A Bio-Fluorometric Acetone Gas Imaging System for the Dynamic Analysis of Lipid Metabolism in Human Breath
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of the Acetone Gas Imaging System
2.2. Enzyme Immobilization Method
2.3. Characterization and Analysis Method of the Imaging System
2.4. Imaging of Acetone Gas in Exhaled Air during Fasting
3. Results and Discussions
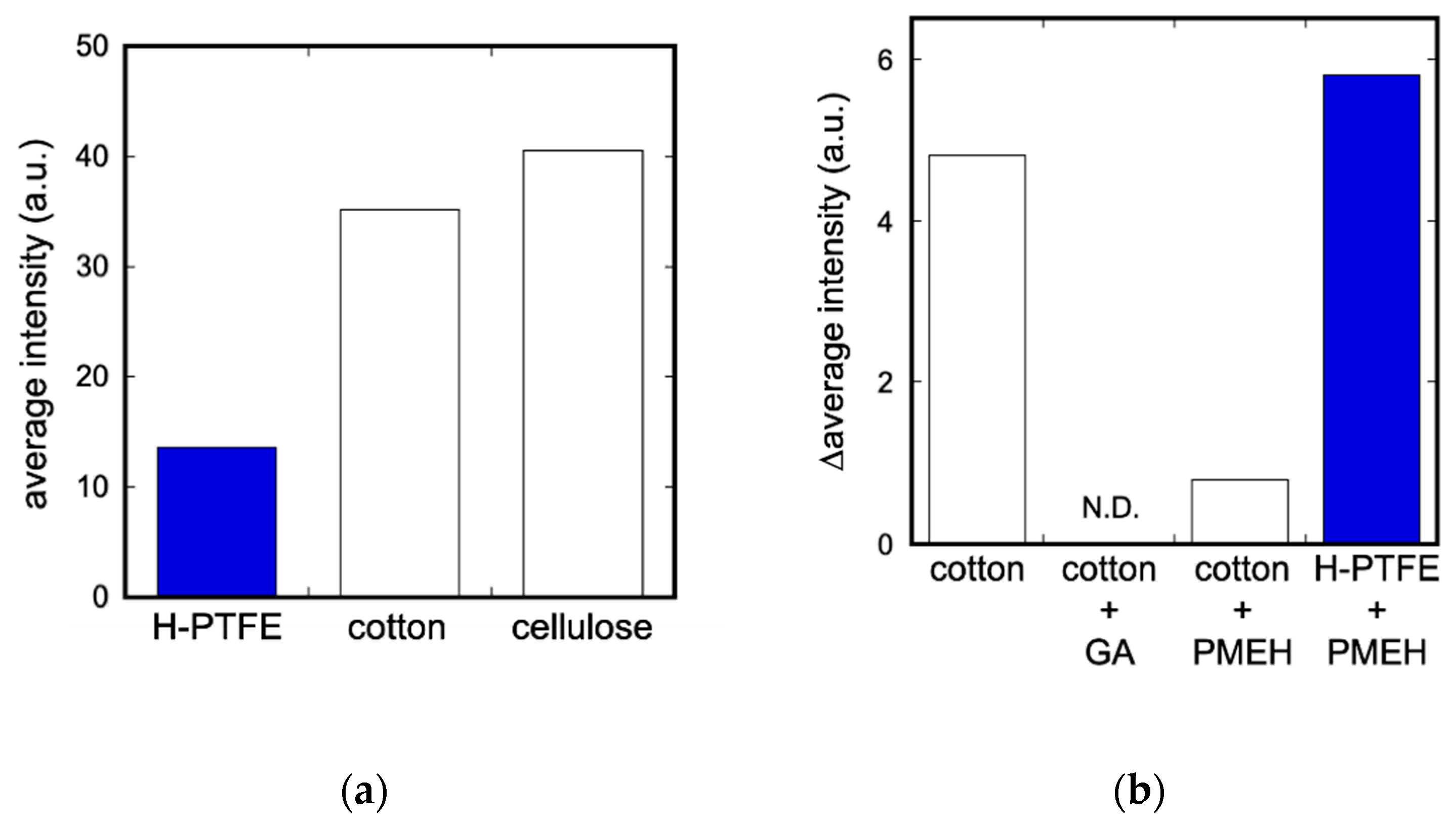
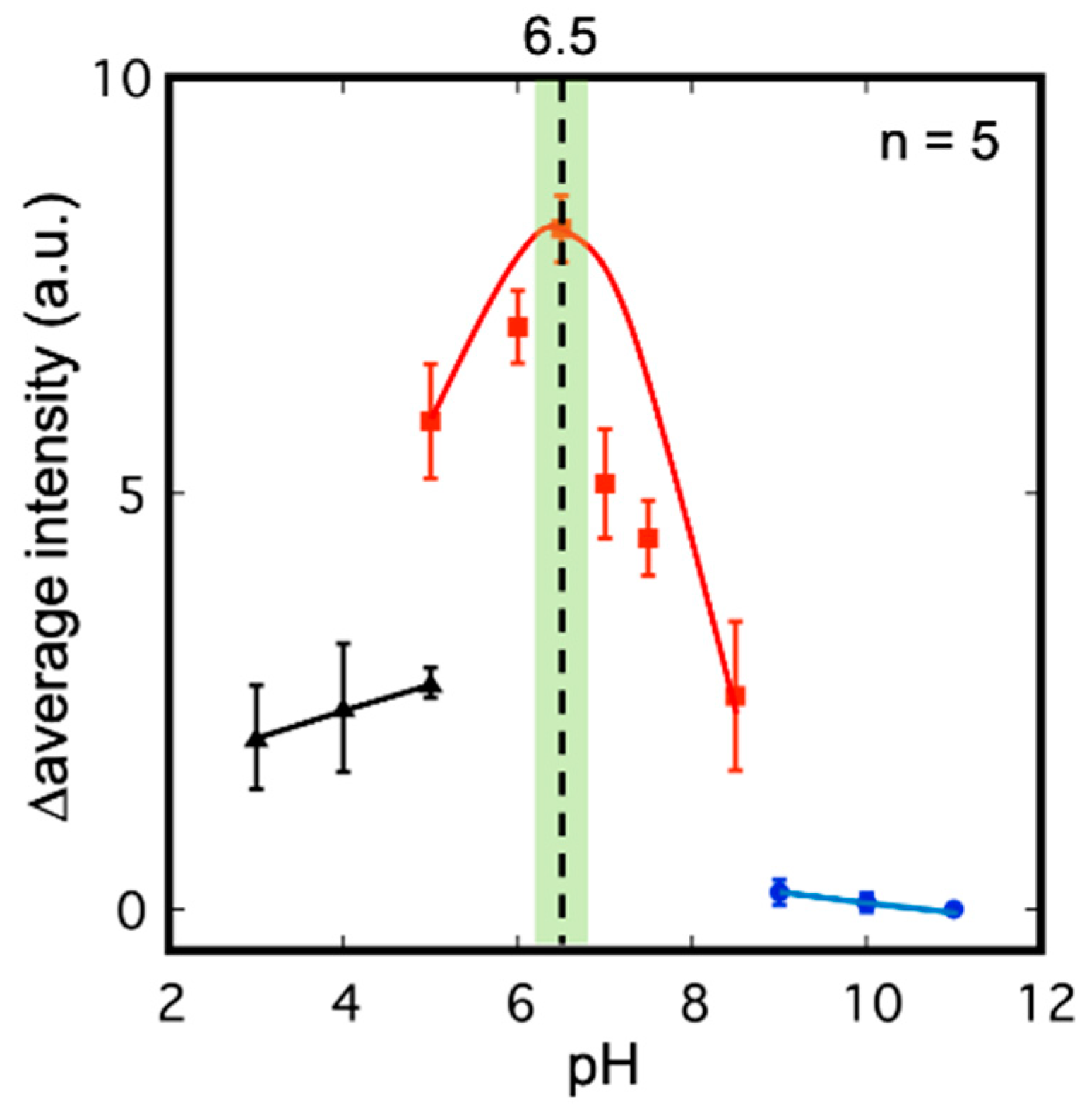
3.1. Evaluation of Enzyme Immobilization Methods and Substrates
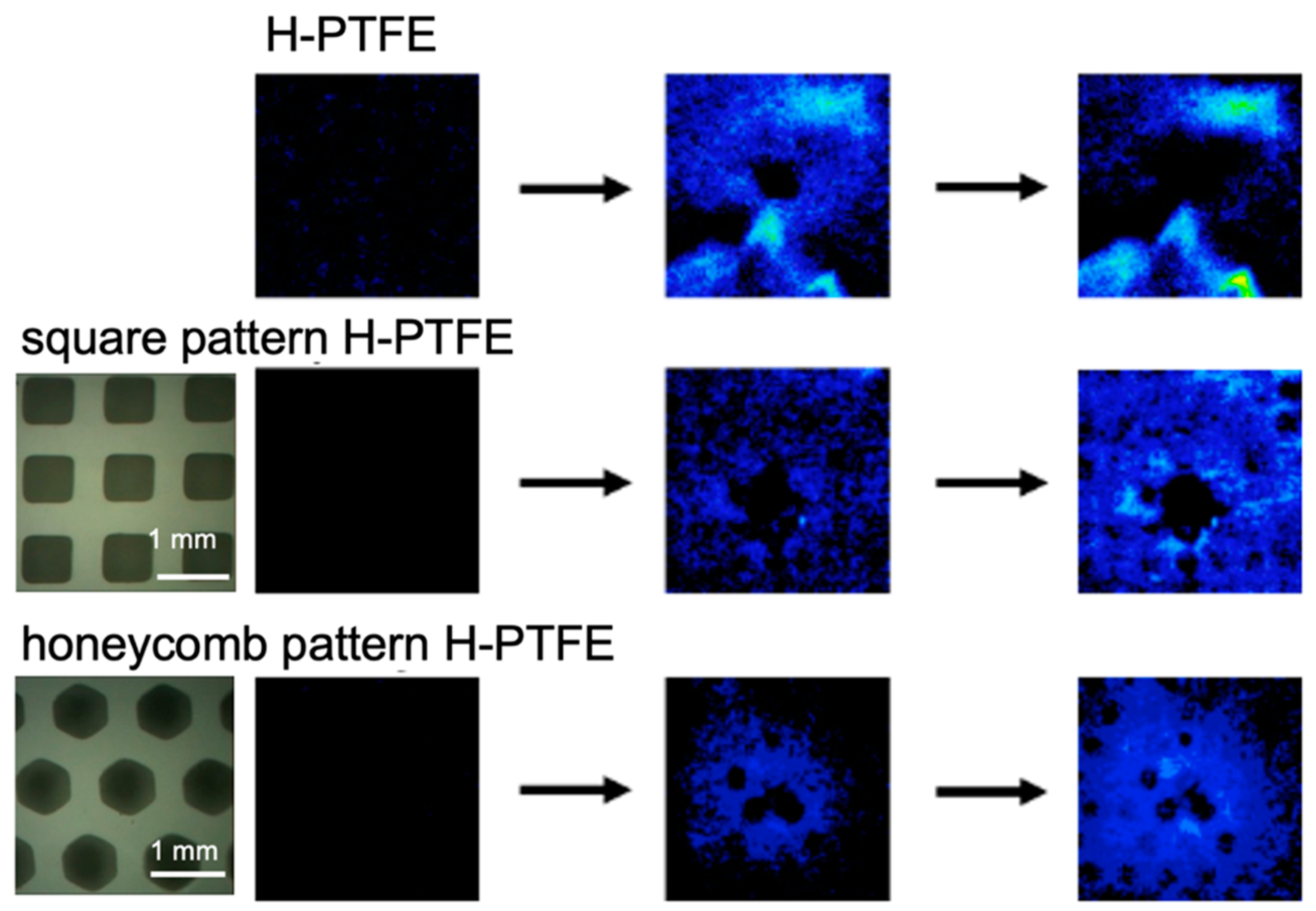
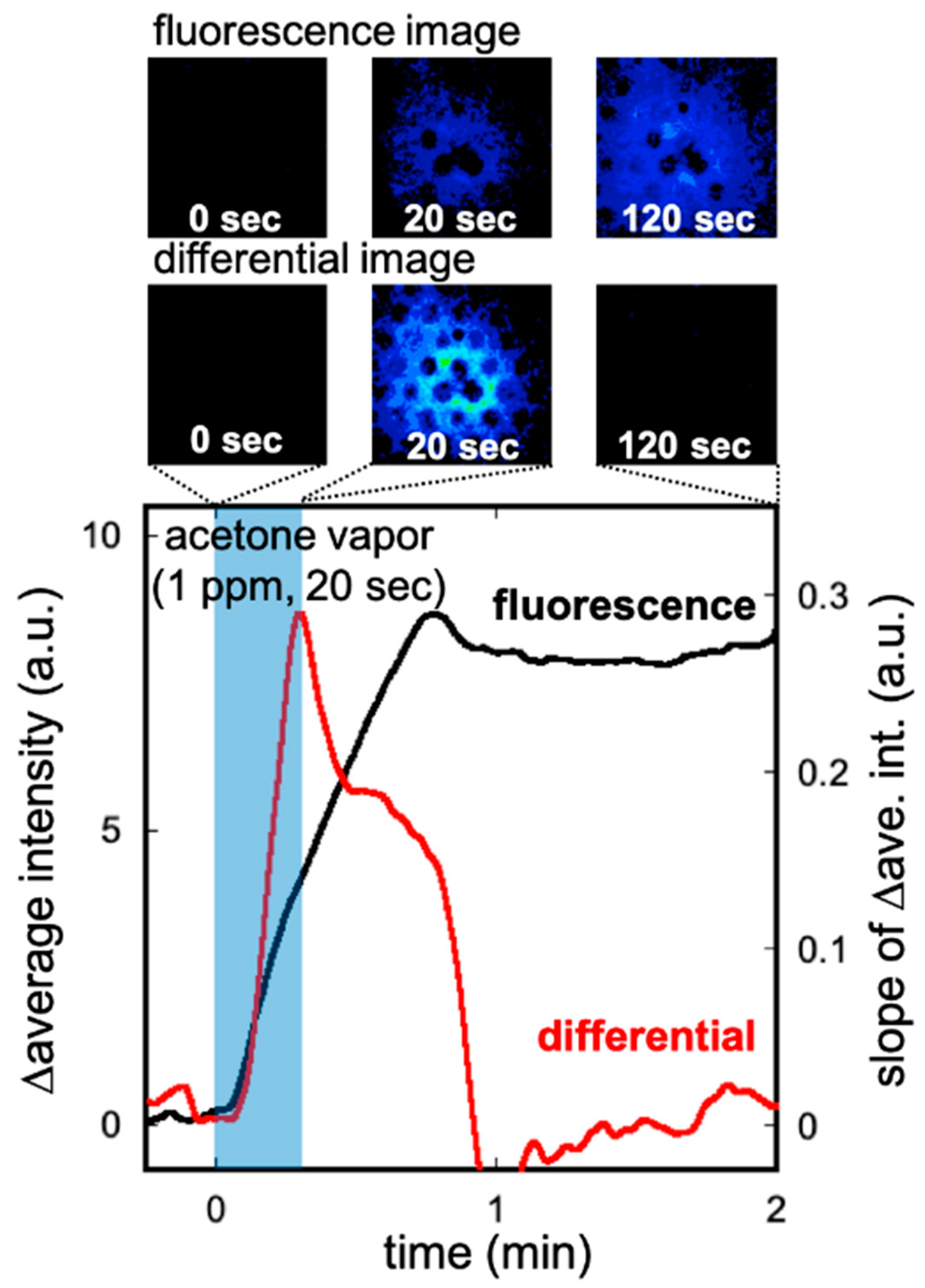
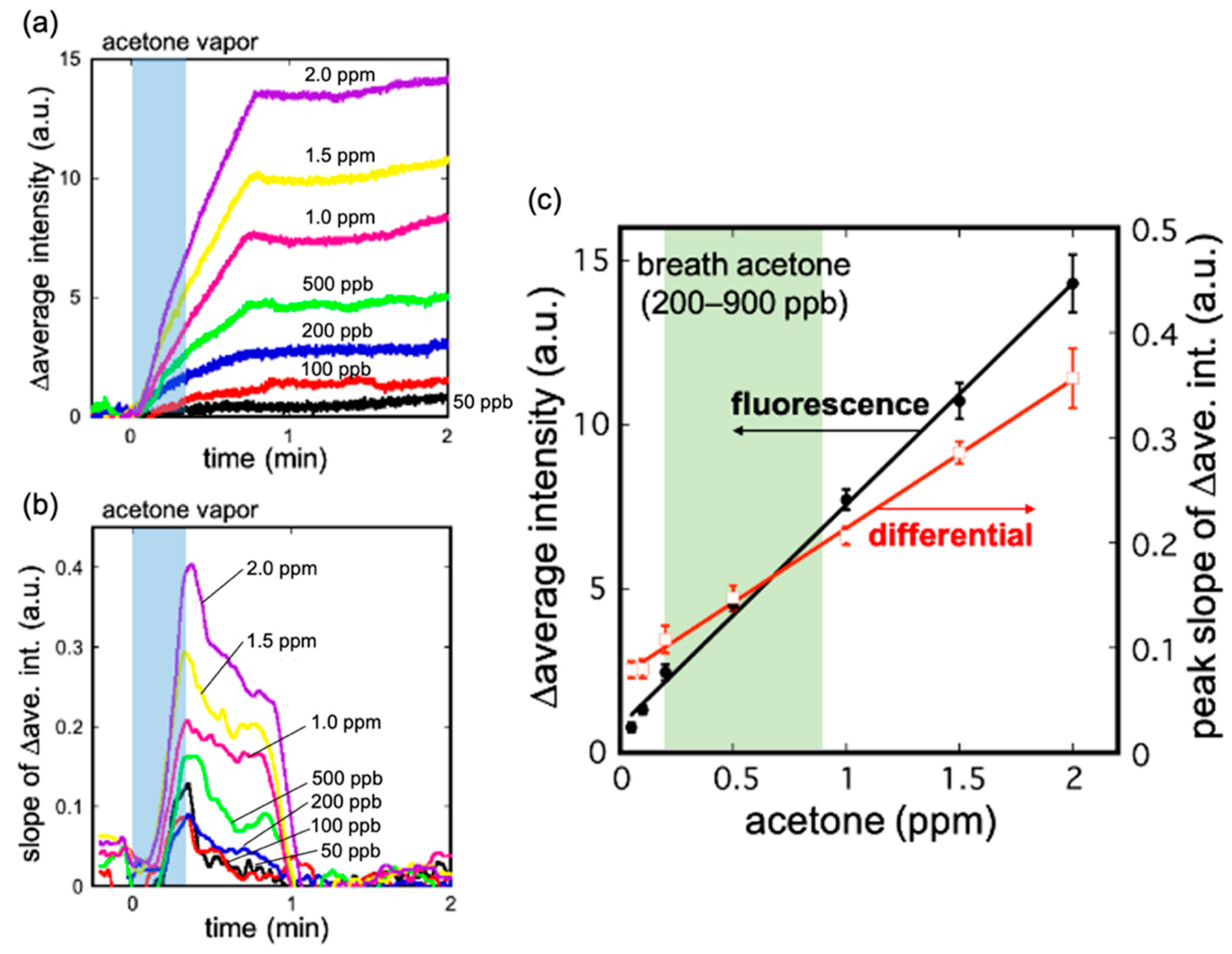
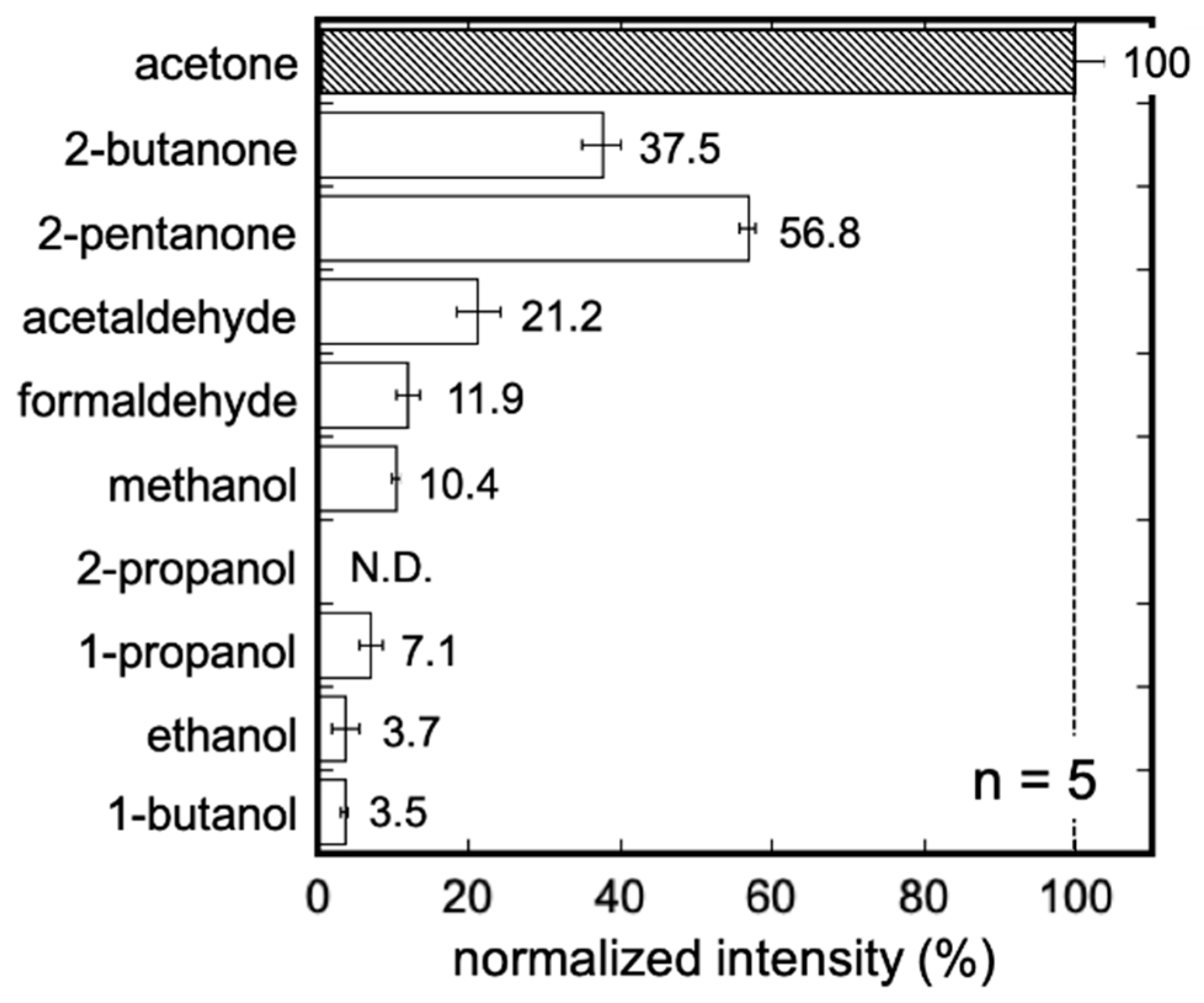
3.2. Characteristics of the Acetone Gas Imaging System
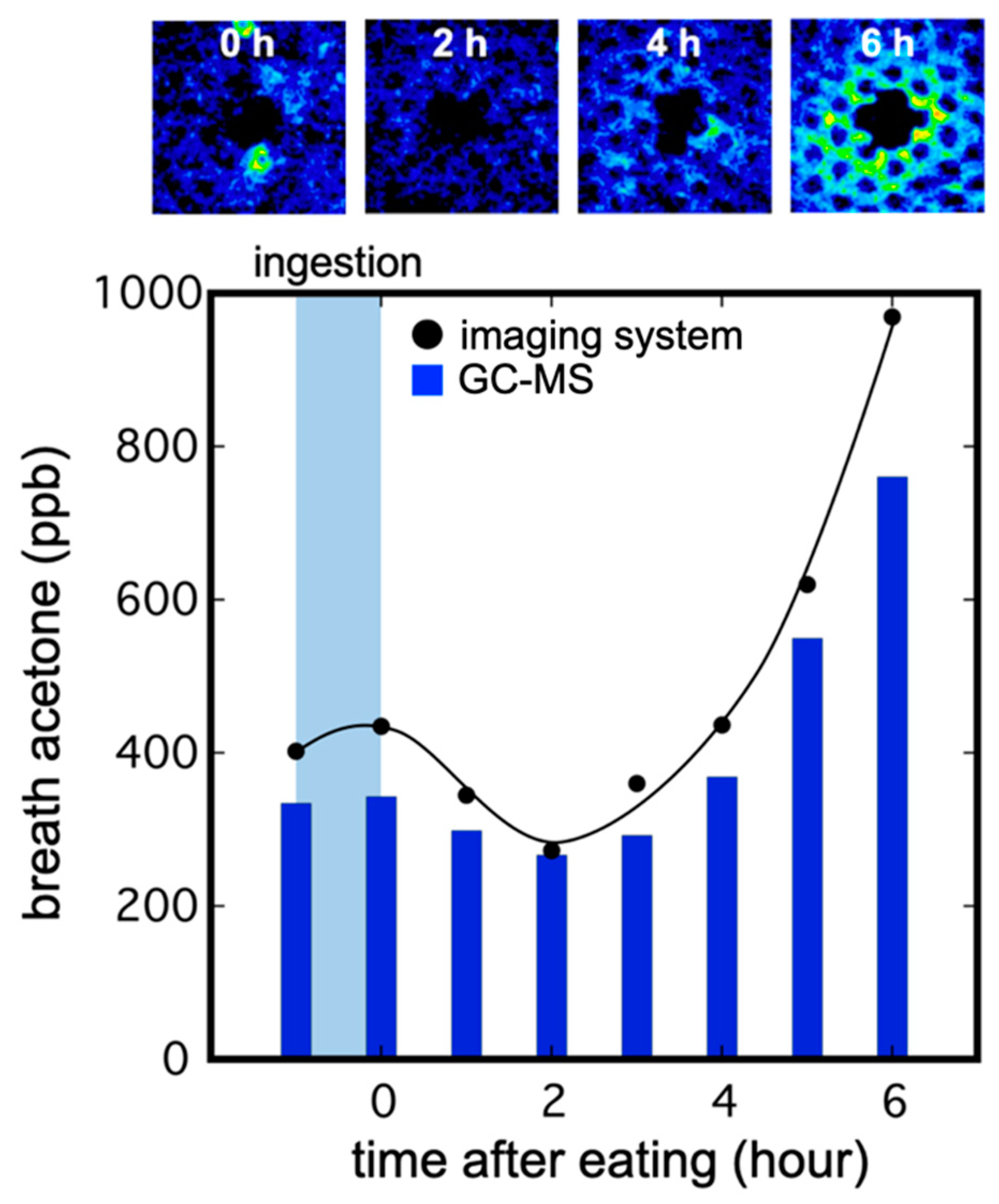
3.3. Imaging of Breath Acetone Gas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dormont, L.; Bessière, J.M.; Cohuet, A. Human Skin Volatiles: A Review. J. Chem. Ecol. 2013, 39, 569–578. [Google Scholar] [CrossRef]
- Dormont, L.; Bessiere, J.-M.; McKey, D.; Cohuet, A. New Methods for Field Collection of Human Skin Volatiles and Perspectives for Their Application in the Chemical Ecology of Human-Pathogen-Vector Interactions. J. Exp. Biol. 2013, 216, 2783–2788. [Google Scholar] [CrossRef] [Green Version]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A Review of the Volatiles from the Healthy Human Body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Lourenço, C.; Turner, C. Breath Analysis in Disease Diagnosis: Methodological Considerations and Applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef]
