Taurine Grafted Micro-Implants Improved Functions without Direct Dependency between Interleukin-6 and the Bile Acid Lithocholic Acid in Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graft Imaging and Confocal Microscopic Analyses
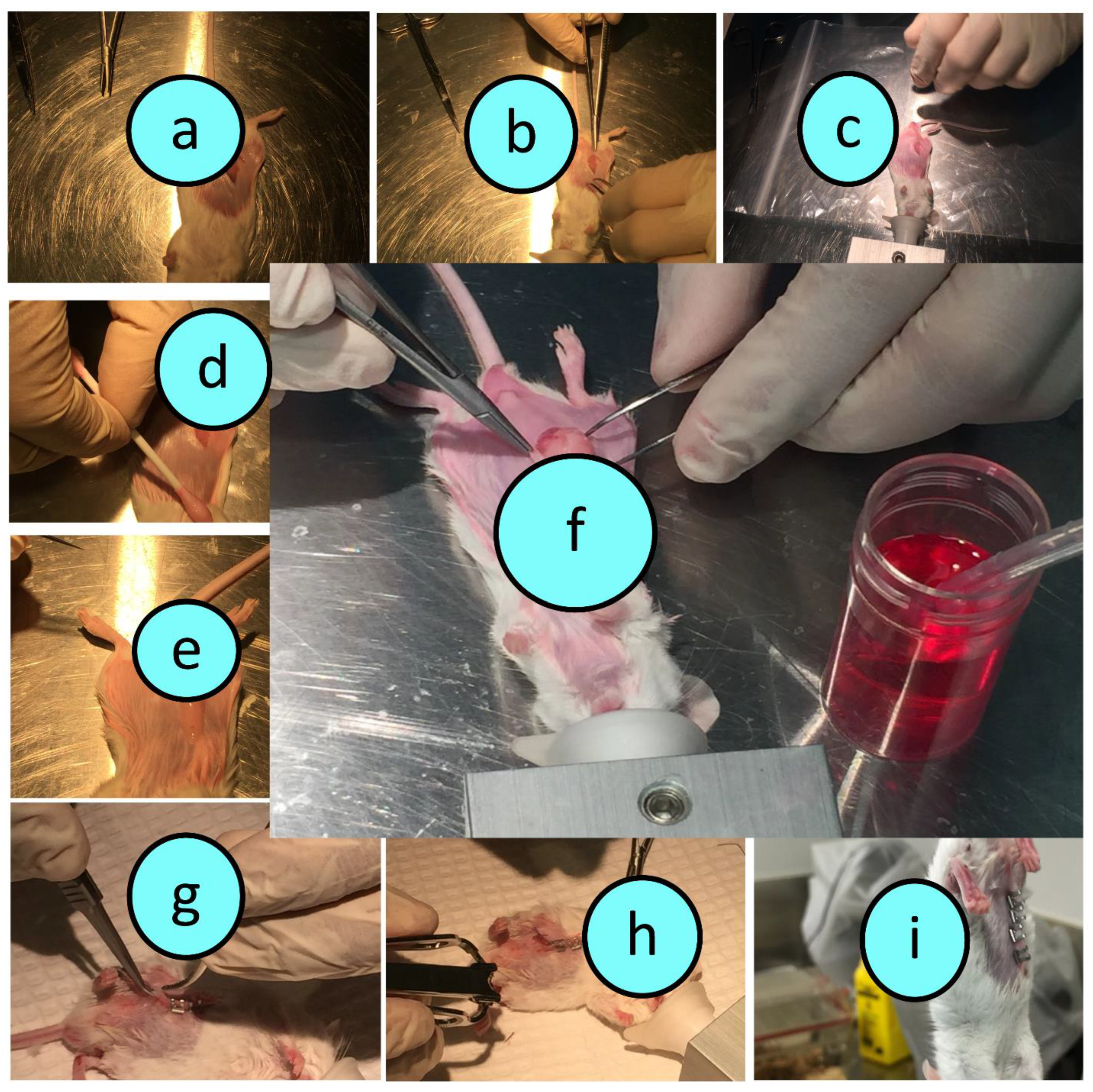
2.2. Graft Preparation and Surgical Transplantation
2.3. Study Design
2.4. Weights Measurements, and Blood Glucose and Plasma Cytokine Analyses
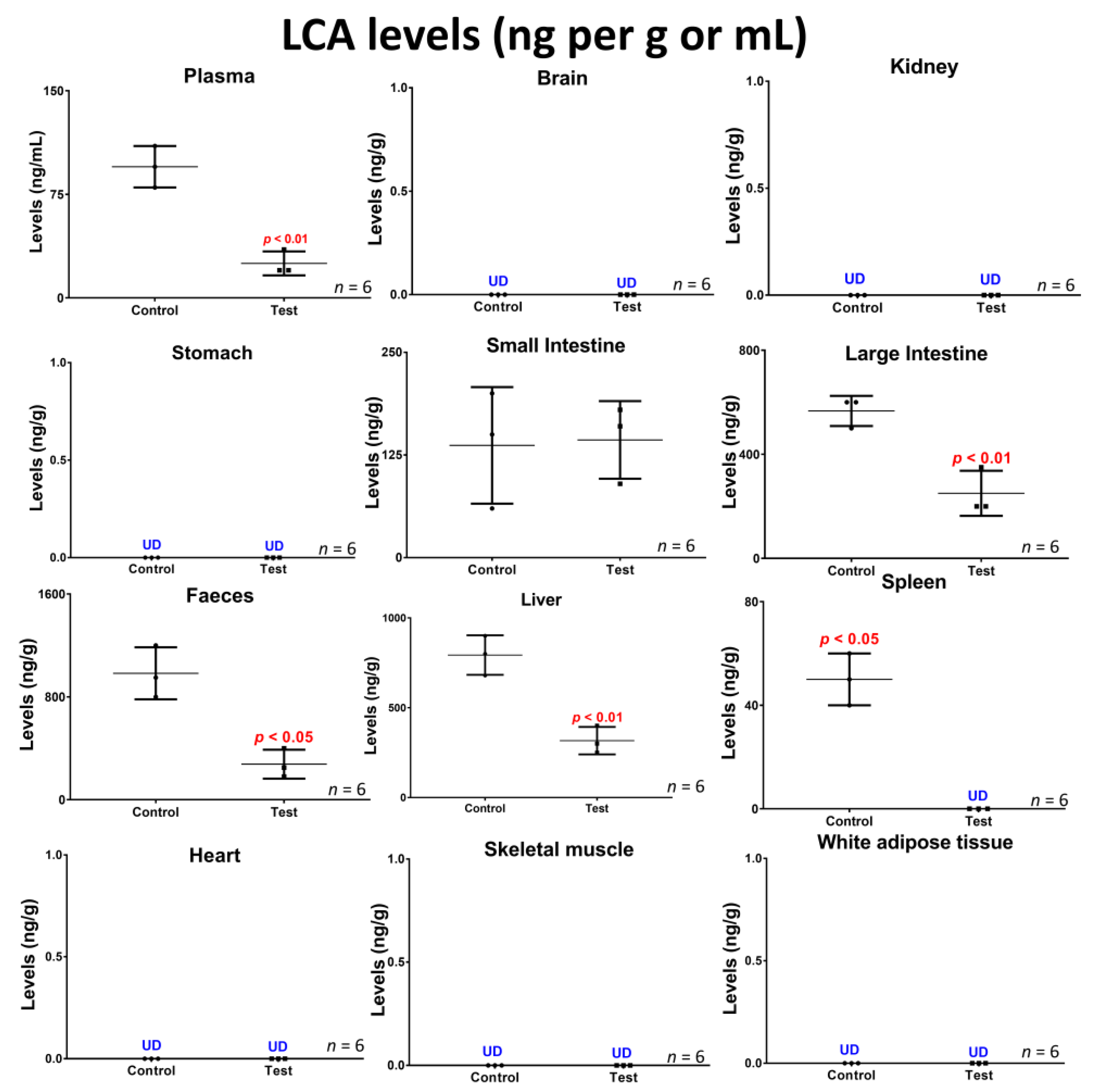
2.5. Lithocholic Acid Quantification in Blood, Tissues and Faeces
2.6. Statistical Analysis
3. Results and Discussion
3.1. Graft Imaging and Microscopic Measurements
3.2. Graft Biological Effects in Diabetic Mice
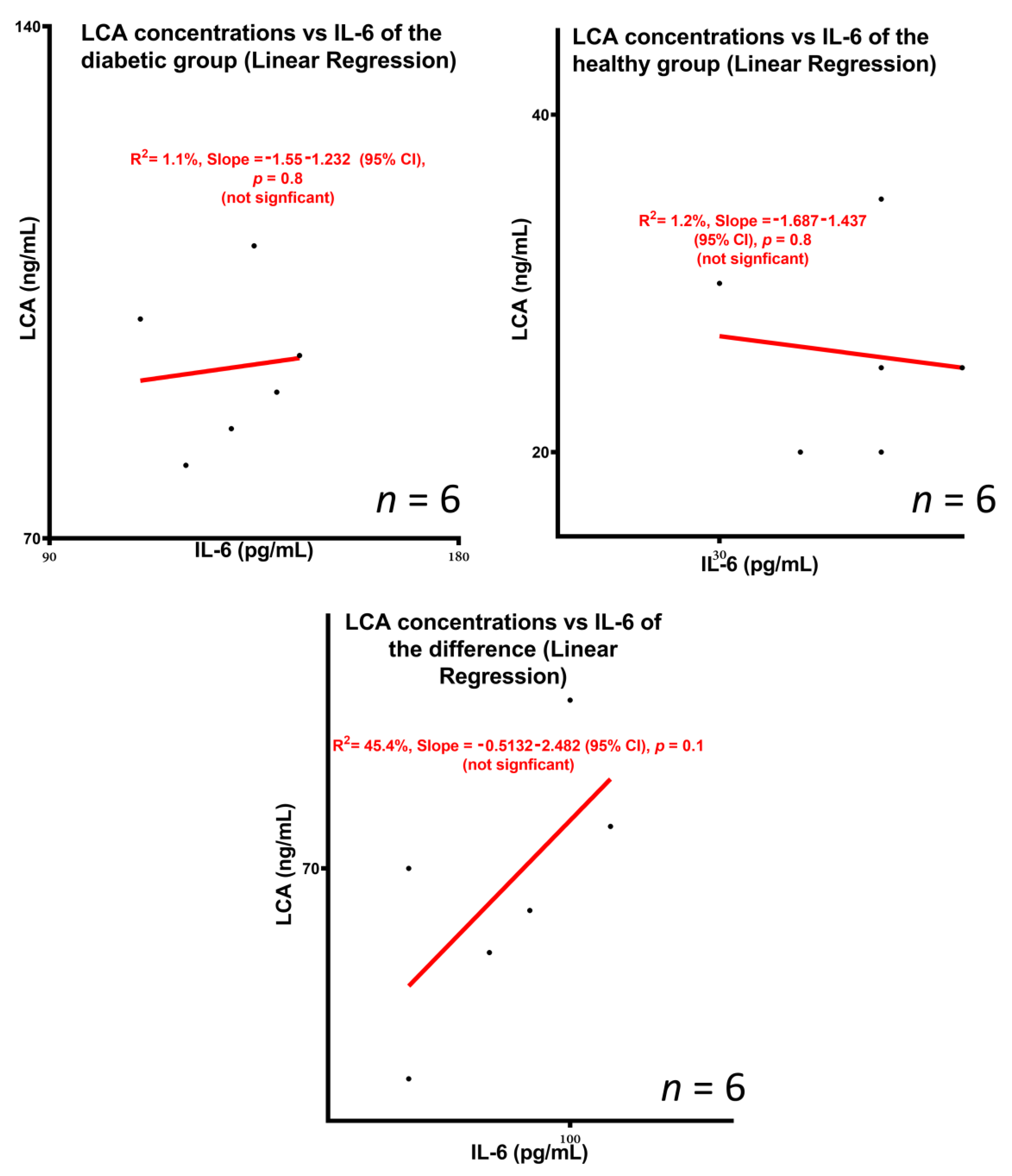
3.3. Concentrations of the Bile Acid Lithocholic Acid and Potential Association with Inflammation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rokstad, A.M.; Lacik, I.; de Vos, P.; Strand, B.L. Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation. Adv. Drug Deliv. Rev. 2013, 67–68, 111–130. [Google Scholar] [CrossRef]
- Rydgren, T.; Sandler, S. Efficacy of 1400 W, a novel inhibitor of inducible nitric oxide synthase, in preventing interleukin-1beta-induced suppression of pancreatic islet function in vitro and multiple low-dose streptozotocin-induced diabetes in vivo. Eur. J. Endocrinol. 2002, 147, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Allison, J.; McClive, P.; Oxbrow, L.; Baxter, A.; Morahan, G.; Miller, J.F. Genetic requirements for acceleration of diabetes in non-obese diabetic mice expressing interleukin-2 in islet beta-cells. Eur. J. Immunol. 1994, 24, 2535–2541. [Google Scholar] [CrossRef]
- Peakman, M.; Wen, L.; McNab, G.L.; Watkins, P.J.; Tan, K.C.; Vergani, D. T cell clones generated from patients with type 1 diabetes using interleukin-2 proliferate to human islet antigens. Autoimmunity 1994, 17, 31–39. [Google Scholar] [CrossRef]
- Park, Y.J.; Warnock, G.L.; Ao, Z.; Safikhan, N.; Meloche, M.; Asadi, A.; Kieffer, T.J.; Marzban, L. Dual role of interleukin-1β in islet amyloid formation and its β-cell toxicity: Implications for type 2 diabetes and islet transplantation. Diabetes Obes. Metab. 2017, 19, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, A.; Suarez-Pinzon, W.L.; Sorensen, O.; Bleackley, R.C.; Power, R.F.; Rajotte, R.V. Combined therapy with interleukin-4 and interleukin-10 inhibits autoimmune diabetes recurrence in syngeneic islet-transplanted nonobese diabetic mice. Analysis of cytokine mRNA expression in the graft. Transplantation 1995, 60, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Ladefoged, M.; Buschard, K.; Hansen, A.M. Increased expression of toll-like receptor 4 and inflammatory cytokines, interleukin-6 in particular, in islets from a mouse model of obesity and type 2 diabetes. APMIS 2013, 121, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.; Moore, F.; Marks, K.; Bouckenooghe, T.; Dayan, C.M.; Planas, R.; Vives-Pi, M.; Powrie, J.; Tree, T.; Marchetti, P.; et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes 2011, 60, 2112–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaas, A.; Pfleger, C.; Hansen, L.; Buschard, K.; Schloot, N.C.; Roep, B.O.; Mortensen, H.B.; Hvidore Study Group on Childhood, D. Association of adiponectin, interleukin (IL)-1ra, inducible protein 10, IL-6 and number of islet autoantibodies with progression patterns of type 1 diabetes the first year after diagnosis. Clin. Exp. Immunol. 