Controlled Growth of WO3 Pyramidal Thin Film via Hot-Filament Chemical Vapor Deposition: Electrochemical Detection of Ethylenediamine
Abstract
:1. Introduction
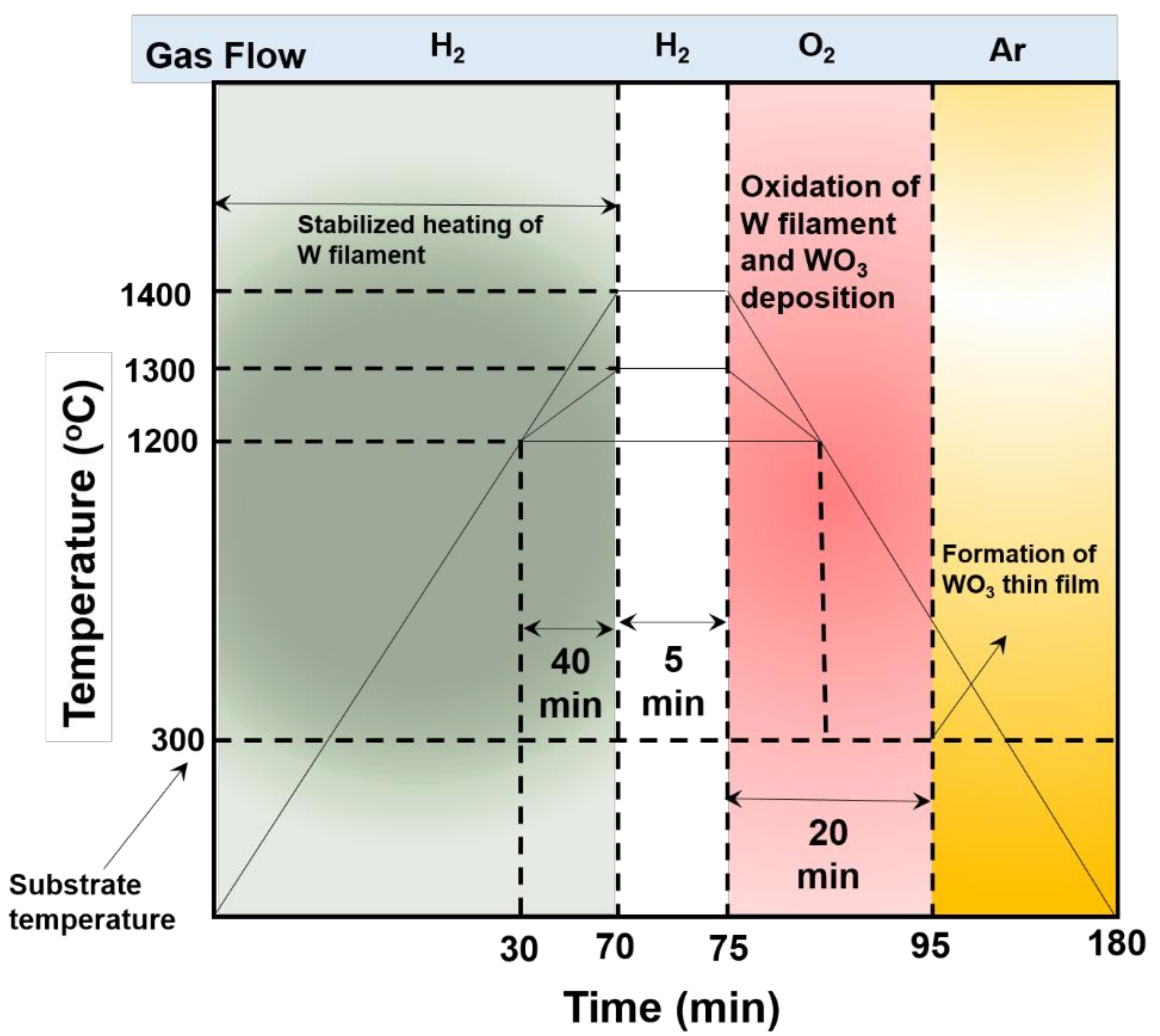
2. Materials and Methods
Materials Characterization
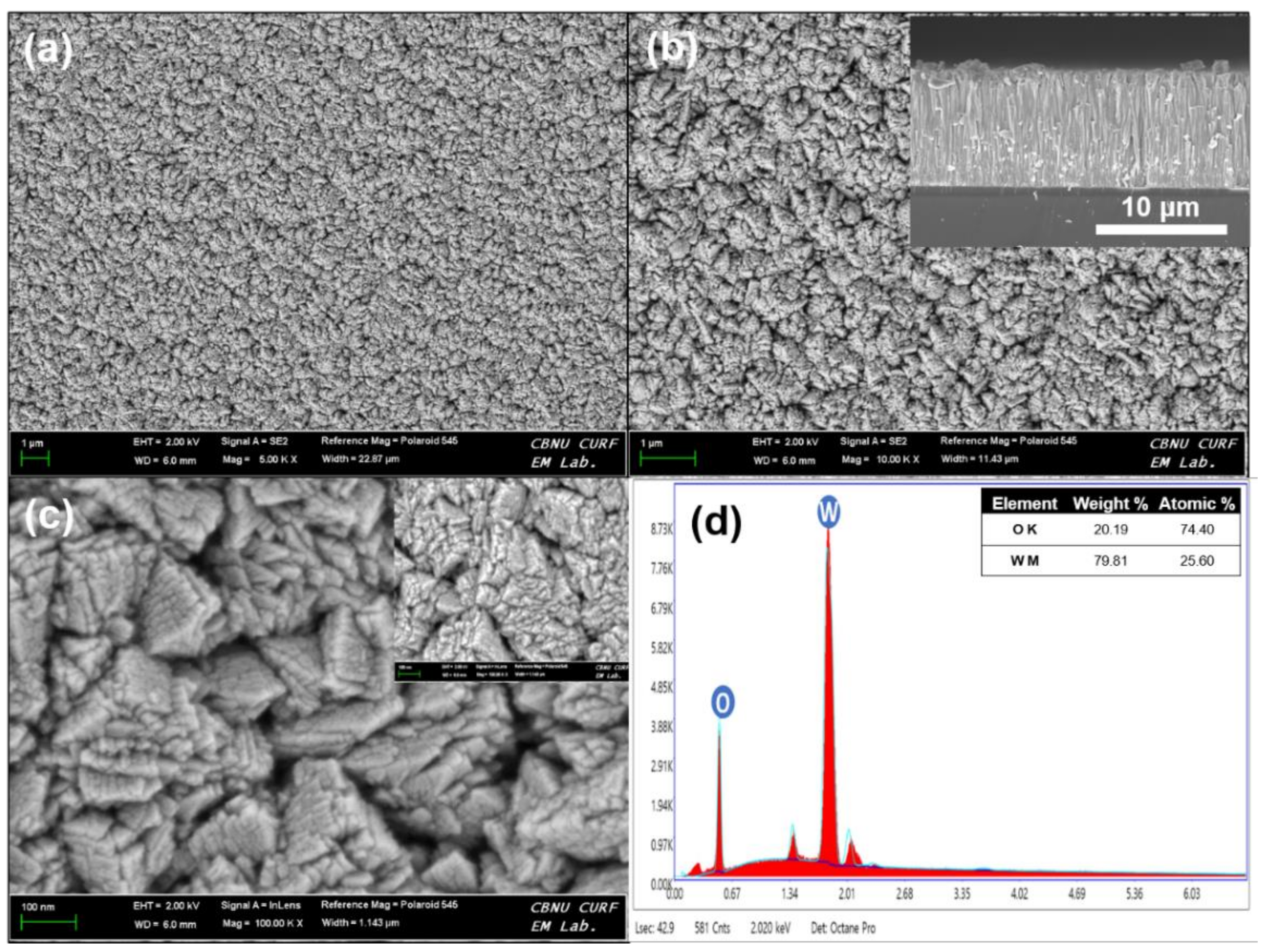
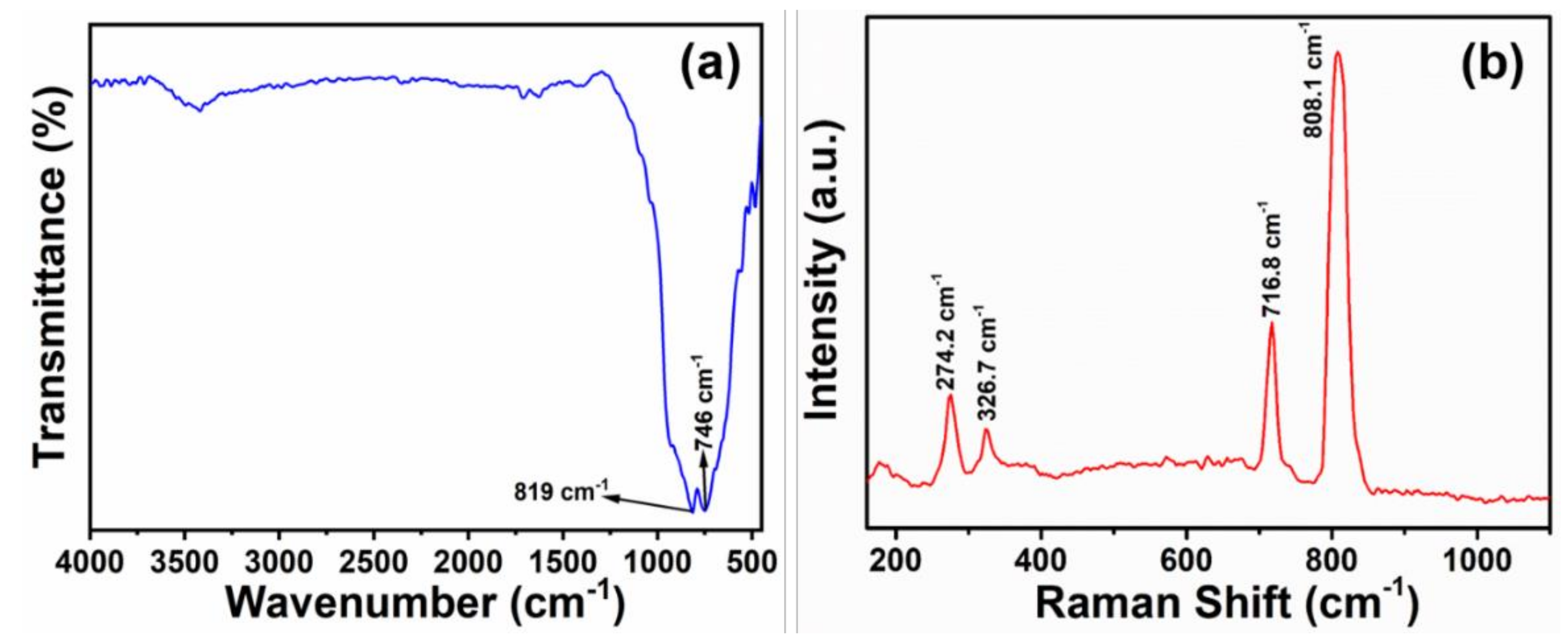
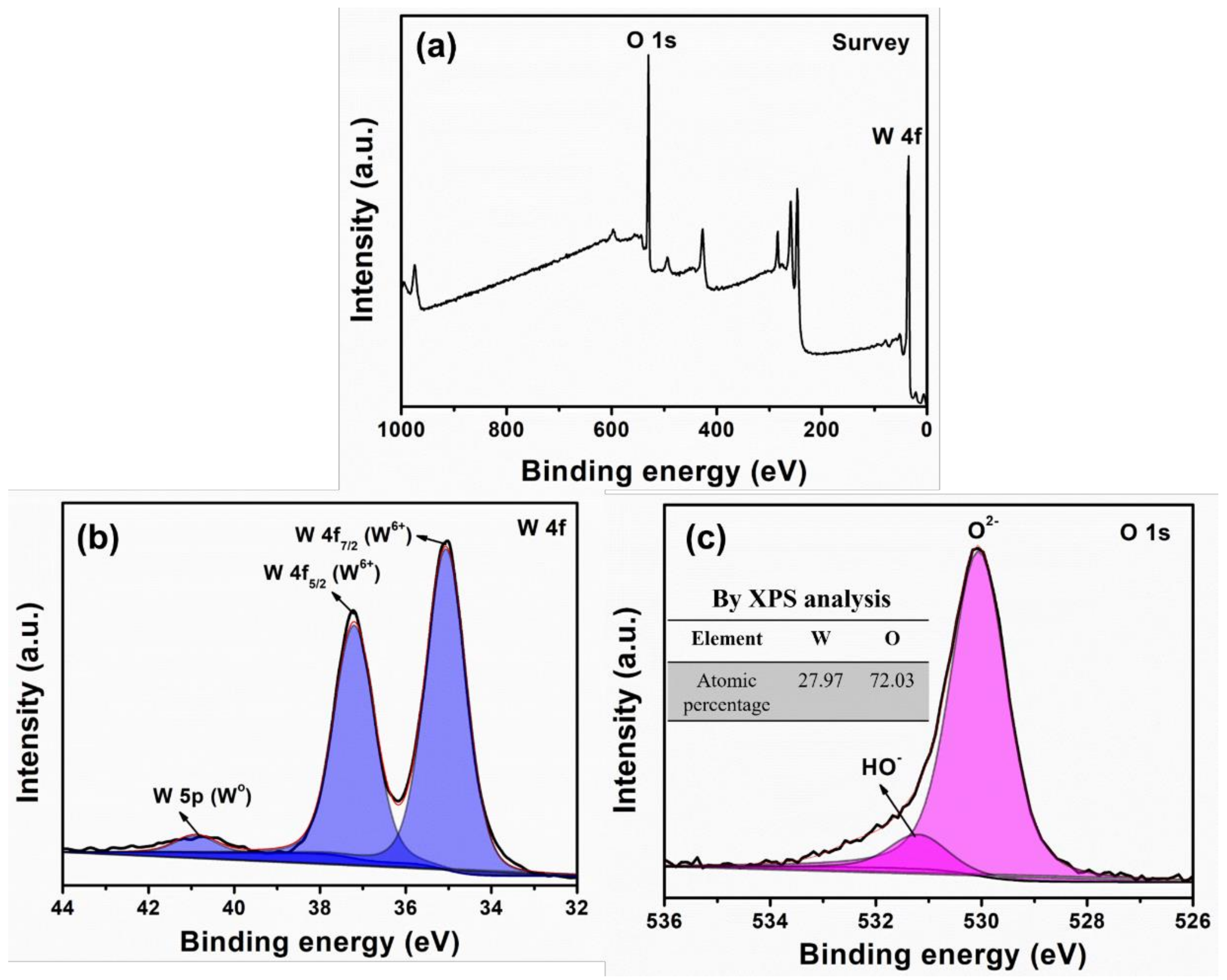
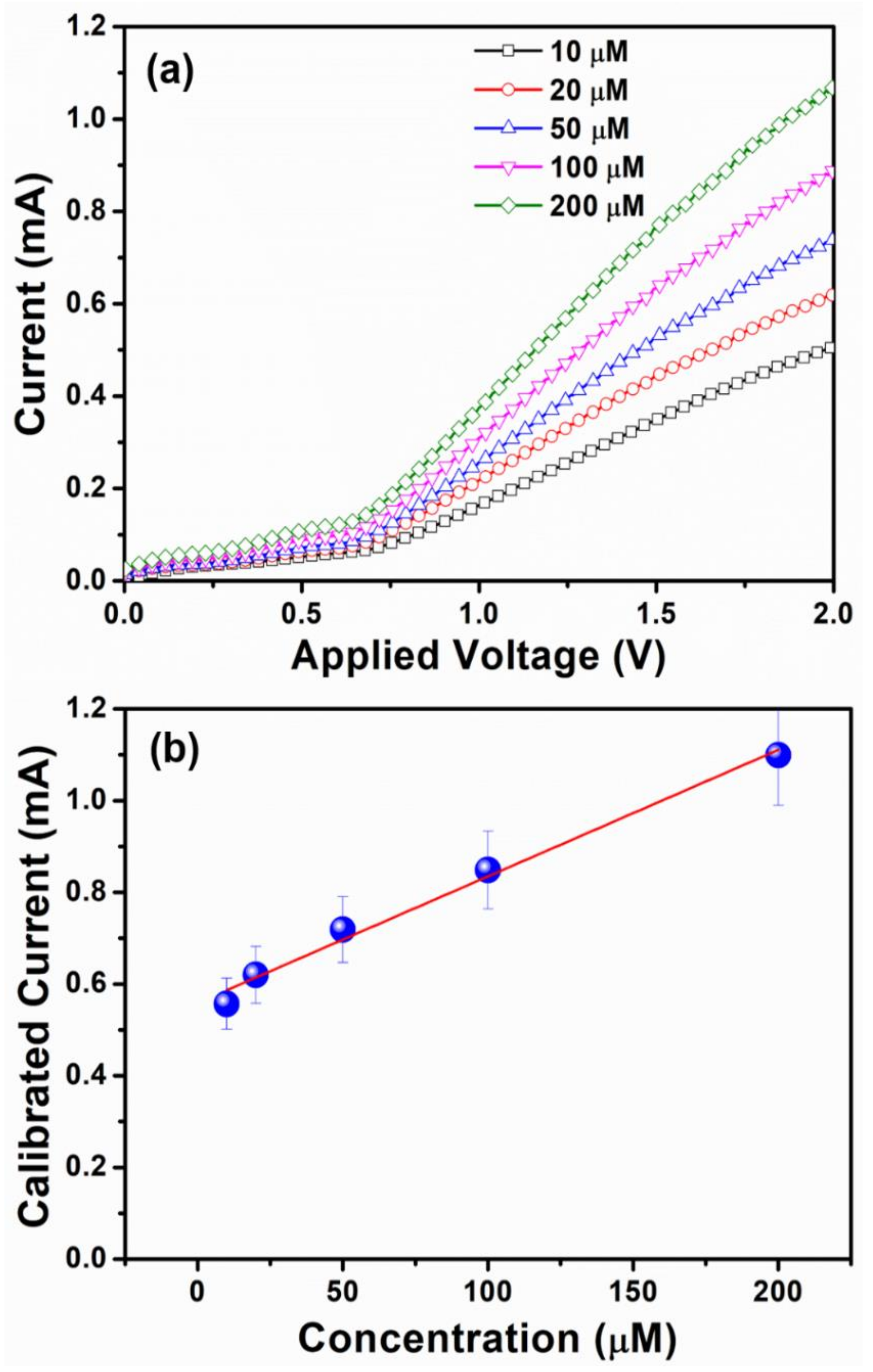
3. Results
| Materials | Chemical | Sensitivity | LOD | Linear Dynamic | Refs. |
|---|---|---|---|---|---|
| Al − ZnO | Phenyl-hydrazine | 1.143 μA·mM−1·cm−2 | 1.215 μM | 10 μM–50 mM | [41] |
| CuO − ZnO | NH4OH | 1.549 μA·mM−1·cm−2 | 8.9 μM | 77 μM–7.7 M | [42] |
| Fe,Ni − ZnO | Hexahydropyridine | 62.28 μA·μM−1·cm−2 | 79 μM | 0.1 μM–1 mM | [43] |
| ZnO nanopencils | Ammonia | 26.58 μA·cm−2·μM−1 | 5 nM | - | [44] |
| ZnO nanonails | Hydrazine | 8.56 μA·cm−2·μM−1 | 0.2 μM | - | [45] |
| WO3 NPys | Ethylenediamine | 161.33μA·μM−1·cm−2 | 9.56 μM | 10–200 μM | This work |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jitan, S.A.; Palmisano, G.; Garlisi, C. Synthesis