Ultrathin Leaf-Shaped CuO Nanosheets Based Sensor Device for Enhanced Hydrogen Sulfide Gas Sensing Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Leaf-Shaped CuO Nanosheets
2.2. Characterization of Leaf-Shaped CuO Nanosheets
2.3. Fabrication of H2S Gas Sensor Based on Leaf-Shaped CuO Nanosheets
3. Results and Discussion
3.1. Characterizations
3.2. H2S Gas Sensing Applications of CuO Nanosheets
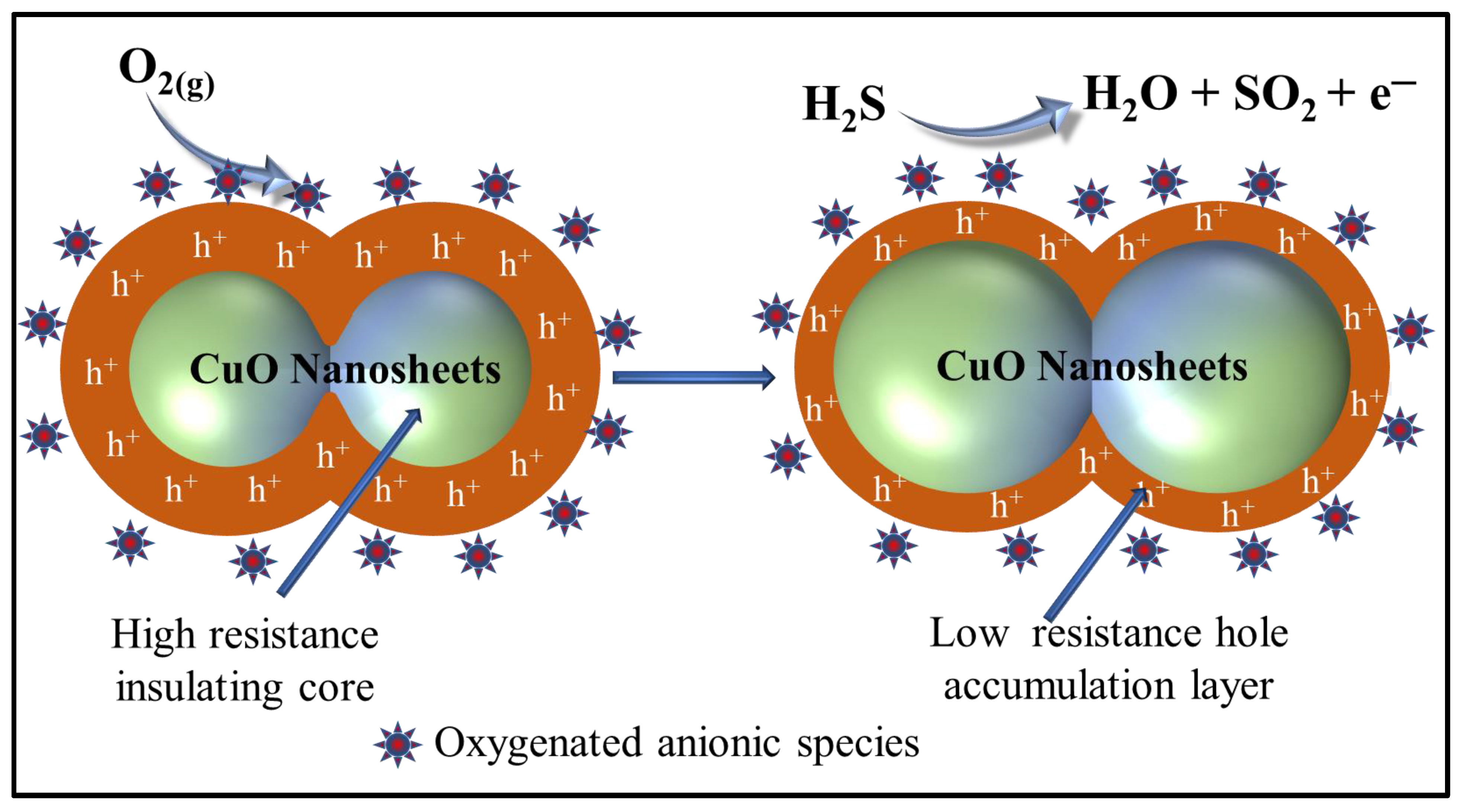
3.3. Proposed Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umar, A.; Alduraibi, M.; Al-Dossary, O. NOx Gas Sensing Properties of Fe-Doped ZnO Nanoparticles. Sci. Adv. Mater. 2020, 12, 908–914. [Google Scholar] [CrossRef]
- Sampaolo, A.; Yu, C.; Wei, T.; Zifarelli, A.; Giglio, M.; Patimisco, P.; Zhu, H.; Zhu, H.; He, L.; Wu, H.; et al. H2S quartz-enhanced photoacoustic spectroscopy sensor employing a liquid-nitrogen-cooled THz quantum cascade laser operating in pulsed mode. Photoacoustics 2021, 21, 100219. [Google Scholar] [CrossRef]
- Wu, C.; Ye, G.; Qi, L.; Wang, Y.; Yuan, C.; Zhang, L. Novel ZnO Sensor and Gas Detection Performance in Tunnel Construction. J. Nanoelectron. Optoelectron. 2020, 15, 1114–1119. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, L. A Novel H 2 S Gas Sensor Applied to the Construction Workers During the Construction Process. Sci. Adv. Mater. 2018, 11, 143–146. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Zhang, L.; Chen, J.; Zhou, Q.; Zhang, H.; Nie, L.; Dong, Z.; Zhang, Z.; Wang, Z.; et al. Construction of a low-temperature, highly sensitive H2S sensor based on surfaces and interfaces reaction triggered by Au-doped hierarchical structured composites. Chem. Phys. Lett. 2021, 763, 138188. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, C.; Qi, L.; Wang, Y.; Guo, Y. A Novel Hydrogen Sulfide Gas Sensor Apply in Tunnel Construction. Sci. Adv. Mater. 2018, 11, 112–115. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Wu, G.; Chen, J.-Y.; Li, W.-C. A Novel Gas Sensor Detection of Hydrogen Sulfide in Tunnel Construction. Sci. Adv. Mater. 2018, 11, 147–151. [Google Scholar] [CrossRef]