- Longo, N.; Li, S.K.; Yan, G.; Kochambilli, R.P.; Papangkorn, K.; Berglund, D.; Ghanem, A.H.; Ashurst, C.L.; Ernst, S.L.; Pasquali, M.; et al. Noninvasive Measurement of Phenylalanine by Iontophoretic Extraction in Patients with Phenylketonuria. J. Inherit. Metab. Dis. 2007, 30, 910–915. [Google Scholar] [CrossRef]
- Rattray, N.J.W.; Hamrang, Z.; Trivedi, D.K.; Goodacre, R.; Fowler, S.J. Taking Your Breath Away: Metabolomics Breathes Life in to Personalized Medicine. Trends Biotechnol. 2014, 32, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Gaugg, M.T.; Engler, A.; Nussbaumer-Ochsner, Y.; Bregy, L.; Stöberl, A.S.; Gaisl, T.; Bruderer, T.; Zenobi, R.; Kohler, M.; Martinez-Lozano Sinues, P. Metabolic Effects of Inhaled Salbutamol Determined by Exhaled Breath Analysis. J. Breath Res. 2017, 11, 46004. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Q.; Li, W.; Zhao, Z.; Yuan, X.; Huang, Y.; Duan, Y. Discovery of Potential Biomarkers in Exhaled Breath for Diagnosis of Type 2 Diabetes Mellitus Based on GC-MS with Metabolomics. RSC Adv. 2014, 4, 25430–25439. [Google Scholar] [CrossRef]
- Jia, Z.; Patra, A.; Kutty, V.; Venkatesan, T. Critical Review of Volatile Organic Compound Analysis in Breath and In Vitro Cell Culture for Detection of Lung Cancer. Metabolites 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tassopoulos, C.N.; Barnett, D.; Russell Fraser, T. Breath-Acetone and Blood-Sugar Measurements in Diabetes. Lancet 1969, 293, 1282–1286. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Is Breath Acetone a Biomarker of Diabetes? A Historical Review on Breath Acetone Measurements. J. Breath Res. 2013, 7, 037109. [Google Scholar] [CrossRef] [Green Version]
