Impedimetric Detection of Albumin-Bound Fatty Acids Using Graphene Oxide Electrode
Abstract
:1. Introduction
2. Materials and Methods
3. Results
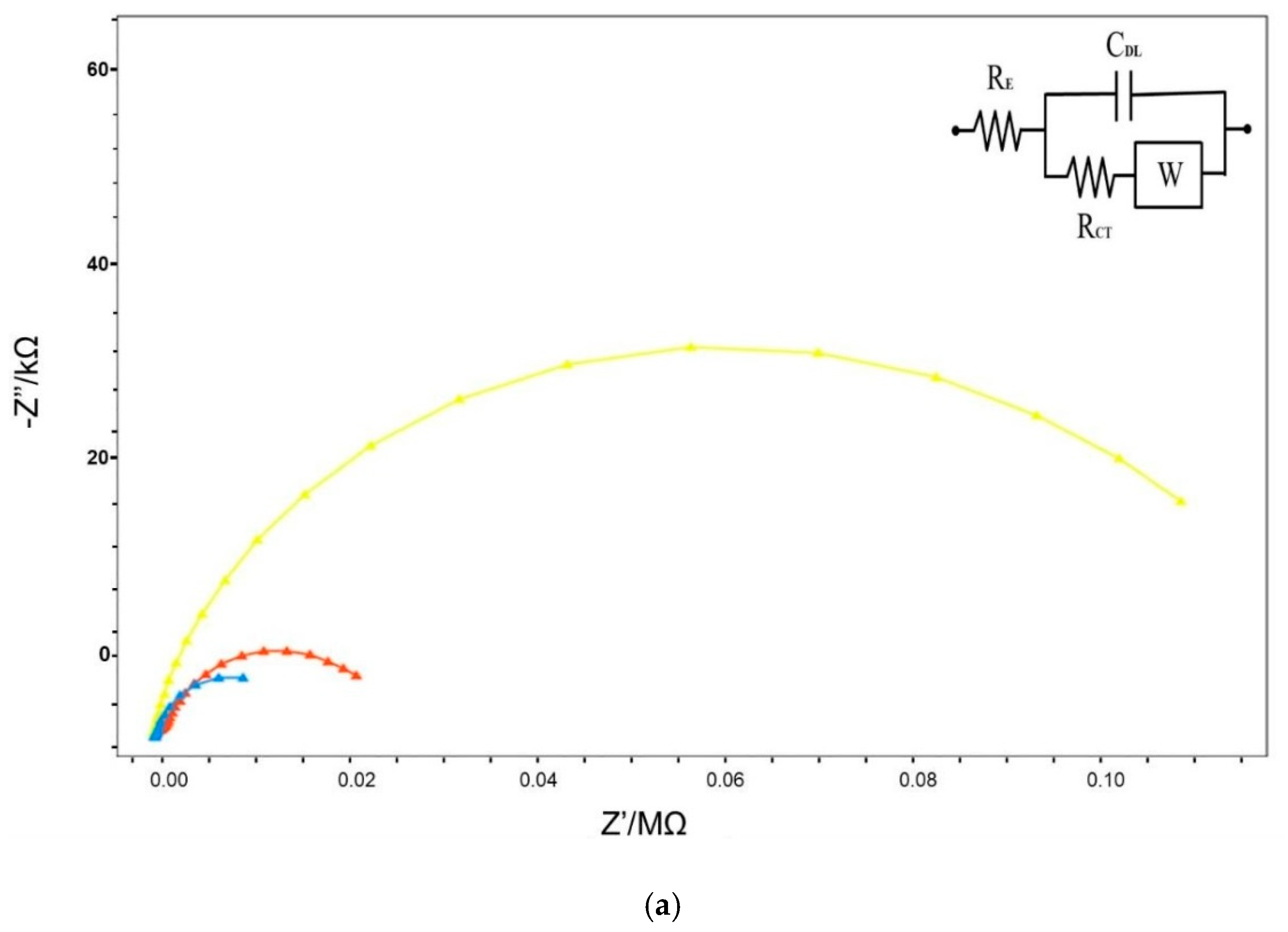
3.1. Frequency-Ranged Faradaic EIS in Characterization
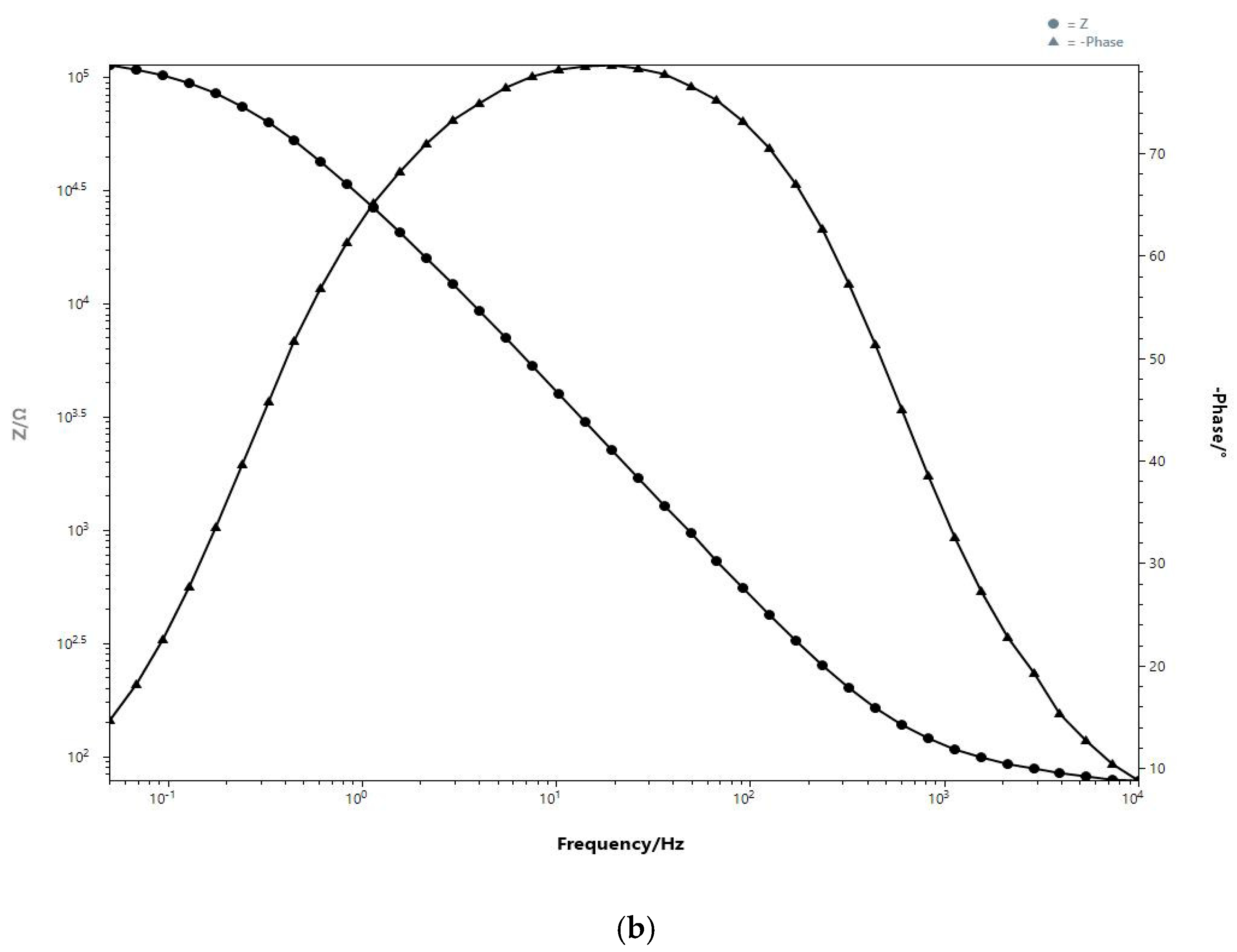
3.2. Single-Frequency Non-Faradaic Impedance (Chronoimpedance)
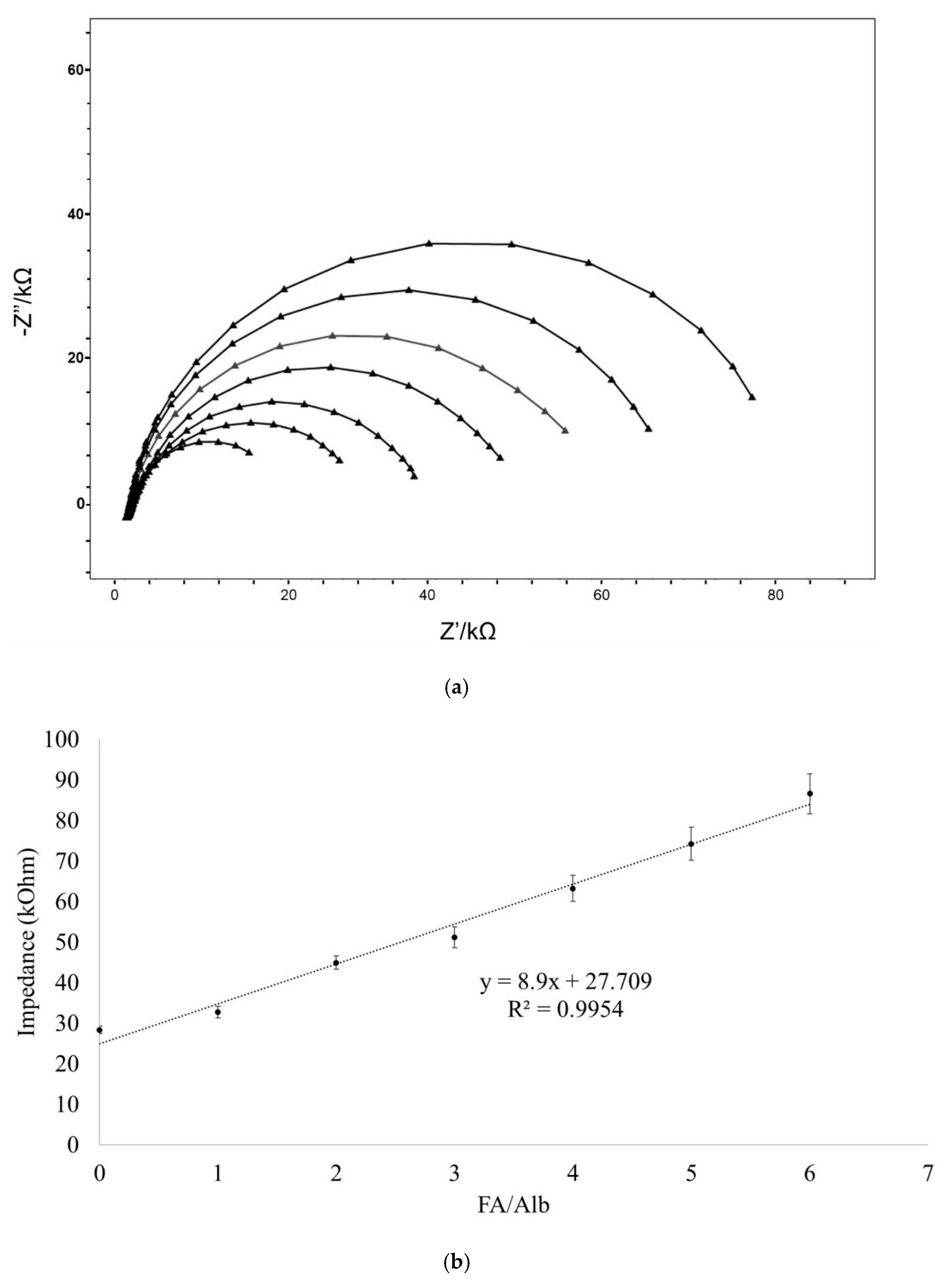
3.3. Frequency-Ranged Faradaic EIS at Different FA/Alb Ratios
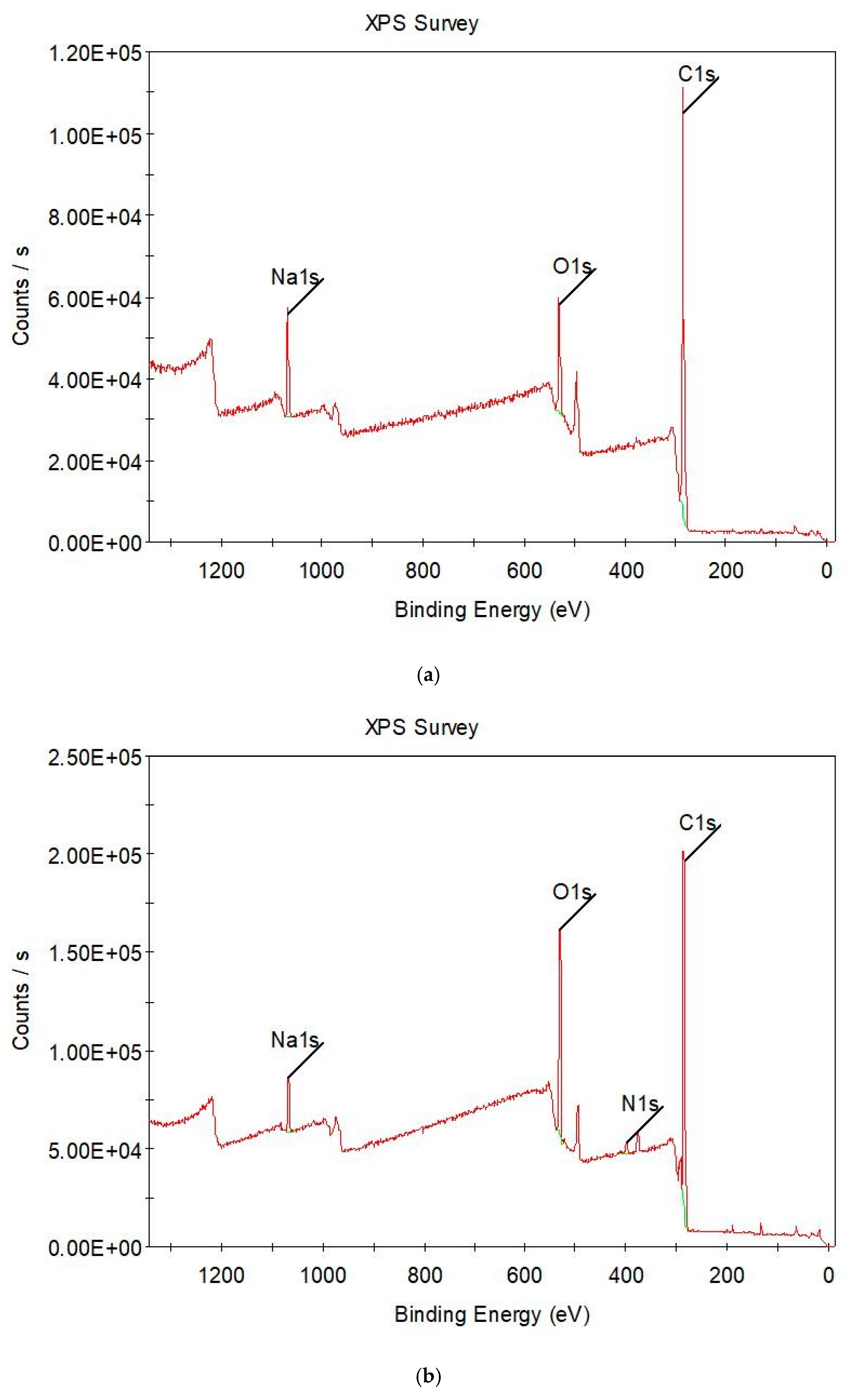
3.4. Surface Characterizations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemi, O.; Bauersachs, J.; Bhatt, D.L. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Malik, A.; Khurshid, R.; Faryal, U.; Qazi, S. The diagnostic value of biochemical cardiac markers in acute myocardial infarction. IntechOpen 2019. [Google Scholar] [CrossRef] [Green Version]
- Huber, A.H.; Kampf, J.P.; Kwan, T.; Zhu, B.; Adams, J.; Kleinfeld, A.M. Usefulness of serum unbound free fatty acid levels to predict death early in patients with st-segment elevation myocardial infarction (from the Thrombolysis in Myocardial Infarction [TIMI] II trial). Am. J. Cardiol. 2014, 113, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Spector, A.A. Fatty acid binding to plasma albumin. J. Lipid Res. 1975, 16, 165–179. [Google Scholar] [CrossRef]
