The Effect of Au Nanoparticle Addition on Humidity Sensing with Ultra-Small TiO2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
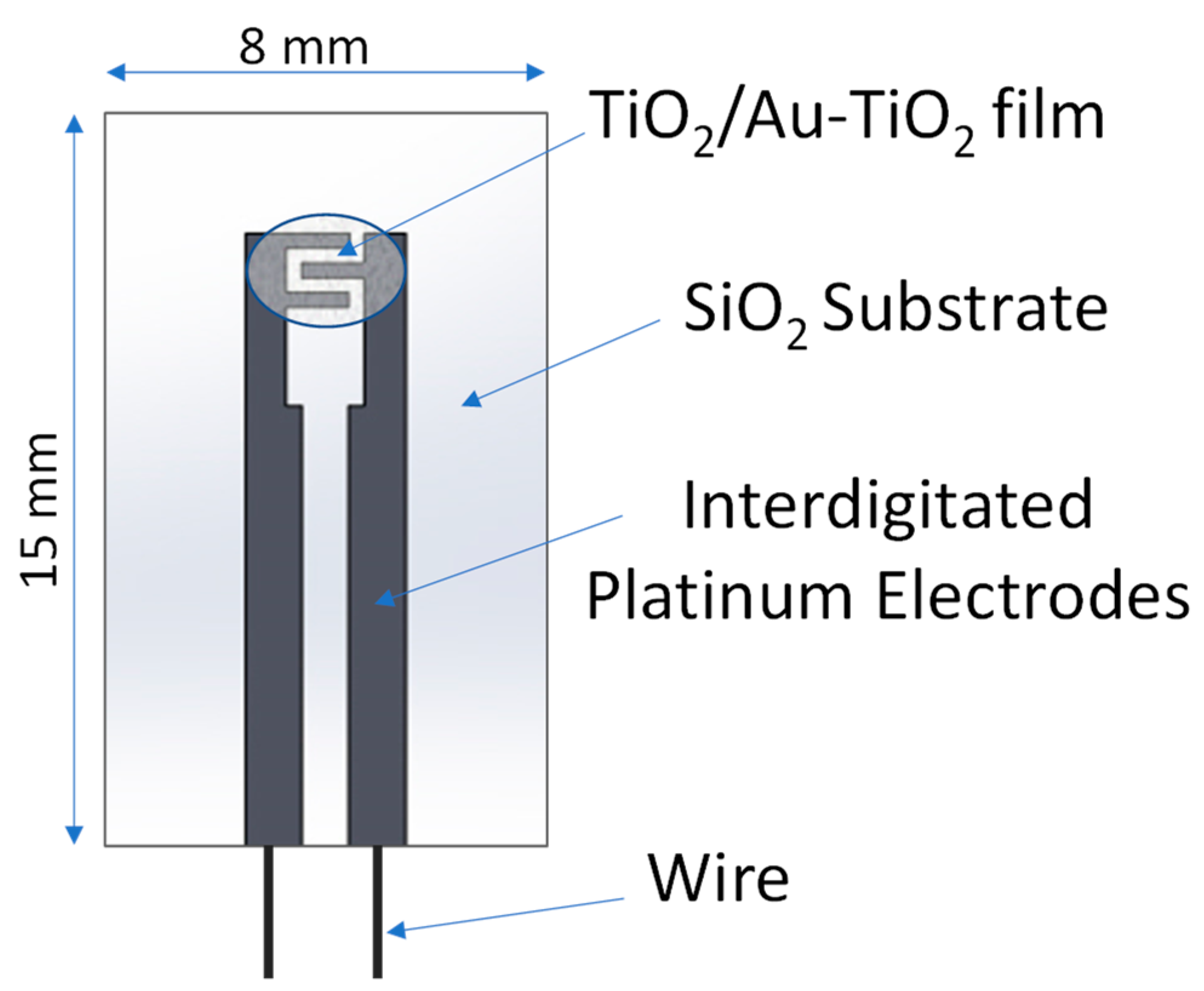
2.2. Sensing Device Preparation
2.3. Instrumentation for Material Characterization
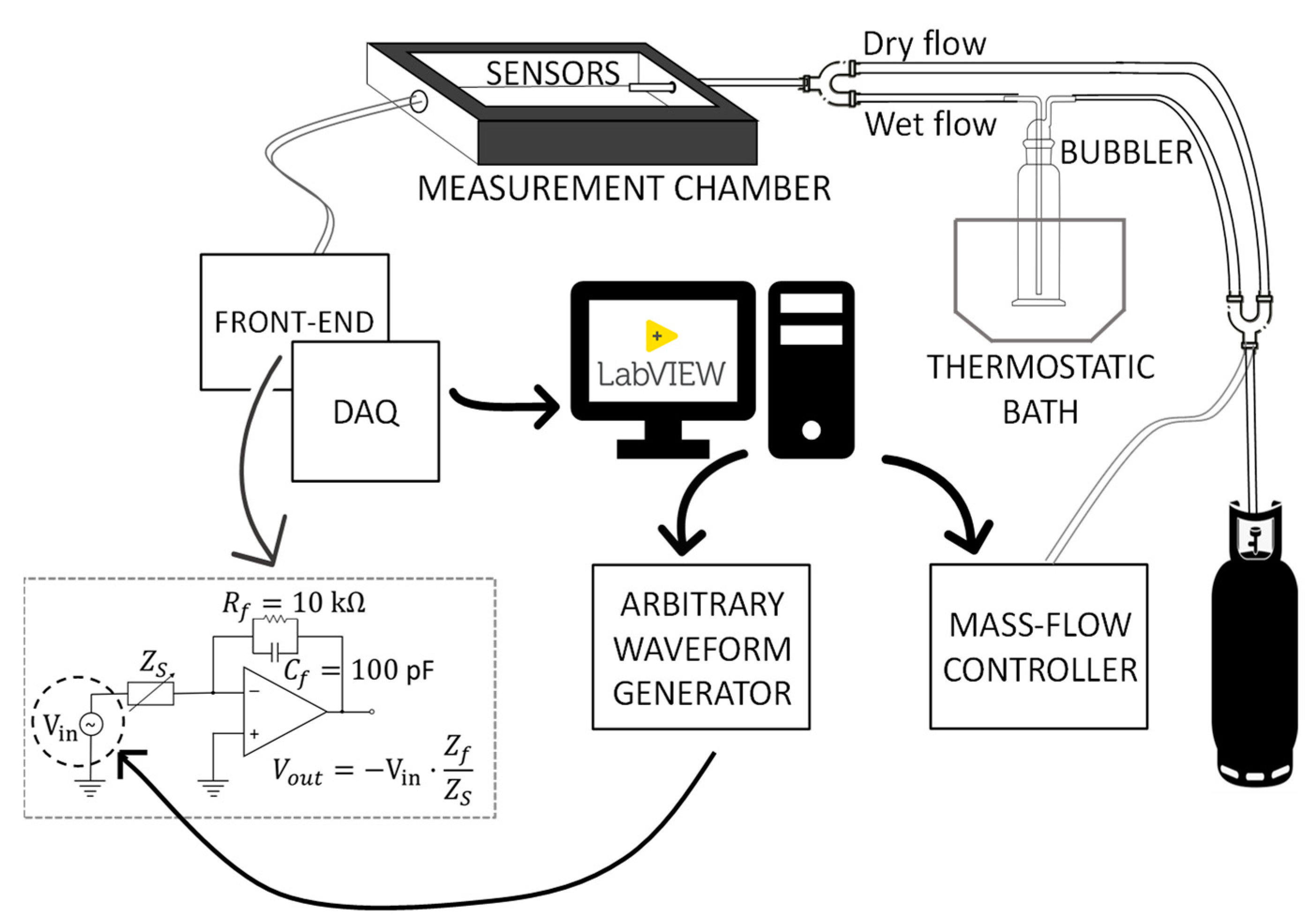
2.4. Setup for the Humidity-Sensing Characterization
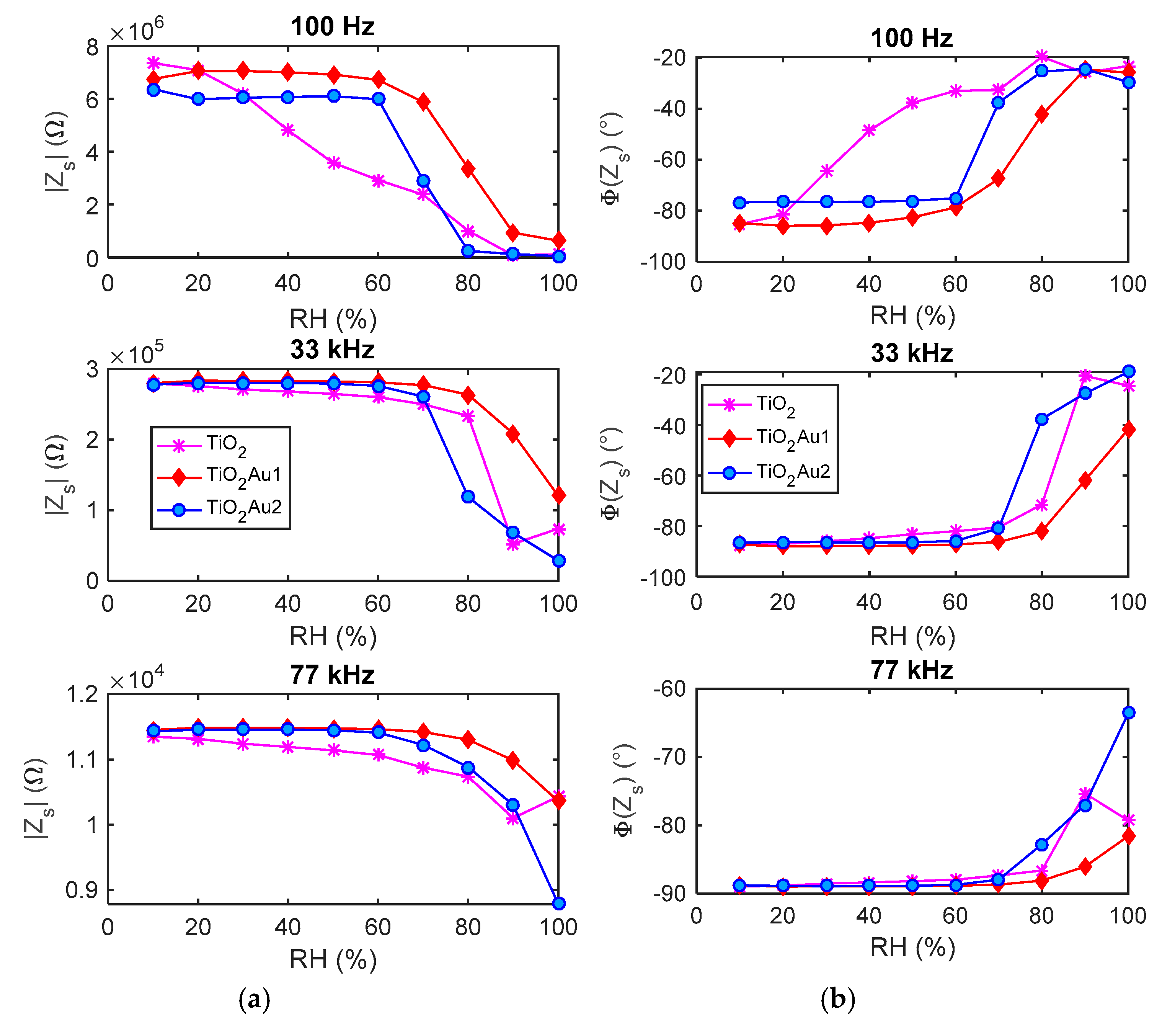
