; Hassan, A.K.; Basova, T.V. Influence of fluorosubstitution on the structure of zinc phthalocyanine thin films. Macroheterocycles 2018, 11, 304–311. [Google Scholar] [CrossRef]
- Pandey, P.A.A.; Rochford, L.S.; Keeble, D.P.; Rourke, J.S.; Jones, T.; Beanland, R.R.; Wilson, N. Resolving the Nanoscale Morphology and Crystallographic Structure of Molecular Thin Films: F16CuPc on Graphene Oxide. Chem. Mater. 2012, 24, 1365–1370. [Google Scholar] [CrossRef]
- Yoon, S.M.; Song, H.J.; Hwang, I.C.; Kim, K.S.; Choi, H.C. Single crystal structure of copper hexadecafluorophthalocyanine (F16CuPc) ribbon. Chem. Commun. 2010, 46, 231–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuprikova, N.M.; Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Mrsic, I.; Basova, T.V. Fluorosubstituted lead phthalocyanines: Crystal structure, spectral and sensing properties. Dyes Pigments 2020, 173, 107939. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Nikolaeva, N.; Morozova, N.; Basova, T. Vanadyl phthalocyanine films and their hybrid structures with Pd nanoparticles: Structure and sensing properties. Sensors 2020, 20, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, E.N.; Şenocak, A.; Klyamer, D.D.; Demirbaş, E.; Basova, T.V.; Durmuş, M. Ammonia sensing performance of thin films of cobalt(II) phthalocyanine bearing fluorinated substituents. J. Mater. Sci. Mater. Electron. 2019, 30, 7543–7551. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, Y.; Wang, H.; Dai, F.; Yang, G.; Chen, Y. A phthalocyanine sensor array based on sensitivity and current changes for highly sensitive identification of three toxic gases at ppb levels. New J. Chem. 2020, 44, 13240–13248. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.; Xue, Y.; Zhang, M.; Li, P.; Lian, K.; Zhuiykov, S.; Zhang, W.; Chen, Y. Highly sensitive and ultra-fast gas sensor based on CeO2-loaded In2O3 hollow spheres for ppb-level hydrogen detection. Sens. Actuators B Chem. 2018, 257, 469–476. [Google Scholar] [CrossRef]
- Şen, Z.; Tarakci, D.K.; Gürol, I.; Ahsen, V.; Harbeck, M. Governing the sorption and sensing properties of titanium phthalocyanines by means of axial ligands. Sens. Actuators B Chem. 2016, 229, 581–586. [Google Scholar] [CrossRef]
- Dong, Z.; Kong, X.; Wu, Y.; Zhang, J.; Chen, Y. High-sensitive room-temperature NO2 sensor based on a soluble n-type phthalocyanine semiconductor. Inorg. Chem. Commun. 2017, 77, 18–22. [Google Scholar] [CrossRef]
- Basova, T.V.; Hassan, A.; Krasnov, P.O.; Gürol, I.; Ahsen, V. Trimethylamine sorption into thin layers of fluoroalkyloxy and alkyloxy substituted phthalocyanines: Optical detection and DFT calculations. Sens. Actuators B Chem. 2015, 216, 204–211. [Google Scholar] [CrossRef]