- Richieri, G.V.; Kleinfeld, A.M. Unbound free fatty acid levels in human serum. J. Lipid Res. 1995, 36, 229–240. [Google Scholar] [CrossRef]
- Huber, A.H.; Kleinfeld, A.M. Unbound free fatty acid profiles in human plasma & the unexpected absence of unbound palmitoleate. J. Lipid Res. 2017, 58, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.F. Removal of fatty acids from serum albumin by charcoal treatment. J. Biol. Chem. 1967, 242, 173–181. [Google Scholar] [CrossRef]
- Nakano, N.I.; Shimamori, Y.; Nakano, M. Activated carbon beads for the removal of highly albumin-bound species. Anal. Biochem. 1983, 129, 64–71. [Google Scholar] [CrossRef]
- Hibino, M.; Sumi, A.; Hatta, I. Scanning tunneling microscopy study on dynamic structural formation in mixed fatty-acid monolayers at liquid/graphite interface. Thin Solid Films. 1996, 281–282, 594–597. [Google Scholar] [CrossRef]
- Wu, S.H.; Pendleton, P. Adsorption of anionic surfactant by activated carbon: Effect of surface chemistry, ionic strength, and hydrophobicity. J. Colloid Interface Sci. 2001, 243, 306–315. [Google Scholar] [CrossRef]