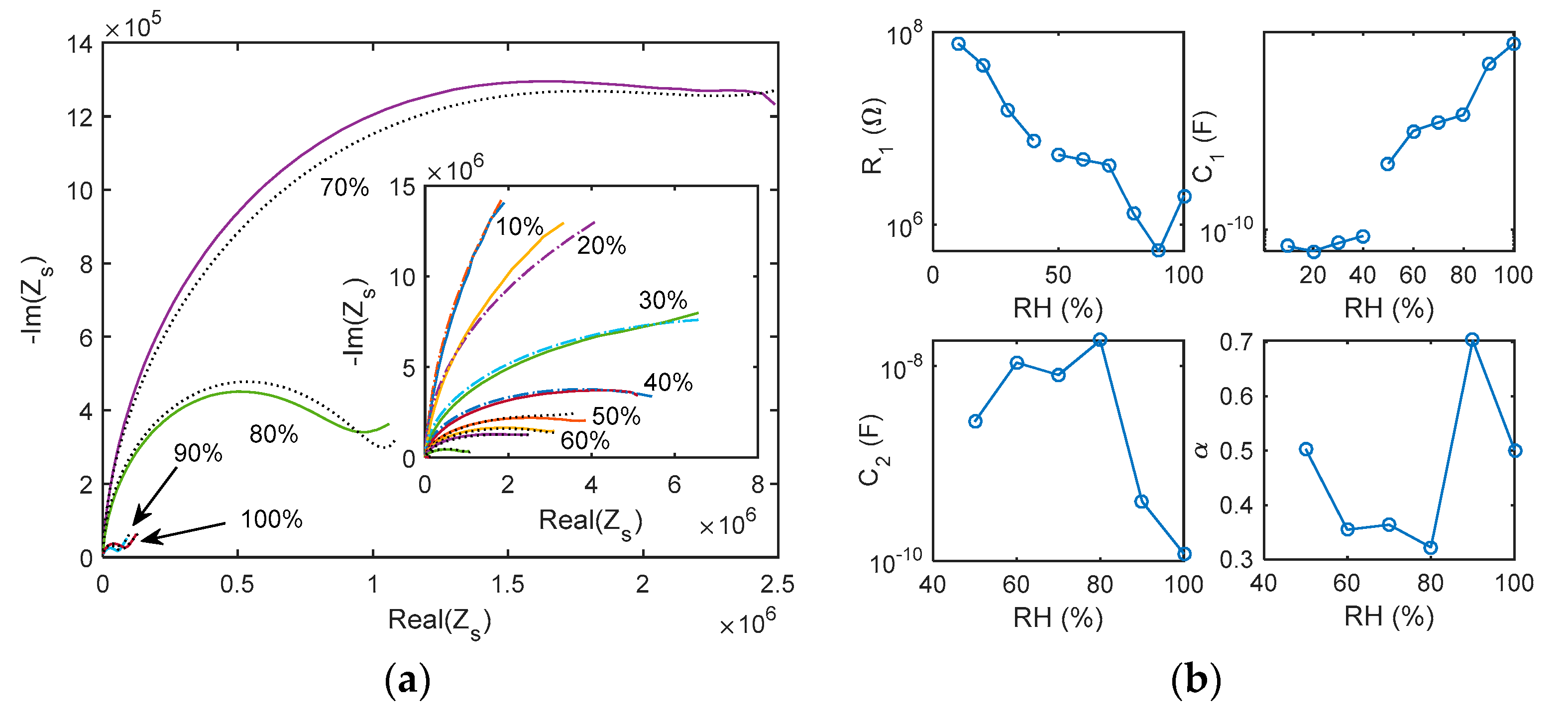
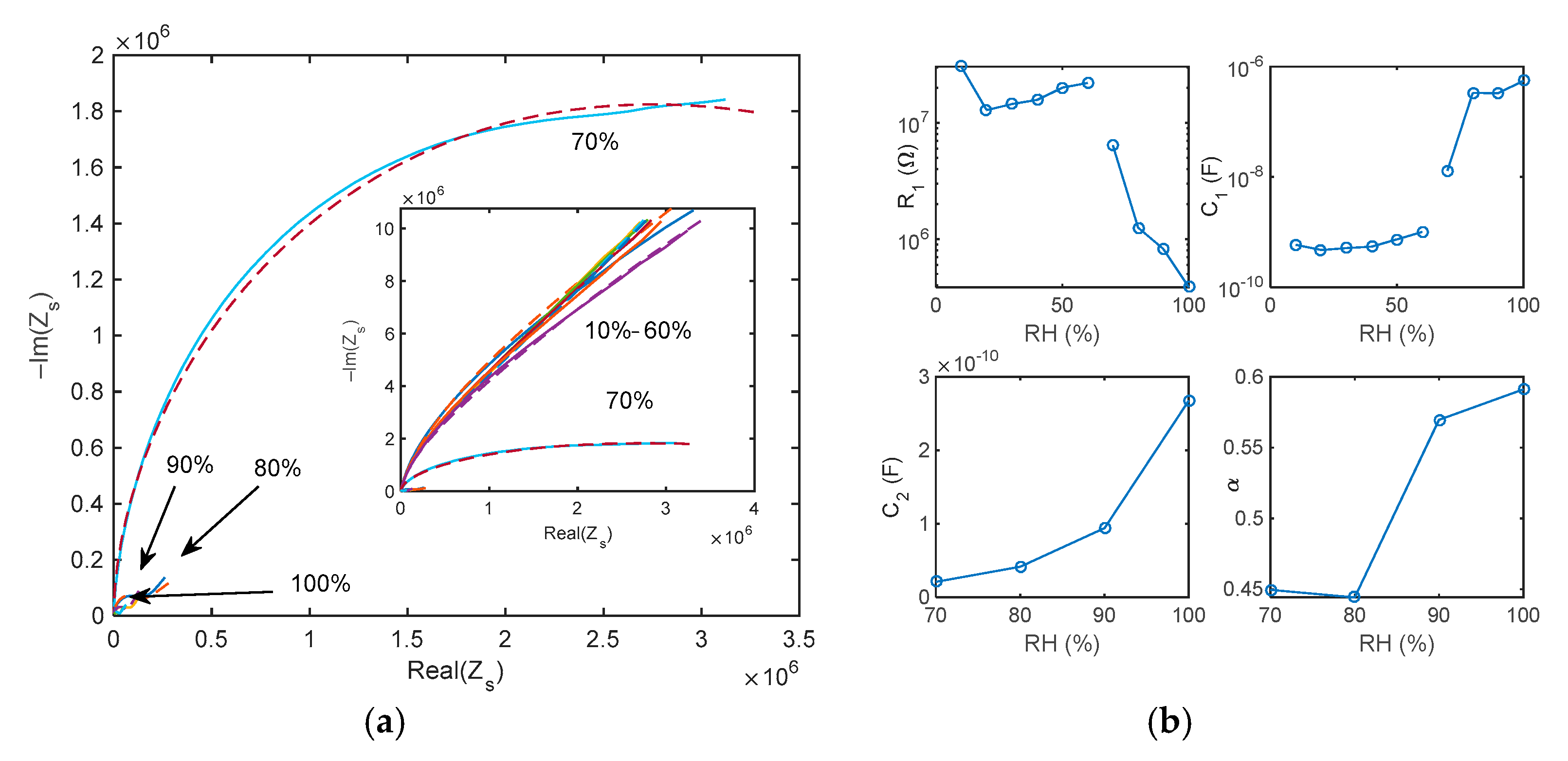
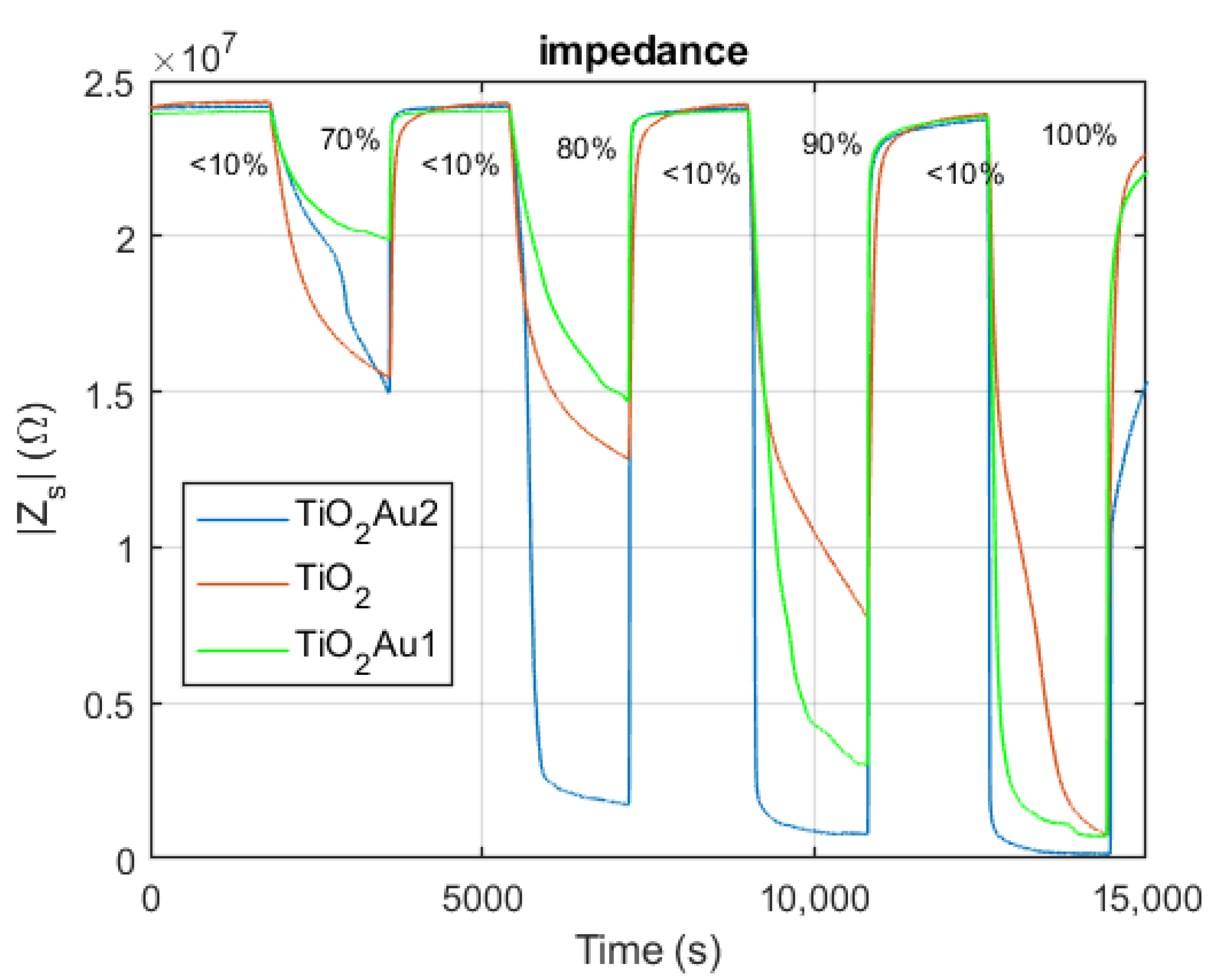
- The impedance spectra of the tested sensing devices in the frequency range 50 Hz–80 kHz, measured at constant RH in the range 0–100%.
- The admittance/impedance magnitude evaluated at fixed frequencies (most of these measurements are performed