- Chien, P.-J.; Suzuki, T.; Tsujii, M.; Ye, M.; Minami, I.; Toda, K.; Otsuka, H.; Toma, K.; Arakawa, T.; Araki, K.; et al. Biochemical Gas Sensors (Biosniffers) Using Forward and Reverse Reactions of Secondary Alcohol Dehydrogenase for Breath Isopropanol and Acetone as Potential Volatile Biomarkers of Diabetes Mellitus. Anal. Chem. 2017, 89, 12261–12268. [Google Scholar] [CrossRef] [PubMed]
- Saasa, V.; Beukes, M.; Lemmer, Y.; Mwakikunga, B. Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus. Diagnostics 2019, 9, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzsányi, V.; Kalapos, M.P. Breath Acetone as a Potential Marker in Clinical Practice. J. Breath Res. 2017, 11, 24002. [Google Scholar] [CrossRef] [PubMed]
- Mörk, A.K.; Johanson, G. A Human Physiological Model Describing Acetone Kinetics in Blood and Breath during Various Levels of Physical Exercise. Toxicol. Lett. 2006, 164, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Liu, Y.; Cheng, S.; Duan, Y. Exhaled Isopropanol: New Potential Biomarker in Diabetic Breathomics and Its Metabolic Correlations with Acetone. RSC Adv. 2017, 7, 17480–17488. [Google Scholar] [CrossRef] [Green Version]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured Metal Oxide-Based Acetone Gas Sensors: A Review. Sensors 2020, 20, 3096. [Google Scholar] [CrossRef]
