PANI-Based Wearable Electrochemical Sensor for pH Sweat Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
2.2. Electrode Fabrication
2.3. Electrochemical Detection of pH
2.4. Sweat Collection and Testing
3. Results and Discussion
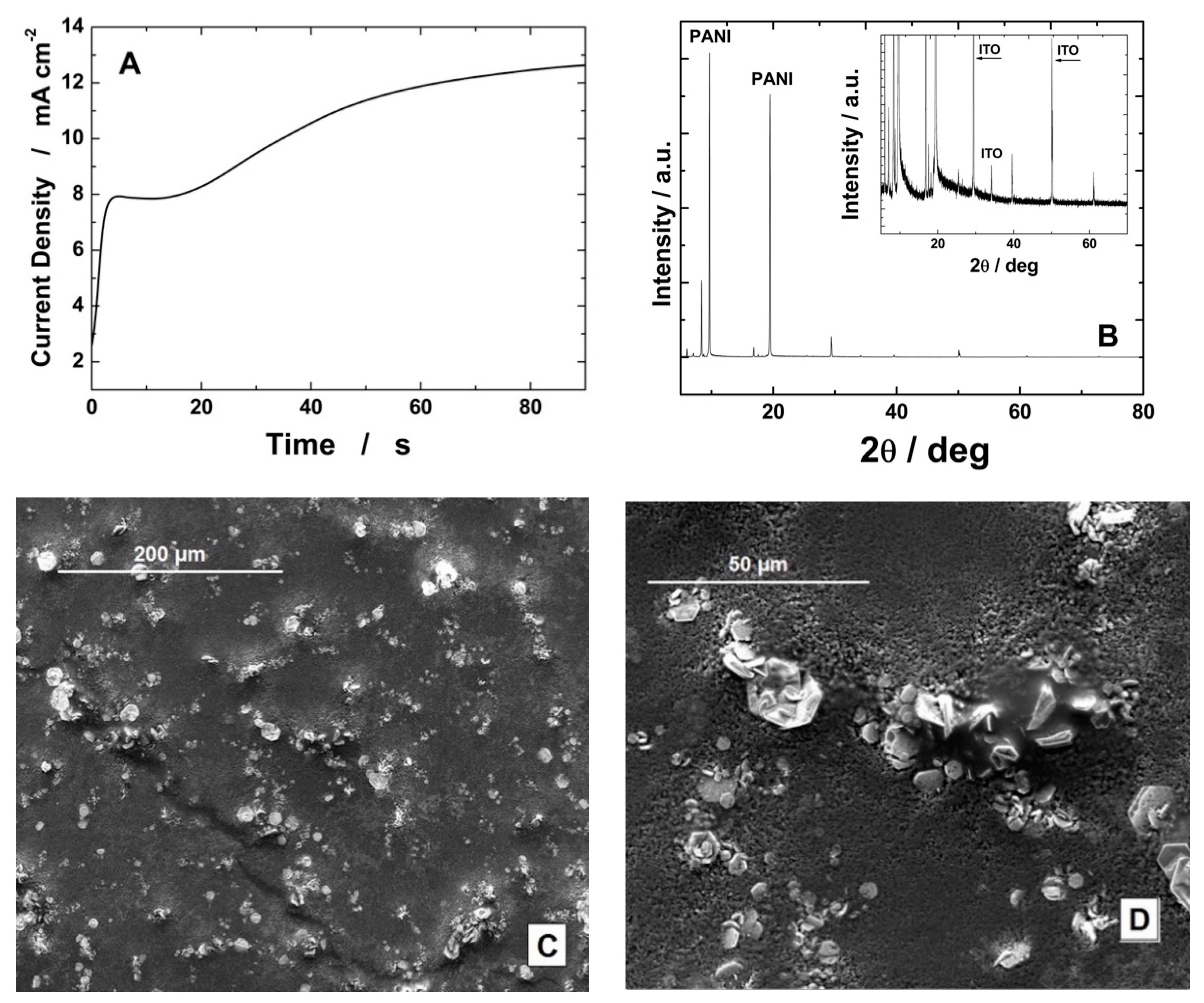
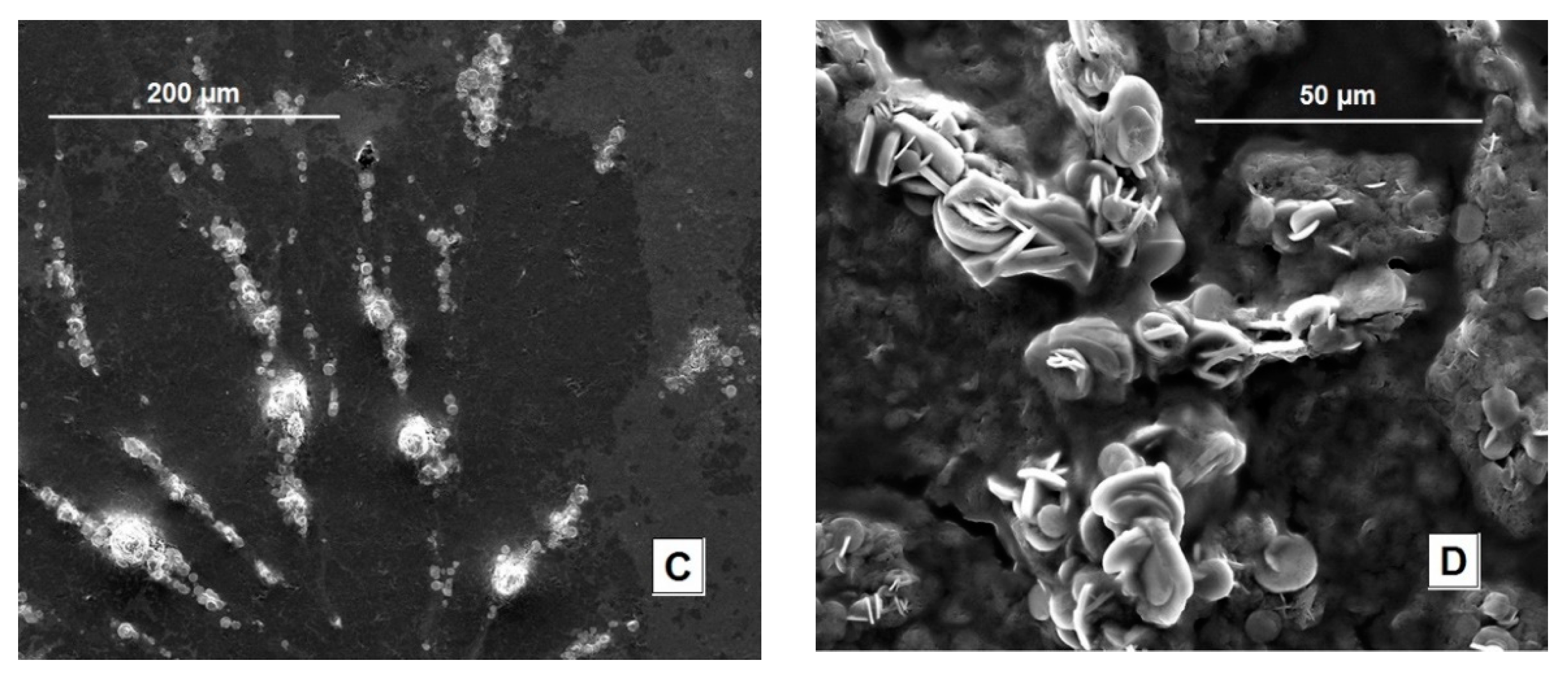
3.1. Electrode Fabrication and Characterization
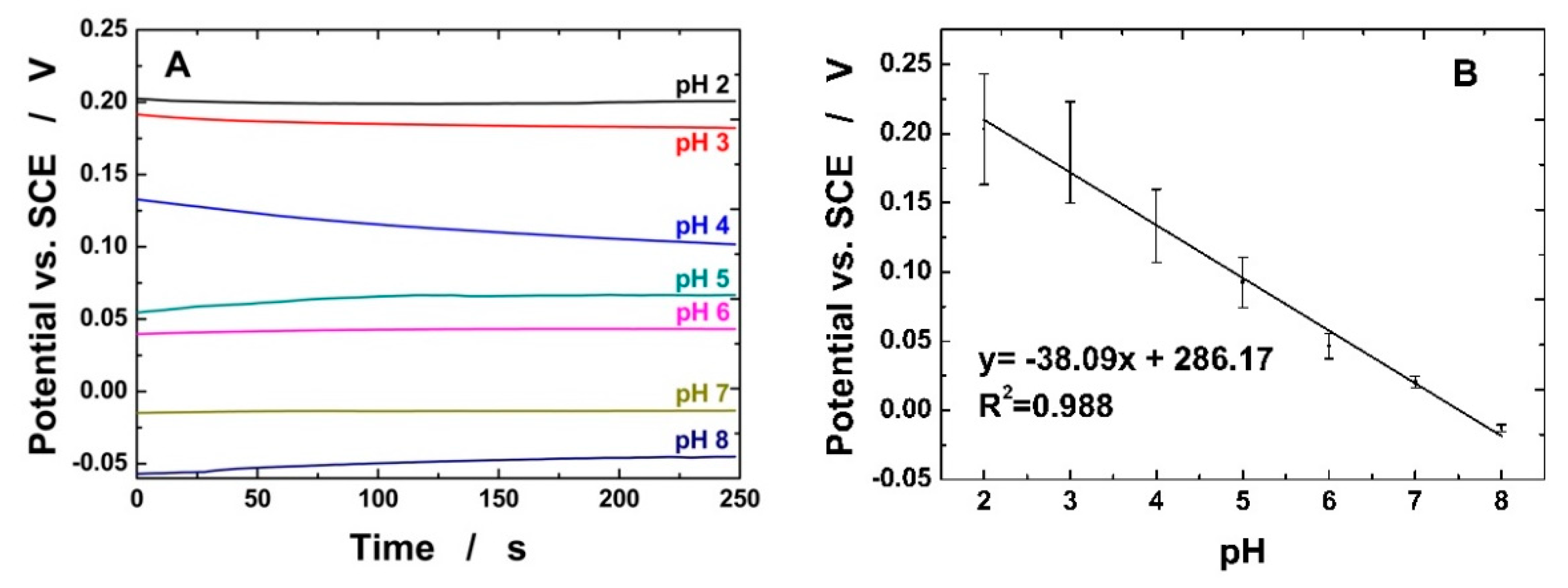
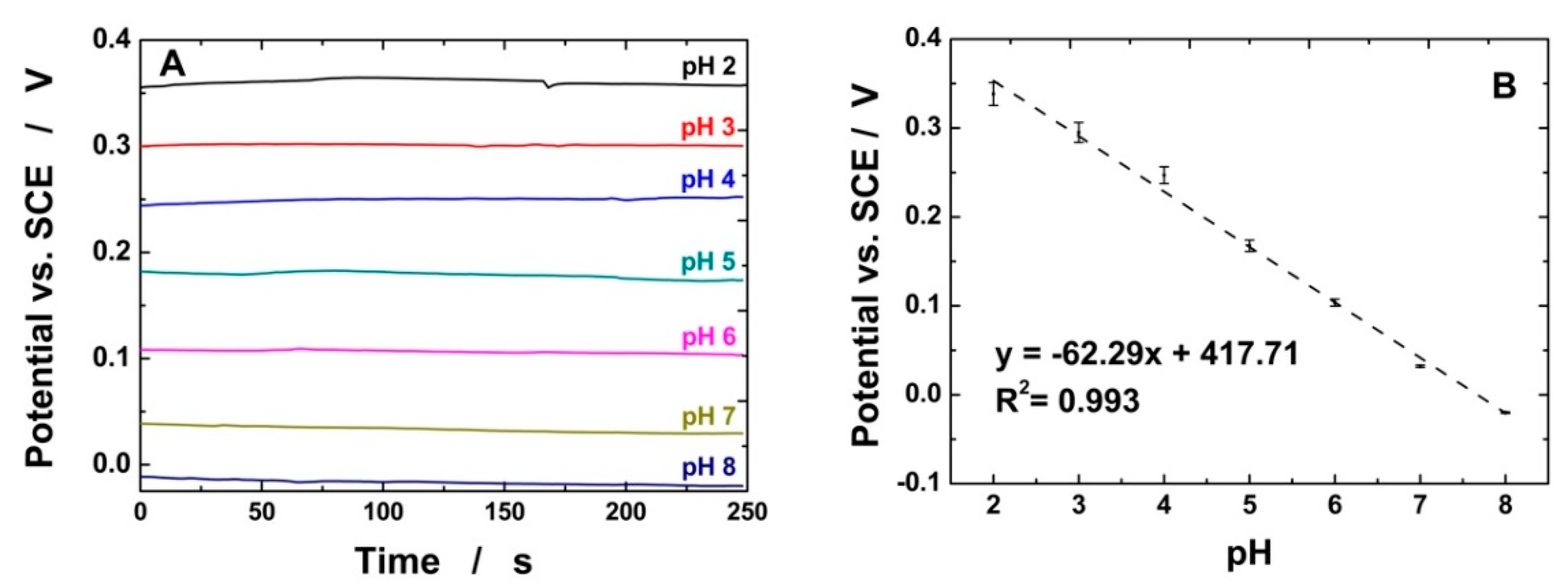
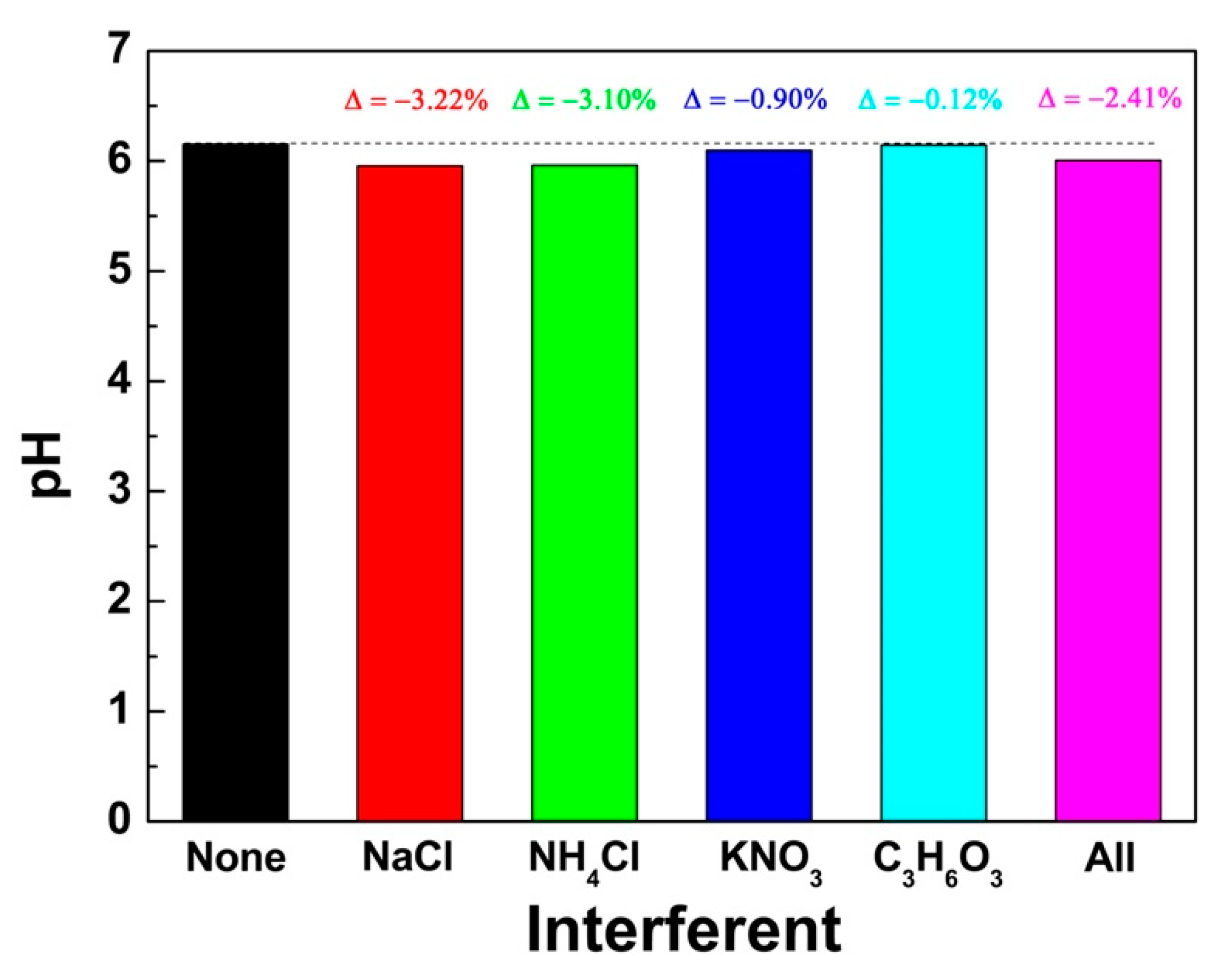
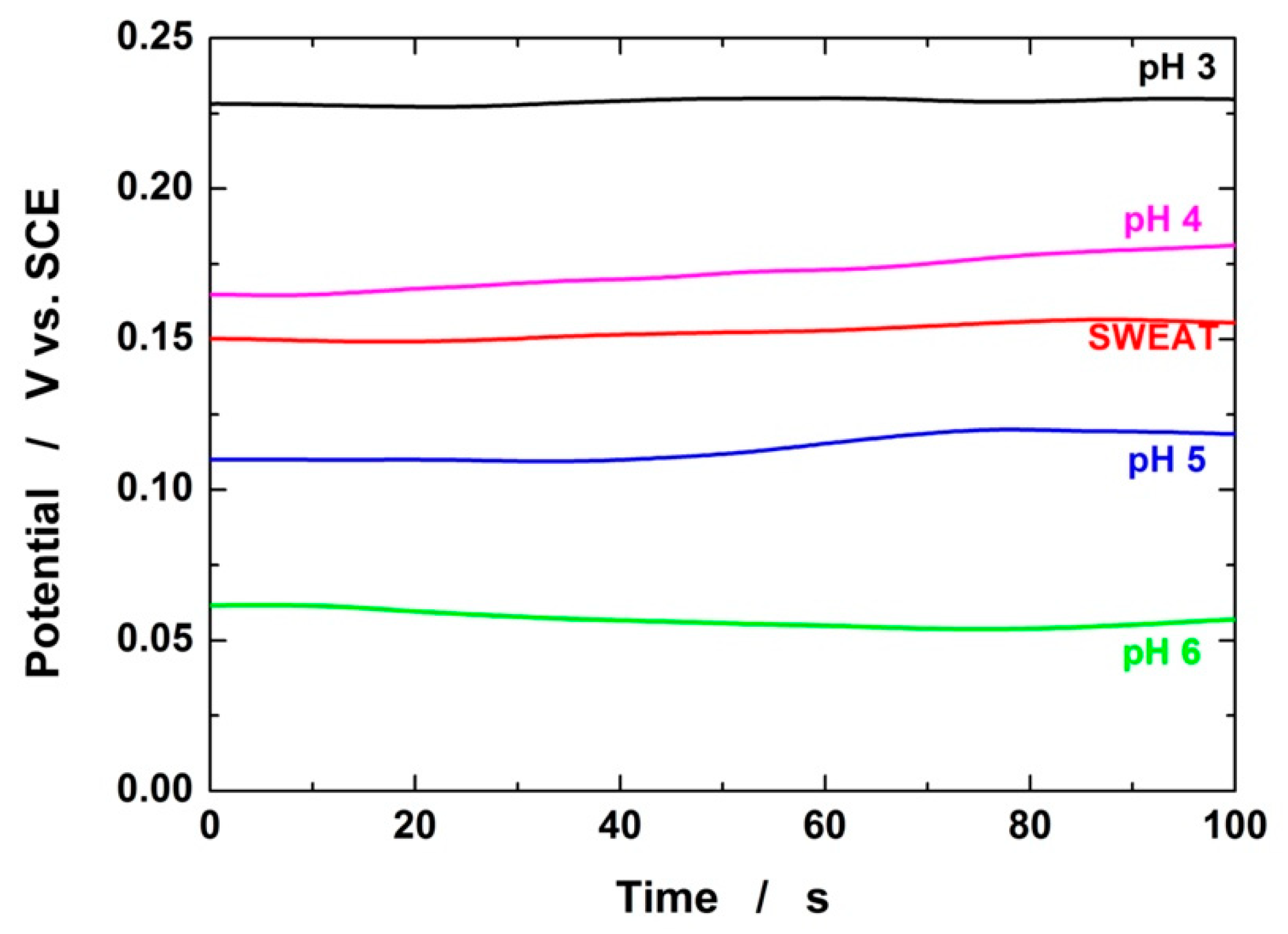
3.2. Electrochemical Detection of pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable Sensors for Monitoring the Physiological and Biochemical Profile of the Athlete. NPJ Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef] [Green Version]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A Review of Wearable Sensors and Systems with Application in Rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, D.R.; Davies, E.V.; Harlow, E.R.; Hsu, J.J.; Knighton, S.C.; Walker, T.A.; Voos, J.E.; Drummond, C.K. Wearable Sensors for COVID-19: A Call to Action to Harness Our Digital Infrastructure for Remote Patient Monitoring and Virtual Assessments. Front. Digit. Health 2020, 2, 8. [Google Scholar] [CrossRef]
- Angelucci, A.; Cavicchioli, M.; Cintorrino, I.A.; Lauricella, G.; Rossi, C.; Strati, S.; Aliverti, A. Smart Textiles and Sensorized Garments for Physiological Monitoring: A Review of Available Solutions and Techniques. Sensors 2021, 21, 814. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Lee, M.; Rhee, Y.; Lee, S.; Jeong, Y.-R.; Kang, S.; Naqi, M.; Hong, S. Smart Patch for Skin Temperature: Preliminary Study to Evaluate Psychometrics and Feasibility. Sensors 2021, 21, 1855. [Google Scholar] [CrossRef]
- Gaubert, V.; Gidik, H.; Koncar, V. Proposal of a Lab Bench for the Unobtrusive Monitoring of the Bladder Fullness with Bioimpedance Measurements. Sensors 2020, 20, 3980. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Dias, D.; Múrias Lopes, E.; Vilas-Boas, M.d.C.; Paulo Silva Cunha, J. SnapKi—An Inertial Easy-to-Adapt Wearable Textile Device for Movement Quantification of Neurological Patients. Sensors 2020, 20, 3875. [Google Scholar] [CrossRef]
- Pham, S.; Yeap, D.; Escalera, G.; Basu, R.; Wu, X.; Kenyon, N.J.; Hertz-Picciotto, I.; Ko, M.J.; Davis, C.E. Wearable Sensor System to Monitor Physical Activity and the Physiological Effects of Heat Exposure. Sensors 2020, 20, 855. [Google Scholar] [CrossRef] [Green Version]
- Davis-Martin, R.E.; Alessi, S.M.; Boudreaux, E.D. Alcohol Use Disorder in the Age of Technology: A Review of Wearable Biosensors in Alcohol Use Disorder Treatment. Front. Psychiatry 2021, 12, 642813. [Google Scholar] [CrossRef]
- Madden, J.; O’Mahony, C.; Thompson, M.; O’Riordan, A.; Galvin, P. Biosensing in Dermal Interstitial Fluid Using Microneedle Based Electrochemical Devices. Sens. Bio-Sens. Res. 2020, 29, 100348. [Google Scholar] [CrossRef]
