Anorectal and Genital Mucosal Melanoma: Diagnostic Challenges, Current Knowledge and Therapeutic Opportunities of Rare Melanomas
Abstract
1. Introduction
2. Data Sources
3. Epidemiology and Risk Factors
4. Biology and Genetic Alterations
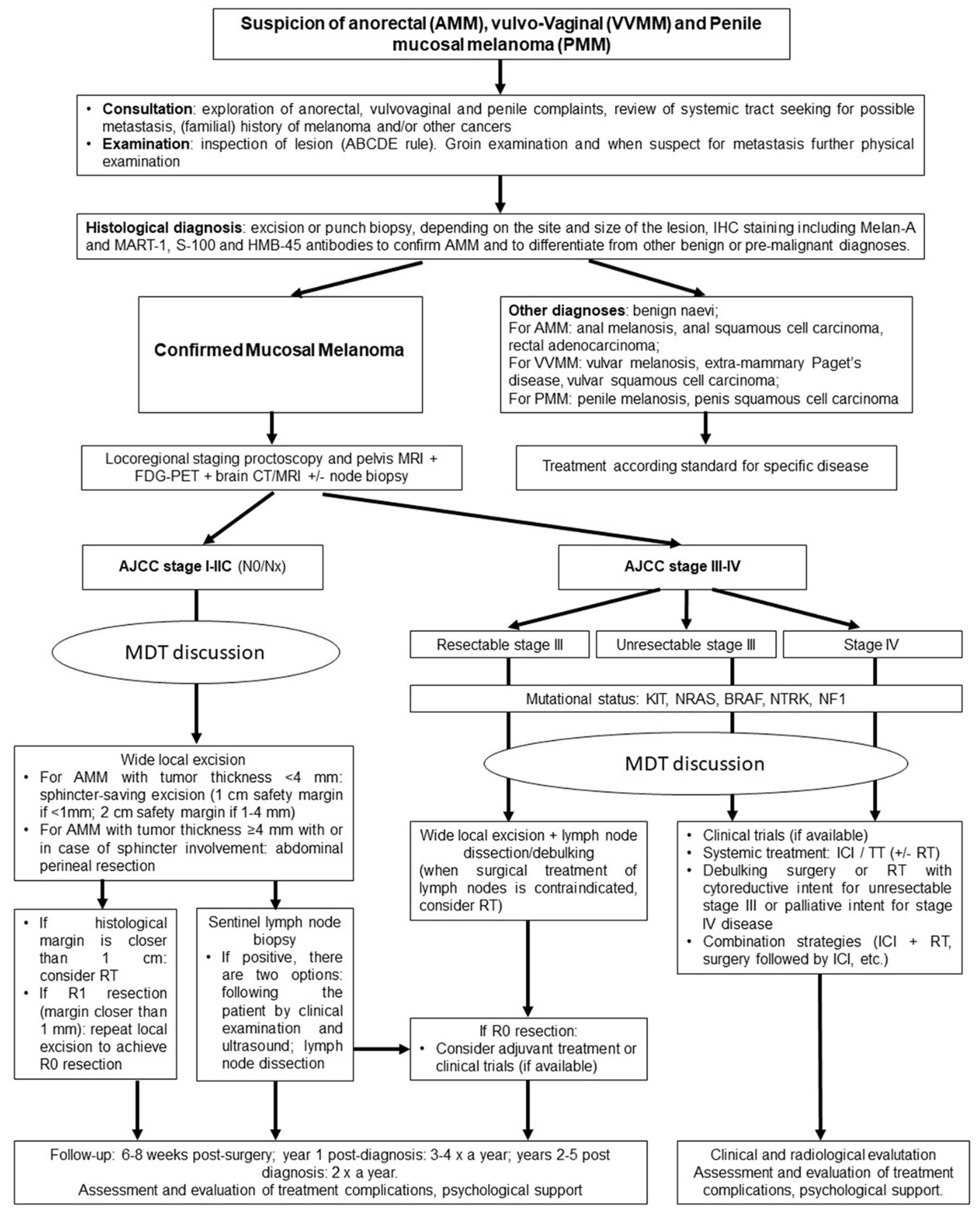
5. Diagnosis, Prognosis and Loco-Regional Treatments
5.1. Anorectal Mucosal Melanoma
5.2. Vulvo-Vaginal Mucosal Melanoma
5.3. Penile Mucosal Melanoma
6. Systemic Treatments for Anorectal and Genital Mucosal Melanoma
6.1. Immune Check-Points Inhibitors
6.2. Targeted Therapy
7. Follow-Up
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyrrell, H.; Payne, M. Combatting mucosal melanoma: Recent advances and future perspectives. Melanoma Manag. 2018, 5, MMT11. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.R.; Mehnert, J.M. Mucosal Melanoma: Epidemiology, Biology and Treatment. Hematopoietic Growth Factors in Oncology 2016, 167, 295–320. [Google Scholar] [CrossRef]
- Ma, Y.; Xia, R.; Ma, X.; Judson-Torres, R.L.; Zeng, H. Mucosal Melanoma: Pathological Evolution, Pathway Dependency and Targeted Therapy. Front. Oncol. 2021, 11, 702287. [Google Scholar] [CrossRef]
- Trzcinski, R.; Kujawski, R.; Mik, M.; Sygut, A.; Dziki, L.; Dziki, A. Malignant melanoma of the anorectum—a rare entity. Langenbeck’s Archives of Surgery 2010, 395, 757–760. [Google Scholar] [CrossRef]
- Ragnarsson-Olding, B.K.; Nilsson, P.J.; Olding, L.B.; Nilsson, B.R. Primary ano-rectal malignant melanomas within a population-based national patient series in Sweden during 40 years. Acta Oncol. 2009, 48, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Coté, T.R.; Sobin, L.H. Primary melanomas of the esophagus and anorectum: Epidemiologic comparison with melanoma of the skin. Melanoma Res. 2009, 19, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Gökaslan, H.; Şişmanoğlu, A.; Pekin, T.; Kaya, H.; Ceyhan, N. Primary malignant melanoma of the vagina: A case report and review of the current treatment options. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 121, 243–248. [Google Scholar] [CrossRef]