- Bickerstaffe, A.K.; Cheah, N.P.; Clarke, A.M.; Parker, J.E.; Perdigon, A.; Messe, L.; Inaba, A. The crystalline structures of carboxylic acid monolayers adsorbed on graphite. J. Phys. Chem. B. 2006, 110, 5570–5575. [Google Scholar] [CrossRef]
- Medina, S.; Benítez, J.J.; Castro, M.A.; Cerrillos, C.; Millán, C.; Alba, M.D. Monolayer arrangement of fatty hydroxystearic acids on graphite: Influence of hydroxyl groups. Thin Solid Films. 2013, 539, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Ertugrul Uygun, H.D.; Uygun, Z.O. Impedimetric biosensors for label-free and enzymless detection, State of the Art in Biosensors-General Aspects, Toonika Rinken. IntechOpen 2013. [CrossRef] [Green Version]
- Uygun, Z.O.; Yeniay, L.; Girgin Sagin, F. CRISPR-dCas9 powered impedimetric biosensor for label-free detection of circulating tumor DNAs. Anal. Chim. Acta. 2020, 1121, 35–41. [Google Scholar] [CrossRef]
- Uygun, Z.O.; Dilgin, Y. A novel impedimetric sensor based on molecularly imprinted polypyrrole modified pencil graphite electrode for trace level determination of chlorpyrifos. Sensors Actuators B Chem. 2013, 188, 78–84. [Google Scholar] [CrossRef]