- Barrett, C.; O’Sullivan, F.; Barry, S.; Grygoryev, K.; O’Gorman, D.; O’Mahony, C.; O’Riordan, A. Novel Surface Modified Polymer Microneedle Based Biosensors for Interstitial Fluid Glucose Detection. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Park, H.; Park, W.; Lee, C.H. Electrochemically Active Materials and Wearable Biosensors for the in Situ Analysis of Body Fluids for Human Healthcare. NPG Asia Mater. 2021, 13, 23. [Google Scholar] [CrossRef]
- Wang, Z.; Shin, J.; Park, J.; Lee, H.; Kim, D.; Liu, H. Engineering Materials for Electrochemical Sweat Sensing. Adv. Funct. Mater. 2021, 31, 2008130. [Google Scholar] [CrossRef]
- Bondioli, M.; Chessa, S.; Narzisi, A.; Pelagatti, S.; Zoncheddu, M. Towards Motor-Based Early Detection of Autism Red Flags: Enabling Technology and Exploratory Study Protocol. Sensors 2021, 21, 1971. [Google Scholar] [CrossRef] [PubMed]
- Wasiewska, L.A.; Seymour, I.; Patella, B.; Inguanta, R.; Burgess, C.M.; Duffy, G.; O’Riordan, A. Reagent Free Electrochemical-Based Detection of Silver Ions at Interdigitated Microelectrodes Using in-Situ PH Control. Sens. Actuators B Chem. 2021, 129531. [Google Scholar] [CrossRef]
- Seymour, I.; O’Sullivan, B.; Lovera, P.; Rohan, J.F.; O’Riordan, A. Electrochemical Detection of Free-Chlorine in Water Samples Facilitated by in-Situ PH Control Using Interdigitated Microelectrodes. Sens. Actuators B Chem. 2020, 325, 128774. [Google Scholar] [CrossRef]
- Wahl, A.J.C.; Seymour, I.P.; Moore, M.; Lovera, P.; O’Riordan, A.; Rohan, J.F. Diffusion Profile Simulations and Enhanced Iron Sensing in Generator-Collector Mode at Interdigitated Nanowire Electrode Arrays. Electrochim. Acta 2018, 277, 235–243. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Kuan, W.-H.; Liu, C.-L. Comparative Study of the Composition of Sweat from Eccrine and Apocrine Sweat Glands during Exercise and in Heat. IJERPH 2020, 17, 3377. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Marson, F.A.L.; Mendonça, R.M.H.; Bertuzzo, C.S.; Paschoal, I.A.; Ribeiro, J.D.; Ribeiro, A.F.; Levy, C.E. Chloride and Sodium Ion Concentrations in Saliva and Sweat as a Method to Diagnose Cystic Fibrosis. J. Pediatr. 2019, 95, 443–450. [Google Scholar] [CrossRef]
- Liamis, G. Diabetes Mellitus and Electrolyte Disorders. WJCC 2014, 2, 488. [Google Scholar] [CrossRef]
- Pascual, E.; Addadi, L.; Andrés, M.; Sivera, F. Mechanisms of Crystal Formation in Gout—A Structural Approach. Nat. Rev. Rheumatol. 2015, 11, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Worthley, L.I.G. Hydrogen Ion Metabolism. Anaesth Intensive Care 1977, 5, 347–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudon, M.; Traxer, O.; Conort, P.; Lacour, B.; Jungers, P. Type 2 Diabetes Increases the Risk for Uric Acid Stones. JASN 2006, 17, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Schmid-Wendtner, M.-H.; Korting, H.C. The PH of the Skin Surface and Its Impact on the Barrier Function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possanzini, L.; Decataldo, F.; Mariani, F.; Gualandi, I.; Tessarolo, M.; Scavetta, E.; Fraboni, B. Textile Sensors Platform for the Selective and Simultaneous Detection of Chloride Ion and PH in Sweat. Sci. Rep. 2020, 10, 17180. [Google Scholar] [CrossRef] [PubMed]