- Sugiyama, V.E.; Chan, J.K.; Shin, J.Y.; Berek, J.S.; Osann, K.; Kapp, D.S. Vulvar Melanoma. Obstet. Gynecol. 2007, 110, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Papeš, D.; Altarac, S.; Arslani, N.; Rajković, Z.; Antabak, A.; Ćaćić, M. Melanoma of the Glans Penis and Urethra. Urology 2014, 83, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Situm, M.; Buljan, M.; Bulić, S.O.; Simić, D. The mechanisms of UV radiation in the development of malignant melanoma. Coll. Antropol. 2007, 31, 13–16. [Google Scholar] [PubMed]
- Edwards, R.H.; Ward, M.R.; Wu, H.; Medina, C.A.; Brose, M.S.; Volpe, P.; Nussen-Lee, S.; Haupt, H.M.; Martin, A.M.; Herlyn, M.; et al. Absence of BRAF mutations in UV-protected mucosal melanomas. J. Med Genet. 2004, 41, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Row, D.; Weiser, M.R. Anorectal Melanoma. Clin. Colon Rectal Surg. 2009, 22, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Heinzelmann-Schwarz, V.A.; Nixdorf, S.; Valadan, M.; Diczbalis, M.; Olivier, J.; Otton, G.; Fedier, A.; Hacker, N.F.; Scurry, J.P. A clinicopathological review of 33 patients with vulvar melanoma identifies c-KIT as a prognostic marker. Int. J. Mol. Med. 2014, 33, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Madeddu, R.; Catania, V.E.; Bertino, G.; Morelli, L.; Perrotta, R.E.; Drago, F.; Malaguarnera, M.; Latteri, S. Anorectal mucosal melanoma. Oncotarget 2018, 9, 8785–8800. [Google Scholar] [CrossRef]
- Newell, F.; Kong, Y.; Wilmott, J.S.; Johansson, P.A.; Ferguson, P.M.; Cui, C.; Li, Z.; Kazakoff, S.H.; Burke, H.; Dodds, T.J.; et al. Whole-genome landscape of mucosal melanoma reveals diverse drivers and therapeutic targets. Nat. Commun. 2019, 10, 3163. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef]
- The AACR Project Genie Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Mucosa Melanoma—My Cancer Genome. Available online: https://www.mycancergenome.org/content/disease/mucosal-melanoma/ (accessed on 10 April 2021).