- Sun, Q.; Feng, W.; Yang, P.; You, G.; Chen, Y. Highly selective room-temperature NO2 sensors based on a fluoroalkoxy-substituted phthalocyanine. New J. Chem. 2018, 42, 6713–6718. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Ding, X.; Wang, X.; Li, X.; Chen, Y. High-performance room-temperature NO2 sensors based on microstructures self-assembled from n-type phthalocyanines: Effect of fluorine–hydrogen bonding and metal–ligand coordination on morphology and sensing performance. Org. Electron. 2017, 50, 389–396. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, L.; Su, X.; Zhou, F.; Duan, G. Interfacial self-assembly of CoPc thin films with their high sensing use as NO2 sensors. Mater. Chem. Phys. 2019, 234, 94–101. [Google Scholar] [CrossRef]
- Liu, C.J.; Peng, C.H.; Ju, Y.H.; Hsieh, J.C. Titanyl phthalocyanine gas sensor for NO2 detection. Sens. Actuators B Chem. 1998, 52, 264–269. [Google Scholar] [CrossRef]
- Nemakal, M.; Aralekallu, S.; Mohammed, I.; Pari, M.; Venugopala Reddy, K.; Sannegowda, L.K. Nanomolar detection of 4-aminophenol using amperometric sensor based on a novel phthalocyanine. Electrochim. Acta 2019, 318, 342–353. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V. Thin films of tetrafluorosubstituted cobalt phthalocyanine: Structure and sensor properties. Appl. Surf. Sci. 2016, 372, 79–86. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Saini, R.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Reversible and fast responding ppb level Cl2 sensor based on noncovalent modified carbon nanotubes with Hexadecafluorinated copper phthalocyanine. Sens. Actuators B Chem. 2018, 255, 87–99. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated zinc phthalocyanine as ppb level chemiresistive chlorine sensor. Appl. Surf. Sci. 2018, 427, 202–209. [Google Scholar] [CrossRef]
- Zhang, B.; Tai, H.L.; Xie, G.Z.; Li, X.; Zhang, H.N. The investigation of a new NO2 OTFT sensor based on heterojunction F16CuPc/CuPc thin films. Adv. Mater. Res. 2013, 721, 159–163. [Google Scholar] [CrossRef]
- Nikolaeva, N.S.; Klyamer, D.D.; Zharkov, S.M.; Tsygankova, A.R.; Sukhikh, A.S.; Morozova, N.B.; Basova, T.V. Heterostructures based on Pd–Au nanoparticles and cobalt phthalocyanine for hydrogen chemiresistive sensors. Int. J. Hydrogen Energy 2021, 46, 19682–19692. [Google Scholar] [CrossRef]
- Sukhikh, A.S.; Klyamer, D.D.; Parkhomenko, R.G.; Krasnov, P.O.; Gromilov, S.A.; Hassan, A.K.; Basova, T.V. Effect of fluorosubstitution on the structure of single crystals, thin films and spectral properties of palladium phthalocyanines. Dyes Pigments 2018, 149, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Parkhomenko, R.G.; Sukhikh, A.S.; Klyamer, D.D.; Krasnov, P.O.; Gromilov, S.; Kadem, B.; Hassan, A.K.; Basova, T.V. Thin Films of Unsubstituted and Fluorinated Palladium Phthalocyanines: Structure and Sensor Response toward Ammonia and Hydrogen. J. Phys. Chem. C 2017, 121, 1200–1209. [Google Scholar] [CrossRef]