- Coyle, S.; Morris, D.; Lau, K.-T.; Diamond, D.; Taccini, N. Textile Sensors to Measure Sweat PH and Sweat-Rate during Exercise. In Proceedings of the 2009 3rd International Conference on Pervasive Computing Technologies for Healthcare, London, UK, 1–3 April 2009. [Google Scholar]
- Mazzaracchio, V.; Fiore, L.; Nappi, S.; Marrocco, G.; Arduini, F. Medium-Distance Affordable, Flexible and Wireless Epidermal Sensor for PH Monitoring in Sweat. Talanta 2021, 222, 121502. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully Printed Wearable Microfluidic Devices for High-Throughput Sweat Sampling and Multiplexed Electrochemical Analysis. ACS Sens. 2021, 6, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhou, Y.; Liu, Y.; Wang, L.; Wang, J. Coaxial Electrospun Flexible PANI//PU Fibers as Highly Sensitive PH Wearable Sensor. J. Mater. Sci. 2020, 55, 16033–16047. [Google Scholar] [CrossRef]
- Wang, R.; Zhai, Q.; Zhao, Y.; An, T.; Gong, S.; Guo, Z.; Shi, Q.; Yong, Z.; Cheng, W. Stretchable Gold Fiber-Based Wearable Electrochemical Sensor toward PH Monitoring. J. Mater. Chem. B 2020, 8, 3655–3660. [Google Scholar] [CrossRef] [PubMed]
- Diculescu, V.C.; Beregoi, M.; Evanghelidis, A.; Negrea, R.F.; Apostol, N.G.; Enculescu, I. Palladium/Palladium Oxide Coated Electrospun Fibers for Wearable Sweat PH-Sensors. Sci. Rep. 2019, 9, 8902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manjakkal, L.; Dang, W.; Yogeswaran, N.; Dahiya, R. Textile-Based Potentiometric Electrochemical PH Sensor for Wearable Applications. Biosensors 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, W.; Manjakkal, L.; Navaraj, W.T.; Lorenzelli, L.; Vinciguerra, V.; Dahiya, R. Stretchable Wireless System for Sweat PH Monitoring. Biosens. Bioelectron. 2018, 107, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) Based Electrode Materials for Energy Storage and Conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef] [Green Version]
- Hatchett, D.W.; Josowicz, M.; Janata, J. Acid Doping of Polyaniline: Spectroscopic and Electrochemical Studies. J. Phys. Chem. B 1999, 103, 10992–10998. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef]
- Gvozdenovic, M.; Jugovic, B.; Stevanovic, J.; Grgur, B. Electrochemical Synthesis of Electroconducting Polymers. Hem Ind 2014, 68, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Yoneyama, H.; Tamura, H. Electrochemical Reactions Concerned with Electrochromism of Polyaniline Film-Coated Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1984, 177, 281–291. [Google Scholar] [CrossRef]
- Lindfors, T.; Ervelä, S.; Ivaska, A. Polyaniline as PH-Sensitive Component in Plasticized PVC Membranes. J. Electroanal. Chem. 2003, 560, 69–78. [Google Scholar] [CrossRef]
- Dispenza, C.; Sabatino, M.A.; Deghiedy, N.; Casaletto, M.P.; Spadaro, G.; Piazza, S.; Abd El-Rehim, H.A. In-Situ Polymerization of Polyaniline in Radiation Functionalized Polypropylene Films. Polymer 2015, 67, 128–138. [Google Scholar] [CrossRef]