- Nassar, K.; Tan, A.C. The mutational landscape of mucosal melanoma. Semin. Cancer Biol. 2020, 61, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Dumaz, N.; Jouenne, F.; Delyon, J.; Mourah, S.; Bensussan, A.; Lebbé, C. Atypical BRAF and NRAS Mutations in Mucosal Melanoma. Cancers 2019, 11, 1133. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, M.; Giunta, E.; Tortora, M.; Curvietto, M.; Attademo, L.; Bosso, D.; Cardalesi, C.; Rosanova, M.; De Placido, P.; Pietroluongo, E.; et al. BRAF Gene and Melanoma: Back to the Future. Int. J. Mol. Sci. 2021, 22, 3474. [Google Scholar] [CrossRef] [PubMed]
- Satzger, I.; Schaefer, T.; Kuettler, U.; Broecker, V.; Voelker, B.; Ostertag, H.; Kapp, A.; Gutzmer, R. Analysis of c-KIT expression and KIT gene mutation in human mucosal melanomas. Br. J. Cancer 2008, 99, 2065–2069. [Google Scholar] [CrossRef]
- Chacón, M.; Pfluger, Y.; Angel, M.; Waisberg, F.; Enrico, D. Uncommon Subtypes of Malignant Melanomas: A Review Based on Clinical and Molecular Perspectives. Cancers 2020, 12, 2362. [Google Scholar] [CrossRef]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F.; Azar, S.; et al. KIT Gene Mutations and Copy Number in Melanoma Subtypes. Clin. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef]
- Omholt, K.; Grafström, E.; Kanter-Lewensohn, L.; Hansson, J.; Ragnarsson-Olding, B.K. KIT Pathway Alterations in Mucosal Melanomas of the Vulva and Other Sites. Clin. Cancer Res. 2011, 17, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, I.; Ugurel, S.; Sucker, A.; Livingstone, E.; Zimmer, L.; Ziemer, M.; Utikal, J.; Mohr, P.; Pfeiffer, C.; Pföhler, C.; et al. Targeted next generation sequencing of mucosal melanomas identifies frequent NF1 and RAS mutations. Oncotarget 2017, 8, 40683–40692. [Google Scholar] [CrossRef]
- Gil, J.; Peters, G. Regulation of the INK4b–ARF–INK4a tumour suppressor locus: All for one or one for all. Nat. Rev. Mol. Cell Biol. 2006, 7, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cheng, Z.; Cui, C.; Wu, X.; Yu, H.; Guo, J.; Kong, Y. Frequent genetic aberrations in the cell cycle related genes in mucosal melanoma indicate the potential for targeted therapy. J. Transl. Med. 2019, 17, 1–15. [Google Scholar] [CrossRef]
- Kong, Y.; Sheng, X.; Wu, X.; Yan, J.; Ma, M.; Yu, J.; Si, L.; Chi, Z.; Cui, C.; Dai, J.; et al. Frequent Genetic Aberrations in the CDK4 Pathway in Acral Melanoma Indicate the Potential for CDK4/6 Inhibitors in Targeted Therapy. Clin. Cancer Res. 2017, 23, 6946–6957. [Google Scholar] [CrossRef] [PubMed]
- Forschner, A.; Hilke, F.-J.; Bonzheim, I.; Gschwind, A.; Demidov, G.; Amaral, T.; Ossowski, S.; Riess, O.; Schroeder, C.; Martus, P.; et al. MDM2, MDM4 and EGFR Amplifications and Hyperprogression in Metastatic Acral and Mucosal Melanoma. Cancers 2020, 12, 540. [Google Scholar] [CrossRef] [PubMed]