2010, 161, 444–452. [Google Scholar] [CrossRef]
- Krishnan, R.; Alexander, M.; Robles, L.; Foster, C.E., 3rd; Lakey, J.R. Islet and stem cell encapsulation for clinical transplantation. Rev. Diabet Stud. 2014, 11, 84–101. [Google Scholar] [CrossRef] [Green Version]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Advanced and multifaceted stability profiling of the first-line antidiabetic drugs metformin, gliclazide and glipizide under various controlled stress conditions. Saudi Pharm. J. 2020, 28, 362–368. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Alginate-combined cholic acid increased insulin secretion of microencapsulated mouse cloned pancreatic beta cells. Ther. Deliv. 2017, 8, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Suciu, M.; Ionescu, C.M.; Ciorita, A.; Tripon, S.C.; Nica, D.; Al-Salami, H.; Barbu-Tudoran, L. Applications of superparamagnetic iron oxide nanoparticles in drug and therapeutic delivery, and biotechnological advancements. Beilstein J. Nanotechnol. 2020, 11, 1092–1109. [Google Scholar] [CrossRef] [PubMed]
- Al-Salami, H.; Kansara, H.; King, J.; Morar, B.; Jayathilaka, B.; Fawcett, P.J.; Mikov, M. Bile acids: A bitter sweet remedy for diabetes. New Zealand Pharm. J. 2007, 27, 17–20. [Google Scholar]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Current status and applications of animal models in pre-clinical development of orally administered insulin-loaded nanoparticles. J. Drug Target. 2020, 28, 882–903. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Takechi, R.; Luna, G.; Mikov, M.; Golocorbin-Kon, S.; Elnashar, M.; Arfuso, F.; Al-Salami, H. An in vivo pharmacological study: Variation in tissue-accumulation for the drug probucol as the result of targeted microtechnology and matrix-acrylic acid optimization and stabilization techniques. PLoS ONE 2019, 14, e0214984. [Google Scholar] [CrossRef] [Green Version]
- Wagle, S.R.; Walker, D.; Kovacevic, B.; Gedawy, A.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Al-Salami, H. Micro-Nano formulation of bile-gut delivery: Rheological, stability and cell survival, basal and maximum respiration studies. Sci. Rep. 2020, 10, 7715. [Google Scholar] [CrossRef]
- Wagle, S.R.; Kovacevic, B.; Walker, D.; Ionescu, C.M.; Shah, U.; Stojanovic, G.; Kojic, S.; Mooranian, A.; Al-Salami, H. Alginate-based drug oral targeting using bio-micro/nano encapsulation technologies. Expert Opin. Drug Deliv. 2020, 17, 1361–1376. [Google Scholar] [CrossRef]