- Dispenza, C.; Fiandaca, G.; Lo Presti, C.; Piazza, S.; Spadaro, G. Electrical Properties of γ-Crosslinked Hydrogels Incorporating Organic Conducting Polymers. Radiat. Phys. Chem. 2007, 76, 1371–1375. [Google Scholar] [CrossRef]
- Brochocka, A.; Nowak, A.; Zajączkowska, H.; Sieradzka, M. Chemosensitive Thin Films Active to Ammonia Vapours. Sensors 2021, 21, 2948. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Iqbal, S.; Jang, H.J.; Jung, E.Y.; Bae, G.T.; Park, C.S.; Shin, B.J.; Tae, H.S. Transparent Polyaniline Thin Film Synthesized Using a Low-Voltage-Driven Atmospheric Pressure Plasma Reactor. Materials 2021, 14, 1278. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Ma, M.; Zhong, Z.; Lin, X.; Chen, M. Interfacial Growth of Free-Standing PANI Films: Toward High-Performance All-Polymer Supercapacitors. Chem. Sci. 2021, 12, 1783–1790. [Google Scholar] [CrossRef]
- Medi, B.; Bahramian, A.; Nazari, V. Synthesis and Characterization of Conducting Polyaniline Nanostructured Thin Films for Solar Cell Applications. JOM 2021, 73, 504–514. [Google Scholar] [CrossRef]
- Akber, H.J.; Ibrahim, I.M.; Razeg, K.H. Hydrothermal Synthesis of Polyaniline Nano-Fibers as H 2 S Gas Sensor. J. Phys. Conf. Ser. 2020, 1664, 012017. [Google Scholar] [CrossRef]
- Demuru, S.; Kunnel, B.P.; Briand, D. Thin Film Organic Electrochemical Transistors Based on Hybrid PANI/PEDOT:PSS Active Layers for Enhanced PH Sensing. Biosens. Bioelectron. X 2021, 7, 100065. [Google Scholar] [CrossRef]
- Silipigni, L.; Barreca, F.; Fazio, E.; Neri, F.; Spanò, T.; Piazza, S.; Sunseri, C.; Inguanta, R. Template Electrochemical Growth and Properties of Mo Oxide Nanostructures. J. Phys. Chem. C 2014, 118, 22299–22308. [Google Scholar] [CrossRef]
- Battaglia, M.; Piazza, S.; Sunseri, C.; Inguanta, R. Amorphous Silicon Nanotubes via Galvanic Displacement Deposition. Electrochem. Commun. 2013, 34, 134–137. [Google Scholar] [CrossRef]
- Battaglia, M.; Inguanta, R.; Piazza, S.; Sunseri, C. Fabrication and Characterization of Nanostructured Ni–IrO2 Electrodes for Water Electrolysis. Int. J. Hydrog. Energy 2014, 39, 16797–16805. [Google Scholar] [CrossRef]
- Ganci, F.; Lombardo, S.; Sunseri, C.; Inguanta, R. Nanostructured Electrodes for Hydrogen Production in Alkaline Electrolyzer. Renew. Energy 2018, 123, 117–124. [Google Scholar] [CrossRef]
- Patella, B.; Piazza, S.; Sunseri, C.; Inguanta, R. Nio Thin Film for Mercury Detection in Water by Square Wave Anodic Stripping Voltammetry. Chem. Eng. Trans. 2017, 60, 1–6. [Google Scholar] [CrossRef]
- Insinga, M.G.; Oliveri, R.L.; Sunseri, C.; Inguanta, R. Template Electrodeposition and Characterization of Nanostructured Pb as a Negative Electrode for Lead-Acid Battery. J. Power Sources 2019, 413, 107–116. [Google Scholar] [CrossRef]
- Sunseri, C.; Cocchiara, C.; Ganci, F.; Moncada, A.; Oliveri, R.L.; Patella, B.; Piazza, S.; Inguanta, R. Nanostructured Electrochemical Devices for Sensing, Energy Conversion and Storage. Chem. Eng. Trans. 2016, 47, 43–48. [Google Scholar] [CrossRef]