at fin = 100 Hz, i.e., at low frequency, to highlight the conduction mechanisms contribution) in the presence of smooth or abrupt RH variations.
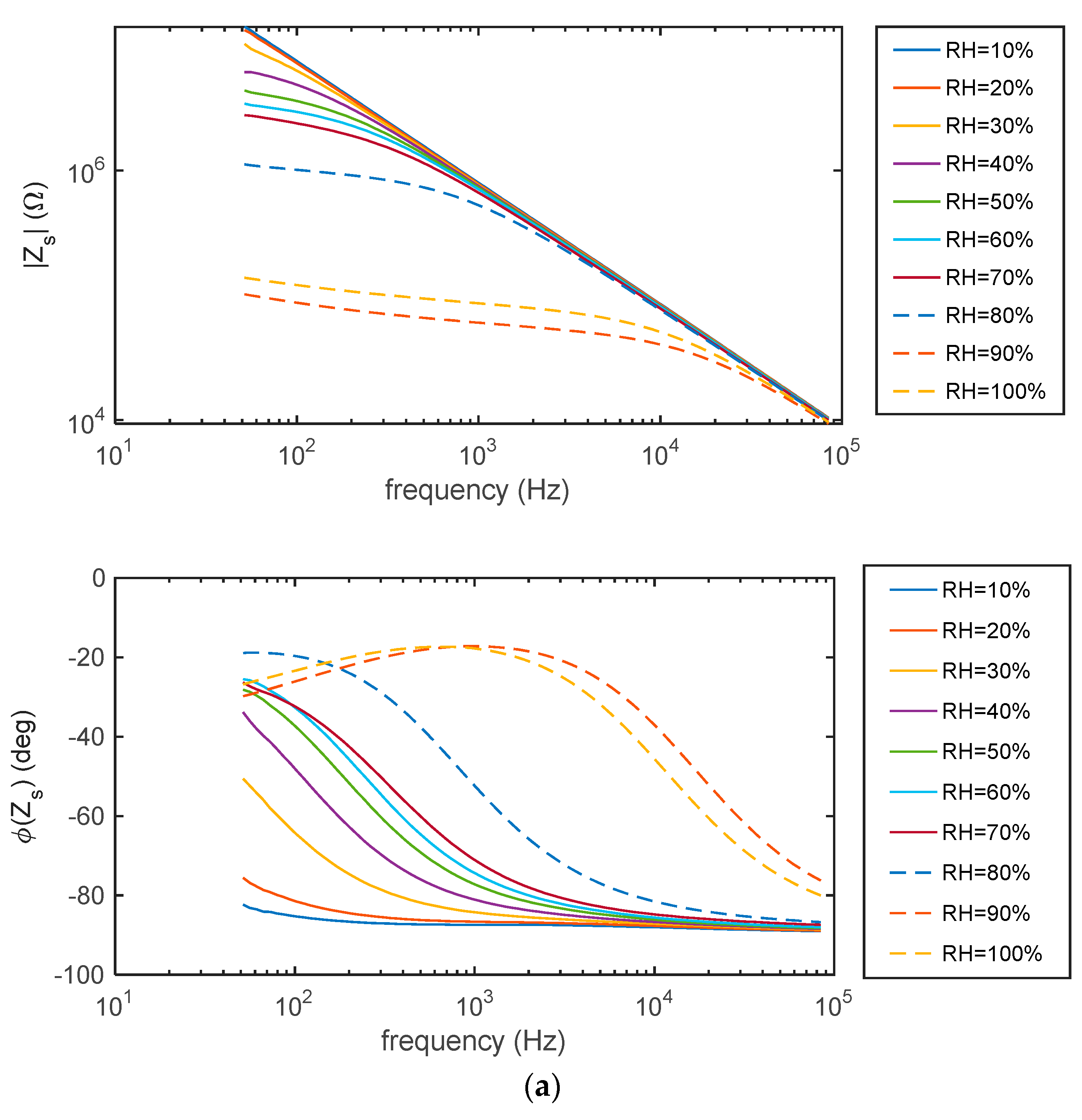
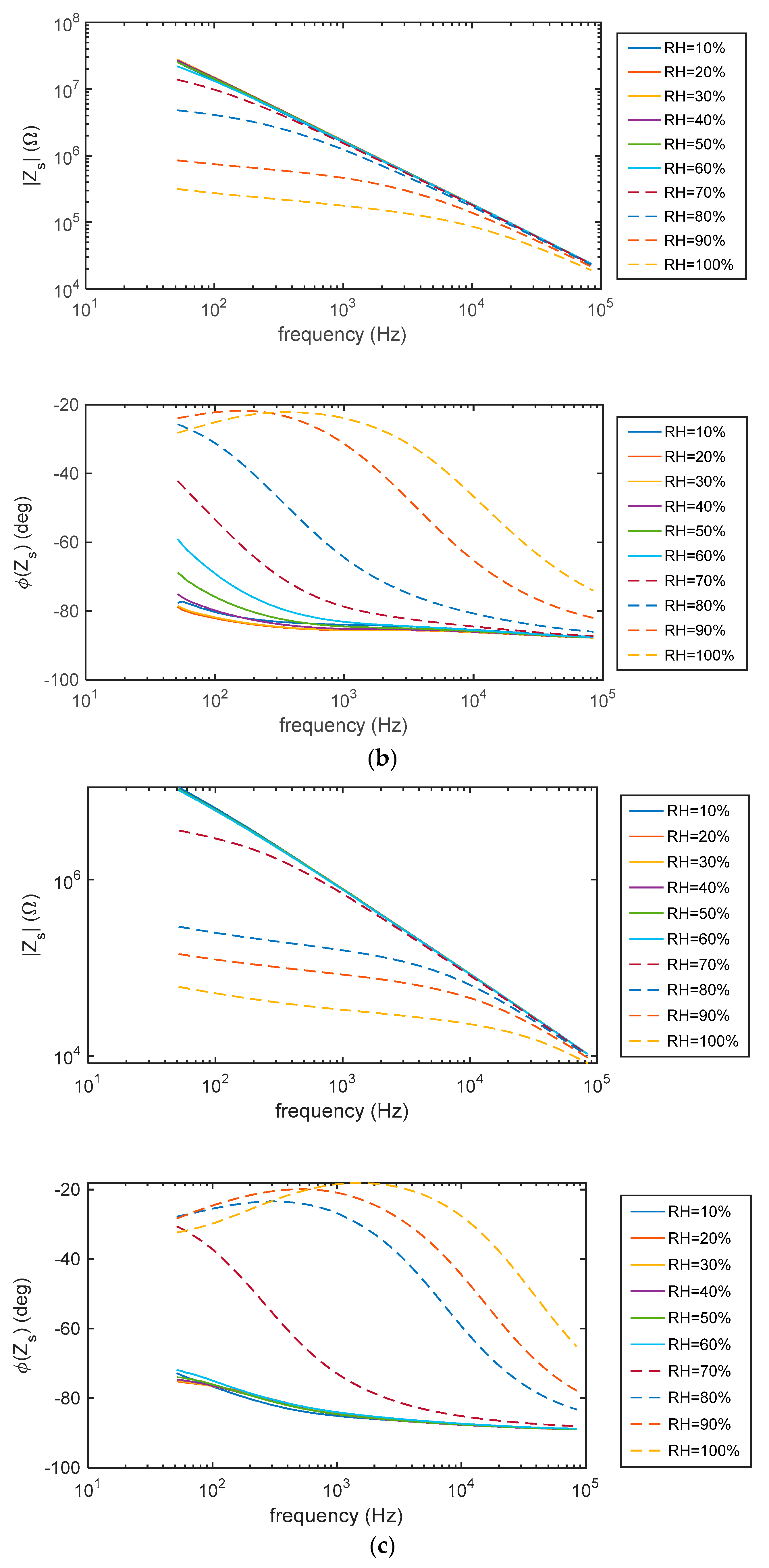
2.4.1. Impedance Spectra at Constant RH
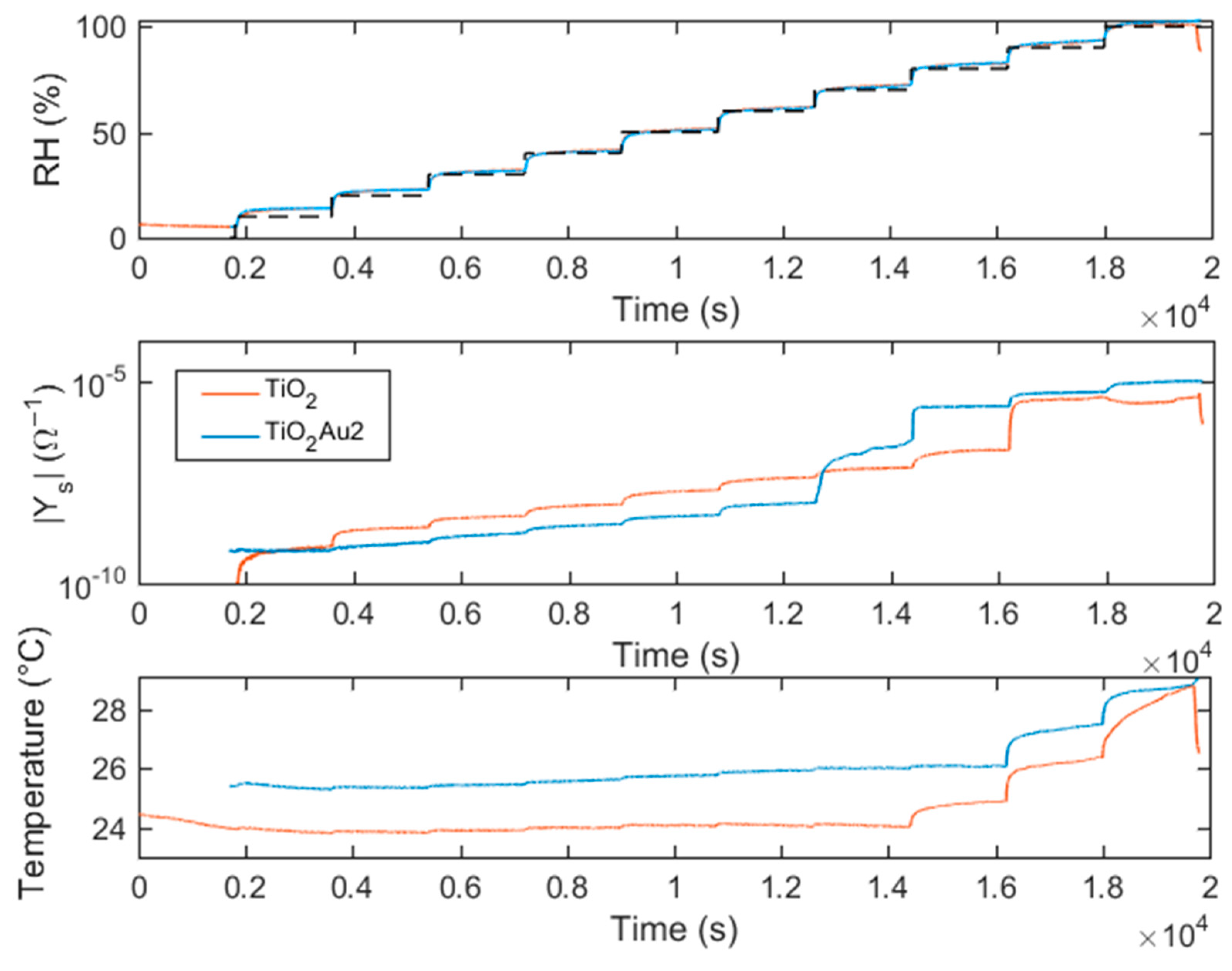
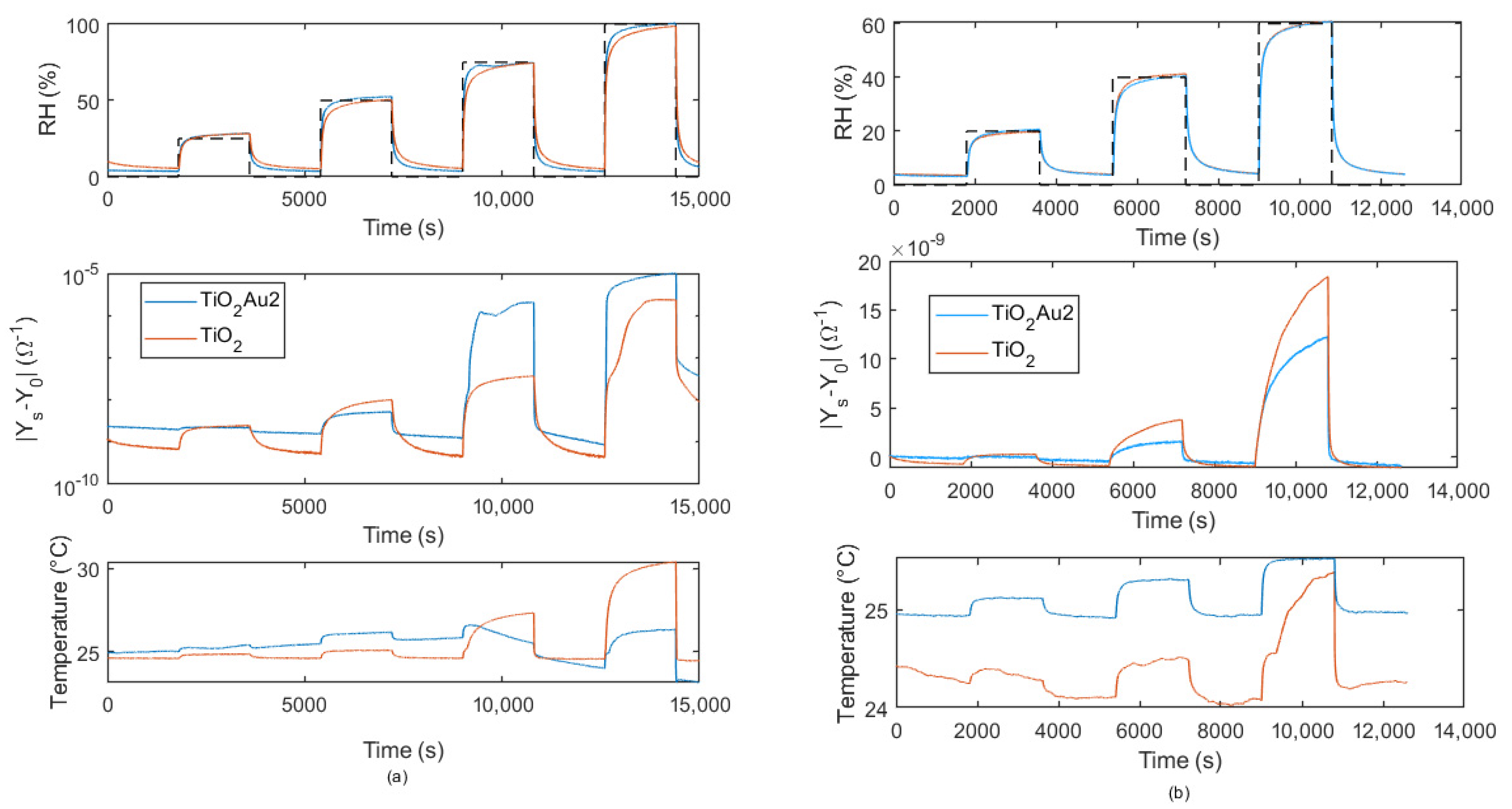
