Targeting Endoplasmic Reticulum Stress as an Effective Treatment for Alcoholic Pancreatitis
Abstract
:1. Acute and Chronic Pancreatitis
2. Alcohol Consumption and Pancreatitis
3. Animal and Cell Culture Models for Alcoholic Pancreatitis
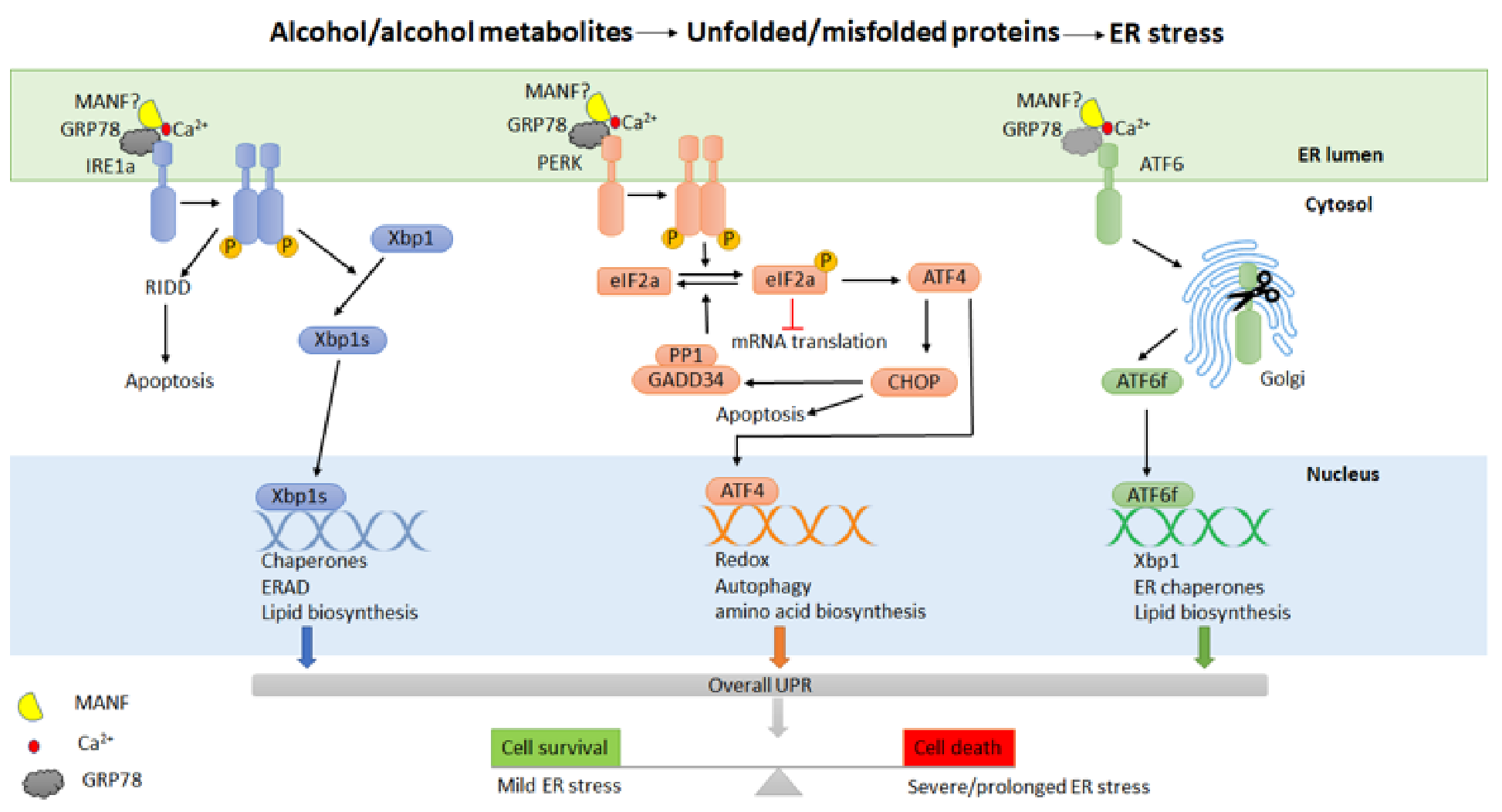
4. Endoplasmic Reticulum (ER) Stress and Unfolded Protein Response (UPR) in Alcohol-Related Pancreatitis
5. Potential Treatment of Alcoholic Pancreatitis by Targeting ER Stress and UPR
5.1. Small Molecules
5.2. Natural-Products-Derived Antioxidants
5.3. Gene Therapy
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | acute pancreatitis |
| CP | chronic pancreatitis |
| ER | endoplasmic reticulum |
| UPR | unfolded protein response |
| EPI | exocrine pancreatic insufficiency |
| PC | pancreatic cancer |
| DM | diabetes mellitus |
| CLDN2 | clauding 2 |
| CTRB1 | chymotrypsin B1 |
| CTRB2 | chymotrypsin B2 |
| ALDH2 | aldehyde dehydrogenase-2 |
| ADH1B | alcohol dehydrogenase-1B |
| ALD | alcoholic liver disease |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| LPS | lipopolysaccharides |
| CPA1 | carboxypeptidase A1 |
| TPP | thiamine pyrophosphate |
| ROS | reactive oxygen species |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| IRE1 | inositol-requiring kinase 1 |
| PERK | protein kinase-like ER kinase |
| GRP78 | 78 kDa glucose-regulated protein |
| eIF2 | eukaryotic translation initiation factor-2 |
| XBP1 | X box-binding protein 1 |
| ERAD | ER-associated degradation |
| RIDD | IRE1-dependent RNA decay |
| ATF6 | activating transcription factor 6 |
| ATF4 | activating transcription factor 4 |
| CHOP | C/EBP homologous protein |
| GADD34 | growth arrest and DNA damage-inducible protein 34 |
| MANF | mesencephalic astrocyte-derived neurotrophic factor |
| hPAC | human pancreatic acinar cells |
| ERAD | ER-associated degradation |
| 4-PBA | sodium phenylbutyrate |
| UDCA | ursodeoxycholic acid |
| TUDCA | tauroursodeoxycholic acid |
| CCK-8 | cholecystokinin-8 |
| GA | guanabenz acetate |
| TZD | trazodone |
| DBM | dibenzoylmethane |
| ALS | amyotrophic lateral sclerosis |
| OPMD | oculopharyngeal muscular dystrophy |
| HSPs | hereditary spastic paraplegias |
| SCI | spinal cord injury |
| AA 147 | compound 147 |
| AA 263 | compound 263 |
| CRAC | calcium release-activated calcium channel |
| AAV | adeno-associated virus |
| DA | nigral dopamine |
| TLCA3S | taurolithocholic acid 3-sulfate |
References
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, A.Y.; Tan, M.L.; Wu, L.M.; Asrani, V.M.; Windsor, J.A.; Yadav, D.; Petrov, M.S. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 2016, 1, 45–55. [Google Scholar] [CrossRef]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R.; et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012, 143, 1179–1187.e1173. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.; Lowenfels, A.B. Trends in the epidemiology of the first attack of acute pancreatitis: A systematic review. Pancreas 2006, 33, 323–330. [Google Scholar] [CrossRef]
- Weiss, F.U.; Laemmerhirt, F.; Lerch, M.M. Etiology and Risk Factors of Acute and Chronic Pancreatitis. Visc. Med. 2019, 35, 73–81. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Lowenfels, A.B.; Maisonneuve, P. What is the risk of alcoholic pancreatitis in heavy drinkers? Pancreas 2002, 25, 411–412. [Google Scholar] [CrossRef] [Green Version]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Bugiantella, W.; Rondelli, F.; Boni, M.; Stella, P.; Polistena, A.; Sanguinetti, A.; Avenia, N. Necrotizing pancreatitis: A review of the interventions. Int. J. Surg. 2016, 28 (Suppl. 1), S163–S171. [Google Scholar] [CrossRef]
- Ahmed Ali, U.; Issa, Y.; Hagenaars, J.C.; Bakker, O.J.; van Goor, H.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; Brink, M.A.; et al. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin. Gastroenterol. Hepatol. 2016, 14, 738–746. [Google Scholar] [CrossRef]
- Vipperla, K.; Papachristou, G.I.; Easler, J.; Muddana, V.; Slivka, A.; Whitcomb, D.C.; Yadav, D. Risk of and factors associated with readmission after a sentinel attack of acute pancreatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 1911–1919. [Google Scholar] [CrossRef]
- Hollemans, R.A.; Hallensleben, N.D.L.; Mager, D.J.; Kelder, J.C.; Besselink, M.G.; Bruno, M.J.; Verdonk, R.C.; van Santvoort, H.C. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology 2018, 18, 253–262. [Google Scholar] [CrossRef]
- Huang, W.; de la Iglesia-García, D.; Baston-Rey, I.; Calviño-Suarez, C.; Lariño-Noia, J.; Iglesias-Garcia, J.; Shi, N.; Zhang, X.; Cai, W.; Deng, L.; et al. Exocrine Pancreatic Insufficiency Following Acute Pancreatitis: Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2019, 64, 1985–2005. [Google Scholar] [CrossRef] [Green Version]
- Das, S.L.; Singh, P.P.; Phillips, A.R.; Murphy, R.; Windsor, J.A.; Petrov, M.S. Newly diagnosed diabetes mellitus after acute pancreatitis: A systematic review and meta-analysis. Gut 2014, 63, 818–831. [Google Scholar] [CrossRef]
- Shen, H.N.; Yang, C.C.; Chang, Y.H.; Lu, C.L.; Li, C.Y. Risk of Diabetes Mellitus after First-Attack Acute Pancreatitis: A National Population-Based Study. Am. J. Gastroenterol. 2015, 110, 1698–1706. [Google Scholar] [CrossRef]
- Sadr-Azodi, O.; Oskarsson, V.; Discacciati, A.; Videhult, P.; Askling, J.; Ekbom, A. Pancreatic Cancer Following Acute Pancreatitis: A Population-based Matched Cohort Study. Am. J. Gastroenterol. 2018, 113, 1711–1719. [Google Scholar] [CrossRef]
- Kirkegård, J.; Cronin-Fenton, D.; Heide-Jørgensen, U.; Mortensen, F.V. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology 2018, 154, 1729–1736. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Yadav, D.; Garg, P.K. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA 2019, 322, 2422–2434. [Google Scholar] [CrossRef]
- Majumder, S.; Chari, S.T. Chronic pancreatitis. Lancet 2016, 387, 1957–1966. [Google Scholar] [CrossRef]
- Lindkvist, B.; Domínguez-Muñoz, J.E.; Luaces-Regueira, M.; Castiñeiras-Alvariño, M.; Nieto-Garcia, L.; Iglesias-Garcia, J. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology 2012, 12, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Layer, P.; Yamamoto, H.; Kalthoff, L.; Clain, J.E.; Bakken, L.J.; DiMagno, E.P. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 1994, 107, 1481–1487. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodovicz, K.G.; Kou, T.D.; Alexander, C.M.; O’Neill, E.A.; Engel, S.S.; Girman, C.J.; Goldstein, B.J. Impact of diabetes duration and chronic pancreatitis on the association between type 2 diabetes and pancreatic cancer risk. Diabetes Obes. Metab. 2012, 14, 1123–1128. [Google Scholar] [CrossRef]
- Malka, D.; Hammel, P.; Sauvanet, A.; Rufat, P.; O’Toole, D.; Bardet, P.; Belghiti, J.; Bernades, P.; Ruszniewski, P.; Lévy, P. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology 2000, 119, 1324–1332. [Google Scholar] [CrossRef]
- Hardt, P.D.; Killinger, A.; Nalop, J.; Schnell-Kretschmer, H.; Zekorn, T.; Klör, H.U. Chronic pancreatitis and diabetes mellitus. A retrospective analysis of 156 ERCP investigations in patients with insulin-dependent and non-insulin-dependent diabetes mellitus. Pancreatology 2002, 2, 30–33. [Google Scholar] [CrossRef]
- Forsmark, C.E.; Vege, S.S.; Wilcox, C.M. Acute Pancreatitis. N. Engl. J. Med. 2016, 375, 1972–1981. [Google Scholar] [CrossRef]
- Kleeff, J.; Whitcomb, D.C.; Shimosegawa, T.; Esposito, I.; Lerch, M.M.; Gress, T.; Mayerle, J.; Drewes, A.M.; Rebours, V.; Akisik, F.; et al. Chronic pancreatitis. Nat. Reviews. Dis. Primers 2017, 3, 17060. [Google Scholar] [CrossRef]
- Bertilsson, S.; Swärd, P.; Kalaitzakis, E. Factors That Affect Disease Progression After First Attack of Acute Pancreatitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2015, 13, 1662–1669.e1663. [Google Scholar] [CrossRef]
- Dreiling, D.A.; Koller, M. The natural history of alcoholic pancreatitis: Update 1985. Mt. Sinai J. Med. N. Y. 1985, 52, 340–342. [Google Scholar]
- Kristiansen, L.; Grønbaek, M.; Becker, U.; Tolstrup, J.S. Risk of pancreatitis according to alcohol drinking habits: A population-based cohort study. Am. J. Epidemiol. 2008, 168, 932–937. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D. Recent advances in the epidemiology of alcoholic pancreatitis. Curr. Gastroenterol. Rep. 2011, 13, 157–165. [Google Scholar] [CrossRef]
- Corrao, G.; Bagnardi, V.; Zambon, A.; La Vecchia, C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev. Med. 2004, 38, 613–619. [Google Scholar] [CrossRef]
- Irving, H.M.; Samokhvalov, A.V.; Rehm, J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. JOP J. Pancreas 2009, 10, 387–392. [Google Scholar]