- Patella, B.; Buscetta, M.; Di Vincenzo, S.; Ferraro, M.; Aiello, G.; Sunseri, C.; Pace, E.; Inguanta, R.; Cipollina, C. Electrochemical Sensor Based on RGO/Au Nanoparticles for Monitoring H2O2 Released by Human Macrophages. Sens. Actuators B Chem. 2021, 327, 128901. [Google Scholar] [CrossRef]
- Patella, B.; Russo, R.R.; O’Riordan, A.; Aiello, G.; Sunseri, C.; Inguanta, R. Copper Nanowire Array as Highly Selective Electrochemical Sensor of Nitrate Ions in Water. Talanta 2021, 221, 121643. [Google Scholar] [CrossRef]
- Belgherbi, O.; Seid, L.; Lakhdari, D.; Chouder, D.; Akhtar, M.S.; Saeed, M.A. Optical and Morphological Properties of Electropolymerized Semiconductor Polyaniline Thin Films: Effect of Thickness. J. Electron. Mater. 2021, 50, 3876–3884. [Google Scholar] [CrossRef]
- Nekrasov, A.A.; Iakobson, O.D.; Gribkova, O.L.; Pozin, S.I. Raman Spectroelectrochemical Monitoring of Conducting Polymer Electrosynthesis on Reflective Metallic Electrode: Effects Due to Double Excitation of the Electrode/Film/Solution Interfaces. J. Electroanal. Chem. 2020, 873, 114415. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Rendón-Enríquez, I.; Tausch, M.; Scherf, U. Thin Functional Polymer Films by Electropolymerization. Nanomaterials 2019, 9, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quijada, C.; Leite-Rosa, L.; Berenguer, R.; Bou-Belda, E. Enhanced Adsorptive Properties and Pseudocapacitance of Flexible Polyaniline-Activated Carbon Cloth Composites Synthesized Electrochemically in a Filter-Press Cell. Materials 2019, 12, 2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzara, F.; Patella, B.; Aiello, G.; O’Riordan, A.; Torino, C.; Vilasi, A.; Inguanta, R. Electrochemical Detection of Uric Acid and Ascorbic Acid Using R-GO/NPs Based Sensors. Electrochim. Acta 2021, 388, 138652. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Liu, Y.; Xin, G.; Wang, M.; Zhang, Z.; Liu, Z. Electrochemical Sensor Based on a Three Dimensional Nanostructured MoS 2 Nanosphere-PANI/Reduced Graphene Oxide Composite for Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid. RSC Adv. 2019, 9, 2997–3003. [Google Scholar] [CrossRef] [Green Version]
- Anand, V.K.; Bukke, A.; Bhatt, K.; Kumar, S.; Sharma, S.; Goyal, R.; Virdi, G.S. Highly Sensitive and Reusable Cu+2/Polyaniline/Reduced Graphene Oxide Nanocomposite Ink-Based Non-Enzymatic Glucose Sensor. Appl. Phys. A 2020, 126, 500. [Google Scholar] [CrossRef]
- Du, X.; Chen, Y.; Dong, W.; Han, B.; Liu, M.; Chen, Q.; Zhou, J. A Nanocomposite-Based Electrochemical Sensor for Non-Enzymatic Detection of Hydrogen Peroxide. Oncotarget 2017, 8, 13039–13047. [Google Scholar] [CrossRef] [Green Version]