- Guterres, A.N.; Villanueva, J. Targeting telomerase for cancer therapy. Oncogene 2020, 39, 5811–5824. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Murali, R.; Puig-Butille, J.A.; Schilling, B.; Livingstone, E.; Potrony, M.; Carrera, C.; Schimming, T.; Möller, I.; Schwamborn, M.; et al. TERT Promoter Mutation Status as an Independent Prognostic Factor in Cutaneous Melanoma. JNC: J. Natl. Cancer Inst. 2014, 106, dju246. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Wang, R.; Ju, H.; Ren, G.; Guo, W.; Lyu, J. TERT promoter mutation is absent in oral mucosal melanoma. Oral Oncol. 2015, 51, e65–e66. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Song, J.; Li, N.; Song, Q.; Li, Y.; Du, Y.; Wang, X.; Jiao, Y.; Wu, L. Telomere Maintenance Associated Mutations in the Genetic Landscape of Gynecological Mucosal Melanoma. Front. Oncol. 2020, 10, 1707. [Google Scholar] [CrossRef]
- Couts, K.L.; Bemis, J.; Turner, J.A.; Bagby, S.M.; Murphy, D.; Christiansen, J.; Hintzsche, J.D.; Danielle, M.; Pitts, T.M.; Wells, K.; et al. ALK Inhibitor Response in Melanomas Expressing EML4-ALK Fusions and Alternate ALK Isoforms. Mol. Cancer Ther. 2018, 17, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, A. Trk receptor tyrosine kinases: A bridge between cancer and neural development. Cancer Lett. 2001, 169, 107–114. [Google Scholar] [CrossRef]
- Wiesner, T.; He, J.; Yelensky, R.; Esteve-Puig, R.; Botton, T.; Yeh, I.; Lipson, D.; Otto, G.; Brennan, K.; Murali, R.; et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat. Commun. 2014, 5, 3116. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Jorgenson, E.; Shen, L.; Xu, M.; North, J.P.; Shain, A.H.; Reuss, D.; Wu, H.; Robinson, W.; Olshen, A.; et al. Targeted Genomic Profiling of Acral Melanoma. JNCI J. Natl. Cancer Inst. 2019, 111, 1068–1077. [Google Scholar] [CrossRef]
- Stransky, N.; Cerami, E.; Schalm, S.; Kim, J.L.; Lengauer, C. The landscape of kinase fusions in cancer. Nat. Commun. 2014, 5, 4846. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, C.; Shoushtari, A.N.; Ariyan, C.; Hollmann, T.J.; Busam, K.J. Primary and Metastatic Melanoma With NTRK Fusions. Am. J. Surg. Pathol. 2018, 42, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Ablain, J.; Xu, M.; Rothschild, H.; Jordan, R.C.; Mito, J.K.; Daniels, B.H.; Bell, C.F.; Joseph, N.M.; Wu, H.; Bastian, B.C.; et al. Human tumor genomics and zebrafish modeling identify SPRED1 loss as a driver of mucosal melanoma. Science 2018, 362, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Hintzsche, J.D.; Gorden, N.T.; Amato, C.M.; Kim, J.; Wuensch, K.E.; Robinson, S.E.; Applegate, A.J.; Couts, K.L.; Medina, T.M.; Wells, K.R.; et al. Whole-exome sequencing identifies recurrent SF3B1 R625 mutation and comutation of NF1 and KIT in mucosal melanoma. Melanoma Res. 2017, 27, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Hsiao, S.J.; Schaeffer, D.F.; Lai, C.; Remotti, H.E.; Horst, D.; Mansukhani, M.M.; Horst, B.A. Identification of recurrent mutational events in anorectal melanoma. Mod. Pathol. 2016, 30, 286–296. [Google Scholar] [CrossRef]