- García-Argumánez, A.; Llorente, I.; Caballero-Calero, O.; González, Z.; Menéndez, R.; Escudero, M.L.; García-Alonso, M.C. Electrochemical reduction of graphene oxide on biomedical grade CoCr alloy. Appl. Surf. Sci. 2019, 465, 1028–1036. [Google Scholar] [CrossRef]
- Ray, S.; Shard, A.G. Quantitative analysis of adsorbed proteins by X-ray photoelectron spectroscopy. Anal. Chem. 2011, 83, 8659–8666. [Google Scholar] [CrossRef]
- Opie, L.H. Metabolism of free fatty acids, glucose and catecholamines in acute myocardial infarction. Relation to myocardial ischemia and infarct size. Am. J. Cardiol. 1975, 36, 938–953. [Google Scholar] [CrossRef]
- Kleinfeld, A.M.; Kleinfeld, K.J.; Adams, J.E. Serum levels of unbound free fatty acids reveal high sensitivity for early detection of acute myocardial infarction in patient samples from the TIMI II Trial. J. Am. Coll. Cardiol. 2002, 39, 312A. [Google Scholar] [CrossRef] [Green Version]
- Apple, F.S.; Kleinfeld, A.M.; Adams, J. Biomarker discovery and profiling unbound free fatty acid concentrations are increased in cardiac ischemia. Clin. Proteomics. 2004, 1, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Roy, V.K.; Kumar, A.; Joshi, P.; Arora, J.; Ahanger, A.M. Plasma free fatty acid concentrations as a marker for acute myocardial infarction. J. Clin. Diagnostic Res. 2013, 7, 2432–2434. [Google Scholar] [CrossRef]
- Oliver, M.F. Fatty acids and the risk of death during acute myocardial ischaemia. Clin. Sci. 2015, 128, 349–355. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Truong, Q.A.; Peacock, W.F.; Yeo, K.T.J.; Storrow, A.; Thomas, S.; Curtis, K.M.; Foote, R.S.; Lee, H.K.; Miller, K.F.; et al. A multicenter comparison of established and emerging cardiac biomarkers for the diagnostic evaluation of chest pain in the emergency department. Am. Heart J. 2011, 162, 276–282. [Google Scholar] [CrossRef]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, M.; Wessman, Y.; Almgren, P.; Groop, L. High levels of nonesterified fatty acids are associated with increased familial risk of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Miedema, M.D.; Maziarz, M.; Biggs, M.L.; Zieman, S.J.; Kizer, J.R.; Ix, J.H. Plasma free fatty acids, fatty acid-binding protein 4, and mortality in older adults (from the Cardiovascular Health Study). Am. J. Cardiol. 2014, 114, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swietlow, A.; Skoog, M.; Johansson, G. Double-layer capacitance measurement of self-assembled layers on gold electrodes. Electroanalysis 1992, 4, 921–928. [Google Scholar] [CrossRef]
- Goryunov, A.; Rozhkov, S.; Rozhkova, N. Fatty acid transfer between serum albumins and shungite carbon nanoparticles and its effect on protein aggregation and association. Eur. Biophys. J. 2020, 49, 85–94. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uygun, Z.O.; Duman, S.; Oran, I. Impedimetric Detection of Albumin-Bound Fatty Acids Using Graphene Oxide Electrode. Chemosensors 2021, 9, 240. https://doi.org/10.3390/chemosensors9090240
Uygun ZO, Duman S, Oran I. Impedimetric Detection of Albumin-Bound Fatty Acids Using Graphene Oxide Electrode. Chemosensors. 2021; 9(9):240. https://doi.org/10.3390/chemosensors9090240
Chicago/Turabian StyleUygun, Zihni Onur, Soner Duman, and Ismail Oran. 2021. "Impedimetric Detection of Albumin-Bound Fatty Acids Using Graphene Oxide Electrode" Chemosensors 9, no. 9: 240. https://doi.org/10.3390/chemosensors9090240