- Pravarthana, D.; Tyagi, A.; Jagadale, T.C.; Prellier, W.; Aswal, D.K. Highly sensitive and selective H2S gas sensor based on TiO2 thin films. Appl. Surf. Sci. 2021, 549, 149281. [Google Scholar] [CrossRef]
- Cho, M.; Eom, T.; Nundy, S.; Park, J.-S.; Lee, H.-J. Conductometric nitrogen dioxide gas sensors based on sol-gel-prepared hafnium-added indium zinc oxide (Hf-IZO). Sens. Actuators B Chem. 2021, 344, 130198. [Google Scholar] [CrossRef]
- Pi, M.; Zheng, C.; Bi, R.; Zhao, H.; Liang, L.; Zhang, Y.; Wang, Y.; Tittel, F.K. Design of a mid-infrared suspended chalcogenide/silica-on-silicon slot-waveguide spectroscopic gas sensor with enhanced light-gas interaction effect. Sens. Actuators B Chem. 2019, 297, 126732. [Google Scholar] [CrossRef]
- Bao, Y.; Xu, P.; Cai, S.; Yu, H.; Li, X. Detection of volatile-organic-compounds (VOCs) in solution using cantilever-based gas sensors. Talanta 2018, 182, 148–155. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, M.S.; Jung, H.; Choe, Y.-S.; Kim, W.; Song, Y.G.; Kang, C.-Y.; Lee, H.-S.; Lee, W. Selective C2H2 detection with high sensitivity using SnO2 nanorod based gas sensors integrated with a gas chromatography. Sens. Actuators B Chem. 2020, 307, 127598. [Google Scholar] [CrossRef]
- Maurya, J.B.; Prajapati, Y.K.; Raikwar, S.; Saini, J.P. A silicon-black phosphorous based surface plasmon resonance sensor for the detection of NO2 gas. Optik 2018, 160, 428–433. [Google Scholar] [CrossRef]
- Grabka, M.; Jasek, K.; Pasternak, M. Application of polymethyl [4-(2,3-difluoro-4-hydroxyphenoxy) butyl] siloxane in surface acoustic wave gas sensors for dimethyl methylphosphonate detection. Sens. Actuators B Chem. 2021, 329, 129216. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, Y.; Zhang, J.; Liu, B.; Ma, X.; Wang, Y. Highly sensitive gas sensing platforms based on field effect Transistor-A review. Anal. Chim. Acta 2021, 1172, 338575. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.-C.; Han, X.; Liu, K.-Y.; Xu, Y.; Lin, J. Rapid and Sensitive Acetylene Gas Sensor Based on TiO 2 Nanoparticles. Sci. Adv. Mater. 2019, 11, 1126–1132. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, X.; Zhang, L.; Zeng, W. High Performance Novel Gas Sensor Device for Site Environmental Protection Using Ti 0.5 Sn 0.5 O 2 Nanomaterials. J. Nanoelectron. Optoelectron. 2021, 15, 1423–1428. [Google Scholar] [CrossRef]
- Shaik, M.; Rao, V.K.; Gupta, M.; Murthy, K.S.R.C.; Jain, R. Chemiresistive gas sensor for the sensitive detection of nitrogen dioxide based on nitrogen doped graphene nanosheets. RSC Adv. 2016, 6, 1527–1534. [Google Scholar] [CrossRef]
- Qiao, X.; Xu, Y.; Yang, K.; Ma, J.; Li, C.; Wang, H.; Jia, L. Mo doped BiVO4 gas sensor with high sensitivity and selectivity towards H2S. Chem. Eng. J. 2020, 395, 125144. [Google Scholar] [CrossRef]
- Li, S.; Xie, L.; He, M.; Hu, X.; Luo, G.; Chen, C.; Zhu, Z. Metal-Organic frameworks-derived bamboo-like CuO/In2O3 Heterostructure for high-performance H2S gas sensor with Low operating temperature. Sens. Actuators B Chem. 2020, 310, 127828. [Google Scholar] [CrossRef]
- Hosseini, Z.S.; Iraji zad, A.; Mortezaali, A. Room temperature H2S gas sensor based on rather aligned ZnO nanorods with flower-like structures. Sens. Actuators B Chem. 2015, 207, 865–871. [Google Scholar] [CrossRef]
- Shewale, P.S.; Yun, K.-S. Synthesis and characterization of Cu-doped ZnO/RGO nanocomposites for room-temperature H2S gas sensor. J. Alloy. Compd. 2020, 837, 155527. [Google Scholar] [CrossRef]
- Haija, M.A.; Abu-Hani, A.F.S.; Hamdan, N.; Stephen, S.; Ayesh, A.I. Characterization of H2S gas sensor based on CuFe2O4 nanoparticles. J. Alloy. Compd. 2017, 690, 461–468. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Sagboye, P.A.; Umenweke, G.; Ajala, O.J.; Omoarukhe, F.O.; Adeyanju, C.A.; Ogunniyi, S.; Adeniyi, A.G. CuO nanoparticles (CuO NPs) for water treatment: A review of recent advances. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100443. [Google Scholar] [CrossRef]