- Yadav, D.; Whitcomb, D.C. The role of alcohol and smoking in pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 131–145. [Google Scholar] [CrossRef]
- Yadav, D.; Hawes, R.H.; Brand, R.E.; Anderson, M.A.; Money, M.E.; Banks, P.A.; Bishop, M.D.; Baillie, J.; Sherman, S.; DiSario, J.; et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch. Intern. Med. 2009, 169, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Jaakkola, M.; Sillanaukee, P.; Löf, K.; Koivula, T.; Nordback, I. Amount of alcohol is an important determinant of the severity of acute alcoholic pancreatitis. Surgery 1994, 115, 31–38. [Google Scholar]
- Phillip, V.; Huber, W.; Hagemes, F.; Lorenz, S.; Matheis, U.; Preinfalk, S.; Schuster, T.; Lippl, F.; Saugel, B.; Schmid, R.M. Incidence of acute pancreatitis does not increase during Oktoberfest, but is higher than previously described in Germany. Clin. Gastroenterol. Hepatol. 2011, 9, 995–1000.e1003. [Google Scholar] [CrossRef]
- Takeyama, Y. Long-term prognosis of acute pancreatitis in Japan. Clin. Gastroenterol. Hepatol. 2009, 7, S15–S17. [Google Scholar] [CrossRef]
- Lankisch, M.R.; Imoto, M.; Layer, P.; DiMagno, E.P. The effect of small amounts of alcohol on the clinical course of chronic pancreatitis. Mayo Clin. Proc. 2001, 76, 242–251. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Pandol, S.J.; Porcel, J.; Wilkens, L.R.; Le Marchand, L.; Pike, M.C.; Monroe, K.R. Prospective Study of Alcohol Drinking, Smoking, and Pancreatitis: The Multiethnic Cohort. Pancreas 2016, 45, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Strum, W.B. Abstinence in alcoholic chronic pancreatitis. Effect on pain and outcome. J. Clin. Gastroenterol. 1995, 20, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Nikkola, J.; Räty, S.; Laukkarinen, J.; Seppänen, H.; Lappalainen-Lehto, R.; Järvinen, S.; Nordback, I.; Sand, J. Abstinence after first acute alcohol-associated pancreatitis protects against recurrent pancreatitis and minimizes the risk of pancreatic dysfunction. Alcohol Alcohol. 2013, 48, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Pelli, H.; Lappalainen-Lehto, R.; Piironen, A.; Sand, J.; Nordback, I. Risk factors for recurrent acute alcohol-associated pancreatitis: A prospective analysis. Scand. J. Gastroenterol. 2008, 43, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Pandol, S.J.; Lugea, A.; Mareninova, O.A.; Smoot, D.; Gorelick, F.S.; Gukovskaya, A.S.; Gukovsky, I. Investigating the pathobiology of alcoholic pancreatitis. Alcohol. Clin. Exp. Res. 2011, 35, 830–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadr-Azodi, O.; Andrén-Sandberg, Å.; Orsini, N.; Wolk, A. Cigarette smoking, smoking cessation and acute pancreatitis: A prospective population-based study. Gut 2012, 61, 262–267. [Google Scholar] [CrossRef]
- Lin, Y.; Tamakoshi, A.; Hayakawa, T.; Ogawa, M.; Ohno, Y. Cigarette smoking as a risk factor for chronic pancreatitis: A case-control study in Japan. Research Committee on Intractable Pancreatic Diseases. Pancreas 2000, 21, 109–114. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; Whitcomb, D.C.; Lerch, M.M.; DiMagno, E.P. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA 2001, 286, 169–170. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Colditz, G.A.; Stampfer, M.J.; Giovannucci, E.L.; Hunter, D.J.; Rimm, E.B.; Willett, W.C.; Speizer, F.E. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch. Intern. Med. 1996, 156, 2255–2260. [Google Scholar] [CrossRef]
- Greer, J.B.; Thrower, E.; Yadav, D. Epidemiologic and Mechanistic Associations Between Smoking and Pancreatitis. Curr. Treat. Options Gastroenterol. 2015, 13, 332–346. [Google Scholar] [CrossRef] [Green Version]
- Talamini, G.; Bassi, C.; Falconi, M.; Frulloni, L.; Di Francesco, V.; Vaona, B.; Bovo, P.; Rigo, L.; Castagnini, A.; Angelini, G.; et al. Cigarette smoking: An independent risk factor in alcoholic pancreatitis. Pancreas 1996, 12, 131–137. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Lowenfels, A.B.; Müllhaupt, B.; Cavallini, G.; Lankisch, P.G.; Andersen, J.R.; Dimagno, E.P.; Andrén-Sandberg, A.; Domellöf, L.; Frulloni, L.; et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut 2005, 54, 510–514. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, X.; Zeng, H.; He, W.; Xia, L.; Liu, P.; Zhu, Y.; Chen, Y.; Lv, N. A Study on the Etiology, Severity, and Mortality of 3260 Patients With Acute Pancreatitis According to the Revised Atlanta Classification in Jiangxi, China Over an 8-Year Period. Pancreas 2017, 46, 504–509. [Google Scholar] [CrossRef]
- Toskes, P.P. Hyperlipidemic pancreatitis. Gastroenterol. Clin. N. Am. 1990, 19, 783–791. [Google Scholar] [CrossRef]
- Vipperla, K.; Somerville, C.; Furlan, A.; Koutroumpakis, E.; Saul, M.; Chennat, J.; Rabinovitz, M.; Whitcomb, D.C.; Slivka, A.; Papachristou, G.I.; et al. Clinical Profile and Natural Course in a Large Cohort of Patients With Hypertriglyceridemia and Pancreatitis. J. Clin. Gastroenterol. 2017, 51, 77–85. [Google Scholar] [CrossRef]
- Klop, B.; do Rego, A.T.; Cabezas, M.C. Alcohol and plasma triglycerides. Curr. Opin. Lipidol. 2013, 24, 321–326. [Google Scholar] [CrossRef]
- Bessembinders, K.; Wielders, J.; van de Wiel, A. Severe hypertriglyceridemia influenced by alcohol (SHIBA). Alcohol Alcohol. 2011, 46, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Garg, R.; Rustagi, T. Management of Hypertriglyceridemia Induced Acute Pancreatitis. BioMed Res. Int. 2018, 2018, 4721357. [Google Scholar] [CrossRef] [Green Version]
- Fortson, M.R.; Freedman, S.N.; Webster, P.D., 3rd. Clinical assessment of hyperlipidemic pancreatitis. Am. J. Gastroenterol. 1995, 90, 2134–2139. [Google Scholar]
- Rosenthal, R.; Günzel, D.; Krug, S.M.; Schulzke, J.D.; Fromm, M.; Yu, A.S. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 2017, 219, 521–536. [Google Scholar] [CrossRef] [Green Version]
- Aung, P.P.; Mitani, Y.; Sanada, Y.; Nakayama, H.; Matsusaki, K.; Yasui, W. Differential expression of claudin-2 in normal human tissues and gastrointestinal carcinomas. Virchows Arch. 2006, 448, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; LaRusch, J.; Krasinskas, A.M.; Klei, L.; Smith, J.P.; Brand, R.E.; Neoptolemos, J.P.; Lerch, M.M.; Tector, M.; Sandhu, B.S.; et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat. Genet. 2012, 44, 1349–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosendahl, J.; Kirsten, H.; Hegyi, E.; Kovacs, P.; Weiss, F.U.; Laumen, H.; Lichtner, P.; Ruffert, C.; Chen, J.M.; Masson, E.; et al. Genome-wide association study identifies inversion in the CTRB1-CTRB2 locus to modify risk for alcoholic and non-alcoholic chronic pancreatitis. Gut 2018, 67, 1855–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.L.; Vadhavkar, S.; Singh, G.; Omary, M.B. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch. Intern. Med. 2008, 168, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.M.; Sandhu, B.S.; Singh, V.; Gelrud, A.; Abberbock, J.N.; Sherman, S.; Cote, G.A.; Al-Kaade, S.; Anderson, M.A.; Gardner, T.B.; et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. Am. J. Gastroenterol. 2016, 111, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Chang, H.Y.; Chiang, Y.T.; Wu, M.S.; Lin, J.T.; Liao, W.C. Smoking, drinking, and pancreatitis: A population-based cohort study in Taiwan. Pancreas 2014, 43, 1117–1122. [Google Scholar] [CrossRef]
- Samokhvalov, A.V.; Rehm, J.; Roerecke, M. Alcohol Consumption as a Risk Factor for Acute and Chronic Pancreatitis: A Systematic Review and a Series of Meta-analyses. EBioMedicine 2015, 2, 1996–2002. [Google Scholar] [CrossRef] [Green Version]
- Kume, K.; Masamune, A.; Ariga, H.; Shimosegawa, T. Alcohol Consumption and the Risk for Developing Pancreatitis: A Case-Control Study in Japan. Pancreas 2015, 44, 53–58. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, C.; Wu, D.C.; Lee, C.H.; Wu, C.I.; Lee, J.M.; Goan, Y.G.; Huang, S.P.; Lin, C.C.; Li, T.C.; et al. Interactive effects of lifetime alcohol consumption and alcohol and aldehyde dehydrogenase polymorphisms on esophageal cancer risks. Int. J. Cancer 2006, 119, 2827–2831. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, J.M.; Wu, D.C.; Goan, Y.G.; Chou, S.H.; Wu, I.C.; Kao, E.L.; Chan, T.F.; Huang, M.C.; Chen, P.S.; et al. Carcinogenetic impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of the esophagus with regard to the consumption of alcohol, tobacco and betel quid. Int. J. Cancer 2008, 122, 1347–1356. [Google Scholar] [CrossRef]