- Wagle, S.R.; Kovacevic, B.; Walker, D.; Ionescu, C.M.; Jones, M.; Stojanovic, G.; Kojic, S.; Mooranian, A.; Al-Salami, H. Pharmacological and advanced cell respiration effects, enhanced by toxic human-bile nano-pharmaceuticals of probucol cell-targeting formulations. Pharmaceutics 2020, 12, 708. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Takechi, R.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Modulatory nano/micro effects of diabetes development on pharmacology of primary and secondary bile acids concentrations. Curr. Diabetes Rev. 2020, 16, 900–909. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Kovacevic, B.; Al-Salami, H. A second-generation micro/nano capsules of an endogenous primary unmetabolised bile acid, stabilized by Eudragit-alginate complex with antioxidant compounds. Saudi Pharm. J. 2020, 28, 165–171. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Kovacevic, B.; Al-Salami, H. Bio micro-nano technologies of antioxidants optimised their pharmacological and cellular effects, ex vivo, in pancreatic β-cells. Nanotechnol. Sci. Appl. 2020, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Zamani, N.; Kovacevic, B.; Ionescu, C.M.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Kojic, S.; Al-Salami, H. Pharmacological effects of secondary bile acid microparticles in diabetic murine model. Curr. Diabetes Rev. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Ionescu, C.M.; Takechi, R.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Kovačević, B.; Al-Salami, H. Oral gavage of nano-encapsulated conjugated acrylic acid-bile acid formulation in type 1 diabetes altered pharmacological profile of bile acids, and improved glycaemia and suppressed inflammation. Pharmacol. Rep. 2020, 72, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Raj Wagle, S.; Kovacevic, B.; Takechi, R.; Mamo, J.; Lam, V.; Watts, G.F.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; et al. Bile acid bio-nanoencapsulation improved drug targeted-delivery and pharmacological effects via cellular flux: 6-months diabetes preclinical study. Sci. Rep. 2020, 10, 106. [Google Scholar] [CrossRef]
- Mathavan, S.; Ionescu, C.M.; Kovacevic, B.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Dass, C.R.; Al-Salami, H. Histological effects of pharmacologically active human bile acid nano/micro-particles in Type-1 diabetes. Ther. Deliv. 2020, 11, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Walker, D.; Ionescu, C.M.; Kovacevic, B.; Wagle, S.R.; Mooranian, A.; Brown, D.; Al-Salami, H. Microencapsulation of Coenzyme Q10 and bile acids using ionic gelation vibrational jet flow technology for oral delivery. Ther. Deliv. 2020, 11, 791–805. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Takechi, R.; Al-Sallami, H.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Probucol-poly(meth)acrylate-bile acid nanoparticles increase IL-10, and primary bile acids in prediabetic mice. Ther. Deliv. 2019, 10, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Stability and biological testing of taurine-conjugated bile acid antioxidant microcapsules for diabetes treatment. Ther. Deliv. 2019, 10, 99–106. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Luna, G.; Al-Sallami, H.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Kovacevic, B.; Al-Salami, H. Bile acid-polymer-probucol microparticles: Protective effect on pancreatic β-cells and decrease in type 1 diabetes development in a murine model. Pharm. Dev. Technol. 2019, 24, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. The role of the bile acid chenodeoxycholic acid in the targeted oral delivery of the anti-diabetic drug gliclazide, and its applications in type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Multicompartmental, multilayered probucol microcapsules for diabetes mellitus: Formulation characterization and effects on production of insulin and inflammation in a pancreatic β-cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1642–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Characterization of a novel bile acid-based delivery platform for microencapsulated pancreatic beta-cells. Artif. Cells Nanomed. Biotechnol. 2016, 44, 194–200. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Fakhoury, M.; Arfuso, F.; Jones, F.; Al-Salami, H. Advanced bile acid-based multi-compartmental microencapsulated pancreatic beta-cells integrating a polyelectrolyte-bile acid formulation, for diabetes treatment. Artif. Cells Nanomed. Biotechnol. 2016, 44, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Biological Assessments of Encapsulated Pancreatic β-Cells: Their Potential Transplantation in Diabetes. Cell. Mol. Bioeng. 2016, 9, 530–537. [Google Scholar] [CrossRef]
- Mathavan, S.; Ionescu, C.M.; Kovacevic, B.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Dass, C.R.; Al-Salami, H. Formulation buoyancy of nanoencapsulated gliclazide using primary, conjugated and deconjugated bile acids. Ther. Deliv. 2019, 10, 573–583. [Google Scholar] [CrossRef]
- Mamo, J.C.L.; Lam, V.; Brook, E.; Mooranian, A.; Al-Salami, H.; Fimognari, N.; Nesbit, M.; Takechi, R. Probucol prevents blood–brain barrier dysfunction and cognitive decline in mice maintained on pro-diabetic diet. Diabetes Vasc. Dis. Res. 2019, 16, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Zamani, N.; Takechi, R.; Al-Sallami, H.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Pharmacological effects of nanoencapsulation of human-based dosing of probucol on ratio of secondary to primary bile acids in gut, during induction and progression of type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2018, 46, S748–S754. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Novel nano-encapsulation of probucol in microgels: Scanning electron micrograph characterizations, buoyancy profiling, and antioxidant assay analyses. Artif. Cells Nanomed. Biotechnol. 2018, 46, S741–S747. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Eudragit®-based microcapsules of probucol with a gut-bacterial processed secondary bile acid. Ther. Deliv. 2018, 9, 811–821. [Google Scholar] [CrossRef]