- Zhu, W.; An, X.; Huangfu, W.; Chi, J.; Xu, L. A Novel CuO flower like nanomaterials and its fault detection performance in power cable. J. Nanoelectron. Optoelectron. 2020, 15, 1146–1150. [Google Scholar]
- Yang, C.; Xiao, F.; Wang, J.; Su, X. 3D flower- and 2D sheet-like CuO nanostructures: Microwave-assisted synthesis and application in gas sensors. Sens. Actuators B Chem. 2015, 207, 177–185. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.; Wang, X.; Wang, Q.; Huang, B.; Li, Y.; Hua, X.; Liu, G.; Li, B.; Zhou, J.; et al. Binder-free CuO nanoneedle arrays based tube-type sensor for H2S gas sensing. Sens. Actuators B Chem. 2021, 326, 128993. [Google Scholar] [CrossRef]
- Yang, C.; Su, X.; Xiao, F.; Jian, J.; Wang, J. Gas sensing properties of CuO nanorods synthesized by a microwave-assisted hydrothermal method. Sens. Actuators B Chem. 2011, 158, 299–303. [Google Scholar] [CrossRef]
- Kim, J.H.; Katoch, A.; Choi, S.W.; Kim, S.S. Growth and sensing properties of networked p-CuO nanowires. Sens. Actuators B Chem. 2015, 212, 190–195. [Google Scholar] [CrossRef]
- Umar, A.; Alshahrani, A.A.; Algarni, H.; Kumar, R. CuO nanosheets as potential scaffolds for gas sensing applications. Sens. Actuators B Chem. 2017, 250, 24–31. [Google Scholar] [CrossRef]
- Chaloeipote, G.; Prathumwan, R.; Subannajui, K.; Wisitsoraat, A.; Wongchoosuk, C. 3D printed CuO semiconducting gas sensor for ammonia detection at room temperature. Mater. Sci. Semicond. Process. 2021, 123, 105546. [Google Scholar] [CrossRef]
- Rezaei, K.; Nasirian, S. A low-level acetone gas sensor based on n-type ZnO/p-type CuO composite nanostructure for the diagnosis of diabetes in dynamic situations. J. Mater. Sci. Mater. Electron. 2021, 32, 5199–5214. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Yuan, Z.; Sun, Y.; Chang, F.; Deng, H.; Xie, L.; Li, H. A highly sensitive gas sensor based on CuO nanoparticles synthetized via a sol–gel method. RSC Adv. 2016, 6, 79343–79349. [Google Scholar] [CrossRef]