- Yang, R.D.; Park, J.; Colesniuc, C.N.; Schuller, I.K.; Royer, J.E.; Trogler, W.C.; Kummel, A.C. Analyte chemisorption and sensing on n- and p-channel copper phthalocyanine thin-film transistors. J. Chem. Phys. 2009, 130, 164703. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chen, P.; Hu, W. Organic field-effect transistor-based gas sensors. Chem. Soc. Rev. 2015, 44, 2087–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, D.; Wang, C. Molecular Interface Effect of Heterogeneous Molecular Junctions Consisting of Nonfluorinated and Fluorinated Phthalocyanines. J. Phys. Chem. B 2006, 110, 20789–20793. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Ouedraogo, S.; Meunier-Prest, R.; Kumar, A.; Bayo-Bangoura, M.; Bouvet, M. Modulating the Electrical Properties of Organic Heterojunction Devices Based on Phthalocyanines for Ambipolar Sensors. ACS Sens. 2020, 5, 1849–1857. [Google Scholar] [CrossRef]
- Ye, R.; Ohta, K.; Baba, M. In-situ study of pn-heterojunction interface states in organic thin film transistors. Thin Solid Films 2014, 554, 137–140. [Google Scholar] [CrossRef]
- Parra, V.; Brunet, J.; Pauly, A.; Bouvet, M. Molecular semiconductor-doped insulator (MSDI) heterojunctions: An alternative transducer for gas chemosensing. Analyst 2009, 134, 1776–1778. [Google Scholar] [CrossRef]
- Kumar, A.; Meunier-Prest, R.; Bouvet, M. Organic heterojunction devices based on phthalocyanines: A new approach to gas chemosensing. Sensors 2020, 20, 4700. [Google Scholar] [CrossRef] [PubMed]
- Schöllhorn, B.; Germain, J.P.; Pauly, A.; Maleysson, C.; Blanc, J.P. Influence of peripheral electron-withdrawing substituents on the conductivity of zinc phthalocyanine in the presence of gases. Part 1: Reducing gases. Thin Solid Films 1998, 326, 245–250. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Shi, M.; Wu, G.; Wang, M.; Huang, J. High gas-sensitivity and selectivity of fluorinated zinc phthalocyanine film to some non-oxidizing gases at room temperature. Thin Solid Films 2005, 489, 257–261. [Google Scholar] [CrossRef]
- Bengasi, G.; Meunier-Prest, R.; Baba, K.; Kumar, A.; Pellegrino, A.L.; Boscher, N.D.; Bouvet, M. Molecular Engineering of Porphyrin-Tapes/Phthalocyanine Heterojunctions for a Highly Sensitive Ammonia Sensor. Adv. Electron. Mater. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Bouvet, M.; Gaudillat, P.; Kumar, A.; Sauerwald, T.; Schüler, M.; Schütze, A.; Suisse, J.M. Revisiting the electronic properties of Molecular Semiconductor–Doped Insulator (MSDI) heterojunctions through impedance and chemosensing studies. Org. Electron. 2015, 26, 345–354. [Google Scholar] [CrossRef]
- Mateos, M.; Meunier-Prest, R.; Suisse, J.M.; Bouvet, M. Modulation of the organic heterojunction behavior, from electrografting to enhanced sensing properties. Sens. Actuators B Chem. 2019, 299, 126968. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ji, S.; Wang, H.; Yan, D. Highly sensitive gas sensor enhanced by tuning the surface potential. Org. Electron. 2011, 12, 2230–2235. [Google Scholar] [CrossRef]