- Yokoyama, A.; Mizukami, T.; Matsui, T.; Yokoyama, T.; Kimura, M.; Matsushita, S.; Higuchi, S.; Maruyama, K. Genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and liver cirrhosis, chronic calcific pancreatitis, diabetes mellitus, and hypertension among Japanese alcoholic men. Alcohol. Clin. Exp. Res. 2013, 37, 1391–1401. [Google Scholar] [CrossRef]
- Gukovsky, I.; Lugea, A.; Shahsahebi, M.; Cheng, J.H.; Hong, P.P.; Jung, Y.J.; Deng, Q.G.; French, B.A.; Lungo, W.; French, S.W.; et al. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G68–G79. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.; Pietschmann, M.; Hartwig, W.; Hackert, T.; Marcos, S.S.; Longerich, T.; Gebhard, M.M.; Büchler, M.W.; Werner, J. Alcohol pretreatment increases hepatic and pulmonary injury in experimental pancreatitis. Pancreatology 2009, 9, 258–266. [Google Scholar] [CrossRef]
- Lugea, A.; Tischler, D.; Nguyen, J.; Gong, J.; Gukovsky, I.; French, S.W.; Gorelick, F.S.; Pandol, S.J. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 2011, 140, 987–997. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Wang, X.; Xu, M.; Yang, F.; Frank, J.A.; Ke, Z.J.; Luo, J. Binge ethanol exposure causes endoplasmic reticulum stress, oxidative stress and tissue injury in the pancreas. Oncotarget 2016, 7, 54303–54316. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Yang, F.; Wang, X.; Wang, Y.; Xu, M.; Frank, J.A.; Ke, Z.J.; Zhang, Z.; Shi, X.; Luo, J. Chronic plus binge ethanol exposure causes more severe pancreatic injury and inflammation. Toxicol. Appl. Pharmacol. 2016, 308, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ni, H.M.; Chao, X.; Ma, X.; Kolodecik, T.; De Lisle, R.; Ballabio, A.; Pacher, P.; Ding, W.X. Critical Role of TFEB-Mediated Lysosomal Biogenesis in Alcohol-Induced Pancreatitis in Mice and Humans. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 59–81. [Google Scholar] [CrossRef] [Green Version]
- Sloan, F.; Grossman, D.; Platt, A. Heavy episodic drinking in early adulthood and outcomes in midlife. J. Stud. Alcohol Drugs 2011, 72, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.; Runyon, B.A. Alcoholic hepatitis: A clinician’s guide. Clin. Liver Dis. 2012, 16, 371–385. [Google Scholar] [CrossRef]
- Mathurin, P.; Lucey, M.R. Management of alcoholic hepatitis. J. Hepatol. 2012, 56 (Suppl. 1), S39–S45. [Google Scholar] [CrossRef]
- Ki, S.H.; Park, O.; Zheng, M.; Morales-Ibanez, O.; Kolls, J.K.; Bataller, R.; Gao, B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: Role of signal transducer and activator of transcription 3. Hepatology 2010, 52, 1291–1300. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Xu, M.J.; Bertola, A.; Wang, H.; Zhou, Z.; Liangpunsakul, S. Animal Models of Alcoholic Liver Disease: Pathogenesis and Clinical Relevance. Gene Expr. 2017, 17, 173–186. [Google Scholar] [CrossRef]
- Lieber, C.S.; DeCarli, L.M. Ethanol oxidation by hepatic microsomes: Adaptive increase after ethanol feeding. Science 1968, 162, 917–918. [Google Scholar] [CrossRef]
- Mello, T.; Ceni, E.; Surrenti, C.; Galli, A. Alcohol induced hepatic fibrosis: Role of acetaldehyde. Mol. Asp. Med. 2008, 29, 17–21. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol research & health: The journal of the National Institute on Alcohol Abuse and Alcoholism 2006, 29, 245–254. [Google Scholar]
- Guerri, C.; Sanchis, R. Acetaldehyde and alcohol levels in pregnant rats and their fetuses. Alcohol 1985, 2, 267–270. [Google Scholar] [CrossRef]
- Eriksson, C.J.; Sippel, H.W. The distribution and metabolism of acetaldehyde in rats during ethanol oxidation-I. The distribution of acetaldehyde in liver, brain, blood and breath. Biochem. Pharmacol. 1977, 26, 241–247. [Google Scholar] [CrossRef]
- Laposata, E.A.; Lange, L.G. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science 1986, 231, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Laposata, M.; Fernández-del Castillo, C.; Saghir, M.; Iozzo, R.V.; Lewandrowski, K.B.; Warshaw, A.L. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology 1997, 113, 286–294. [Google Scholar] [CrossRef]
- Werner, J.; Saghir, M.; Warshaw, A.L.; Lewandrowski, K.B.; Laposata, M.; Iozzo, R.V.; Carter, E.A.; Schatz, R.J.; Fernández-Del Castillo, C. Alcoholic pancreatitis in rats: Injury from nonoxidative metabolites of ethanol. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G65–G73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Booth, D.M.; Cane, M.C.; Chvanov, M.; Javed, M.A.; Elliott, V.L.; Armstrong, J.A.; Dingsdale, H.; Cash, N.; Li, Y.; et al. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut 2014, 63, 1313–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampel, M.; Kern, H.F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Archiv. A Pathol. Anat. Histol. 1977, 373, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Wisner, J.; Green, D.; Ferrell, L.; Renner, I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut 1988, 29, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Pandol, S.J.; Periskic, S.; Gukovsky, I.; Zaninovic, V.; Jung, Y.; Zong, Y.; Solomon, T.E.; Gukovskaya, A.S.; Tsukamoto, H. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology 1999, 117, 706–716. [Google Scholar] [CrossRef]
- Rongione, A.J.; Kusske, A.M.; Kwan, K.; Ashley, S.W.; Reber, H.A.; McFadden, D.W. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology 1997, 112, 960–967. [Google Scholar] [CrossRef]
- Virlos, I.; Mazzon, E.; Serraino, I.; Di Paola, R.; Genovese, T.; Britti, D.; Thiemerman, C.; Siriwardena, A.; Cuzzocrea, S. Pyrrolidine dithiocarbamate reduces the severity of cerulein-induced murine acute pancreatitis. Shock 2003, 20, 544–550. [Google Scholar] [CrossRef]
- Niederau, C.; Ferrell, L.D.; Grendell, J.H. Caerulein-induced acute necrotizing pancreatitis in mice: Protective effects of proglumide, benzotript, and secretin. Gastroenterology 1985, 88, 1192–1204. [Google Scholar] [CrossRef]
- Demols, A.; Van Laethem, J.L.; Quertinmont, E.; Legros, F.; Louis, H.; Le Moine, O.; Devière, J. N-acetylcysteine decreases severity of acute pancreatitis in mice. Pancreas 2000, 20, 161–169. [Google Scholar] [CrossRef]
- Genovese, T.; Mazzon, E.; Di Paola, R.; Muià, C.; Crisafulli, C.; Menegazzi, M.; Malleo, G.; Suzuki, H.; Cuzzocrea, S. Hypericum perforatum attenuates the development of cerulein-induced acute pancreatitis in mice. Shock 2006, 25, 161–167. [Google Scholar] [CrossRef]
- Bartholomew, C. Acute scorpion pancreatitis in Trinidad. Br. Med. J. 1970, 1, 666–668. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Bhardwaj, U.; Verma, S.K.; Bhalla, A.; Gill, K. Hyperamylasemia and acute pancreatitis following anticholinesterase poisoning. Hum. Exp. Toxicol. 2007, 26, 467–471. [Google Scholar] [CrossRef]
- Becerril, B.; Marangoni, S.; Possani, L.D. Toxins and genes isolated from scorpions of the genus Tityus. Toxicon 1997, 35, 821–835. [Google Scholar] [CrossRef]
- Fletcher, P.L., Jr.; Fletcher, M.D.; Possani, L.D. Characteristics of pancreatic exocrine secretion produced by venom from the Brazilian scorpion, Tityus serrulatus. Eur. J. Cell Biol. 1992, 58, 259–270. [Google Scholar]
- Quon, M.G.; Kugelmas, M.; Wisner, J.R., Jr.; Chandrasoma, P.; Valenzuela, J.E. Chronic alcohol consumption intensifies caerulein-induced acute pancreatitis in the rat. Int. J. Pancreatol. 1992, 12, 31–39. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Burton, F.R.; Presti, M.E.; Britton, R.S.; Janney, C.G.; Garvin, P.R.; Brunt, E.M.; Galvin, N.J.; Poulos, J.E. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig. Dis. Sci. 2000, 45, 665–674. [Google Scholar] [CrossRef]
- Ulmasov, B.; Oshima, K.; Rodriguez, M.G.; Cox, R.D.; Neuschwander-Tetri, B.A. Differences in the degree of cerulein-induced chronic pancreatitis in C57BL/6 mouse substrains lead to new insights in identification of potential risk factors in the development of chronic pancreatitis. Am. J. Pathol. 2013, 183, 692–708. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Wang, L.; Elm, M.S.; Gabazadeh, D.; Diorio, G.J.; Eagon, P.K.; Whitcomb, D.C. Chronic alcohol consumption accelerates fibrosis in response to cerulein-induced pancreatitis in rats. Am. J. Pathol. 2005, 166, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Perides, G.; Tao, X.; West, N.; Sharma, A.; Steer, M.L. A mouse model of ethanol dependent pancreatic fibrosis. Gut 2005, 54, 1461–1467. [Google Scholar] [CrossRef] [Green Version]
- Vonlaufen, A.; Xu, Z.; Daniel, B.; Kumar, R.K.; Pirola, R.; Wilson, J.; Apte, M.V. Bacterial endotoxin: A trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology 2007, 133, 1293–1303. [Google Scholar] [CrossRef]
- Farhana, A.; Khan, Y.S. Biochemistry, Lipopolysaccharide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bode, C.; Kugler, V.; Bode, J.C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 1987, 4, 8–14. [Google Scholar] [CrossRef]