- Aryal, K.P.; Jeong, H.K. Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid with Polyaniline/Hemin/Reduced Graphite Oxide Composite. Chem. Phys. Lett. 2021, 768, 138405. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Zhu, W.; Han, Q.; Zhu, C.; Hong, J.; Zhou, X.; Jiang, H. Electrochemical Serotonin Sensing Interface Based on Double-Layered Membrane of Reduced Graphene Oxide/Polyaniline Nanocomposites and Molecularly Imprinted Polymers Embedded with Gold Nanoparticles. Sens. Actuators B Chem. 2014, 196, 57–63. [Google Scholar] [CrossRef]
- Wirth, D.M.; Waldman, L.J.; Petty, M.; LeBlanc, G. Communication—Polyaniline Electrodeposition on Flexible ITO Substrates and the Effect of Curved Electrochemical Conditions. J. Electrochem. Soc. 2019, 166, D635–D637. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Fang, Y.; Han, L.; Wang, E.; Dong, S. One-Step Electrochemical Approach to the Synthesis of Graphene/MnO2 Nanowall Hybrids. Nano Res. 2011, 4, 648–657. [Google Scholar] [CrossRef]
- Erez, M.; Zaykovsky, Z. Sweat Collectors and Methods of Collecting Sweat. U.S. Patent 8,215,192, 10 July 2012. [Google Scholar]
- Inguanta, R.; Garlisi, C.; Spanò, T.; Piazza, S.; Sunseri, C. Growth and Photoelectrochemical Behaviour of Electrodeposited ZnO Thin Films for Solar Cells. J. Appl. Electrochem. 2013, 43, 199–208. [Google Scholar] [CrossRef]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and Structural Analysis of Polyaniline and Poly(o-Anisidine) Layers Generated in a DC Glow Discharge Plasma by Using an Oblique Angle Electrode Deposition Configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, T.V.; Sousa, E.A.; Fuzari Jr, G.C.; Arlindo, E.P.S. Different Morphologies of Polyaniline Nanostructures Synthesized by Interfacial Polymerization. Mater. Lett. 2018, 224, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Komathi, S.; Gopalan, A.I.; Muthuchamy, N.; Lee, K.P. Polyaniline Nanoflowers Grafted onto Nanodiamonds via a Soft Template-Guided Secondary Nucleation Process for High-Performance Glucose Sensing. RSC Adv. 2017, 7, 15342–15351. [Google Scholar] [CrossRef] [Green Version]
- Patella, B.; Sortino, A.; Aiello, G.; Sunseri, C.; Inguanta, R. Reduced Graphene Oxide Decorated with Metals Nanoparticles Electrode as Electrochemical Sensor for Dopamine. In Proceedings of the 2019 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Glasgow, UK, 8–10 July 2019; pp. 1–3. [Google Scholar]
- Mazzara, F.; Patella, B.; Aiello, G.; Sunseri, C.; Inguanta, R. Ascorbic Acid Determination Using Linear Sweep Voltammetry on Flexible Electrode Modified with Gold Nanoparticles and Reduced Graphene Oxide. In Proceedings of the 2020 IEEE 20th Mediterranean Electrotechnical Conference (MELECON), Palermo, Italy, 16–18 June 2020; pp. 406–410. [Google Scholar]
- Tran, H.D.; D’Arcy, J.M.; Wang, Y.; Beltramo, P.J.; Strong, V.A.; Kaner, R.B. The Oxidation of Aniline to Produce “Polyaniline”: A Process Yielding Many Different Nanoscale Structures. J. Mater. Chem. 2011, 21, 3534–3550. [Google Scholar] [CrossRef]
- Strankowski, M.; Włodarczyk, D.; Piszczyk, Ł.; Strankowska, J. Polyurethane Nanocomposites Containing Reduced Graphene Oxide, FTIR, Raman, and XRD Studies. J. Spectrosc. 2016, 2016, 7520741. [Google Scholar] [CrossRef] [Green Version]