- Zhao, S.; Kong, X.; Wang, X.; Li, X.; Yang, G.; Chen, Y. Fine-tuning intermolecular and intramolecular interactions to build the films of tris(phthalocyaninato) rare earth complexes and their comparative performances in ambipolar gas sensing. IEEE Trans. Electron Devices 2019, 66, 1930–1936. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A.K.; Sohal, M.K.; Sharma, D.P.; Debnath, A.K.; Aswal, D.K.; Mahajan, A. Room temperature highly sensitive chlorine sensor based on reduced graphene oxide anchored with substituted copper phthalocyanine. Sens. Actuators B Chem. 2021, 327, 128925. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. CNTs based improved chlorine sensor from non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated cobalt phthalocyanines. RSC Adv. 2017, 7, 49675–49683. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Mahajan, A.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Tailoring of the chlorine sensing properties of substituted metal phthalocyanines non-covalently anchored on single-walled carbon nanotubes. RSC Adv. 2018, 8, 32719–32730. [Google Scholar] [CrossRef] [Green Version]
- Bonegardt, D.; Klyamer, D.; Köksoy, B.; Durmuş, M.; Basova, T. Hybrid materials of carbon nanotubes with fluoroalkyl–and alkyl-substituted zinc phthalocyanines. J. Mater. Sci. Mater. Electron. 2020, 31, 11021–11028. [Google Scholar] [CrossRef]
- Hesse, K.; Schlettwein, D. Spectroelectrochemical investigations on the reduction of thin films of hexadecafluorophthalocyaninatozinc (F16PcZn). J. Electroanal. Chem. 1999, 476, 148–158. [Google Scholar] [CrossRef]
- Engel, M.K. Single-Crystal Structures of Phthalocyanine Complexes and Related Macrocycles. In The Porphyrin Handbook: Phthalocyanines: Structural Characterization; Elsevier: Amsterdam, The Netherlands, 2012; Volume 20, pp. 122–142. [Google Scholar]
- Brinkmann, H.; Kelting, C.; Makarov, S.; Tsaryova, O.; Schnurpfeil, G.; Wöhrle, D.; Schlettwein, D. Fluorinated phthalocyanines as molecular semiconductor thin films. Phys. Status Solidi Appl. Mater. Sci. 2008, 205, 409–420. [Google Scholar] [CrossRef]
- Schlettwein, D.; Hesse, K.; Gruhn, N.E.; Lee, P.A.; Nebesny, K.W.; Armstrong, N.R. Electronic Energy Levels in Individual Molecules, Thin Films, and Organic Heterojunctions of Substituted Phthalocyanines. J. Phys. Chem. B 2001, 105, 4791–4800. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Kerp, H.R.; Westerduin, K.T.; van Veen, A.T.; van Faassen, E.E. Quantification and effects of molecular oxygen and water in zinc phthalocyanine layers. J. Mater. Res. 2001, 16, 503–511. [Google Scholar] [CrossRef]
- de Haan, A.; Debliquy, M.; Decroly, A. Influence of atmospheric pollutants on the conductance of phthalocyanine films. Sens. Actuators B Chem. 1999, 57, 69–74. [Google Scholar] [CrossRef]
- Gould, R.D. Structure and electrical conduction properties of phthalocyanine thin films. Coord. Chem. Rev. 1996, 156, 237–274. [Google Scholar] [CrossRef]
- Basova, T.V.; Mikhaleva, N.S.; Hassan, A.K.; Kiselev, V.G. Thin films of fluorinated 3d-metal phthalocyanines as chemical sensors of ammonia: An optical spectroscopy study. Sens. Actuators B Chem. 2016, 227, 634–642. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, B.; Zhang, H.; Qin, J. Theoretical insight into two-dimensional M-Pc monolayer as an excellent material for formaldehyde and phosgene sensing. Appl. Surf. Sci. 2021, 543, 148805. [Google Scholar] [CrossRef]