- Singer, M.; Mutch, M.G. Anal Melanoma. Clin. Colon Rectal Surg. 2006, 19, 078–087. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.S.; Kavolius, J.P.; Quan, S.H.Q. Anorectal melanoma. Dis. Colon Rectum 1995, 38, 146–151. [Google Scholar] [CrossRef]
- Droesch, J.T.; Flum, D.R.; Mann, G.N. Wide local excision or abdominoperineal resection as the initial treatment for anorectal melanoma? Am. J. Surg. 2005, 189, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Podnos, Y.D.; Tsai, N.-C.; Smith, D.; Ellenhorn, J.D. Factors Affecting Survival in Patients with Anal Melanoma. Am. Surg. 2006, 72, 917–920. [Google Scholar] [CrossRef]
- Thibault, C.; Sagar, P.; Nivatvongs, S.; Ilstrup, D.M.; Wolff, B.G. Anorectal melanoma—An incurable disease? Dis. Colon Rectum 1997, 40, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Glimelius, B.; Påhlman, L. Anorectal malignant melanoma in Sweden. Dis. Colon Rectum 1990, 33, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Pessaux, P.; Pocard, M.; Elias, D.; Duvillard, P.; Avril, M.; Zimmerman, P.; Lasser, P. Surgical management of primary anorectal melanoma. Br. J. Surg. 2004, 91, 1183–1187. [Google Scholar] [CrossRef]
- Yeh, J.J.; Shia, J.; Hwu, W.J.; Busam, K.J.; Paty, P.B.; Guillem, J.G.; Coit, D.G.; Wong, W.D.; Weiser, M.R. The Role of Abdominoperineal Resection as Surgical Therapy for Anorectal Melanoma. Ann. Surg. 2006, 244, 1012–1017. [Google Scholar] [CrossRef]
- Falch, C.; Stojadinovic, A.; Hann-von-Weyhern, C.; Protic, M.; Nissan, A.; Faries, M.B.; Daumer, M.; Bilchik, A.J.; Itzhak, A.; Brücher, B.L. Anorectal malignant melanoma: Extensive 45-year review and proposal for a novel staging classification. J. Am. Coll. Surg. 2013, 217, 324e35. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, F.; Wan, D. Effect of misdiagnosis on the prognosis of anorectal malignant melanoma. J. Cancer Res. Clin. Oncol. 2010, 136, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.G.; Bagwan, I.; Board, R.E.; Capper, S.; Coupland, S.E.; Glen, J.; Lalondrelle, S.; Mayberry, A.; Muneer, A.; Nugent, K.; et al. Ano-uro-genital mucosal melanoma UK national guidelines. Eur. J. Cancer 2020, 135, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Kim, C.W.; Baek, S.J.; Hur, H.; Min, B.S.; Baik, S.H.; Kim, N.-K. The clinical features and optimal treatment of anorectal malignant melanoma. Ann. Surg. Treat. Res. 2014, 87, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Iddings, D.M.; Fleisig, A.J.; Chen, S.L.; Faries, M.B.; Morton, D.L. Practice Patterns and Outcomes for Anorectal Melanoma in the USA, Reviewing Three Decades of Treatment: Is More Extensive Surgical Resection Beneficial in All Patients? Ann. Surg. Oncol. 2010, 17, 40–44. [Google Scholar] [CrossRef]
- Stoidis, C.N.; Spyropoulos, B.G.; Misiakos, E.P.; Fountzilas, C.K.; Paraskeva, P.P.; Fotiadis, C.I. Diffuse anorectal melanoma; review of the current diagnostic and treatment aspects based on a case report. World J. Surg. Oncol. 2009, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Sayari, S.; Moussi, A.; Salah, R.B.H.; Gherib, S.B.; Haouet, K.; Zaouche, A. Primary anorectal melanoma: A case report. La Tunis. Med. 2010, 88, 430–432. [Google Scholar]