- Mooranian, A.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. The effect of molecular weights of microencapsulating polymers on viability of mouse-cloned pancreatic β-cells: Biomaterials, osmotic forces and potential applications in diabetes treatment. Pharm. Dev. Technol. 2018, 23, 145–150. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Mamo, J.; Al-Sallami, H.; Al-Salami, H. The biological effects of the hypolipidaemic drug probucol microcapsules fed daily for 4 weeks, to an insulin-resistant mouse model: Potential hypoglycaemic and anti-inflammatory effects. Drug Deliv. Transl. Res. 2018, 8, 543–551. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Influence of Biotechnological Processes, Speed of Formulation Flow and Cellular Concurrent Stream-Integration on Insulin Production from β-cells as a Result of Co-Encapsulation with a Highly Lipophilic Bile Acid. Cell. Mol. Bioeng. 2018, 11, 65–75. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Electrokinetic potential-stabilization by bile acid-microencapsulating formulation of pancreatic β-cells cultured in high ratio poly-L-ornithine-gel hydrogel colloidal dispersion: Applications in cell-biomaterials, tissue engineering and biotechnological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1156–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamo, J.C.; Lam, V.; Al-Salami, H.; Brook, E.; Mooranian, A.; Nesbit, M.; Graneri, L.; D’Alonzo, Z.; Fimognari, N.; Stephenson, A.; et al. Sodium alginate capsulation increased brain delivery of probucol and suppressed neuroinflammation and neurodegeneration. Ther. Deliv. 2018, 9, 703–709. [Google Scholar] [CrossRef]
- Takechi, R.; Lam, V.; Brook, E.; Giles, C.; Fimognari, N.; Mooranian, A.; Al-Salami, H.; Coulson, S.H.; Nesbit, M.; Mamo, J.C.L. Blood-brain barrier dysfunction precedes cognitive decline and neurodegeneration in diabetic insulin resistant mouse model: An implication for causal link. Front. Aging Neurosci. 2017, 9, 399. [Google Scholar] [CrossRef]
- Mooranian, A.; Tackechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Innovative Microcapsules for Pancreatic β-Cells Harvested from Mature Double-Transgenic Mice: Cell Imaging, Viability, Induced Glucose-Stimulated Insulin Measurements and Proinflammatory Cytokines Analysis. Pharm. Res. 2017, 34, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. New Biotechnological Microencapsulating Methodology Utilizing Individualized Gradient-Screened Jet Laminar Flow Techniques for Pancreatic β-Cell Delivery: Bile Acids Support Cell Energy-Generating Mechanisms. Mol. Pharm. 2017, 14, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The effects of Ionic Gelation- Vibrational Jet Flow technique in fabrication of microcapsules incorporating β-cell: Applications in Type-1 Diabetes. Curr. Diabetes Rev. 2017, 13, 91–96. [Google Scholar] [CrossRef]
- Mamo, J.C.L.; Lam, V.; Giles, C.; Coulson, S.H.; Fimognari, N.; Mooranian, A.; Al-Salami, H.; Takechi, R. Antihypertensive agents do not prevent blood-brain barrier dysfunction and cognitive deficits in dietary-induced obese mice. Int. J. Obes. 2017, 41, 926–934. [Google Scholar] [CrossRef]