- Soury, R.; Chaabene, M.; Jabli, M.; Saleh, T.A.; Ben Chaabane, R.; Saint-Aman, E.; Loiseau, F.; Philouze, C.; Allouche, A.R.; Nasri, H. Meso-tetrakis(3,4,5-trimethoxyphenyl)porphyrin derivatives: Synthesis, spectroscopic characterizations and adsorption of NO2. Chem. Eng. J. 2019, 375, 122005. [Google Scholar] [CrossRef]
- Rana, M.K.; Sinha, M.; Panda, S. Gas sensing behavior of metal-phthalocyanines: Effects of electronic structure on sensitivity. Chem. Phys. 2018, 513, 23–34. [Google Scholar] [CrossRef]
- Saini, R.; Mahajan, A.; Bedi, R.K.; Aswal, D.K.; Debnath, A.K. Room temperature ppb level Cl2 detection and sensing mechanism of highly selective and sensitive phthalocyanine nanowires. Sens. Actuators B Chem. 2014, 203, 17–24. [Google Scholar] [CrossRef]
- Kim, H.; Meihui, Z.; Battaglini, N.; Lang, P.; Horowitz, G. Large enhancement of hole injection in pentacene by modification of gold with conjugated self-assembled monolayers. Org. Electron. 2013, 14, 2108–2113. [Google Scholar] [CrossRef]
- Bouvet, M.; Parra, V.; Suisse, J.M. Molecular semiconductor-doped insulator (MSDI) heterojunctions as new transducers for chemical sensors. EPJ Appl. Phys. 2011, 56, 34103. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Van Den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. TrAC Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Basova, T.V.; Polyakov, M.S. Hybrid materials based on carbon nanotubes and polyaromatic molecules: Methods of functionalization and sensor properties. Macroheterocycles 2020, 13, 91–112. [Google Scholar] [CrossRef]
- Basova, T.V.; Ray, A.K. Review—Hybrid Materials Based on Phthalocyanines and Metal Nanoparticles for Chemiresistive and Electrochemical Sensors: A Mini-Review. ECS J. Solid State Sci. Technol. 2020, 9, 061001. [Google Scholar] [CrossRef]
- Basova, T.V.; Hassan, A. Ammonia sorption studies into thin layers of hexadecafluorinated cobalt phthalocyanine using optical techniques. J. Porphyr. Phthalocyanines 2013, 17, 934–940. [Google Scholar] [CrossRef]
- Kumar, A.; Brunet, J.; Varenne, C.; Ndiaye, A.; Pauly, A. Phthalocyanines based QCM sensors for aromatic hydrocarbons monitoring: Role of metal atoms and substituents on response to toluene. Sens. Actuators B Chem. 2016, 230, 320–329. [Google Scholar] [CrossRef]
- Harbeck, M.; Erbahar, D.D.; Gürol, I.; Musluolu, E.; Ahsen, V.; Öztürk, Z.Z. Phthalocyanines as sensitive coatings for QCM sensors: Comparison of gas and liquid sensing properties. Sens. Actuators B Chem. 2011, 155, 298–303. [Google Scholar] [CrossRef]
- Tasaltin, C.; Gurol, I.; Harbeck, M.; Musluoglu, E.; Ahsen, V.; Ozturk, Z.Z. Synthesis and DMMP sensing properties of fluoroalkyloxy and fluoroaryloxy substituted phthalocyanines in acoustic sensors. Sens. Actuators B Chem. 2010, 150, 781–787. [Google Scholar] [CrossRef]