2.4.2. Admittance/Impedance Magnitude at fin = 100 Hz in Presence of RH Variations
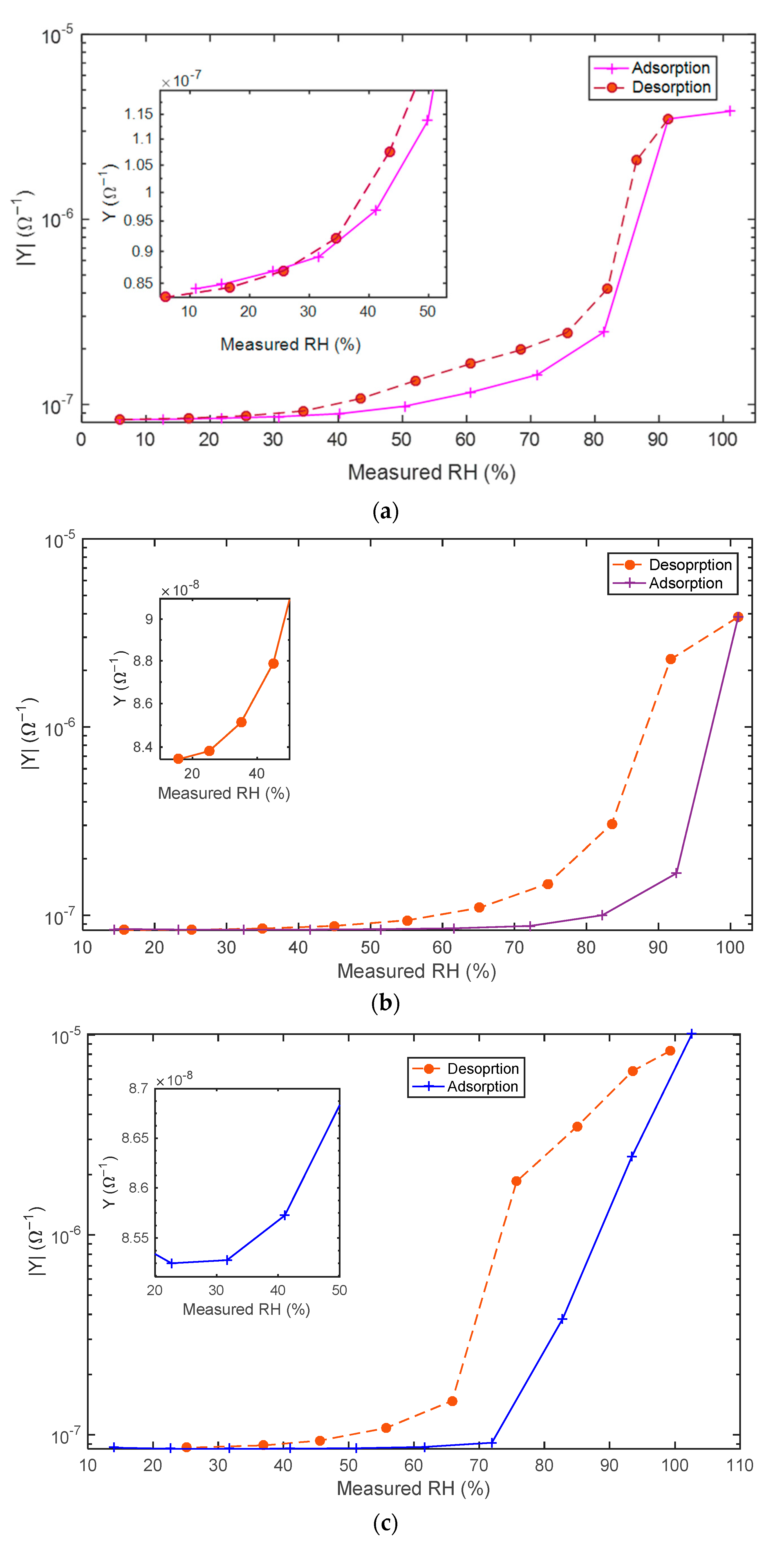
- Slowly increased from RH = 10% to RH = 100% and decreased from RH = 100% to RH = 10%. The monitoring of the sensing material real-time response gives information about the different water adsorption and desorption phenomena and about their effect on the electronic conduction mechanisms of the sensing film as a function of the RH.
- Changed in a pulsed way. Increasing/decreasing RH pulses, whether or not followed by recovery phases, were applied. Despite the sudden steps in humidity, the small chamber volume and the fast flow velocity ensured that the transient response of the chamber ran out quickly and did not affect the sensor transient response.