- Al-Salami, H.; Mamo, J.C.; Mooranian, A.; Negrulj, R.; Lam, V.; Elahy, M.; Takechi, R. Long-Term Supplementation of Microencapsulated ursodeoxycholic Acid Prevents Hypertension in a Mouse Model of Insulin Resistance. Exp. Clin. Endocrinol. Diabetes 2017, 125, 28–32. [Google Scholar] [CrossRef]
- Negrulj, R.; Mooranian, A.; Chen-Tan, N.; Al-Salami, H.S.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F.; Arfuso, F.; Al-Salami, H. Swelling, mechanical strength, and release properties of probucol microcapsules with and without a bile acid, and their potential oral delivery in diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Viability and topographical analysis of microencapsulated β-cells exposed to a biotransformed tertiary bile acid: An ex vivo study. Int. J. Nano Biomater. 2016, 6, 74–82. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H.; Morahan, G.; Jamieson, E. Designing anti-diabetic β-cells microcapsules using polystyrenic sulfonate, polyallylamine, and a tertiary bile acid: Morphology, bioenergetics, and cytokine analysis. Biotechnol. Prog. 2016, 32, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The incorporation of water-soluble gel matrix into bile acid-based microcapsules for the delivery of viable β-cells of the pancreas, in diabetes treatment: Biocompatibility and functionality studies. Drug Deliv. Transl. Res. 2016, 6, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Flow vibration-doubled concentric system coupled with low ratio amine to produce bile acid-macrocapsules of β-cells. Ther. Deliv. 2016, 7, 171–178. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The impact of allylamine-bile acid combinations on cell delivery microcapsules in diabetes. J. Microencapsul. 2016, 33, 569–574. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The Influence of Stabilized Deconjugated Ursodeoxycholic Acid on Polymer-Hydrogel System of Transplantable NIT-1 Cells. Pharm. Res. 2016, 33, 1182–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gvoic, M.; Vukmirovic, S.; Al-Salami, H.; Mooranian, A.; Mikov, M.; Stankov, K. Bile acids as novel enhancers of CNS targeting antitumor drugs: A comprehensive review. Pharm. Dev. Technol. 2021, 26, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Leach, M.C. Using the mouse grimace scale to assess pain associated with routine ear notching and the effect of analgesia in laboratory mice. Lab. Anim. 2015, 49, 117–120. [Google Scholar] [CrossRef]
- Leach, M.C.; Klaus, K.; Miller, A.L.; Scotto di Perrotolo, M.; Sotocinal, S.G.; Flecknell, P.A. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS ONE 2012, 7, e35656. [Google Scholar] [CrossRef]