- Fukui, H.; Brauner, B.; Bode, J.C.; Bode, C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: Reevaluation with an improved chromogenic assay. J. Hepatol. 1991, 12, 162–169. [Google Scholar] [CrossRef]
- Ammori, B.J.; Leeder, P.C.; King, R.F.; Barclay, G.R.; Martin, I.G.; Larvin, M.; McMahon, M.J. Early increase in intestinal permeability in patients with severe acute pancreatitis: Correlation with endotoxemia, organ failure, and mortality. J. Gastrointest. Surg. 1999, 3, 252–262. [Google Scholar] [CrossRef]
- Fortunato, F.; Deng, X.; Gates, L.K.; McClain, C.J.; Bimmler, D.; Graf, R.; Whitcomb, D.C. Pancreatic response to endotoxin after chronic alcohol exposure: Switch from apoptosis to necrosis? Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G232–G241. [Google Scholar] [CrossRef] [Green Version]
- Vonlaufen, A.; Phillips, P.A.; Xu, Z.; Zhang, X.; Yang, L.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Withdrawal of alcohol promotes regression while continued alcohol intake promotes persistence of LPS-induced pancreatic injury in alcohol-fed rats. Gut 2011, 60, 238–246. [Google Scholar] [CrossRef]
- Gu, H.; Werner, J.; Bergmann, F.; Whitcomb, D.C.; Büchler, M.W.; Fortunato, F. Necro-inflammatory response of pancreatic acinar cells in the pathogenesis of acute alcoholic pancreatitis. Cell Death Dis. 2013, 4, e816. [Google Scholar] [CrossRef] [Green Version]
- Grauvogel, J.; Daemmrich, T.D.; Ryschich, E.; Gebhard, M.M.; Werner, J. Chronic alcohol intake increases the severity of pancreatitis induced by acute alcohol administration, hyperlipidemia and pancreatic duct obstruction in rats. Pancreatology 2010, 10, 603–612. [Google Scholar] [CrossRef]
- Tanaka, T.; Miura, Y.; Matsugu, Y.; Ichiba, Y.; Ito, H.; Dohi, K. Pancreatic duct obstruction is an aggravating factor in the canine model of chronic alcoholic pancreatitis. Gastroenterology 1998, 115, 1248–1253. [Google Scholar] [CrossRef]
- Orekhova, A.; Geisz, A.; Sahin-Tóth, M. Ethanol feeding accelerates pancreatitis progression in CPA1 N256K mutant mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G694–G704. [Google Scholar] [CrossRef]
- Sun, J.; Fu, J.; Zhong, Y.; Li, L.; Chen, C.; Wang, X.; Wang, L.; Hou, Y.; Wang, H.; Zhao, R.; et al. NRF2 mitigates acute alcohol-induced hepatic and pancreatic injury in mice. Food Chem. Toxicol. 2018, 121, 495–503. [Google Scholar] [CrossRef]
- Amsterdam, A.; Jamieson, J.D. Structural and functional characterization of isolated pancreatic exocrine cells. Proc. Natl. Acad. Sci. USA 1972, 69, 3028–3032. [Google Scholar] [CrossRef] [Green Version]
- Saluja, A.; Dudeja, V.; Dawra, R.; Sah, R.P. Early Intra-Acinar Events in Pathogenesis of Pancreatitis. Gastroenterology 2019, 156, 1979–1993. [Google Scholar] [CrossRef]
- Steer, M.L. Early events in acute pancreatitis. Best Pract. Res.. Clin. Gastroenterol. 1999, 13, 213–225. [Google Scholar] [CrossRef]
- Gorelick, F.S.; Thrower, E. The acinar cell and early pancreatitis responses. Clin. Gastroenterol. Hepatol 2009, 7, S10–S14. [Google Scholar] [CrossRef] [Green Version]
- Subramanya, S.B.; Subramanian, V.S.; Sekar, V.T.; Said, H.M. Thiamin uptake by pancreatic acinar cells: Effect of chronic alcohol feeding/exposure. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G896–G904. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, P.; Nabokina, S.; Said, H.M. Chronic alcohol exposure affects pancreatic acinar mitochondrial thiamin pyrophosphate uptake: Studies with mouse 266-6 cell line and primary cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G750–G758. [Google Scholar] [CrossRef] [Green Version]
- Singh, M. Effect of thiamin deficiency on pancreatic acinar cell function. Am. J. Clin. Nutr. 1982, 36, 500–504. [Google Scholar] [CrossRef]
- Rathanaswami, P.; Pourany, A.; Sundaresan, R. Effects of thiamine deficiency on the secretion of insulin and the metabolism of glucose in isolated rat pancreatic islets. Biochem. Int. 1991, 25, 577–583. [Google Scholar]
- Rathanaswami, P.; Sundaresan, R. Effects of thiamine deficiency on the biosynthesis of insulin in rats. Biochem. Int. 1991, 24, 1057–1062. [Google Scholar]
- Wu, H.; Bhopale, K.K.; Ansari, G.A.; Kaphalia, B.S. Ethanol-induced cytotoxicity in rat pancreatic acinar AR42J cells: Role of fatty acid ethyl esters. Alcohol Alcohol. 2008, 43, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lugea, A.; Gerloff, A.; Su, H.Y.; Xu, Z.; Go, A.; Hu, C.; French, S.W.; Wilson, J.S.; Apte, M.V.; Waldron, R.T.; et al. The Combination of Alcohol and Cigarette Smoke Induces Endoplasmic Reticulum Stress and Cell Death in Pancreatic Acinar Cells. Gastroenterology 2017, 153, 1674–1686. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Pariente, J.A.; Salido, G.M. Ethanol impairs calcium homeostasis following CCK-8 stimulation in mouse pancreatic acinar cells. Alcohol 2008, 42, 565–573. [Google Scholar] [CrossRef]
- Fernández-Sánchez, M.; del Castillo-Vaquero, A.; Salido, G.M.; González, A. Ethanol exerts dual effects on calcium homeostasis in CCK-8-stimulated mouse pancreatic acinar cells. BMC Cell Biol. 2009, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Núñez, A.M.; Granados, M.P.; Pariente, J.A.; Salido, G.M. Ethanol impairs CCK-8-evoked amylase secretion through Ca2+-mediated ROS generation in mouse pancreatic acinar cells. Alcohol 2006, 38, 51–57. [Google Scholar] [CrossRef]
- Orabi, A.I.; Shah, A.U.; Muili, K.; Luo, Y.; Mahmood, S.M.; Ahmad, A.; Reed, A.; Husain, S.Z. Ethanol enhances carbachol-induced protease activation and accelerates Ca2+ waves in isolated rat pancreatic acini. J. Biol. Chem. 2011, 286, 14090–14097. [Google Scholar] [CrossRef] [Green Version]
- Hegyi, P.; Pandol, S.; Venglovecz, V.; Rakonczay, Z., Jr. The acinar-ductal tango in the pathogenesis of acute pancreatitis. Gut 2011, 60, 544–552. [Google Scholar] [CrossRef]
- Wilschanski, M.; Novak, I. The cystic fibrosis of exocrine pancreas. Cold Spring Harb. Perspect. Med. 2013, 3, a009746. [Google Scholar] [CrossRef] [PubMed]
- Larusch, J.; Whitcomb, D.C. Genetics of pancreatitis with a focus on the pancreatic ducts. Minerva Gastroenterol. E Dietol. 2012, 58, 299–308. [Google Scholar]
- Sarles, H.; Sarles, J.C.; Camatte, R.; Muratore, R.; Gaini, M.; Guien, C.; Pastor, J.; Le Roy, F. Observations on 205 confirmed cases of acute pancreatitis, recurring pancreatitis, and chronic pancreatitis. Gut 1965, 6, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Sharer, N.; Schwarz, M.; Malone, G.; Howarth, A.; Painter, J.; Super, M.; Braganza, J. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N. Engl. J. Med. 1998, 339, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Maléth, J.; Balázs, A.; Pallagi, P.; Balla, Z.; Kui, B.; Katona, M.; Judák, L.; Németh, I.; Kemény, L.V.; Rakonczay, Z., Jr.; et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology 2015, 148, 427–439.e416. [Google Scholar] [CrossRef] [Green Version]
- Schröder, M. Endoplasmic reticulum stress responses. Cell. Mol. Life Sci. CMLS 2008, 65, 862–894. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Parra, V.; Gatica, D.; Rodriguez, A.E.; Torrealba, N.; Paredes, F.; Wang, Z.V.; Zorzano, A.; Hill, J.A.; Jaimovich, E.; et al. Endoplasmic reticulum and the unfolded protein response: Dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 2013, 301, 215–290. [Google Scholar] [CrossRef] [Green Version]
- Kubisch, C.H.; Sans, M.D.; Arumugam, T.; Ernst, S.A.; Williams, J.A.; Logsdon, C.D. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G238–G245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, B.; Chen, X.Q.; Misek, D.E.; Kuick, R.; Hanash, S.; Ernst, S.; Najarian, R.; Logsdon, C.D. Pancreatic gene expression during the initiation of acute pancreatitis: Identification of EGR-1 as a key regulator. Physiol. Genom. 2003, 14, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Sah, R.P.; Garg, S.K.; Dixit, A.K.; Dudeja, V.; Dawra, R.K.; Saluja, A.K. Endoplasmic reticulum stress is chronically activated in chronic pancreatitis. J. Biol. Chem. 2014, 289, 27551–27561. [Google Scholar] [CrossRef] [Green Version]
- Kereszturi, E.; Szmola, R.; Kukor, Z.; Simon, P.; Weiss, F.U.; Lerch, M.M.; Sahin-Tóth, M. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: A novel disease mechanism. Hum. Mutat. 2009, 30, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Le Maréchal, C.; Bretagne, J.F.; Raguénès, O.; Quéré, I.; Chen, J.M.; Ferec, C. Identification of a novel pancreatitis-associated missense mutation, R116C, in the human cationic trypsinogen gene (PRSS1). Mol. Genet. Metab. 2001, 74, 342–344. [Google Scholar] [CrossRef]