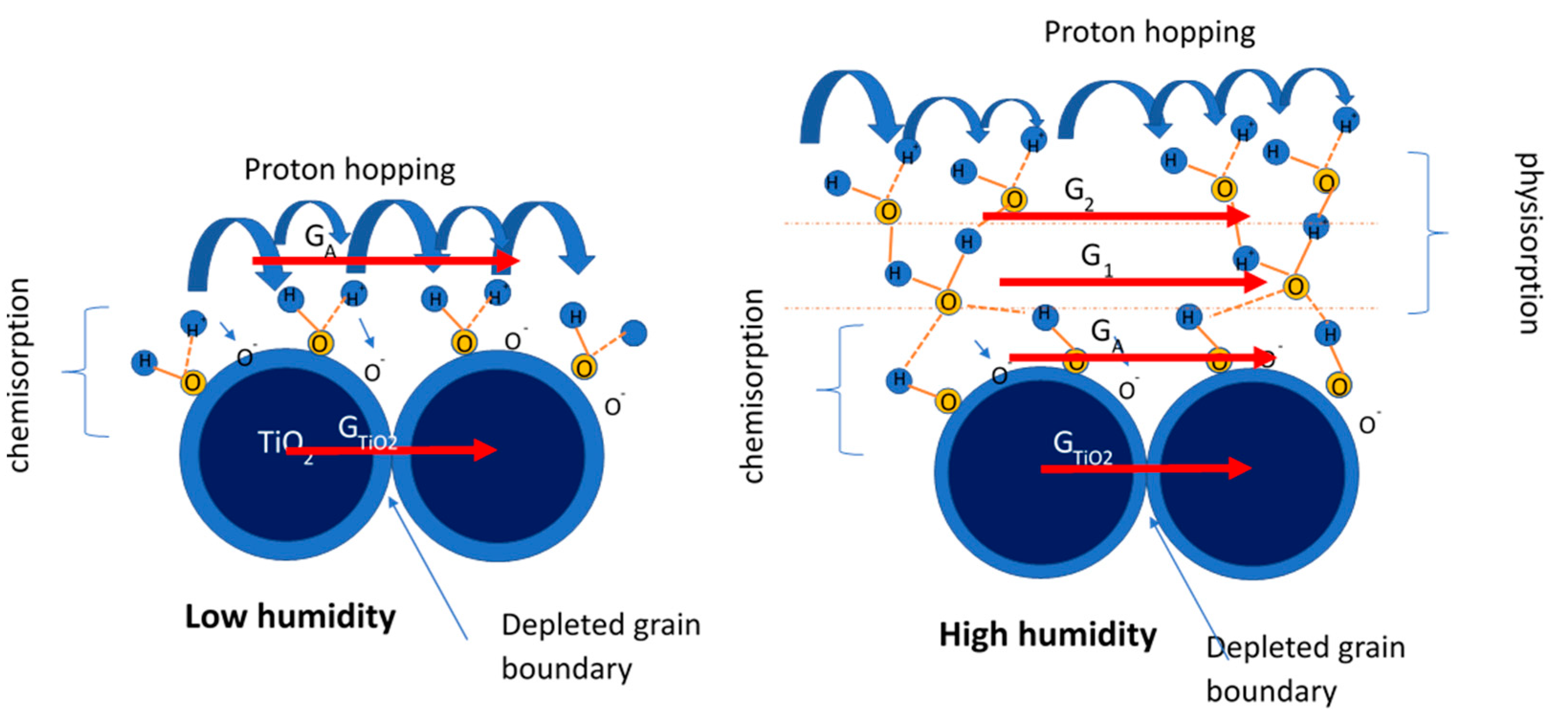
3. Sensing Mechanism
4. Material Characterization: Experimental Results and Discussion
5. Humidity Sensing: Experimental Results and Discussion
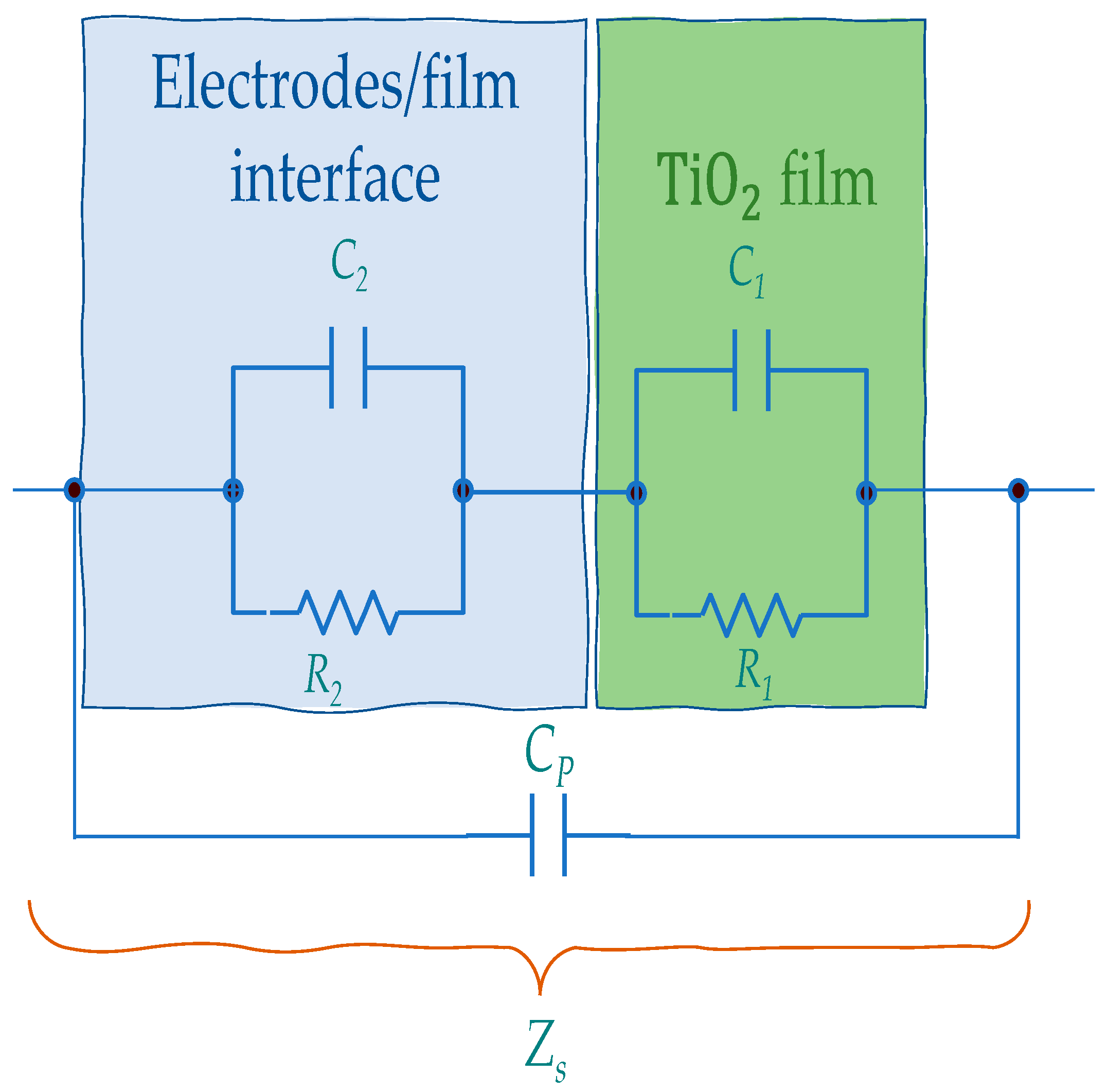
5.1. Impedance Spectra
5.2. Adsorption/Desorption Response: Experimental Results and Discusssion
5.3. Transient Response
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S.M.A. Gas Sensors Based on One Dimensional Nanostructured Metal-Oxides: A Review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef]
- Dubourg, G.; Segkos, A.; Katona, J.; Radović, M.; Savić, S.; Niarchos, G.; Tsamis, C.; Crnojević-Bengin, V. Fabrication and Characterization of Flexible and Miniaturized Humidity Sensors Using Screen-Printed TiO2 Nanoparticles as Sensitive Layer. Sensors 2017, 17, 1854. [Google Scholar] [CrossRef] [Green Version]
- Fort, A.; Panzardi, E.; Al-Hamry, A.; Vignoli, V.; Mugnaini, M.; Addabbo, T.; Kanoun, O. Highly Sensitive Detection of NO2 by Au and TiO2 Nanoparticles Decorated SWCNTs Sensors. Sensors 2020, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Rzaij, J.; Abass, A. Review on: TiO2 Thin Film as a Metal Oxide Gas Sensor. J. Chem. Rev. 2020, 2, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yu, S.; Zhang, H. Preparation and Performance Analysis of Ag/ZnO Humidity Sensor. Sensors 2021, 21, 857. [Google Scholar] [CrossRef]
- Arunachalam, S.; Izquierdo, R.; Nabki, F. Low-hysteresis and fast response time humidity sensors using suspended functionalized carbon nanotubes. Sensors 2019, 19, 680. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, J.; Ding, C.; Bai, G.; Xu, J.; Ren, Q.; Li, J. Fabrication of ordered SnO2 nanostructures with enhanced humidity sensing performance. Sensors 2017, 17, 2392. [Google Scholar] [CrossRef] [Green Version]
- Platonov, V.; Rumyantseva, M.; Khmelevsky, N.; Gaskov, A. Electrospun ZnO/Pd Nanofibers: CO Sensing and Humidity Effect. Sensors 2020, 20, 7333. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, E.; Tan, S.P.; Saraji, S.; Piri, M.; Chen, J.H. A review on capillary condensation in nanoporous media: Implications for hydrocarbon recovery from tight reservoirs. Fuel 2016, 184, 344–361. [Google Scholar] [CrossRef]
- Fort, A.; Panzardi, E.; Vignoli, V.; Landi, E.; Mugnaini, M.; Carlo, T. Performance Analysis of an AlN Humidity Sensor based on TiO2 nanoparticles. In Proceedings of the 2019 IEEE International Symposium on Measurements & Networking (M&N), Catania, Italy, 8–10 July 2019; pp. 1–5. [Google Scholar]
- Cappelli, I.; Fort, A.; Lo Grasso, A.; Panzardi, E.; Mugnaini, M.; Vignoli, V. RH Sensing by Means of TiO2 Nanoparticles: A Comparison among Different Sensing Techniques Based on Modeling and Chemical/Physical Interpretation. Chemosensors 2020, 8, 89. [Google Scholar] [CrossRef]
- Farzaneh, A.; Mohammadzadeh, A.; Esrafili, M.D.; Mermer, O. Experimental and theoretical study of TiO2 based nanostructured semiconducting humidity sensor. Ceram. Int. 2019, 45, 8362–8369. [Google Scholar] [CrossRef]
- Mele, G.; Del Sole, R.; Lü, X. Applications of TiO2 in sensor devices. Titanium Dioxide (TiO2) and Its Applications. In Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 527–581. [Google Scholar]
- Feng, H.; Li, C.; Li, T.; Diao, F.; Xin, T.; Liu, B.; Wang, Y. Three-dimensional hierarchical SnO2 dodecahedral nanocrystals with enhanced humidity sensing properties. Sens. Actuators B Chem. 2017, 243, 704–714. [Google Scholar] [CrossRef]