- Matsumiya, L.C.; Sorge, R.E.; Sotocinal, S.G.; Tabaka, J.M.; Wieskopf, J.S.; Zaloum, A.; King, O.D.; Mogil, J.S. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 42–49. [Google Scholar] [PubMed]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. Morphological, Stability, and Hypoglycemic Effects of New Gliclazide-Bile Acid Microcapsules for Type 1 Diabetes Treatment: The Microencapsulation of Anti-diabetics Using a Microcapsule-Stabilizing Bile Acid. AAPS PharmSciTech 2018, 19, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Wagle, S.R.; Kovacevic, B.; Ionescu, C.M.; Walker, D.; Jones, M.; Carey, L.; Takechi, R.; Mikov, M.; Mooranian, A.; Al-Salami, H. pharmacological and Biological Study of Microencapsulated Probucol-Secondary Bile Acid in a Diseased Mouse Model. Pharmaceutics 2021, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Al-Salami, H.; Butt, G.; Tucker, I.; Mikov, M. Influence of the semisynthetic bile acid (MKC) on the ileal permeation of gliclazide in healthy and diabetic rats. Pharmacol. Rep. 2008, 60, 532–541. [Google Scholar] [PubMed]
- Mikov, M.; Boni, N.S.; Al-Salami, H.; Kuhajda, K.; Kevresan, S.; Golocorbin-Kon, S.; Fawcett, J.P. Bioavailability and hypoglycemic activity of the semisynthetic bile acid salt, sodium 3alpha,7alpha-dihydroxy-12-oxo-5beta-cholanate, in healthy and diabetic rats. Eur. J. Drug Metab. Pharm. 2007, 32, 7–12. [Google Scholar] [CrossRef] [PubMed]
- de Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; De Haan, B.J.; Wolters, G.H.; Strubbe, J.H.; Van Schilfgaarde, R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia 1997, 40, 262–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooraniana, A.; Negrulja, R.; Chen-Tanb, N.; Al-Sallamic, H.S.; Fangd, Z.; Mikov, M.; Golocorbin-Kong, S.; Fakhouri, M.; Arfusoe, F.; Al-Salamia, H. Novel artificial cell microencapsulation of a complex gliclazide-deoxycholic bile acid formulation: A Characterisation Study. Drug Des. Dev. Ther. 2014; in press. [Google Scholar]
- Al-Salami, H.; Butt, G.; Fawcett, J.P.; Tucker, I.G.; Golocorbin-Kon, S.; Mikov, M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur. J. Drug Metab. Pharm. 2008, 33, 101–106. [Google Scholar] [CrossRef]
- Al-Salami, H.; Butt, G.; Tucker, I.; Fawcett, P.J.; Golocorbin-Kon, S.; Mikov, I.; Mikov, M. Gliclazide reduces MKC intestinal transport in healthy but not diabetic rats. Eur. J. Drug Metab. Pharmacokinet. 2009, 34, 43–50. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Alginate-deoxycholic Acid Interaction and Its Impact on Pancreatic Β-Cells and Insulin Secretion and Potential Treatment of Type 1 Diabetes. J. Pharm. Innov. 2016, 11, 156–161. [Google Scholar] [CrossRef]
- Jin, S.M.; Oh, S.H.; Oh, B.J.; Suh, S.; Bae, J.C.; Lee, J.H.; Lee, M.S.; Lee, M.K.; Kim, K.W.; Kim, J.H. Benefits of PEGylation in the early post-transplant period of intraportal islet transplantation as assessed by magnetic resonance imaging of labeled islets. Islets 2014, 6, e27827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loganathan, G.; Graham, M.L.; Radosevich, D.M.; Soltani, S.M.; Tiwari, M.; Anazawa, T.; Papas, K.K.; Sutherland, D.E.; Hering, B.J.; Balamurugan, A.N. Factors affecting transplant outcomes in diabetic nude mice receiving human, porcine, and nonhuman primate islets: Analysis of 335 transplantations. Transplantation 2013, 95, 1439–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, R.A.; Cheng, K.; Deters, N.; Lau, S.M.; Hawthorne, W.J.; O’Connell, P.J.; Stolp, J.; Grey, S.; Loudovaris, T.; Kay, T.W.; et al. Hypoxia-inducible factor-1alpha (HIF-1alpha) potentiates beta-cell survival after islet transplantation of human and mouse islets. Cell Transpl. 