- Tautermann, G.; Ruebsamen, H.; Beck, M.; Dertinger, S.; Drexel, H.; Lohse, P. R116C mutation of cationic trypsinogen in a Turkish family with recurrent pancreatitis illustrates genetic microheterogeneity of hereditary pancreatitis. Digestion 2001, 64, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Pho-Iam, T.; Thongnoppakhun, W.; Yenchitsomanus, P.T.; Limwongse, C. A Thai family with hereditary pancreatitis and increased cancer risk due to a mutation in PRSS1 gene. World J. Gastroenterol. 2005, 11, 1634–1638. [Google Scholar] [CrossRef]
- Kubisch, C.H.; Gukovsky, I.; Lugea, A.; Pandol, S.J.; Kuick, R.; Misek, D.E.; Hanash, S.M.; Logsdon, C.D. Long-term ethanol consumption alters pancreatic gene expression in rats: A possible connection to pancreatic injury. Pancreas 2006, 33, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Waldron, R.T.; Su, H.Y.; Piplani, H.; Capri, J.; Cohn, W.; Whitelegge, J.P.; Faull, K.F.; Sakkiah, S.; Abrol, R.; Yang, W.; et al. Ethanol Induced Disordering of Pancreatic Acinar Cell Endoplasmic Reticulum: An ER Stress/Defective Unfolded Protein Response Model. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 479–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, R.J. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 2002, 110, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Reviews. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Reviews. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H. ER stress and diseases. FEBS J. 2007, 274, 630–658. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Reviews. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Howell, S.H. Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 2013, 64, 477–499. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.S.; Shamu, C.E.; Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 1993, 73, 1197–1206. [Google Scholar] [CrossRef]
- Wang, X.Z.; Harding, H.P.; Zhang, Y.; Jolicoeur, E.M.; Kuroda, M.; Ron, D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998, 17, 5708–5717. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.H.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003, 23, 7448–7459. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Alvear, D.; Zhou, Y.; Blais, A.; Tsikitis, M.; Lents, N.H.; Arias, C.; Lennon, C.J.; Kluger, Y.; Dynlacht, B.D. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 2007, 27, 53–66. [Google Scholar] [CrossRef]
- Maurel, M.; Chevet, E.; Tavernier, J.; Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014, 39, 245–254. [Google Scholar] [CrossRef]
- Han, D.; Lerner, A.G.; Vande Walle, L.; Upton, J.P.; Xu, W.; Hagen, A.; Backes, B.J.; Oakes, S.A.; Papa, F.R. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 2009, 138, 562–575. [Google Scholar] [CrossRef] [Green Version]
- Hollien, J.; Weissman, J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 2006, 313, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.H.; Chu, G.C.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005, 24, 4368–4380. [Google Scholar] [CrossRef] [Green Version]
- Iwawaki, T.; Akai, R.; Kohno, K. IRE1α disruption causes histological abnormality of exocrine tissues, increase of blood glucose level, and decrease of serum immunoglobulin level. PLoS ONE 2010, 5, e13052. [Google Scholar] [CrossRef] [Green Version]
- Hess, D.A.; Humphrey, S.E.; Ishibashi, J.; Damsz, B.; Lee, A.H.; Glimcher, L.H.; Konieczny, S.F. Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice. Gastroenterology 2011, 141, 1463–1472. [Google Scholar] [CrossRef] [Green Version]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef]
- Shi, Y.; Vattem, K.M.; Sood, R.; An, J.; Liang, J.; Stramm, L.; Wek, R.C. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 1998, 18, 7499–7509. [Google Scholar] [CrossRef] [Green Version]
- Harding, H.P.; Zhang, Y.; Bertolotti, A.; Zeng, H.; Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 2000, 5, 897–904. [Google Scholar] [CrossRef]
- Ameri, K.; Harris, A.L. Activating transcription factor 4. Int. J. Biochem. Cell Biol. 2008, 40, 14–21. [Google Scholar] [CrossRef]
- Luhr, M.; Torgersen, M.L.; Szalai, P.; Hashim, A.; Brech, A.; Staerk, J.; Engedal, N. The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress. J. Biol. Chem. 2019, 294, 8197–8217. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef] [Green Version]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.L.; Flockhart, R.; Veal, G.J.; Lovat, P.E.; Redfern, C.P. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J. Biol. Chem. 2010, 285, 6091–6100. [Google Scholar] [CrossRef] [Green Version]
- Novoa, I.; Zeng, H.; Harding, H.P.; Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 2001, 153, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; McGrath, B.; Li, S.; Frank, A.; Zambito, F.; Reinert, J.; Gannon, M.; Ma, K.; McNaughton, K.; Cavener, D.R. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 2002, 22, 3864–3874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, H.P.; Zeng, H.; Zhang, Y.; Jungries, R.; Chung, P.; Plesken, H.; Sabatini, D.D.; Ron, D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol. Cell 2001, 7, 1153–1163. [Google Scholar] [CrossRef]
- Casini, A.; Galli, A.; Pignalosa, P.; Frulloni, L.; Grappone, C.; Milani, S.; Pederzoli, P.; Cavallini, G.; Surrenti, C. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J. Pathol. 2000, 192, 81–89. [Google Scholar] [CrossRef]
- Iida, K.; Li, Y.; McGrath, B.C.; Frank, A.; Cavener, D.R. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 2007, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senkal, C.E.; Ponnusamy, S.; Bielawski, J.; Hannun, Y.A.; Ogretmen, B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010, 24, 296–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Rutkowski, D.T.; Dubois, M.; Swathirajan, J.; Saunders, T.; Wang, J.; Song, B.; Yau, G.D.; Kaufman, R.J. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 2007, 13, 351–364. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Sato, T.; Matsui, T.; Sato, M.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell 2007, 13, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Tan, J.H.; Cao, R.C.; Xu, J.; Chen, X.M.; Qi, Z.C.; Zhou, S.Y.; Li, S.B.; Mo, Q.X.; Li, Z.W.; et al. ATF6 regulates the development of chronic pancreatitis by inducing p53-mediated apoptosis. Cell Death Dis. 2019, 10, 662. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Matsui, T.; Hosokawa, N.; Kaufman, R.J.; Nagata, K.; Mori, K. A time-dependent phase shift in the mammalian unfolded protein response. Dev. Cell 2003, 4, 265–271. [Google Scholar] [CrossRef] [Green Version]
- DuRose, J.B.; Tam, A.B.; Niwa, M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol. Biol. Cell 2006, 17, 3095–3107. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, M.P.; Bhopale, K.K.; Caracheo, A.A.; Amer, S.M.; Khan, S.; Kaphalia, L.; Loganathan, G.; Balamurugan, A.N.; Kaphalia, B.S. Activation of AMP-activated protein kinase attenuates ethanol-induced ER/oxidative stress and lipid phenotype in human pancreatic acinar cells. Biochem. Pharmacol. 2020, 180, 114174. [Google Scholar] [CrossRef]
- Halliday, M.; Radford, H.; Zents, K.A.M.; Molloy, C.; Moreno, J.A.; Verity, N.C.; Smith, E.; Ortori, C.A.; Barrett, D.A.; Bushell, M.; et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain 2017, 140, 1768–1783. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C.; Axten, J.M.; Patterson, J.B. Pharmacological targeting of the unfolded protein response for disease intervention. Nat. Chem. Biol. 2019, 15, 764–775. [Google Scholar] [CrossRef]
- Jian, L.; Lu, Y.; Lu, S.; Lu, C. Chemical Chaperone 4-Phenylbutyric Acid Reduces Cardiac Ischemia/Reperfusion Injury by Alleviating Endoplasmic Reticulum Stress and Oxidative Stress. Med. Sci. Monit. 2016, 22, 5218–5227. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.P.; Deng, W.H.; Guo, W.Y.; Shi, Q.; Zhao, L.; You, Y.D.; Mei, F.C.; Zhou, Y.; Wang, C.Y.; Chen, C.; et al. Inhibition of endoplasmic reticulum stress by 4-phenylbutyric acid prevents vital organ injury in rat acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G838–G847. [Google Scholar] [CrossRef]
- Poupon, R.E.; Balkau, B.; Eschwège, E.; Poupon, R.; UDCA-PBC Study Group. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. N. Engl. J. Med. 1997, 324, 1548–1554. [Google Scholar] [CrossRef]
- Ros, E.; Navarro, S.; Bru, C.; Garcia-Pugés, A.; Valderrama, R. Occult microlithiasis in ‘idiopathic’ acute pancreatitis: Prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 1991, 101, 1701–1709. [Google Scholar] [CrossRef]