- Wiesenhofer, H.; Allers, M.; Zimmermann, S.; Güntner, A.T.; Pineau, N.J.; Lederer, W.; Agapiou, A.; Mayhew, C.A.; Ruzsanyi, V. Monitoring of Selected Skin- and Breath-Borne Volatile Organic Compounds Emitted from the Human Body Using Gas Chromatography Ion Mobility Spectrometry ( GC-IMS ). J. Chromatogr. B 2018, 1076, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Arasaradnam, R.P.; McFarlane, M.; Ling, K.; Wurie, S.; O’Connell, N.; Nwokolo, C.U.; Bardhan, K.D.; Skinner, J.; Savage, R.S.; Covington, J.A. Breathomics—Exhaled Volatile Organic Compound Analysis to Detect Hepatic Encephalopathy: A Pilot Study. J. Breath Res. 2016, 10, 016012. [Google Scholar] [CrossRef]
- Shimouchi, A.; Nose, K.; Shirai, M. Analysis of Breath and Skin Gases Emanating During Exercise Using an Original Biogas Sampling System Connected to an Atmospheric Pressure Ionization Mass Spectrometer. IEEE Sens. J. 2010, 10, 85–91. [Google Scholar] [CrossRef]
- Yamane, N.; Tsuda, T.; Nose, K.; Yamamoto, A.; Ishiguro, H.; Kondo, T. Relationship between Skin Acetone and Blood β-Hydroxybutyrate Concentrations in Diabetes. Clin. Chim. Acta 2006, 365, 325–329. [Google Scholar] [CrossRef]
- Sekine, Y.; Toyooka, S.; Watts, S.F. Determination of Acetaldehyde and Acetone Emanating from Human Skin Using a Passive Flux Sampler-HPLC System. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 859, 201–207. [Google Scholar] [CrossRef]
- Mochalski, P.; King, J.; Unterkofler, K.; Hinterhuber, H.; Amann, A. Emission Rates of Selected Volatile Organic Compounds from Skin of Healthy Volunteers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 959, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Fenske, J.D.; Paulson, S.E. Human Breath Emissions of VOCs. J. Air Waste Manag. Assoc. 