2013, 22, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Moreno, R.; Samikannu, B.; Bretzel, R.G.; Schmitz, M.L.; Linn, T. Improved intraportal islet transplantation outcome by systemic IKK-beta inhibition: NF-kappaB activity in pancreatic islets depends on oxygen availability. Am. J. Transpl. 2011, 11, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Feilen, P.J.; Brunnenmeier, F.; Minnemann, T.; Zimmermann, H.; Zimmermann, U.; Weber, M.M. Long-term graft function of adult rat and human islets encapsulated in novel alginate-based microcapsules after transplantation in immunocompetent diabetic mice. Diabetes 2005, 54, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Adcock, J.; Kuhtreiber, W.; Qiang, D.; Salleng, K.J.; Trenary, I.; Williams, P. Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation 2008, 85, 331–337. [Google Scholar] [CrossRef]
- Beger, C.; Cirulli, V.; Vajkoczy, P.; Halban, P.A.; Menger, M.D. Vascularization of purified pancreatic islet-like cell aggregates (pseudoislets) after syngeneic transplantation. Diabetes 1998, 47, 559–565. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, X.; Li, J.; Zhang, Y.; Zhong, H.; Liu, R.; Zhang, D.; Feng, Q.; Xie, X.; Hong, J.; et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 2017, 8, 1785. [Google Scholar] [CrossRef]
- Engin, F.; Yermalovich, A.; Nguyen, T.; Hummasti, S.; Fu, W.; Eizirik, D.L.; Mathis, D.; Hotamisligil, G.S. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci. Transl. Med. 2013, 5, 211ra156. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mooranian, A.; Ionescu, C.M.; Wagle, S.R.; Kovacevic, B.; Walker, D.; Jones, M.; Chester, J.; Foster, T.; Johnston, E.; Kojic, S.; et al. Taurine Grafted Micro-Implants Improved Functions without Direct Dependency between Interleukin-6 and the Bile Acid Lithocholic Acid in Plasma. Biomedicines 2022, 10, 111. https://doi.org/10.3390/biomedicines10010111
Mooranian A, Ionescu CM, Wagle SR, Kovacevic B, Walker D, Jones M, Chester J, Foster T, Johnston E, Kojic S, et al. Taurine Grafted Micro-Implants Improved Functions without Direct Dependency between Interleukin-6 and the Bile Acid Lithocholic Acid in Plasma. Biomedicines. 2022; 10(1):111. https://doi.org/10.3390/biomedicines10010111
Chicago/Turabian StyleMooranian, Armin, Corina Mihaela Ionescu, Susbin Raj Wagle, Bozica Kovacevic, Daniel Walker, Melissa Jones, Jacqueline Chester, Thomas Foster, Edan Johnston, Sanja Kojic, and et al. 2022. "Taurine Grafted Micro-Implants Improved Functions without Direct Dependency between Interleukin-6 and the Bile Acid Lithocholic Acid in Plasma" Biomedicines 10, no. 1: 111. https://doi.org/10.3390/biomedicines10010111