- Singh, S.; Verma, N.; Singh, A.; Yadav, B.C. Synthesis and characterization of CuO–SnO2 nanocomposite and its application as liquefied petroleum gas sensor. Mater. Sci. Semicond. Process. 2014, 18, 88–96. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Z.; Yan, B.; Zheng, Z.; Bao, Q.L.; Wu, T.; Li, C.M.; Shen, Z.X.; Zhang, J.X.; Gong, H.; et al. Multifunctional CuO nanowire devices: P-type field effect transistors and CO gas sensors. Nanotechnology 2009, 20, 085203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, B.C.; Lee, C.W.; Lee, Y.-S.; Im, J.S. Modification of textural properties of CuO-supported activated carbon fibers for SO2 adsorption based on electrical investigation. Mater. Chem. Phys. 2017, 200, 361–367. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Guo, D.; Guo, J.; Su, Y. Room-temperature synthesis of CuO/reduced graphene oxide nanohybrids for high-performance NO2 gas sensor. Sens. Actuators B Chem. 2018, 271, 306–310. [Google Scholar] [CrossRef]
- Cunniff, C.Z.; Specht, S.E.; Love, A.M.; Uchupalanun, P.; Venegas, J.M.; Kruszynski, C.E.; Hermans, I. Investigation of supported metal oxide species with shell-isolated nanoparticle-enhanced Raman spectroscopy. J. Phys. Chem. C 2019, 123, 25220–25227. [Google Scholar] [CrossRef]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Krüger, P. Vibrational Properties of CuO and Cu4O3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Ammar, H.Y.; Nakate, U.T.; Albargi, H.B.; Hahn, Y.B. Urchin like CuO hollow microspheres for selective high response ethanol sensor application: Experimental and theoretical studies. Ceram. Int. 2021, 47, 12084–12095. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Nakate, U.T.; Albargi, H.; Alsaiari, M.A.; Ahmed, F.; Alharthi, F.A.; Ali Alghamdi, A.; Al-Zaqri, N. Fabrication and characterization of CuO nanoplates based sensor device for ethanol gas sensing application. Chem. Phys. Lett. 2021, 763, 138204. [Google Scholar] [CrossRef]