and surface modification of TiO2-based photocatalysts for the conversion of CO2. Catalysts 2020, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Shi, Z.; Liang, L.; Wei, S.; Wang, H.; Dastan, D.; Sun, K.; Fan, R. Layer-structured BaTiO3/P(VDF–HFP) composites with concurrently improved dielectric permittivity and breakdown strength toward capacitive energy-storage applications. J. Mater. Chem. C 2020, 8, 10257–10265. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Wang, H.; Wang, X.; Hao, C.; Fan, R.; Dastan, D.; Shi, Z. Achieving excellent dielectric performance in polymer composites with ultralow filler loadings via constructing hollow-structured filler frameworks. Compos. Part A 2020, 131, 105814. [Google Scholar] [CrossRef]
- Yin, X.T.; Li, J.; Dastan, D.; Zhou, W.D.; Garmestani, H.; Alamgir, F.M. Ultra-high selectivity of H2 over CO with A P-N nanojunction based gas sensors and its mechanism. Sens. Actuator B Chem. 2020, 319, 128330. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic tungsten oxide films: Review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- White, C.M.; Gillaspie, D.T.; Whitney, E.; Lee, S.H.; Dillon, A.C. Flexible electrochromic devices based on crystalline WO3 nanostructures produced with hot-wire chemical vapor deposition. Thin Solid Films 2009, 517, 3596–3599. [Google Scholar] [CrossRef]
- Yin, X.T.; Lv, P.; Lia, J.; Jafari, A.; Wu, F.Y.; Wang, Q.; Dastan, D.; Shi, Z.; Yue, S.; Garmestani, H. Nanostructured tungsten trioxide prepared at various growth temperatures for sensing applications. J. Alloys Comp. 2020, 825, 154105. [Google Scholar] [CrossRef]
- Franke, E.B.; Trimble, C.L.; Hale, J.S.; Schubert, M.; Woollam, J.A. Infrared switching electrochromic devices based on tungsten oxide. J. Appl. Phys. 2000, 88, 5777–5784. [Google Scholar] [CrossRef] [Green Version]
- Trimble, C.; DeVries, M.; Hale, J.S.; Thompson, D.W.; Tiwald, T.E.; Woollam, J.A. Infrared emittance modulation devices using electrochromic crystalline tungsten oxide, polymer conductor, and nickel oxide. Thin Solid Films 1999, 355–356, 26–34. [Google Scholar] [CrossRef]