- Testoni, P.A.; Caporuscio, S.; Bagnolo, F.; Lella, F. Idiopathic recurrent pancreatitis: Long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am. J. Gastroenterol. 2000, 95, 1702–1707. [Google Scholar] [CrossRef]
- Seyhun, E.; Malo, A.; Schäfer, C.; Moskaluk, C.A.; Hoffmann, R.T.; Göke, B.; Kubisch, C.H. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, acinar cell damage, and systemic inflammation in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G773–G782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, M.; Bryant, K.F.; Jousse, C.; Long, K.; Harding, H.P.; Scheuner, D.; Kaufman, R.J.; Ma, D.; Coen, D.M.; Ron, D.; et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 2005, 307, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Mallucci, G.R. The unfolded protein response in neurodegenerative disorders—Therapeutic modulation of the PERK pathway. FEBS J. 2019, 286, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Popko, B.; Tixier, E.; Roos, R.P. Guanabenz, which enhances the unfolded protein response, ameliorates mutant SOD1-induced amyotrophic lateral sclerosis. Neurobiol. Dis. 2014, 71, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Ohri, S.S.; Mullins, A.; Hetman, M.; Whittemore, S.R. Inhibition of GADD34, the stress-inducible regulatory subunit of the endoplasmic reticulum stress response, does not enhance functional recovery after spinal cord injury. PLoS ONE 2014, 9, e109703. [Google Scholar] [CrossRef]
- Julien, C.; Lissouba, A.; Madabattula, S.; Fardghassemi, Y.; Rosenfelt, C.; Androschuk, A.; Strautman, J.; Wong, C.; Bysice, A.; O’Sullivan, J.; et al. Conserved pharmacological rescue of hereditary spastic paraplegia-related phenotypes across model organisms. Hum. Mol. Genet. 2016, 25, 1088–1099. [Google Scholar] [CrossRef] [Green Version]
- Abdulkarim, B.; Hernangomez, M.; Igoillo-Esteve, M.; Cunha, D.A.; Marselli, L.; Marchetti, P.; Ladriere, L.; Cnop, M. Guanabenz Sensitizes Pancreatic β Cells to Lipotoxic Endoplasmic Reticulum Stress and Apoptosis. Endocrinology 2017, 158, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Chien, W.; Ding, L.W.; Sun, Q.Y.; Torres-Fernandez, L.A.; Tan, S.Z.; Xiao, J.; Lim, S.L.; Garg, M.; Lee, K.L.; Kitajima, S.; et al. Selective inhibition of unfolded protein response induces apoptosis in pancreatic cancer cells. Oncotarget 2014, 5, 4881–4894. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Li, H.; Wen, W.; Wang, Y.; Xu, H.; Xu, M.; Frank, J.A.; Wei, W.; Luo, J. MANF protects pancreatic acinar cells against alcohol-induced endoplasmic reticulum stress and cellular injury. J. Hepato-Biliary-Pancreat. Sci. 2021, 28, 883–892. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Zheng, J.; Li, T.; Gao, L.; Lenahan, C.; Shao, A.; Zhang, J.; Yu, J. Melatonin Protects Against Neuronal Apoptosis via Suppression of the ATF6/CHOP Pathway in a Rat Model of Intracerebral Hemorrhage. Front. Neurosci. 2018, 12, 638. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, J.; Zhao, Q.; Chen, Q.; Sun, Y.; Jin, Y.; Wu, J. Melatonin Induces Anti-Inflammatory Effects to Play a Protective Role via Endoplasmic Reticulum Stress in Acute Pancreatitis. Cell. Physiol. Biochem. 2016, 40, 1094–1104. [Google Scholar] [CrossRef]
- Kudo, T.; Kanemoto, S.; Hara, H.; Morimoto, N.; Morihara, T.; Kimura, R.; Tabira, T.; Imaizumi, K.; Takeda, M. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008, 15, 364–375. [Google Scholar] [CrossRef] [Green Version]
- Oida, Y.; Izuta, H.; Oyagi, A.; Shimazawa, M.; Kudo, T.; Imaizumi, K.; Hara, H. Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in gerbil. Brain Res. 2008, 1208, 217–224. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, H.; Huang, J.; Yu, H.; Li, J.; Che, Q.; Sun, Y.; Jin, Y.; Wu, J. Melatonin attenuates the inflammatory response via inhibiting the C/EBP homologous protein-mediated pathway in taurocholate-induced acute pancreatitis. Int. J. Mol. Med. 2018, 42, 3513–3521. [Google Scholar] [CrossRef] [Green Version]
- Petersen, O.H.; Tepikin, A.V.; Gerasimenko, J.V.; Gerasimenko, O.V.; Sutton, R.; Criddle, D.N. Fatty acids, alcohol and fatty acid ethyl esters: Toxic Ca2+ signal generation and pancreatitis. Cell Calcium 2009, 45, 634–642. [Google Scholar] [CrossRef]
- Gerasimenko, J.V.; Gryshchenko, O.; Ferdek, P.E.; Stapleton, E.; Hébert, T.O.; Bychkova, S.; Peng, S.; Begg, M.; Gerasimenko, O.V.; Petersen, O.H. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc. Natl. Acad. Sci. USA 2013, 110, 13186–13191. [Google Scholar] [CrossRef] [Green Version]
- Waldron, R.T.; Chen, Y.; Pham, H.; Go, A.; Su, H.Y.; Hu, C.; Wen, L.; Husain, S.Z.; Sugar, C.A.; Roos, J.; et al. The Orai Ca(2+) channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J. Physiol. 2019, 597, 3085–3105. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, T. Unveiling some FDA-approved drugs as inhibitors of the store-operated Ca(2+) entry pathway. Sci. Rep. 2017, 7, 12881. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C.; Chevet, E.; Harding, H.P. Targeting the unfolded protein response in disease. Nat. Reviews. Drug Discov. 2013, 12, 703–719. [Google Scholar] [CrossRef]
- Perlmutter, D.H. Chemical chaperones: A pharmacological strategy for disorders of protein folding and trafficking. Pediatric Res. 2002, 52, 832–836. [Google Scholar] [CrossRef]
- Lichter-Konecki, U.; Diaz, G.A.; Merritt, J.L., 2nd; Feigenbaum, A.; Jomphe, C.; Marier, J.F.; Beliveau, M.; Mauney, J.; Dickinson, K.; Martinez, A.; et al. Ammonia control in children with urea cycle disorders (UCDs); phase 2 comparison of sodium phenylbutyrate and glycerol phenylbutyrate. Mol. Genet. Metab. 2011, 103, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, J.P.; Mokhtarani, M.; Diaz, G.A.; Rhead, W.; Lichter-Konecki, U.; Berry, S.A.; Lemons, C.; Dickinson, K.; Coakley, D.; Lee, B.; et al. Population pharmacokinetic modeling and dosing simulations of nitrogen-scavenging compounds: Disposition of glycerol phenylbutyrate and sodium phenylbutyrate in adult and pediatric patients with urea cycle disorders. J. Clin. Pharmacol. 2013, 53, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wen, W.; Xu, H.; Wu, H.; Xu, M.; Frank, J.A.; Luo, J. 4-Phenylbutyric Acid Protects Against Ethanol-Induced Damage in the Developing Mouse Brain. Alcohol. Clin. Exp. Res. 2019, 43, 69–78. [Google Scholar] [CrossRef]
- Reddy, S.S.; Shruthi, K.; Joy, D.; Reddy, G.B. 4-PBA prevents diabetic muscle atrophy in rats by modulating ER stress response and ubiquitin-proteasome system. Chem.-Biol. Interact. 2019, 306, 70–77. [Google Scholar] [CrossRef]
- Malo, A.; Krüger, B.; Göke, B.; Kubisch, C.H. 4-Phenylbutyric acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Pancreas 2013, 42, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.F. Medical dissolution of gallstones by oral bile acid therapy. Am. J. Surg. 1989, 158, 198–204. [Google Scholar] [CrossRef]
- Jüngst, C.; Kullak-Ublick, G.A.; Jüngst, D. Gallstone disease: Microlithiasis and sludge. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 1053–1062. [Google Scholar] [CrossRef]
- Malo, A.; Krüger, B.; Seyhun, E.; Schäfer, C.; Hoffmann, R.T.; Göke, B.; Kubisch, C.H. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G877–G886. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Rozpędek-Kamińska, W.; Siwecka, N.; Wawrzynkiewicz, A.; Wojtczak, R.; Pytel, D.; Diehl, J.A.; Majsterek, I. The PERK-Dependent Molecular Mechanisms as a Novel Therapeutic Target for Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 2108. [Google Scholar] [CrossRef] [Green Version]
- Aoi, K.; Nishio, A.; Okazaki, T.; Takeo, M.; Masuda, M.; Fukui, T.; Uchida, K.; Okazaki, K. Inhibition of the dephosphorylation of eukaryotic initiation factor 2α ameliorates murine experimental pancreatitis. Pancreatology 2019, 19, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Ladriere, L.; Hekerman, P.; Ortis, F.; Cardozo, A.K.; Dogusan, Z.; Flamez, D.; Boyce, M.; Yuan, J.; Eizirik, D.L. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J. Biol. Chem. 2007, 282, 3989–3997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malerba, A.; Roth, F.; Harish, P.; Dhiab, J.; Lu-Nguyen, N.; Cappellari, O.; Jarmin, S.; Mahoudeau, A.; Ythier, V.; Lainé, J.; et al. Pharmacological modulation of the ER stress response ameliorates oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 2019, 28, 1694–1708. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Ninov, N.; Delawary, M.; Osman, S.; Roh, A.S.; Gut, P.; Stainier, D.Y. Whole organism high content screening identifies stimulators of pancreatic beta-cell proliferation. PLoS ONE 2014, 9, e104112. [Google Scholar] [CrossRef] [Green Version]