1999, 49, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Suzuki, T.; Tsujii, M.; Iitani, K.; Chien, P.J.; Ye, M.; Toma, K.; Iwasaki, Y.; Mitsubayashi, K. Real-Time Monitoring of Skin Ethanol Gas by a High-Sensitivity Gas Phase Biosensor (Bio-Sniffer) for the Non-Invasive Evaluation of Volatile Blood Compounds. Biosens. Bioelectron. 2019, 129, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Aota, T.; Iitani, K.; Toma, K.; Iwasaki, Y.; Mitsubayashi, K. Skin Ethanol Gas Measurement System with a Biochemical Gas Sensor and Gas Concentrator toward Monitoring of Blood Volatile Compounds. Talanta 2020, 219, 121187. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W. Safety Evaluation of Topical Applications of Ethanol on the Skin and inside the Oral Cavity. J. Occup. Med. Toxicol. 2008, 3, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hair, M.E.; Gerkman, R.; Mathis, A.I.; Halámková, L.; Halámek, J. Noninvasive Concept for Optical Ethanol Sensing on the Skin Surface with Camera-Based Quantification. Anal. Chem. 2019, 91, 15860–15865. [Google Scholar] [CrossRef] [PubMed]
- Giles, H.G.; Meggiorini, S.; Renaud, G.E.; Thiessen, J.J.; Vidins, E.I.; Compton, K.V.; Saldivia, V.; Orrego, H.; Israel, Y. Ethanol Vapor above Skin: Determination by a Gas Sensor Instrument and Relationship with Plasma Concentration. Alcohol. Clin. Exp. Res. 1987, 11, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Zhou, X.; Yuan, M.; Qu, D.; Zheng, Y.; Vishinkin, R.; Khatib, M.; Wu, W.; Haick, H. Disease Detection with Molecular Biomarkers: From Chemistry of Body Fluids to Nature-Inspired Chemical Sensors. Chem. Rev. 2019, 119, 11761–11817. [Google Scholar] [CrossRef]