- Bussjager, R.; Chaiken, J.; Getbehead, M.; Grucza, D.; Hinkel, D.; McEwen, T.; Osman, J.; Voss, E. Using tungsten oxide based thin films for optical memory, and the effects of using IR combined with blue/blue-green wavelengths. Jpn. J. Appl. Phys. 2000, 39, 789–796. [Google Scholar] [CrossRef]
- Turyan, I.; Krasovec, U.O.; Orel, B.; Saraidorov, T.; Reisfeld, R.; Mandler, D. “Writing–reading–erasing” on tungsten oxide films using the scanning electrochemical microscope. Adv. Mater. 2000, 12, 330–333. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, X.; Liang, L.; Yin, P.; Xie, P.; Dastan, D.; Sun, K.; Fan, R.; Shi, Z. Significantly enhanced dielectric permittivity and low loss in epoxy composites incorporating 3d W-WO3/BaTiO3 foams. J. Mat. Sci. 2021, 56, 4254–4265. [Google Scholar] [CrossRef]
- Gillet, M.; Aguir, K.; Bendahan, M.; Mennini, P. Grain size effect in sputtered tungsten trioxide thin films on the sensitivity to ozone. Thin Solid Films 2005, 484, 358–363. [Google Scholar] [CrossRef]
- Fukuda, H.; Zohnishi, R.; Nomura, S. Highly sensitive metal-insulator-semiconductor field-effect transistor sensors for detecting carbon monoxide gas using porous platinum and tungsten oxide thin films. Jpn. J. Appl. Phys. 2001, 40, 2782–2786. [Google Scholar] [CrossRef]
- Fang, L.; Baik, S.J.; Lim, K.S.; Yoo, S.H.; Seo, M.S.; Kang, S.J.; Seo, J.W. Tungsten oxide as a buffer layer inserted at the SnO2/P-A-Sic interface of pin-type amorphous silicon based solar cells. Appl. Phys. Lett. 2010, 96, 193501. [Google Scholar] [CrossRef]
- Saito, Y.; Uchida, S.; Kubo, T.; Segawa, H. Surface-oxidized tungsten for energy-storable dye-sensitized solar cells. Thin Solid Films 2010, 518, 3033–3036. [Google Scholar] [CrossRef]
- Mendoza, F.; Limbu, T.B.; Weiner, B.R.; Morell, G. Hot filament chemical vapor deposition: Enabling the scalable synthesis of bilayer graphene and other carbon materials. ACS Appl. Nano Mater. 2018, 4, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- Godbole, R.; Ameen, S.; Nakate, U.T.; Akhtar, M.S.; Shin, H.S. Low temperature HFCVD synthesis of tungsten oxide thin film for high response hydrogen gas sensor application. Mater. Lett. 2019, 254, 398–401. [Google Scholar] [CrossRef]