- Janiri, L.; Hadjichristos, A.; Buonanno, A.; Rago, R.; Mannelli, P.; de Risio, S. Adjuvant trazodone in the treatment of alcoholism: An open study. Alcohol Alcohol. 1998, 33, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Roccatagliata, G.; Albano, C.; Maffini, M.; Farelli, S. Alcohol withdrawal syndrome: Treatment with trazodone. Int. Pharm. 1980, 15, 105–110. [Google Scholar] [CrossRef]
- Friedmann, P.D.; Rose, J.S.; Swift, R.; Stout, R.L.; Millman, R.P.; Stein, M.D. Trazodone for sleep disturbance after alcohol detoxification: A double-blind, placebo-controlled trial. Alcohol. Clin. Exp. Res. 2008, 32, 1652–1660. [Google Scholar] [CrossRef] [Green Version]
- Lugea, A.; Waldron, R.T.; French, S.W.; Pandol, S.J. Drinking and driving pancreatitis: Links between endoplasmic reticulum stress and autophagy. Autophagy 2011, 7, 783–785. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Niwa, M.; Koong, A.C. Targeting the IRE1α-XBP1 branch of the unfolded protein response in human diseases. Semin. Cancer Biol. 2015, 33, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, R.; Wang, L.; Wang, E.S.; Perera, B.G.; Igbaria, A.; Morita, S.; Prado, K.; Thamsen, M.; Caswell, D.; Macias, H.; et al. Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 2014, 158, 534–548. [Google Scholar] [CrossRef] [Green Version]
- Maly, D.J.; Papa, F.R. Druggable sensors of the unfolded protein response. Nat. Chem. Biol. 2014, 10, 892–901. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, C.M.; Garri, C.; Cain, E.L.; Ang, K.K.; Wilson, C.G.; Chen, S.; Hearn, B.R.; Jaishankar, P.; Aranda-Diaz, A.; Arkin, M.R.; et al. Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6α branch. eLife 2016, 5. [Google Scholar] [CrossRef]
- Blackwood, E.A.; Azizi, K.; Thuerauf, D.J.; Paxman, R.J.; Plate, L.; Kelly, J.W.; Wiseman, R.L.; Glembotski, C.C. Pharmacologic ATF6 activation confers global protection in widespread disease models by reprograming cellular proteostasis. Nat. Commun. 2019, 10, 187. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.K.; Blackwood, E.A.; Azizi, K.; Thuerauf, D.J.; Fahem, A.G.; Hofmann, C.; Kaufman, R.J.; Doroudgar, S.; Glembotski, C.C. ATF6 Decreases Myocardial Ischemia/Reperfusion Damage and Links ER Stress and Oxidative Stress Signaling Pathways in the Heart. Circ. Res. 2017, 120, 862–875. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Sheng, H.; Liu, S.; Zhao, S.; Glembotski, C.C.; Warner, D.S.; Paschen, W.; Yang, W. Activation of the ATF6 branch of the unfolded protein response in neurons improves stroke outcome. J. Cereb. Blood Flow Metab. 2017, 37, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C.; Chevet, E.; Oakes, S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Plate, L.; Wiseman, R.L. Regulating Secretory Proteostasis through the Unfolded Protein Response: From Function to Therapy. Trends Cell Biol. 2017, 27, 722–737. [Google Scholar] [CrossRef]
- Plate, L.; Cooley, C.B.; Chen, J.J.; Paxman, R.J.; Gallagher, C.M.; Madoux, F.; Genereux, J.C.; Dobbs, W.; Garza, D.; Spicer, T.P.; et al. Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. eLife 2016, 5. [Google Scholar] [CrossRef]
- Prachasilchai, W.; Sonoda, H.; Yokota-Ikeda, N.; Ito, K.; Kudo, T.; Imaizumi, K.; Ikeda, M. The protective effect of a newly developed molecular chaperone-inducer against mouse ischemic acute kidney injury. J. Pharmacol. Sci. 2009, 109, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Braakman, I.; Bulleid, N.J. Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 2011, 80, 71–99. [Google Scholar] [CrossRef]
- Petersen, O.H.; Sutton, R. Ca2+ signalling and pancreatitis: Effects of alcohol, bile and coffee. Trends Pharmacol. Sci. 2006, 27, 113–120. [Google Scholar] [CrossRef]
- Wen, L.; Voronina, S.; Javed, M.A.; Awais, M.; Szatmary, P.; Latawiec, D.; Chvanov, M.; Collier, D.; Huang, W.; Barrett, J.; et al. Inhibitors of ORAI1 Prevent Cytosolic Calcium-Associated Injury of Human Pancreatic Acinar Cells and Acute Pancreatitis in 3 Mouse Models. Gastroenterology 2015, 149, 481–492.e487. [Google Scholar] [CrossRef] [Green Version]
- Pevarello, P.; Cainarca, S.; Liberati, C.; Tarroni, P.; Piscitelli, F.; Severi, E. Ca(2+) release-activated Ca(2+) channel inhibitors. Pharm. Pat. Anal. 2014, 3, 171–182. [Google Scholar] [CrossRef]
- Pandol, S.J.; Gorelick, F.S.; Gerloff, A.; Lugea, A. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig. Dis. 2010, 28, 776–782. [Google Scholar] [CrossRef] [Green Version]
- Gukovsky, I.; Reyes, C.N.; Vaquero, E.C.; Gukovskaya, A.S.; Pandol, S.J. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G85–G95. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bu, C.; Wu, K.; Wang, R.; Wang, J. Curcumin protects the pancreas from acute pancreatitis via the mitogen-activated protein kinase signaling pathway. Mol. Med. Rep. 2019, 20, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.G.; Xu, G.; Ren, G.J.; Xu, X.; Yuan, H.Q.; Qi, X.L.; Tian, K.L. Preventive action of curcumin in experimental acute pancreatitis in mouse. Indian J. Med. Res. 2011, 134, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Kanai, M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J. Gastroenterol. 2014, 20, 9384–9391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [Green Version]
- Rose, P.; Fraine, E.; Hunt, L.P.; Acheson, D.W.; Braganza, J.M. Dietary antioxidants and chronic pancreatitis. Hum. Nutr. Clin. Nutr. 1986, 40, 151–164. [Google Scholar]
- De Waele, B.; Vierendeels, T.; Willems, G. Vitamin status in patients with acute pancreatitis. Clin. Nutr. 1992, 11, 83–86. [Google Scholar] [CrossRef]
- Kalvaria, I.; Labadarios, D.; Shephard, G.S.; Visser, L.; Marks, I.N. Biochemical vitamin E deficiency in chronic pancreatitis. Int. J. Pancreatol. 1986, 1, 119–128. [Google Scholar] [CrossRef]
- Du, W.D.; Yuan, Z.R.; Sun, J.; Tang, J.X.; Cheng, A.Q.; Shen, D.M.; Huang, C.J.; Song, X.H.; Yu, X.F.; Zheng, S.B. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J. Gastroenterol. 2003, 9, 2565–2569. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, T.H.; Silva, C.S.; Cordeiro Simões Ambrosio, L.M.; Zucoloto, S.; Vannucchi, H. Vitamin E alters inflammatory gene expression in alcoholic chronic pancreatitis. J. Nutr. Nutr. 2012, 5, 94–105. [Google Scholar] [CrossRef]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Reviews. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Elfiky, A.A.; Baghdady, A.M.; Ali, S.A.; Ahmed, M.I. GRP78 targeting: Hitting two birds with a stone. Life Sci. 2020, 260, 118317. [Google Scholar] [CrossRef]
- Gorbatyuk, M.S.; Gorbatyuk, O.S. The Molecular Chaperone GRP78/BiP as a Therapeutic Target for Neurodegenerative Disorders: A Mini Review. J. Genet. Syndr. Gene Ther. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Ha, Y.; Liu, W.; Liu, H.; Zhu, S.; Xia, F.; Gerson, J.E.; Azhar, N.A.; Tilton, R.G.; Motamedi, M.; Kayed, R.; et al. AAV2-mediated GRP78 Transfer Alleviates Retinal Neuronal Injury by Downregulating ER Stress and Tau Oligomer Formation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4670–4682. [Google Scholar] [CrossRef] [Green Version]
- Gorbatyuk, M.S.; Knox, T.; LaVail, M.M.; Gorbatyuk, O.S.; Noorwez, S.M.; Hauswirth, W.W.; Lin, J.H.; Muzyczka, N.; Lewin, A.S. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc. Natl. Acad. Sci. USA 2010, 107, 5961–5966. [Google Scholar] [CrossRef] [Green Version]
- Ghaderi, S.; Ahmadian, S.; Soheili, Z.S.; Ahmadieh, H.; Samiei, S.; Kheitan, S.; Pirmardan, E.R. AAV delivery of GRP78/BiP promotes adaptation of human RPE cell to ER stress. J. Cell. Biochem. 2018, 119, 1355–1367. [Google Scholar] [CrossRef]
- Gorbatyuk, M.S.; Shabashvili, A.; Chen, W.; Meyers, C.; Sullivan, L.F.; Salganik, M.; Lin, J.H.; Lewin, A.S.; Muzyczka, N.; Gorbatyuk, O.S. Glucose regulated protein 78 diminishes α-synuclein neurotoxicity in a rat model of Parkinson disease. Mol. Ther. 2012, 20, 1327–1337. [Google Scholar] [CrossRef]
- Teodoro-Morrison, T.; Schuiki, I.; Zhang, L.; Belsham, D.D.; Volchuk, A. GRP78 overproduction in pancreatic beta cells protects against high-fat-diet-induced diabetes in mice. Diabetologia 2013, 56, 1057–1067. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; Mareninova, O.A.; Barron, E.; Wang, M.; Hinton, D.R.; Pandol, S.J.; Lee, A.S. Grp78 heterozygosity regulates chaperone balance in exocrine pancreas with differential response to cerulein-induced acute pancreatitis. Am. J. Pathol. 2010, 177, 2827–2836. [Google Scholar] [CrossRef]