- Trchová, M.; Šeděnková, I.; Tobolková, E.; Stejskal, J. FTIR Spectroscopic and Conductivity Study of the Thermal Degradation of Polyaniline Films. Polymer Degrad. Stab. 2004, 86, 179–185. [Google Scholar] [CrossRef]
- Mitra, M.; Kulsi, C.; Chatterjee, K.; Kargupta, K.; Ganguly, S.; Banerjee, D.; Goswami, S. Reduced Graphene Oxide-Polyaniline Composites—Synthesis, Characterization and Optimization for Thermoelectric Applications. RSC Adv. 2015, 5, 31039–31048. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Y.; Chu, J.; Wang, X.; Zhang, R.; Gong, M.; Wu, B.; Li, Z. One-Pot Hydrothermal Synthesis of Polyaniline Nanofibers/Reduced Graphene Oxide Nanocomposites and Their Supercapacitive Properties. High Perform. Polym. 2019, 31, 1238–1247. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J. Control of Polyaniline Conductivity and Contact Angles by Partial Protonation. Polym. Int. 2008, 57, 66–69. [Google Scholar] [CrossRef]
- Akin, I.; Zor, E.; Bingol, H.; Ersoz, M. Green Synthesis of Reduced Graphene Oxide/Polyaniline Composite and Its Application for Salt Rejection by Polysulfone-Based Composite Membranes. J. Phys. Chem. B 2014, 118, 5707–5716. [Google Scholar] [CrossRef]
- Patterson, M.J.; Galloway, S.D.R.; Nimmo, M.A. Variations in Regional Sweat Composition in Normal Human Males. Exp. Physiol. 2000, 85, 869–875. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of Sweat Gland Function: The Roles of Sweating and Sweat Composition in Human Health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Mao, Y.; Xiao, C.; Xu, X.; Li, X. Flexible PH Sensor Based on a Conductive PANI Membrane for PH Monitoring. RSC Adv. 2020, 10, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.H.; Hong, S.B.; Yun, S.-O.; Lee, S.J.; Lee, T.J.; Lee, K.G.; Choi, B.G. High Performance Flexible PH Sensor Based on Polyaniline Nanopillar Array Electrode. J. Colloid Interface Sci. 2017, 490, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Yoon, J.H.; Lee, K.G.; Choi, B.G. Potentiometric Performance of Flexible PH Sensor Based on Polyaniline Nanofiber Arrays. Nano Converg. 2019, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.D.; Pscheidt, J.; Santos, C.S.; Ferreira, R.T.; Marciniuk, G.; Garcia, J.R.; Vidotti, M.; Marchesi, L.F.; Pessoa, C.A. Electrochemical Performance of PH Sensor Based on LbL Films of Polyaniline-Gum Arabic Nanocomposite and Graphene Oxide. J. Electrochem. Soc. 2020, 167, 047505. [Google Scholar] [CrossRef]
- Su, W.; Xu, J.; Ding, X. An Electrochemical PH Sensor Based on the Amino-Functionalized Graphene and Polyaniline Composite Film. IEEE Trans. Nanobioscience 2016, 15, 812–819. [Google Scholar] [CrossRef]
- Ge, C.; Orosz, K.S.; Armstrong, N.R.; Saavedra, S.S. Poly(Aniline) Nanowires in SolÀGel Coated ITO: A PH-Responsive Substrate for Planar Supported Lipid Bilayers. ACS Appl. Mater. Interfaces 2011, 9, 2677–2685. [Google Scholar] [CrossRef] [Green Version]
- Lakard, B. Optimization of the Structural Parameters of New Potentiometric PH and Urea Sensors Based on Polyaniline and a Polysaccharide Coupling Layer. Sens. Actuators B Chem. 2012, 8, 794–801. [Google Scholar] [CrossRef]