- Rashad, M.; Rüsing, M.; Berth, G.; Lischka, K.; Pawlis, A. CuO and Co3O4 nanoparticles: Synthesis, characterizations, and raman spectroscopy. J. Nanomater. 2013, 2013, 714853. [Google Scholar] [CrossRef] [Green Version]
- Veisi, H.; Karmakar, B.; Tamoradi, T.; Hemmati, S.; Hekmati, M.; Hamelian, M. Biosynthesis of CuO nanoparticles using aqueous extract of herbal tea (Stachys Lavandulifolia) flowers and evaluation of its catalytic activity. Sci. Rep. 2021, 11, 1983. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, M.S.; Kulkarni, S.; Raikar, P.; Barretto, D.A.; Vootla, S.K.; Raikar, U.S. Green biosynthesis of CuO & Ag–CuO nanoparticles from Malus domestica leaf extract and evaluation of antibacterial, antioxidant and DNA cleavage activities. New J. Chem. 2018, 42, 204–213. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chatterjee, A.; Liu, S.B.; Wu, S.Y.; Cheng, C.-L. Photoluminescence properties of a single tapered CuO nanowire. Appl. Surf. Sci. 2010, 256, 3688–3692. [Google Scholar] [CrossRef]
- Sagadevan, S.; Pal, K.; Chowdhury, Z.Z. Fabrication of CuO nanoparticles for structural, optical and dielectric analysis using chemical precipitation method. J. Mater. Sci. Mater. Electron. 2017, 28, 12591–12597. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Cretu, V.; Wolff, N.; Duppel, V.; Kienle, L.; Adelung, R. Single and networked CuO nanowires for highly sensitive p-type semiconductor gas sensor applications. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 260–266. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, A.; Luo, Y.; Tian, Y.; Yang, J.; Qin, Y. CuO nanosheets for sensitive and selective determination of H2S with high recovery ability. J. Phys. Chem. C 2010, 114, 19214–19219. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. Room-temperature high performance H2S sensor based on porous CuO nanosheets prepared by hydrothermal method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Zheng, Y.; Lee, J.-H.; Kim, J.-Y.; Kim, H.W.; Kim, S.S. Enhancement of H2S sensing performance of p-CuO nanofibers by loading p-reduced graphene oxide nanosheets. Sens. Actuators B Chem. 2019, 281, 453–461. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Wang, N.; Yan, S.; Liu, W.; Fu, Y.Q.; Wang, Z. Hydrothermal synthesis of hierarchically flower-like CuO nanostructures with porous nanosheets for excellent H2S sensing. J. Alloy. Compd. 2017, 725, 1136–1143. [Google Scholar] [CrossRef]
- Kim, S.-J.; Na, C.W.; Hwang, I.-S.; Lee, J.-H. One-pot hydrothermal synthesis of CuO–ZnO composite hollow spheres for selective H2S detection. Sens. Actuators B Chem. 2012, 168, 83–89. [Google Scholar] [CrossRef]
- Sui, L.; Yu, T.; Zhao, D.; Cheng, X.; Zhang, X.; Wang, P.; Xu, Y.; Gao, S.; Zhao, H.; Gao, Y.; et al. In Situ deposited hierarchical CuO/NiO nanowall arrays film sensor with enhanced gas sensing performance to H2S. J. Hazard. Mater. 2020, 385, 121570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, J.; Cao, Y. Ultrasensitive H2S gas detection at room temperature based on copper oxide/molybdenum disulfide nanocomposite with synergistic effect. Sens. Actuators B Chem. 2019, 287, 346–355. [Google Scholar] [CrossRef]

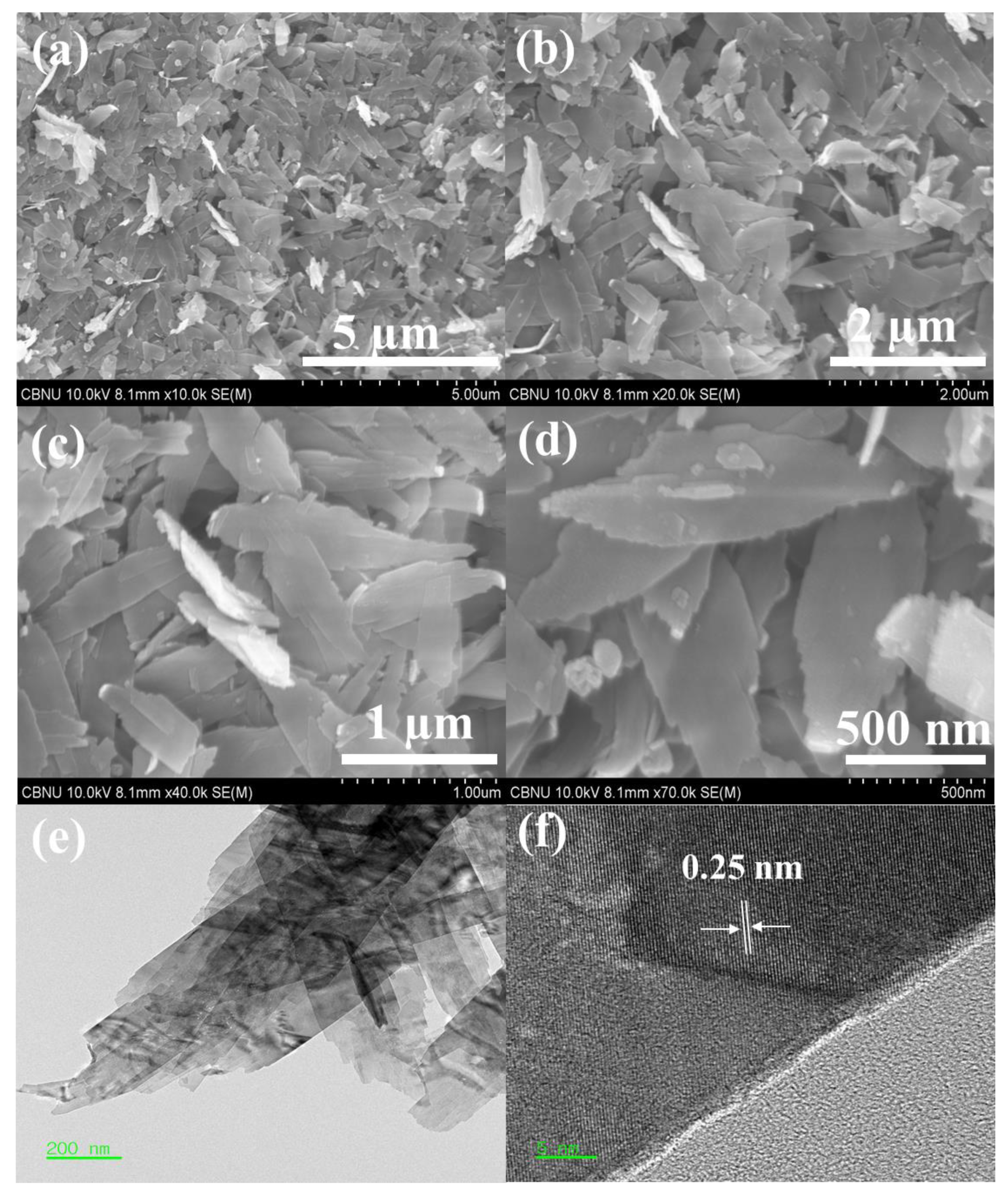
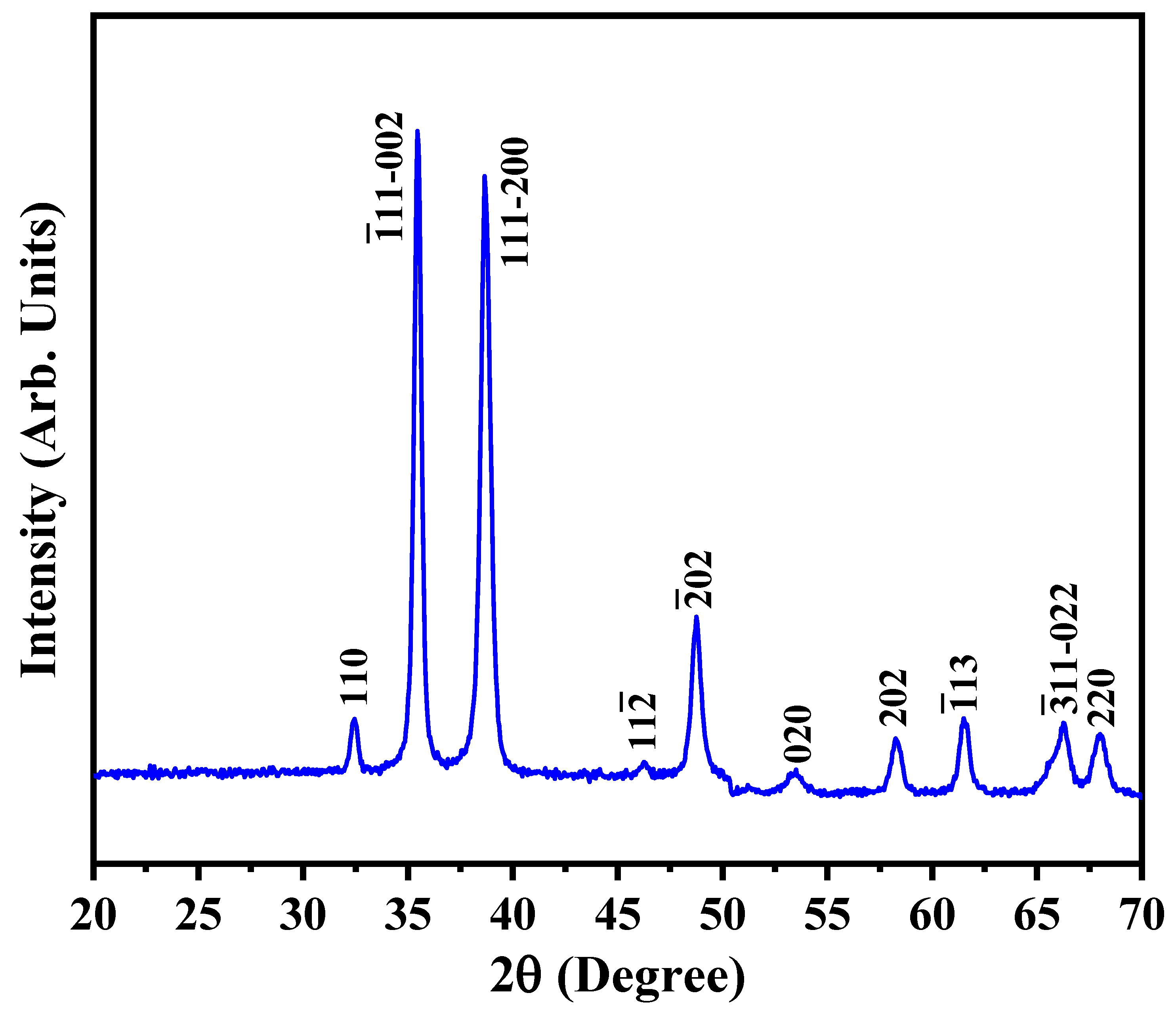
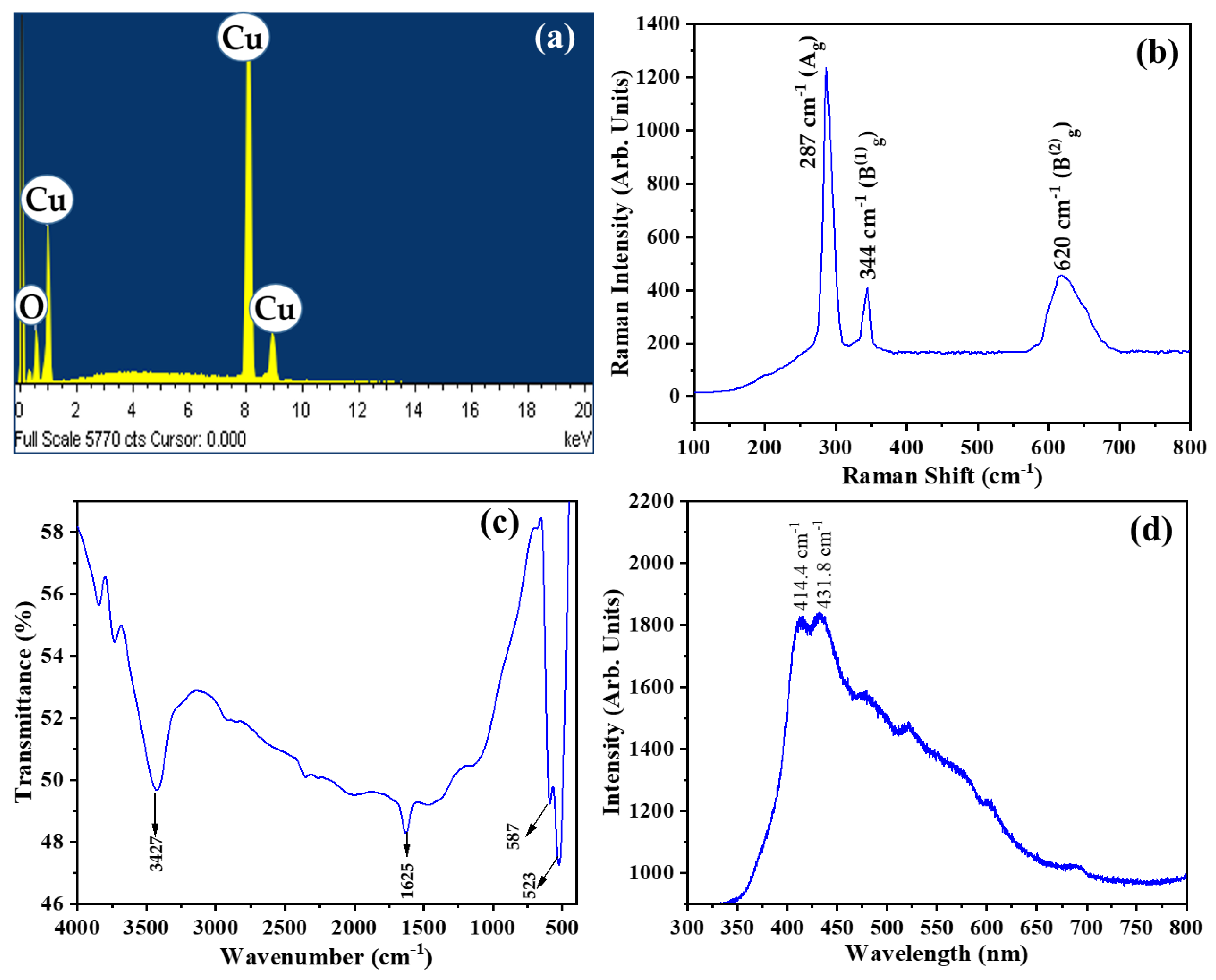
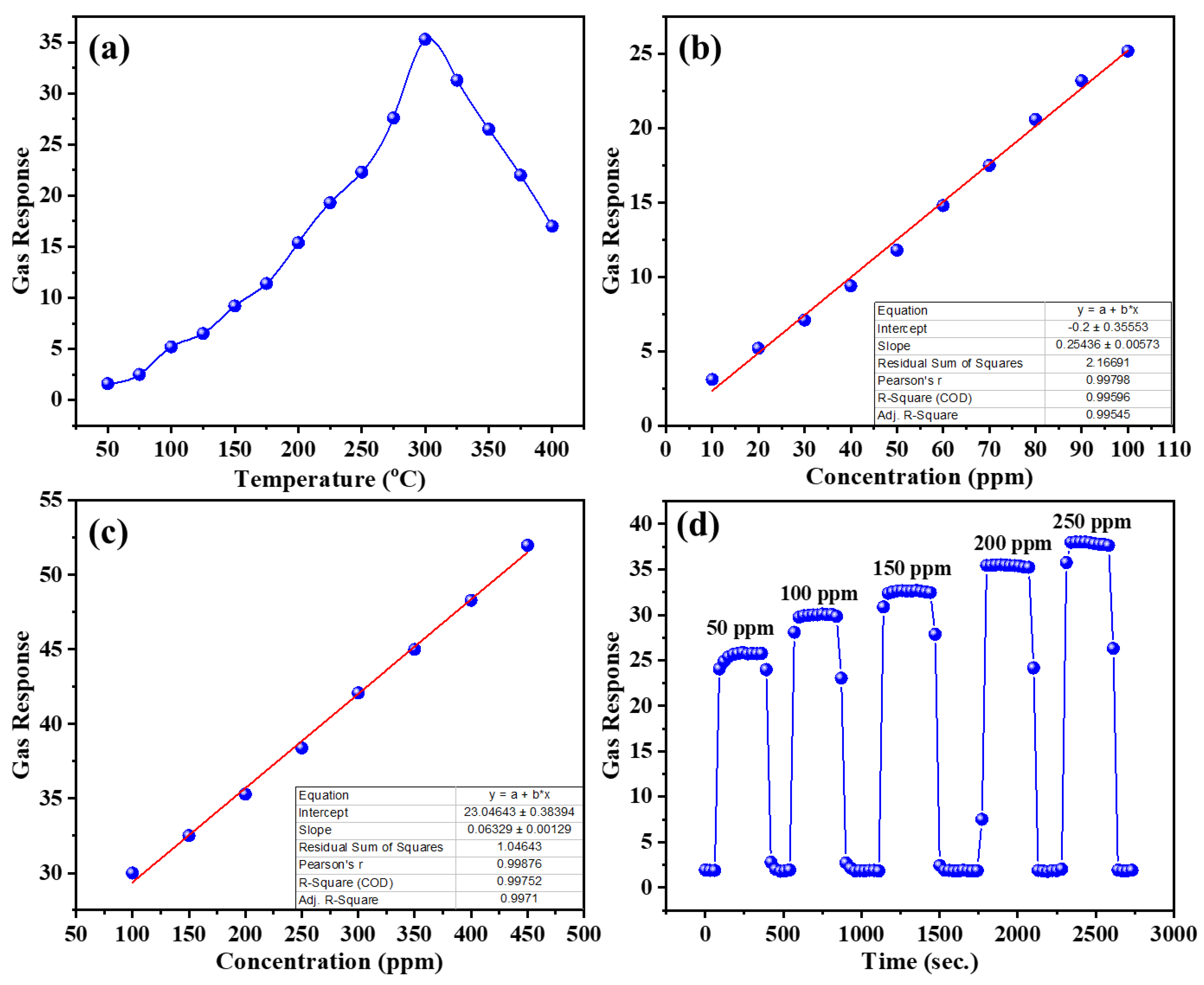
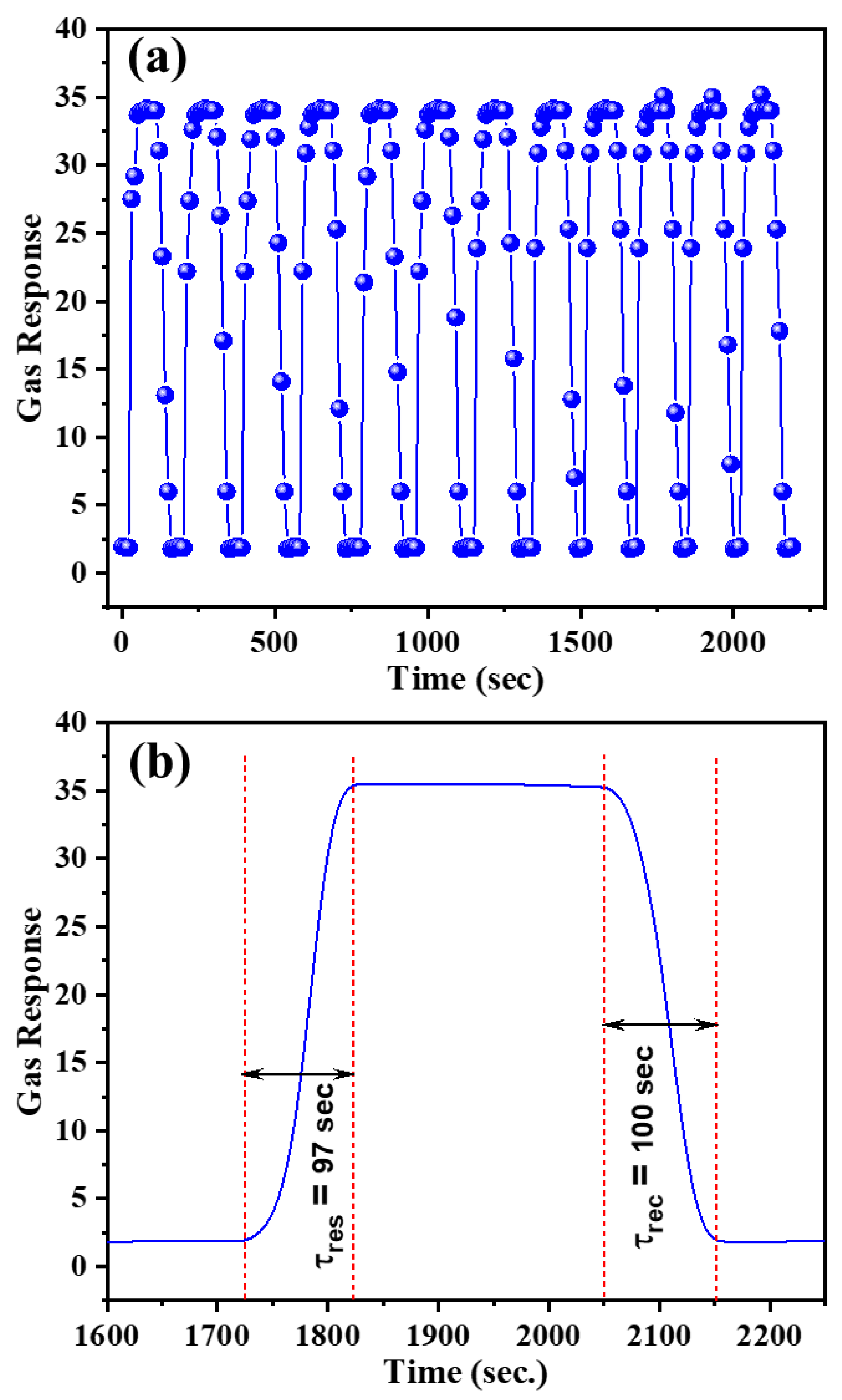

| Diffraction Planes (hkl) | Diffraction Angles (°) | FWHM (β) | The Crystallite Size (nm) |
|---|---|---|---|
| (1 1 0) | 32.45 | 0.7597 | 10.78 |
| 35.45 | 0.4275 | 19.31 | |
| (111-200) | 38.65 | 0.5713 | 14.58 |
| () | 48.75 | 0.6051 | 14.26 |
| (202) | 58.25 | 0.7340 | 12.26 |
| 61.50 | 0.6320 | 14.48 |
| Sensor Material | Conc. | Gas Response | Response Time (s) | Recovery Time (s) | T (°C) | Ref. |