- Saidi, T.; Zaim, O.; Moufid, M.; el Bari, N.; Ionescu, R.; Bouchikhi, B. Exhaled Breath Analysis Using Electronic Nose and Gas Chromatography–Mass Spectrometry for Non-Invasive Diagnosis of Chronic Kidney Disease, Diabetes Mellitus and Healthy Subjects. Sens. Actuators B Chem. 2018, 257, 178–188. [Google Scholar] [CrossRef]
- le Maout, P.; Wojkiewicz, J.L.; Redon, N.; Lahuec, C.; Seguin, F.; Dupont, L.; Mikhaylov, S.; Noskov, Y.; Ogurtsov, N.; Pud, A. Polyaniline Nanocomposites Based Sensor Array for Breath Ammonia Analysis. Portable e-Nose Approach to Non-Invasive Diagnosis of Chronic Kidney Disease. Sens. Actuators B Chem. 2018, 274, 616–626. [Google Scholar] [CrossRef]
- El Manouni el Hassani, S.; Berkhout, D.J.C.; Bosch, S.; Benninga, M.A.; de Boer, N.K.H.; de Meij, T.G.J. Application of Fecal Volatile Organic Compound Analysis in Clinical Practice: Current State and Future Perspectives. Chemosensors 2018, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Kinoyama, M.; Nitta, H.; Watanabe, A.; Ueda, H. Acetone and Isoprene Concentrations in Exhaled Breath in Healthy Subjects. J. Health Sci. 2008, 54, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Liu, Y.; Mao, Y.; Chen, W.; Xiao, Z.; Yu, Y. Relationship between Glycated Haemoglobin Concentration and Erythrocyte Survival in Type 2 Diabetes Mellitus Determined by a Modified Carbon