- Saikrithika, S. A Selective Voltammetric PH Sensor Using Graphitized Mesoporous Carbon/Polyaniline Hybrid System. J. Chem. Sci. 2021, 10, 46. [Google Scholar] [CrossRef]
- Yoon, J.H. Highly Self-Healable and Flexible Cable-Type PH Sensors for Real-Time Monitoring of Human Fluids. Biosens. Bioelectron. 2020, 7, 111946. [Google Scholar] [CrossRef]
- Guinovart, T.; ValdØs-Ramírez, G.; Windmiller, J.R.; Andrade, F.J.; Wang, J. Bandage-Based Wearable Potentiometric Sensor for Monitoring Wound PH. Electroanalysis 2014, 9, 1345–1353. [Google Scholar] [CrossRef]


| pH Range | Sensitivity | Flexible | Easy Fabrication | Interference | Real Samples | Ref | |
|---|---|---|---|---|---|---|---|
| PI-PANI | 5.5–8.5 | 58.6 | Y | N | N.S. | N.S. | [86] |
| PET-PANI | 2–12 | 60.3 | Y | N | Na+, K+, NH4+, Ca2+, Mg2+ | coke, coffee, water, orange juice | [87] |
| CPE-PANI | 3–10 | 62.4 | Y | Y | Ca2+, Mg2+, K+, Na+, NH4+ | milk and apples | [88] |
| LbL/(PANI-GA/GO) | 2–7 | 35.1 | Y | Y | N.S. | N.S. | [89] |
| NH2-G/PANI | 1–11 | 50.7 | N | Y | N.S. | N.S. | [90] |
| PANI-NWs-ITO | 3–9 | 48 | N | Y | N.S. | N.S. | [91] |
| PANI-PS | 4–8 | 59 | N | Y | N.S. | N.S. | [92] |
| PANI/PU | 2–7 | 60 | Y | Y | N.S. | sweat | [30] |
| GCE/GMC@PANI | 2–11 | 58 | N | Y | NaCl, NH4Cl, KNO3, AA, UA, GLU, NaNO3, NaNO2, HYD, DA, CYS | urine, saliva | [93] |
| PANI Cable | 4–10 | 58.7 | Y | Y | Na+, K+, NH4+, Ca2+, | urine, sweat, saliva, tears | [94] |
| Bandage-PANI | 5.5–8 | 58 | Y | Y | NaCl, KCl, Na2SO4 | serum, SWS | [95] |
| IrO2/G | 3–8 | 79 | Y | N | Na+, Cl-, K+, SA | sweat | [28] |
| ITO-rGO-PANI | 2–8 | 62.3 | Y | Y | NaCl, NH4Cl, KNO3, LA | sweat | ThisWork |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzara, F.; Patella, B.; D’Agostino, C.; Bruno, M.G.; Carbone, S.; Lopresti, F.; Aiello, G.; Torino, C.; Vilasi, A.; O’Riordan, A.; et al. PANI-Based Wearable Electrochemical Sensor for pH Sweat Monitoring. Chemosensors 2021, 9, 169. https://doi.org/10.3390/chemosensors9070169
Mazzara F, Patella B, D’Agostino C, Bruno MG, Carbone S, Lopresti F, Aiello G, Torino C, Vilasi A, O’Riordan A, et al. PANI-Based Wearable Electrochemical Sensor for pH Sweat Monitoring. Chemosensors. 2021; 9(7):169. https://doi.org/10.3390/chemosensors9070169
Chicago/Turabian StyleMazzara, Francesca, Bernardo Patella, Chiara D’Agostino, Maria Giuseppina Bruno, Sonia Carbone, Francesco Lopresti, Giuseppe Aiello, Claudia Torino, Antonio Vilasi, Alan O’Riordan, and et al. 2021. "PANI-Based Wearable Electrochemical Sensor for pH Sweat Monitoring" Chemosensors 9, no. 7: 169. https://doi.org/10.3390/chemosensors9070169
APA StyleMazzara, F., Patella, B., D’Agostino, C., Bruno, M. G., Carbone, S., Lopresti, F., Aiello, G., Torino, C., Vilasi, A., O’Riordan, A., & Inguanta, R. (2021). PANI-Based Wearable Electrochemical Sensor for pH Sweat Monitoring. Chemosensors, 9(7), 169. https://doi.org/10.3390/chemosensors9070169