- Ahmetali, E.; Karaoğlu, H.P.; Urfa, Y.; Altındal, A.; Koçak, M.B. A series of asymmetric zinc (II) phthalocyanines containing fluoro and alkynyl groups: Synthesis and examination of humidity sensing performance by using QCM based sensor. Mater. Chem. Phys. 2020, 254, 123477. [Google Scholar] [CrossRef]
- Arwin, H.; Poksinski, M.; Johansen, K. Total internal reflection ellipsometry: Principles and applications. Appl. Opt. 2004, 43, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Basova, T.; Hassan, A.; Yuksel, F.; Gürek, A.G.; Ahsen, V. Optical detection of pentachlorophenol in water using thin films of octa-tosylamido substituted zinc phthalocyanine. Sens. Actuators B Chem. 2010, 150, 523–528. [Google Scholar] [CrossRef]
- Nabok, A.; Tsargorodskaya, A. The method of total internal reflection ellipsometry for thin film characterisation and sensing. Thin Solid Films 2008, 516, 8993–9001. [Google Scholar] [CrossRef]
- Tong, W.Y.B.; Djurišić, A.H.; Xie, M.C.M.; Ng, A.Y.; Cheung, K.K.; Chan, W.H.; Leung, Y.W.; Lin, H.; Gwo, S. Metal Phthalocyanine Nanoribbons and Nanowires. J. Phys. Chem. B 2006, 110, 17406–17413. [Google Scholar] [CrossRef] [PubMed]
- Ballantine, D.S., Jr.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohltjen, H. Acoustic Wave Sens.: Theory, Design and Physico-Chemical Applications, 1st ed.; Levy, M., Stern, R., Eds.; Academic Press: New York, NY, USA, 1997. [Google Scholar]
- Toniolo, R.; Pizzariello, A.; Dossi, N.; Lorenzon, S.; Abollino, O.; Bontempelli, G. Room temperature ionic liquids as useful overlayers for estimating food quality from their odor analysis by quartz crystal microbalance measurements. Anal. Chem. 2013, 85, 7241–7247. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, J.M.; Van Der Zwaag, D.; Walish, J.J.; Weizmann, Y.; Swager, T.M. Sensory arrays of covalently functionalized single-walled carbon nanotubes for explosive detection. Adv. Funct. Mater. 2013, 23, 5285–5291. [Google Scholar] [CrossRef] [Green Version]
- Queralto, N.; Berliner, A.N.; Goldsmith, B.; Martino, R.; Rhodes, P.; Lim, S.H. Detecting cancer by breath volatile organic compound analysis: A review of array-based sensors. J. Breath Res. 2014, 8, 027112. [Google Scholar] [CrossRef] [PubMed]



















 | ||
|---|---|---|
| Number | Abbreviation and Central Metals | Substituents |
| 1 | MPcF4 (M = Co, Cu, Zn, Pb, VO, Ni) | R2 = F, R1,3,4 = H |
| 2 | MPcF8 (M = Cu) | R2,3 = F, R1,4 = H |
| 3 | MPcF16 (M = Zn, Co, Pd, Pb, Cu, Ni) | R1,2,3,4 = F |
| 4 | (a) TiOPc(OCH2CF2CHF2)4 (b) TiOPc(OCH2CF2CHF2)8 | (a) R2 =  R1,3,4 = H R1,3,4 = H(b) R2,3 =  R1,4 = H R1,4 = H |
| 5 | ZnPcF64 | R2,3 =  R1,4 = F R1,4 = F |
| 6 | (a) MPc[β-O(4-CF3-Ph)4] (b) H2Pc[β-O(4-CF3-Ph)4] (c) CoPc[α-O(4-CF3-Ph)4] (d) H2Pc[α-O(4-CF3-Ph)4] | (a), (b) R2 =  R1,3,4 = H R1,3,4 = H(c), (d) R1 =  R2,3,4 = H R2,3,4 = H |
| 7 | ZnPcCF |  R1,3,4 = H R1,3,4 = H |
| 8 | H2Pc(OCH2CF3)4 | R2 =  |
| 9 | H2[Pc(OCH2(CF2)6CF3)4] | R2 =  R1,3,4 = H R1,3,4 = H |
| 10 | H2[Pc(OCH2C3F7)8] Zn[Pc(OCH2C3F7)8] | R2,3 =  R1,4 = H R1,4 = H |
| 11 | Gd2[Pc(OPhF)8]3 Tb2[Pc(OPhF)8]3 | R2,3 =  R1,4 = H R1,4 = H |