- Tan, G.L.; Tang, D.; Dastan, D.; Jafari, A.; Silva, J.P.B.; Yin, X.T. Effect of heat treatment on electrical and surface properties of tungsten oxide thin films grown by HFCVD technique. Mater. Sci. Semicon. Process. 2021, 122, 105506. [Google Scholar] [CrossRef]
- Jacob, S.P.C. The influence of substrate temperature variation on tungsten oxide thin film growth in an HFCVD system. Appl. Surf. Sci. 2007, 253, 3317–3325. [Google Scholar]
- Jafari, A.; Ghoranneviss, M.; Elahi, A.S. HFCVD application for growth of monoclinic tungsten trioxide crystal nano-walls. J. Inorg. Organomet. Polym. Mater. 2016, 26, 254–258. [Google Scholar] [CrossRef]
- Feng, P.X.; Wang, X.P.; Zhang, H.X.; Yang, B.Q.; Wang, Z.B.; Berríos, A.G. Study of the structural evolutions of crystalline tungsten oxide films prepared using hot-filament CVD. J. Phys. D Appl. Phys. 2007, 40, 5239. [Google Scholar] [CrossRef]
- Ethylenediamine. Available online: https://en.wikipedia.org/wiki/Ethylenediamine (accessed on 20 August 2021).