- Sikarwar, S.; Yadav, B.C.; Dzhardimalieva, G.I.; Golubeva, N.D.; Srivastava, P. Synthesis and characterization of nanostructured MnO 2–CoO and its relevance as an opto-electronic humidity sensing device. RSC Adv. 2018, 8, 20534–20542. [Google Scholar] [CrossRef] [Green Version]
- Paramasivam, I.; Macak, J.M.; Schmuki, P. Photocatalytic activity of TiO2 nanotube layers loaded with Ag and Au nanoparticles. Electrochem. Commun. 2018, 10, 71–75. [Google Scholar] [CrossRef]
- Rodriíguez, J.; Evans, J.; Graciani, J.; Park, J.B.; Liu, P.; Hrbek, J.; Sanz, J.F. High Water−Gas Shift Activity in TiO2(110) Supported Cu and Au Nanoparticles: Role of the Oxide and Metal Particle Size. J. Phys. Chem. C 2009, 113, 7364–7370. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liu, S.L. Synthesis and enhanced humidity detection response of nanoscale Au-particle-decorated ZnS spheres. Nanoscale Res. Lett. 2014, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mintcheva, N.; Srinivasan, P.; Rayappan, J.B.B.; Kuchmizhak, A.A.; Gurbatov, S.; Kulinich, S.A. Room-temperature gas sensing of laser-modified anatase TiO2 decorated with Au nanoparticles. Appl. Surf. Sci. 2020, 507, 145169. [Google Scholar] [CrossRef]
- Buso, D.; Post, M.; Cantalini, C.; Mulvaney, P.; Martucci, A. Gold nanoparticle-doped TiO2 semiconductor thin films: Gas sensing properties. Adv. Funct. Mater. 2008, 18, 3843–3849. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.; Sardroodi, J.J. A novel nitrogen dioxide gas sensor based on TiO2-supported Au nanoparticles: A van der Waals corrected DFT study. J. Nanostructure Chem. 2017, 7, 121–132. [Google Scholar] [CrossRef]
- Chomkitichai, W.; Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Sriwichai, S.; Phanichphant, S. H2 Sensor Based on Au/TiO2 Nanoparticles by Flame-Made. Eng. J. 2012, 16, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, I.; Fort, A.; Mugnaini, M.; Panzardi, E.; Pozzebon, A.; Tani, M.; Vignoli, V. Battery-Less HF RFID Sensor Tag for Soil Moisture Measurements. IEEE Trans. Instrum. Meas. 2020, 70, 1–13. [Google Scholar] [CrossRef]
- Fort, A.; Panzardi, E.; Vignoli, V.; Landi, E.; Trigona, C.; Mugnaini, M. Performance Analysis of a Humidity Sensor Based on a Microresonator Functionalized with TiO2 Nanoparticles. IEEE Trans. Instrum. Meas. 2020, 69, 7999–8008. [Google Scholar]
- Chu, Y.L.; Young, S.J.; Ding, R.J.; Chu, T.T.; Lu, T.S.; Ji, L.W. Improving ZnO nanorod humidity sensors with Pt nanoparticle adsorption. ECS J. Solid State Sci. Technol. 2021, 10, 037003. [Google Scholar] [CrossRef]
- Fort, A.; Panzardi, E.; Vignoli, V.; Hjiri, M.; Aida, M.S.; Mugnaini, M.; Addabbo, T. Co3O4/Al-ZnO nano-composites: Gas sensing properties. Sensors 2019, 19, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addabbo, T.; Fort, A.; Mugnaini, M.; Vignoli, V.; Baldi, A.; Bruzzi, M. Quartz-crystal microbalance gas sensors based on TiO2 nanoparticles. IEEE Trans. Instrum. Meas. 2018, 67, 722–730. [Google Scholar] [CrossRef]
- Gao, J.; Meng, Y.; Benton, A.; He, J.; Jacobsohn, L.G.; Tong, J.; Brinkman, K.S. Insights into the Proton Transport Mechanism in TiO2 Simple Oxides by In Situ Raman Spectroscopy. ACS Appl. Mater. Interfaces 2020, 12, 38012–38018. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Do, D.D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface Sci. 2011, 169, 40–58. [Google Scholar] [CrossRef]
- Rouquerol, J.; Sing, K.S.W.; Maurin, G.; Llewellyn, P. Introduction. In Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Oxford, UK, 2014; pp. 1–24. [Google Scholar]