| 12 | CoPcR8 | R2,3 =  R1,4 = H R1,4 = H |
| 13 | ZnPc(OCH2CF2CHF2)8 | R2,3 =  |
| 14 | (a) TiLR4 (See Section 4.2.) (b) TiLR8 | (a) R2 =  R1,3,4 = H R1,3,4 = H(b) R2,3 =  R1,4 = H R1,4 = H |
| 15 | (a) MPc(OCH2CF2CHF2)4 (M = Zn, Ni) (b) MPc(OCH2CF2CHF2)4 (M = Zn, Ni) (c) MPc(OCH2CF2CHF2)8 (M = Zn, Ni, Cu) (d) MPc(OCH2CF2CHF2)4Cl4 (M = Zn, Ni) (e) MPc(OPhF5)4Cl4 (M = Zn, Ni) | (a) R1 =  R2,3,4 = H R2,3,4 = H (b) R2 =  R1,3,4 = H R1,3,4 = H(c) R2,3 =  R1,4 = H R1,4 = H(d) R2 =  R3 = Cl R1,4 = H R3 = Cl R1,4 = H(e) R2 =  R3 = Cl R1,4 = H R3 = Cl R1,4 = H |
| 16 | (a) ZnPc(Ph(CF3)2)6(CC(CH)3)(OH)) (b) ZnPc(Ph(CF3)2)6(CCH) (c) ZnPc(Ph(CF3)2)6(CC(PhNO2)) | (a) R2,3,6,7,10,11 =  R14 = R14 =  (b) R2,3,6,7,10,11 =  R14 = R14 =  (c) R2,3,6,7,10,11 =  R14 = R14 =  |
| Active Layer | Sensor Type | Analyte | Investigated Range, Ppm | LOD, Ppm | Recovery Time, S | Ref. |
|---|---|---|---|---|---|---|
| ZnPcF16 | Chemiresistive | NH3 in N2 H2 in N2 | 0–1000 0–2000 | n/a | Several hours | [76] |
| ZnPcF16 | Chemiresistive | NH3, N(CH3)3, NH2CH3, N(C2H5)3 | 0.72 (NH3) | n/a | n/a | [77] |
| CoPcF4 | Chemiresistive | NH3 | 10–50 | 2.5 | 30 (20 ppm) | [62] |
| PdPcF16 | Chemiresistive | NH3 H2 | 10–50 1000–5000 | n/a 500 | 55 (10 ppm) 60 (1000 ppm) | [68] |
| MPcFx (M = Cu, Co, Zn, x = 4, 16) | Chemiresistive | NH3 | 0.1–50 | 0.1 (for ZnPcF4) | 110 (10 ppm) (for ZnPcF4) | [21] |
| PbPcF4 PbPcF16 | Chemiresistive | NH3 | 1–5 | n/a | 90–220 | [49] |
| VOPcF4 | Chemiresistive | NH3 H2 | 10–50 10–500 | n/a | 230 (30 ppm) 50 (300 ppm) | [50] |
| CuPcF16/pNIDPP CuPcF16/pNIDMP | MSDI | NH3 | 1–50 | ~1 0.228 | n/a | [78] |
| CuPcF16/LuPc2 | MSDI | NH3 | 30–90 | n/a | n/a | [79] |
| CuPcF16/LuPc2 on bare ITO CuPcF16/LuPc2 on DMBz/ITO | MSDI | NH3 | 1–90 | 0.28 (RH 50%) 0.14 (RH 50%) | n/a | [80] |
| CuPcFn/LuPc2, n = 8, 16) | MSDI | O3 in air NH3 in Ar | 0.09 (O3) 35 (NH3) | n/a | n/a | [74] |
| CuPcF16 | OFET | DMMP Methanol | 5–60 200–1650 | n/a | >1 h | [69] |
| TiOPc/CuPcF16 | OTFT | NO2 | <5 | 0.25 | n/a | [81] |
| CuPcF16/CuPc | OTFT | NO2 | 20 | 20 | n/a | [65] |
| CoPc[β-O(4-CF3-Ph)4] H2Pc[β-O(4-CF3-Ph)4] CoPc[α-O(4-CF3-Ph)4] H2Pc[α-O(4-CF3-Ph)4] (Compounds 6, Table 1) | Chemiresistive | NO2, NH3, H2S | Dependent on the analyte | 0.003 0.03 0.198 0.25 (to NO2) | n/a | [52] |
| H2Pc(OCH2CF3)4 (Compound 8, Table 1) | Chemiresistive | NO2 | 0.1–0.5 | n/a | 420 (500 ppb) | [55] |
| H2[Pc(OCH2C3F7)8] Zn[Pc(OCH2C3F7)8] (Compounds 10, Table 1) | Chemiresistive | NO2 | 50–900 100–750 | 50 100 | n/a | [58] |
| H2[Pc(OCH2(CF2)6CF3)4] (Compound 9, Table 1) | Chemiresistive | NO2 | 0.1–1 | n/a | 180 (0.5 ppm) | [57] |
| CoPcR8 (Compound 12, Table 1) | Chemiresistive | NH3 | 0.3–50 | 0.3 | 40 (5 ppm) | [51] |
| Gd2[Pc(OPhF)8]3 Tb2[Pc(OPhF)8]3 (Compounds 11, Table 1) | Chemiresistive | NH3 | 1–20 | - 0.151 | 126 103 (20 ppm) | [82] |
| CuPcF16/rGO | Chemiresistive | Cl2 | 0.06–3 | 1.41·10−3 | 551 | [83] |
| CoPcF16/SWCNT | Chemiresistive | Cl2 | 0.04–2 | 0.05·10−3 | 150 (500 ppb) | [84] |