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Highly sensitive hydrazine chemical sensor fabricated by modified electrode of vertically aligned zinc oxide nanorods. Talanta 2012, 100, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Mehta, S.K. Utilization of ZnO nanoflowers as efficient electrochemical catalyst for the oxidation of hydrazine. Sens. Lett. 2015, 13, 1002–1006. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Dar, G.N.; Zaidi, S.A.; Umar, A.; Abaker, M.; Bouzid, H.; Baskoutas, S. Growth and properties of Ag-doped ZnO nanoflowers for highly sensitive phenyl hydrazine chemical sensor application. Talanta 2012, 93, 257–263. [Google Scholar] [CrossRef]
- Tan, G.-L.; Tang, D.; Dastan, D.; Jafari, A.; Shi, Z.; Chu, Q.Q.; Silva, J.P.B.; Yin, X.T. Structures, morphological control, and antibacterial performance of tungsten oxide thin films. Ceram. Int. 2021, 47, 17153–17160. [Google Scholar] [CrossRef]
- Anderson, A. Raman study of ceramic tungsten trioxide at low temperatures. Spectrosc. Lett. 1976, 9, 809–819. [Google Scholar] [CrossRef]
- Souza-Filho, A.G.; Freire, V.N.; Sasaki, J.M.; Mendes, F.J.; Julio, J.F.; Gomes, U.U. Coexistence of triclinic and monoclinic phases in WO3 ceramics. J. Raman Spectrosc. 2000, 31, 451–454. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chakraborty, A.K. Band-gap engineering of tungsten oxide nanoplates by cobalt ferrite Co-catalyst for solar water oxidation. Opt. Mater. 2021, 111, 110610. [Google Scholar] [CrossRef]
- Asres, G.A.; Dombovari, A.; Sipola, T.; Puskás, R.; Kukovecz, A.; Kónya, Z.; Popov, A.; Lin, J.F.; Lorite, G.S.; Mohl, M.; et al. A novel WS2 nanowire-nanoflake hybrid material synthesized from WO3 nanowires in sulfur vapor. Sci. Rep. 2016, 6, 25610. [Google Scholar] [CrossRef] [Green Version]
- Lozzi, L.; Ottaviano, L.; Passacantando, M.; Santucci, S.; Cantalini, C. The influence of air and vacuum thermal treatments on the NO2 gas sensitivity of WO3 thin films prepared by thermal evaporation. Thin Solid Films 2001, 391, 224–228. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Papaefthimiou, S.; Yianoulis, P.; Siokou, A. Effect of the tungsten oxidation states in the thermal coloration and bleaching of amorphous WO3 films. Thin Solid Films 2001, 384, 298–306. [Google Scholar] [CrossRef]
- Soto, G.; De La Cruz, W.; Díaz, J.A.; Machorro, R.; Castillón, F.F.; Farías, M.H. Characterization of tungsten oxide films produced by reactive pulsed laser deposition. Appl. Surf. Sci. 2003, 218, 281–289. [Google Scholar] [CrossRef]
- Joshi, N.; da Silva, L.F.; Jadhav, H.S.; Shimizu, F.M.; Suman, P.H.; M’Peko, J.C.; Orlandie, M.O.; Seo, J.G.; Mastelaro, V.R.; Oliveira, O.N., Jr. Yolk-shelled ZnCo2O4 microspheres: Surface properties and gas sensing application. Sens. Act. B Chem. 2018, 257, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Fan, Y.; Shi, Y.; Xiang, Q.; Wang, X.; Xu, J. A low temperature formaldehyde gas sensor based on hierarchical SnO/SnO2 nano-flowers assembled from ultrathin nanosheets: Synthesis, sensing performance and mechanism. Sen. Act. B Chem. 2019, 294, 106–115. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, B.; Ye, J.; Ye, J. A sensitive and selective amperometric hydrazine sensor based on palladium nanoparticles loaded on cobalt-wrapped nitrogen-doped carbon nanotubes. J. Electroanal. Chem. 2017, 801, 215–223. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Hao, P.; Tian, J.; Cui, H.; Wang, X. Soft-templated formation of double-shelled ZnO hollow microspheres for acetone gas sensing at low concentration/near room temperature. Sens. Actuators B Chem. 2018, 273, 751–759. [Google Scholar] [CrossRef]