- Morishige, K.; Nakamura, Y. Nature of adsorption and desorption branches in cylindrical pores. Langmuir 2004, 20, 4503–4506. [Google Scholar] [CrossRef]
- Kano, S.; Mekaru, H. Nonporous Inorganic Nanoparticle-Based Humidity Sensor: Evaluation of Humidity Hysteresis and Response Time. Sensors 2020, 20, 3858. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, I.; Takahashi, A. H2O Dissociation at the Perimeter Interface between Gold Nanoparticles and TiO2 Is Crucial for Oxidation of CO. ACS Catal. 2020, 10, 2517–2521. [Google Scholar] [CrossRef]
- Koga, H.; Tada, K.; Okumura, M. Density Functional Theory Study of Active Oxygen at the Perimeter of Au/TiO2 Catalysts. J. Phys. Chem. C 2015, 119, 25907–25916. [Google Scholar] [CrossRef]
- Uner, D.; Tapan, N.A.; Özen, I.; Üner, M. Oxygen adsorption on Pt/TiO2 catalysts. Appl. Catal. A Gen. 2003, 251, 225–234. [Google Scholar] [CrossRef]
- Li, Z.; Haidry, A.A.; Gao, B.; Wang, T.; Yao, Z. The effect of Co-doping on the humidity sensing properties of ordered mesoporous TiO2. Appl. Surf. Sci. 2017, 412, 638–647. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, S.; Chen, C.; Zhang, J.; Liu, J.; Li, P. Effects on structure, surface oxygen defects and humidity performance of Au modified ZnO via hydrothermal method. Appl. Surf. Sci. 2019, 486, 482–489. [Google Scholar] [CrossRef]
- Maurya, D.K.; Sikarwar, S.; Chaudhary, P.; Angaiah, S.; Yadav, B.C. Synthesis and Characterization of Nanostructured Copper Zinc Tin Sulphide (CZTS) for Humidity Sensing Applications. IEEE Sens. J. 2019, 19, 2837–2846. [Google Scholar] [CrossRef]

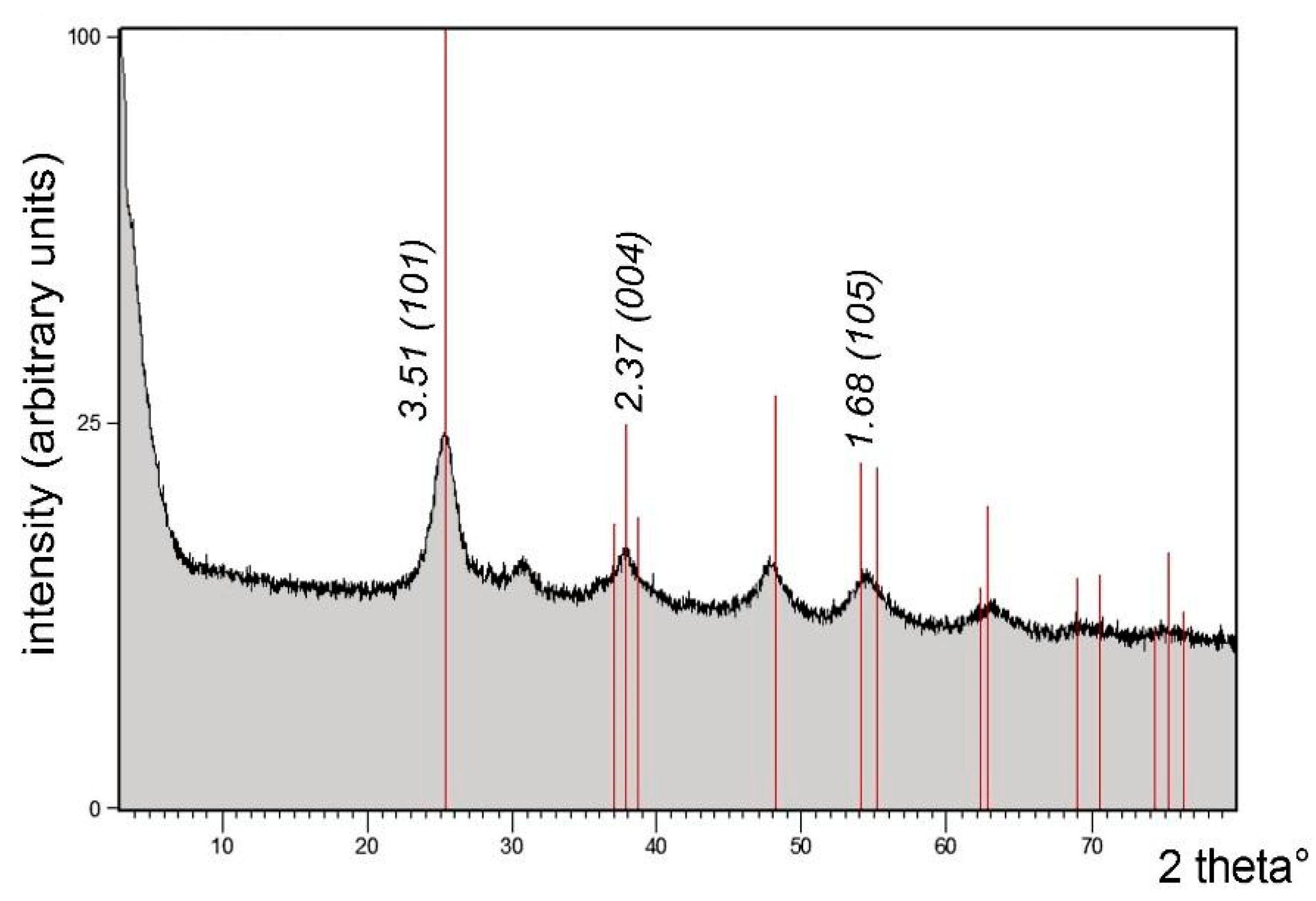

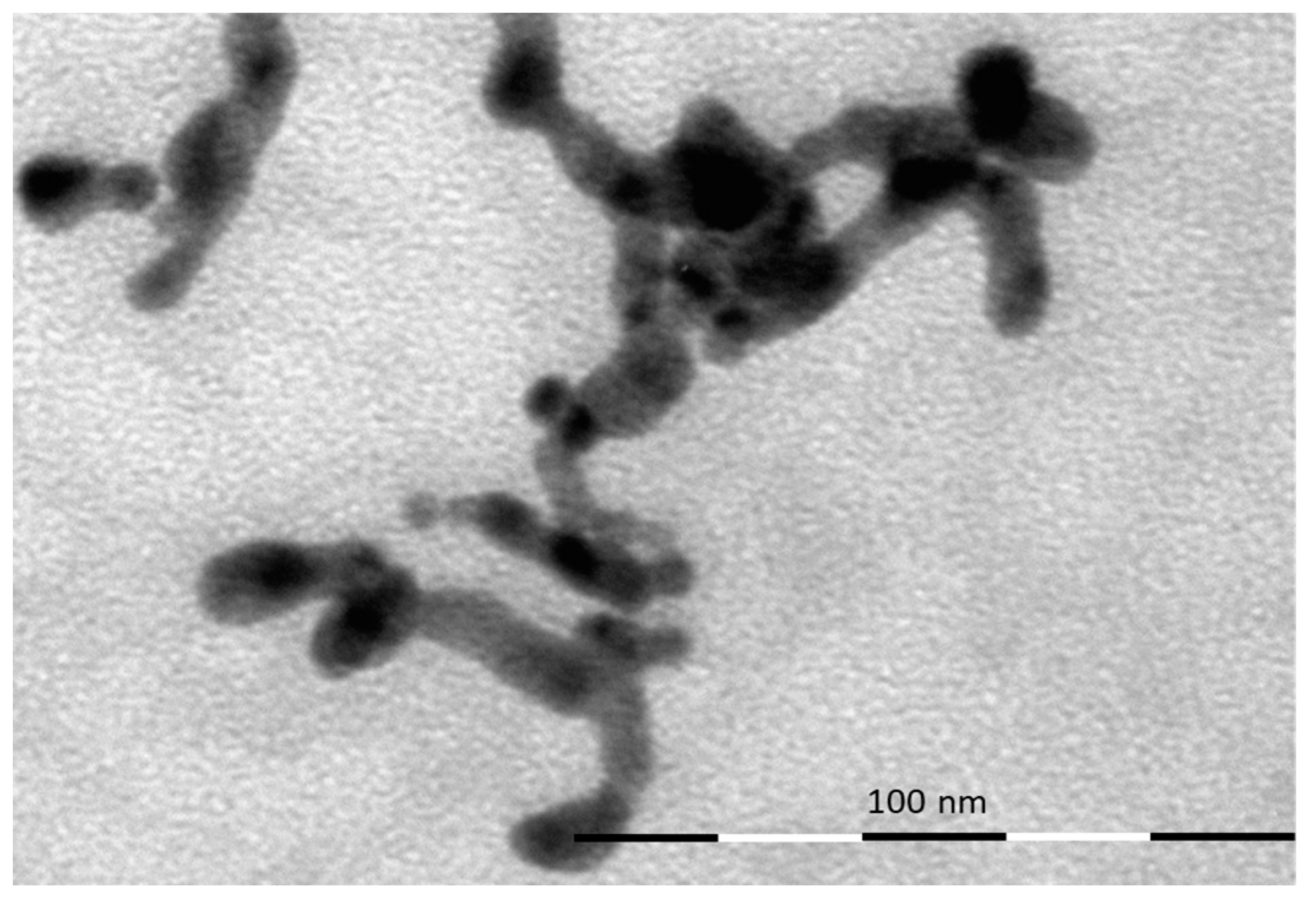
| Pos. [2Th.] | Height [cts] | FWHM [2Th.] | D-Spacing [Å] | Rel. Int. [%] |
|---|---|---|---|---|
| 25.3725 | 843.94 | 0.6022 | 3.51045 | 100.00 |
| 25.6883 | 716.44 | 0.2676 | 3.46800 | 84.89 |
| 28.3571 | 59.38 | 0.2007 | 3.14739 | 7.04 |
| 29.3509 | 64.42 | 0.1004 | 3.04306 | 7.63 |
| 32.4512 | 35.44 | 0.1004 | 2.75906 | 4.20 |
| 35.8230 | 26.97 | 0.5353 | 2.50672 | 3.20 |
| 38.0000 | 169.83 | 0.4015 | 2.36798 | 20.12 |
| 39.8060 | 101.83 | 0.0502 | 2.26461 | 12.07 |
| 42.2773 | 16.16 | 0.8029 | 2.13777 | 1.91 |
| 54.4178 | 117.46 | 0.9368 | 1.68608 | 13.92 |
| 62.9751 | 28.88 | 0.5353 | 1.47600 | 3.42 |
| 67.9895 | 18.53 | 0.3346 | 1.37885 | 2.20 |
| 68.9189 | 51.98 | 0.2448 | 1.36138 | 6.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addabbo, T.; Cappelli, I.; Fort, A.; Mugnaini, M.; Panzardi, E.; Vignoli, V.; Viti, C. The Effect of Au Nanoparticle Addition on Humidity Sensing with Ultra-Small TiO2 Nanoparticles. Chemosensors 2021, 9, 170. https://doi.org/10.3390/chemosensors9070170
Addabbo T, Cappelli I, Fort A, Mugnaini M, Panzardi E, Vignoli V, Viti C. The Effect of Au Nanoparticle Addition on Humidity Sensing with Ultra-Small TiO2 Nanoparticles. Chemosensors. 2021; 9(7):170. https://doi.org/10.3390/chemosensors9070170
Chicago/Turabian StyleAddabbo, Tommaso, Irene Cappelli, Ada Fort, Marco Mugnaini, Enza Panzardi, Valerio Vignoli, and Cecilia Viti. 2021. "The Effect of Au Nanoparticle Addition on Humidity Sensing with Ultra-Small TiO2 Nanoparticles" Chemosensors 9, no. 7: 170. https://doi.org/10.3390/chemosensors9070170
APA StyleAddabbo, T., Cappelli, I., Fort, A., Mugnaini, M., Panzardi, E., Vignoli, V., & Viti, C. (2021). The Effect of Au Nanoparticle Addition on Humidity Sensing with Ultra-Small TiO2 Nanoparticles. Chemosensors, 9(7), 170. https://doi.org/10.3390/chemosensors9070170