Monoxide Breath Test. J. Breath Res. 2018, 12, 26004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, A.; Kim, H.W.; Kim, S.S.; Neri, G. Nanostructured Semiconducting Metal Oxide Gas Sensors for Acetaldehyde Detection. Chemosensors 2019, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Iitani, K.; Chien, P.J.; Suzuki, T.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Fiber-Optic Bio-Sniffer (Biochemical Gas Sensor) Using Reverse Reaction of Alcohol Dehydrogenase for Exhaled Acetaldehyde. ACS Sens. 2018, 3, 425–431. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Meriaudeau, F.; Ayodele, O.B.; Rabih, A.A.S. A Review of Biosensors for Non-Invasive Diabetes Monitoring and Screening in Human Exhaled Breath. IEEE Access 2019, 7, 5963–5974. [Google Scholar] [CrossRef]
- Stefanuto, P.H.; Zanella, D.; Vercammen, J.; Henket, M.; Schleich, F.; Louis, R.; Focant, J.F. Multimodal Combination of GC × GC-HRTOFMS and SIFT-MS for Asthma Phenotyping Using Exhaled Breath. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Ye, M.; Chien, P.J.; Toma, K.; Arakawa, T.; Mitsubayashi, K. An Acetone Bio-Sniffer (Gas Phase Biosensor) Enabling Assessment of Lipid Metabolism from Exhaled Breath. Biosens. Bioelectron. 2015, 73, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, T.; Kita, K.; Wang, X.; Miyajima, K.; Toma, K.; Mitsubayashi, K. Chemiluminescent Imaging of Transpired Ethanol from the Palm for Evaluation of Alcohol Metabolism. Biosens. Bioelectron. 2015, 67, 570–575. [Google Scholar] [CrossRef]