- Wang, P.; Dong, T.; Jia, C.; Yang, P. Ultraselective acetone-gas sensor based ZnO flowers functionalized by au nanoparticle loading on certain facet. Sens. Actuat. B Chem. 2019, 288, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Liu, L.-Z.; Zhang, X.-R.; Zhang, S.; Meng, F.-N. Microwave-assisted solvothermal synthesis of shape-controlled CoFe2O4 nanoparticles for acetone sensor. J. Alloys Compd. 2019, 788, 1103–1112. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bahadar Khan, S.; Jamal, A.; Faisal, M.; Abdullah, M. Asiri, fabrication of phenyl-hydrazine chemical sensor based on Al-doped ZnO nanoparticles. Sens. Trans. J. 2011, 134, 32–44. [Google Scholar]
- Rahman, M.M.; Jamal, A.; Khan, S.B.; Faisal, M. CuO codoped ZnO based nanostructured materials for sensitive chemical sensor applications. ACS Appl. Mater. Interfaces 2011, 3, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.B.; Ameen, S.; Akhtar, M.S.; Shin, H.S. Iron-nickel co-doped ZnO nanoparticles as scaffold for field effect transistor sensor: Application in electrochemical detection of hexahydropyridine chemical. Sens. Actuators B Chem. 2018, 275, 422–431. [Google Scholar] [CrossRef]
- Dar, G.N.; Umar, A.; Zaidi, S.A.; Baskoutas, S.; Hwang, S.W.; Abaker, M.; Al-Hajry, A.; Al-Sayari, S.A. Ultra-high sensitive ammonia chemical sensor based on ZnO nanopencils. Talanta 2012, 89, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Rahman, M.M.; Kim, S.H.; Hahn, Y.B. Zinc oxide nanonail based chemical sensor for hydrazine detection. Chem. Commun. 2008, 2, 166–168. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Kim, E.-B.; Kwak, D.-H.; Akhtar, M.S.; Ameen, S. Controlled Growth of WO3 Pyramidal Thin Film via Hot-Filament Chemical Vapor Deposition: Electrochemical Detection of Ethylenediamine. Chemosensors 2021, 9, 257. https://doi.org/10.3390/chemosensors9090257
Imran M, Kim E-B, Kwak D-H, Akhtar MS, Ameen S. Controlled Growth of WO3 Pyramidal Thin Film via Hot-Filament Chemical Vapor Deposition: Electrochemical Detection of Ethylenediamine. Chemosensors. 2021; 9(9):257. https://doi.org/10.3390/chemosensors9090257
Chicago/Turabian StyleImran, Mohammad, Eun-Bi Kim, Dong-Heui Kwak, Mohammad Shaheer Akhtar, and Sadia Ameen. 2021. "Controlled Growth of WO3 Pyramidal Thin Film via Hot-Filament Chemical Vapor Deposition: Electrochemical Detection of Ethylenediamine" Chemosensors 9, no. 9: 257. https://doi.org/10.3390/chemosensors9090257
APA StyleImran, M., Kim, E.-B., Kwak, D.-H., Akhtar, M. S., & Ameen, S. (2021). Controlled Growth of WO3 Pyramidal Thin Film via Hot-Filament Chemical Vapor Deposition: Electrochemical Detection of Ethylenediamine. Chemosensors, 9(9), 257. https://doi.org/10.3390/chemosensors9090257