| CuPcF16/SWCNT CuPcF16/MWCNT | Chemiresistive | Cl2 | 0.1–2 | 0.27·10−3 0.85·10−3 | n/a | [63] |
| MPcF16/SWCNT (M = Co, Zn, Cu) | Chemiresistive | Cl2 | 0.04–2 | 0.04·10−3 | n/a | [85] |
| ZnPcF16/MWCNT | Chemiresistive | Cl2 | 0.01–2 | 0.06·10−3 | n/a | [64] |
| Compound 7 (Table 1)/SWCNT | Chemiresistive | NH3 | 1–50 | 0.78 | 170 (10 ppm) | [86] |
| Active Layer | Sensor Type | Analyte | Investigated Range, Ppm | LOD, Ppm | Recovery Time, S | Ref. |
|---|---|---|---|---|---|---|
| MPcF16 (M = Zn, Co, Cu, Ni) | SPR | NH3 | 100, 200 ppm | n/a | 15–30 | [96] |
| CoPcF16 | TIRE | NH3 | 50–1000 | n/a | n/a | [107] |
| ZnPc(OCH2CF2CHF2)8 (Compound 13, Table 1) | TIRE | Trimethylamine | 10–300 | 20 | n/a | [56] |
| MPcF16, (M = Zn, Cu) | QCM | Toluene | 500 | n/a | n/a | [108] |
| TiLR4 TiLR8 (Compounds 14, Table 1) (See Section 4.2.) | QCM | MeOH n-Propanol etylacetate triethylamine acetonitrile DMMP n-heptane ethyl benzene toluene o-xylene C2Cl4 chlorobenzene | 1000–5000 130–650 900–4500 10–50 950–4700 3–15 380–1900 70–350 240–1200 40–200 150–730 100–480 | n/a | n/a | [54] |
| Compounds 15, Table 1 | QCM | dichloromethane (DCM), chloroform, tetrachloroetylene, chlorobenzene, toluene, o-xylene, p-xylene | 0.1–300 (for DCM) | For Compound 15c (Table 1): 5 (for DCM) 0.05 (for o-xylene) | 90 | [109] |
| Compounds described in Section 4.2. | QCM SAW | DMMP | 1.5–12 ppm | 0.6 (for 1b) 0.8 (for 2c, see Section 4.2.) | 40 (for 1b) | [110] |
| Compounds 16, Table 1 | QCM | Humidity | 10–90% RH | n/a | ~60 | [111] |
| Compounds 15, Table 1 | QCM | methanol propanol acetonitrile ethyl acetate toluene ethylbenzene o-xylene chlorobenzene chloroform trichloroethylene n-heptane trimethylamine | 1000–5000 131–653 944–4719 902–4509 243–1213 69–345 40–198 95–477 2145–10,726 788–3939 385–1927 721–3606 | 144 34 58 36 13 3 2 3 10,030 60 37 | n/a | [33] |
| ZnPc(OCH2CH2CH3)8 | ZnPc(OCH2CF2CHF2)8 | |||
|---|---|---|---|---|
| Initial Film | After Exposure with Trimethylamine | Initial Film | After Exposure with Trimethylamine | |
| n (λ = 633 nm) | 1.33 | 1.33 | 1.32 | 1.34 |
| k (λ = 633 nm) | 0.15 | 0.15 | 0.19 | 0.21 |
| d, nm | 19.4 | 19.7 | 18.9 | 20.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyamer, D.; Bonegardt, D.; Basova, T. Fluoro-Substituted Metal Phthalocyanines for Active Layers of Chemical Sensors. Chemosensors 2021, 9, 133. https://doi.org/10.3390/chemosensors9060133
Klyamer D, Bonegardt D, Basova T. Fluoro-Substituted Metal Phthalocyanines for Active Layers of Chemical Sensors. Chemosensors. 2021; 9(6):133. https://doi.org/10.3390/chemosensors9060133
Chicago/Turabian StyleKlyamer, Darya, Dmitry Bonegardt, and Tamara Basova. 2021. "Fluoro-Substituted Metal Phthalocyanines for Active Layers of Chemical Sensors" Chemosensors 9, no. 6: 133. https://doi.org/10.3390/chemosensors9060133
APA StyleKlyamer, D., Bonegardt, D., & Basova, T. (2021). Fluoro-Substituted Metal Phthalocyanines for Active Layers of Chemical Sensors. Chemosensors, 9(6), 133. https://doi.org/10.3390/chemosensors9060133