- Zuleta, A.; Vidal, R.L.; Armentano, D.; Parsons, G.; Hetz, C. AAV-mediated delivery of the transcription factor XBP1s into the striatum reduces mutant Huntingtin aggregation in a mouse model of Huntington’s disease. Biochem. Biophys. Res. Commun. 2012, 420, 558–563. [Google Scholar] [CrossRef]
- Valenzuela, V.; Collyer, E.; Armentano, D.; Parsons, G.B.; Court, F.A.; Hetz, C. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 2012, 3, e272. [Google Scholar] [CrossRef]
- Sado, M.; Yamasaki, Y.; Iwanaga, T.; Onaka, Y.; Ibuki, T.; Nishihara, S.; Mizuguchi, H.; Momota, H.; Kishibuchi, R.; Hashimoto, T.; et al. Protective effect against Parkinson’s disease-related insults through the activation of XBP1. Brain Res. 2009, 1257, 16–24. [Google Scholar] [CrossRef]
- Valdés, P.; Mercado, G.; Vidal, R.L.; Molina, C.; Parsons, G.; Court, F.A.; Martinez, A.; Galleguillos, D.; Armentano, D.; Schneider, B.L.; et al. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc. Natl. Acad. Sci. USA 2014, 111, 6804–6809. [Google Scholar] [CrossRef] [Green Version]
- Vidal, R.L.; Sepulveda, D.; Troncoso-Escudero, P.; Garcia-Huerta, P.; Gonzalez, C.; Plate, L.; Jerez, C.; Canovas, J.; Rivera, C.A.; Castillo, V.; et al. Enforced dimerization between XBP1s and ATF6f enhances the protective effects of the UPR in models of neurodegeneration. Mol. Ther. 2021, 29, 1862–1882. [Google Scholar] [CrossRef]
- Tan, J.H.; Cao, R.C.; Zhou, L.; Zhou, Z.T.; Chen, H.J.; Xu, J.; Chen, X.M.; Jin, Y.C.; Lin, J.Y.; Zeng, J.L.; et al. ATF6 aggravates acinar cell apoptosis and injury by regulating p53/AIFM2 transcription in Severe Acute Pancreatitis. Theranostics 2020, 10, 8298–8314. [Google Scholar] [CrossRef] [PubMed]
- Pitale, P.M.; Gorbatyuk, O.; Gorbatyuk, M. Neurodegeneration: Keeping ATF4 on a Tight Leash. Front. Cell. Neurosci. 2017, 11, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhootada, Y.; Kotla, P.; Zolotukhin, S.; Gorbatyuk, O.; Bebok, Z.; Athar, M.; Gorbatyuk, M. Limited ATF4 Expression in Degenerating Retinas with Ongoing ER Stress Promotes Photoreceptor Survival in a Mouse Model of Autosomal Dominant Retinitis Pigmentosa. PLoS ONE 2016, 11, e0154779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; He, Y.; Miao, X.; Yu, B. ATF4-mediated histone deacetylase HDAC1 promotes the progression of acute pancreatitis. Cell Death Dis. 2021, 12, 5. [Google Scholar] [CrossRef]
- Lindholm, P.; Peränen, J.; Andressoo, J.O.; Kalkkinen, N.; Kokaia, Z.; Lindvall, O.; Timmusk, T.; Saarma, M. MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol. Cell. Neurosci. 2008, 39, 356–371. [Google Scholar] [CrossRef]
- Lindahl, M.; Saarma, M.; Lindholm, P. Unconventional neurotrophic factors CDNF and MANF: Structure, physiological functions and therapeutic potential. Neurobiol. Dis. 2017, 97, 90–102. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, W.; Li, H.; Clementino, M.; Xu, H.; Xu, M.; Ma, M.; Frank, J.; Luo, J. MANF is neuroprotective against ethanol-induced neurodegeneration through ameliorating ER stress. Neurobiol. Dis. 2021, 148, 105216. [Google Scholar] [CrossRef]
- Lindahl, M.; Danilova, T.; Palm, E.; Lindholm, P.; Võikar, V.; Hakonen, E.; Ustinov, J.; Andressoo, J.O.; Harvey, B.K.; Otonkoski, T.; et al. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep. 2014, 7, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Danilova, T.; Belevich, I.; Li, H.; Palm, E.; Jokitalo, E.; Otonkoski, T.; Lindahl, M. MANF Is Required for the Postnatal Expansion and Maintenance of Pancreatic β-Cell Mass in Mice. Diabetes 2019, 68, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Hakonen, E.; Chandra, V.; Fogarty, C.L.; Yu, N.Y.; Ustinov, J.; Katayama, S.; Galli, E.; Danilova, T.; Lindholm, P.; Vartiainen, A.; et al. MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia 2018, 61, 2202–2214. [Google Scholar] [CrossRef] [Green Version]
- Montaser, H.; Patel, K.A.; Balboa, D.; Ibrahim, H.; Lithovius, V.; Näätänen, A.; Chandra, V.; Demir, K.; Acar, S.; Ben-Omran, T.; et al. Loss of MANF Causes Childhood-Onset Syndromic Diabetes Due to Increased Endoplasmic Reticulum Stress. Diabetes 2021, 70, 1006–1018. [Google Scholar] [CrossRef]
- Galli, E.; Härkönen, T.; Sainio, M.T.; Ustav, M.; Toots, U.; Urtti, A.; Yliperttula, M.; Lindahl, M.; Knip, M.; Saarma, M.; et al. Increased circulating concentrations of mesencephalic astrocyte-derived neurotrophic factor in children with type 1 diabetes. Sci. Rep. 2016, 6, 29058. [Google Scholar] [CrossRef]
- Airavaara, M.; Shen, H.; Kuo, C.C.; Peränen, J.; Saarma, M.; Hoffer, B.; Wang, Y. Mesencephalic astrocyte-derived neurotrophic factor reduces ischemic brain injury and promotes behavioral recovery in rats. J. Comp. Neurol. 2009, 515, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Airavaara, M.; Chiocco, M.J.; Howard, D.B.; Zuchowski, K.L.; Peränen, J.; Liu, C.; Fang, S.; Hoffer, B.J.; Wang, Y.; Harvey, B.K. Widespread cortical expression of MANF by AAV serotype 7: Localization and protection against ischemic brain injury. Exp. Neurol. 2010, 225, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Shen, Y.; Chen, Y.; Chen, L.; Wang, L.; Wang, H.; Xu, S.; Fang, S.; Fu, Y.; Yu, Y.; et al. Mesencephalic astrocyte-derived neurotrophic factor prevents neuron loss via inhibiting ischemia-induced apoptosis. J. Neurol. Sci. 2014, 344, 129–138. [Google Scholar] [CrossRef]
- Wang, X.Y.; Song, M.M.; Bi, S.X.; Shen, Y.J.; Shen, Y.X.; Yu, Y.Q. MRI Dynamically Evaluates the Therapeutic Effect of Recombinant Human MANF on Ischemia/Reperfusion Injury in Rats. Int. J. Mol. Sci. 2016, 17, 1476. [Google Scholar] [CrossRef] [Green Version]
- Teppo, J.; Vaikkinen, A.; Stratoulias, V.; Mätlik, K.; Anttila, J.E.; Smolander, O.P.; Pöhö, P.; Harvey, B.K.; Kostiainen, R.; Airavaara, M. Molecular profile of the rat peri-infarct region four days after stroke: Study with MANF. Exp. Neurol. 2020, 329, 113288. [Google Scholar] [CrossRef]


| Molecule | Experimental Model/Clinical Settings | Effects | Ref. |
|---|---|---|---|
| Chemical chaperones | |||
| Sodium phenylbutyrate (4-PBA) | CCK-stimulated rat pancreatic acini; taurocholate-induced AP rats | Rescue cell death; protect pancreas | [198,199] |
| UDCA | Idiopathic AP and recurrent pancreatitis patients | Remove gallstones and prevent pancreatitis relapse | [200,201] |
| Tauroursodeoxycholic acid (TUDCA) | CCK-8-stimulated rat pancreatic acini; caerulein-induced AP rats | Reduce cell death, trypsin activation and edema formation | [202,203] |
| PERK/eIF2α inhibitors | |||
| Salubrinal | Caerulein/LPS-induced-AP mice | Reduce serum amylase level, inflammation and cell death | [204] |
| Guanabenz acetate (GA) | Mouse model of neurological diseases | Exert neuroprotection | [205,206,207,208] |
| Trazodone (TZD) | Small-molecule-screen for β-cell proliferation in transgenic zebrafish | Stimulate proliferation of pancreatic β cells | [209] |
| Dibenzoylmethane (DBM) | Mouse models of dementia | Improve neuroprotection and cognition; no toxicity to pancreas | [195] |
| IRE1α Inhibitors | |||
| STF-083010 | Alcohol-treated mouse pancreas 266-6 acinar cells | Reduce cell death | [210] |
| Kinase-Inhibiting RNase-Attenuator 6 (KIRA6) | Akita diabetic mice | Reduce cells death of pancreatic islets in vitro and in vivo | [211] |
| ATF6 inhibitors | |||
| Melatonin | LPS-treated rat AR42J acinar cells; taurocholate-induced AP rats | Attenuate inflammation, reduce apoptosis | [212,213] |
| Bix | Gerbil model of forebrain ischemia; mouse model of renal I/R injury | Rescue cell death | [214,215] |
| Compound 147 (AA 147) | Mouse model of acute myocardial infarction | Cytoprotective effects in heart, brain, kidney and liver | [216] |
| Inhibitors of CRAC channels | |||
| GSK-7975A | AP mice induced by TLCA3S, caerulein or ethanol and palmitoleic acid | Reduce serum amylase level, cell death and inflammation | [217] |
| CM_128/CM4620 | Phase I clinical trials for AP | [218,219,220] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wen, W.; Luo, J. Targeting Endoplasmic Reticulum Stress as an Effective Treatment for Alcoholic Pancreatitis. Biomedicines 2022, 10, 108. https://doi.org/10.3390/biomedicines10010108
Li H, Wen W, Luo J. Targeting Endoplasmic Reticulum Stress as an Effective Treatment for Alcoholic Pancreatitis. Biomedicines. 2022; 10(1):108. https://doi.org/10.3390/biomedicines10010108
Chicago/Turabian StyleLi, Hui, Wen Wen, and Jia Luo. 2022. "Targeting Endoplasmic Reticulum Stress as an Effective Treatment for Alcoholic Pancreatitis" Biomedicines 10, no. 1: 108. https://doi.org/10.3390/biomedicines10010108
APA StyleLi, H., Wen, W., & Luo, J. (2022). Targeting Endoplasmic Reticulum Stress as an Effective Treatment for Alcoholic Pancreatitis. Biomedicines, 10(1), 108. https://doi.org/10.3390/biomedicines10010108