|---|---|---|---|---|---|---|
| CuO nanosheets | 2 ppb | 320 * | 4 | 9 | 240 | [47] |
| Porous CuO nanosheet | 10 ppb | 1.25 # | 234 | 76 | RT | [48] |
| rGO/CuO nanofibers | 10 ppm | 11.7 $ | - | - | 300 | [49] |
| Hierarchically flower-like CuO | 1 ppm | 2.1 $ | 240 | 1341 | RT | [50] |
| CuO–ZnO hollow spheres | 5 ppm | 13.3 # | 270 | 720 | 336 | [51] |
| Hierarchical CuO/NiO nanowall arrays | 5 ppm | 36.9 $ | - | 13 | 133 | [52] |
| CuO/MoS2 film | 30 ppm | 61 * | 26 | 11 | RT | [53] |
| CuO nanosheet monolayer | 200 | 350 * | 20 | 120 | 250 | [54] |
| Leaf-shaped CuO nanosheets | 200 | 35.3 $ | 97 | 100 | 300 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, A.; Algadi, H.; Kumar, R.; Akhtar, M.S.; Ibrahim, A.A.; Albargi, H.; Alhamami, M.A.M.; Alsuwian, T.; Zeng, W. Ultrathin Leaf-Shaped CuO Nanosheets Based Sensor Device for Enhanced Hydrogen Sulfide Gas Sensing Application. Chemosensors 2021, 9, 221. https://doi.org/10.3390/chemosensors9080221
Umar A, Algadi H, Kumar R, Akhtar MS, Ibrahim AA, Albargi H, Alhamami MAM, Alsuwian T, Zeng W. Ultrathin Leaf-Shaped CuO Nanosheets Based Sensor Device for Enhanced Hydrogen Sulfide Gas Sensing Application. Chemosensors. 2021; 9(8):221. https://doi.org/10.3390/chemosensors9080221
Chicago/Turabian StyleUmar, Ahmad, Hassan Algadi, Rajesh Kumar, Mohammad Shaheer Akhtar, Ahmed A. Ibrahim, Hasan Albargi, Mohsen A. M. Alhamami, Turki Alsuwian, and Wen Zeng. 2021. "Ultrathin Leaf-Shaped CuO Nanosheets Based Sensor Device for Enhanced Hydrogen Sulfide Gas Sensing Application" Chemosensors 9, no. 8: 221. https://doi.org/10.3390/chemosensors9080221
APA StyleUmar, A., Algadi, H., Kumar, R., Akhtar, M. S., Ibrahim, A. A., Albargi, H., Alhamami, M. A. M., Alsuwian, T., & Zeng, W. (2021). Ultrathin Leaf-Shaped CuO Nanosheets Based Sensor Device for Enhanced Hydrogen Sulfide Gas Sensing Application. Chemosensors, 9(8), 221. https://doi.org/10.3390/chemosensors9080221