- Arakawa, T.; Sato, T.; Iitani, K.; Toma, K.; Mitsubayashi, K. Fluorometric Biosniffer Camera “Sniff-Cam” for Direct Imaging of Gaseous Ethanol in Breath and Transdermal Vapor. Anal. Chem. 2017, 89, 4495–4501. [Google Scholar] [CrossRef]
- Iitani, K.; Toma, K.; Arakawa, T.; Mitsubayashi, K. Ultrasensitive Sniff-Cam for Biofluorometric-Imaging of Breath Ethanol Caused by Metabolism of Intestinal Flora. Anal. Chem. 2019, 91, 9458–9465. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Ishihara, K. Cell Membrane-Inspired Phospholipid Polymers for Developing Medical Devices with Excellent Biointerfaces. Sci. Technol. Adv. Mater. 2012, 13, 064101. [Google Scholar] [CrossRef] [PubMed]
- Konvalina, G.; Haick, H. Sensors for Breath Testing: From Nanomaterials to Comprehensive Disease Detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arakawa, T.; Mizukoshi, N.; Iitani, K.; Toma, K.; Mitsubayashi, K. A Bio-Fluorometric Acetone Gas Imaging System for the Dynamic Analysis of Lipid Metabolism in Human Breath. Chemosensors 2021, 9, 258. https://doi.org/10.3390/chemosensors9090258
Arakawa T, Mizukoshi N, Iitani K, Toma K, Mitsubayashi K. A Bio-Fluorometric Acetone Gas Imaging System for the Dynamic Analysis of Lipid Metabolism in Human Breath. Chemosensors. 2021; 9(9):258. https://doi.org/10.3390/chemosensors9090258
Chicago/Turabian StyleArakawa, Takahiro, Naoki Mizukoshi, Kenta Iitani, Koji Toma, and Kohji Mitsubayashi. 2021. "A Bio-Fluorometric Acetone Gas Imaging System for the Dynamic Analysis of Lipid Metabolism in Human Breath" Chemosensors 9, no. 9: 258. https://doi.org/10.3390/chemosensors9090258