- Bullard, K.M.; Tuttle, T.M.; Rothenberger, D.A.; Madoff, R.D.; Baxter, N.N.; Finne, C.O.; Spencer, M.P. Surgical therapy for anorectal melanoma. J. Am. Coll. Surg. 2003, 196, 206–211. [Google Scholar] [CrossRef]
- Kiran, R.P.; Rottoli, M.; Pokala, N.; Fazio, V.W. Long-term Outcomes After Local Excision and Radical Surgery for Anal Melanoma: Data From a Population Database. Dis. Colon Rectum 2010, 53, 402–408. [Google Scholar] [CrossRef]
- Weyandt, G.H.; Eggert, A.O.; Houf, M.; Raulf, F.; Bröcker, E.B.; Becker, J.C. Anorectal melanoma: Surgical management guidelines according to tumour thickness. Br. J. Cancer 2003, 89, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.J.; Ragnarsson-Olding, B.K. Importance of clear resection margins in anorectal malignant melanoma. Br. J. Surg. 2009, 97, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Zagars, G.K.; Cormier, J.N.; Ross, M.I.; Guadagnolo, B.A. Sphincter-sparing local excision and hypofractionated radiation therapy for anorectal melanoma. Cancer 2011, 117, 4747–4755. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M.; Cheng, X.; Hamilton, A.L.; Siddiqui, N.A.; Jurgenliemk-Schulz, I.; Mahner, S.; Åvall-Lundqvist, E.; Kim, K.; Freyer, G. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Vulvovaginal Melanomas. Int. J. Gynecol. Cancer 2014, 24, S117–S122. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.A. Malignant melanoma of the vulva and vagina in the United States: Patterns of incidence and population-based estimates of survival. Am. J. Obstet. Gynecol. 1994, 171, 1225–1230. [Google Scholar] [CrossRef]
- Ragnarsson-Olding, B.; Johansson, H.; Rutqvist, L.E.; Ringborg, U. Malignant melanoma of the vulva and vagina. Trends in inci-dence, age distribution, and long-term survival among 245 consecutive cases in Sweden 1960–1984. Cancer. 1993, 71, 1893–1897. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Wu, X.-C.; Jemal, A.; Martin, H.J.; Roche, L.M.; Chen, V.W. Incidence of noncutaneous melanomas in the U.S. Cancer 2005, 103, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, R.; Fukuda, T.; Wada, T.; Kawanishi, M.; Imai, K.; Kasai, M.; Hashiguchi, Y.; Ichimura, T.; Yasui, T.; Sumi, T. A retrospective clinical analysis of 5 cases of vaginal melanoma. Mol. Clin. Oncol. 2017, 6, 373–376. [Google Scholar] [CrossRef]
- Aulmann, S.; Sinn, H.-P.; Penzel, R.; Gilks, C.B.; Schott, S.; Hassel, J.C.; Schmidt, D.; Kommoss, F.; Schirmacher, P.; Kommoss, S. Comparison of molecular abnormalities in vulvar and vaginal melanomas. Mod. Pathol. 2014, 27, 1386–1393. [Google Scholar] [CrossRef]
- Rouzbahman, M.; Kamel-Reid, S.; Al Habeeb, A.; Butler, M.; Dodge, J.; Laframboise, S.; Murphy, J.; Rasty, G.; Ghazarian, D. Malignant Melanoma of Vulva and Vagina. J. Low. Genit. Tract Dis. 2015, 19, 350–353. [Google Scholar] [CrossRef]
- Chung, A.F.; Woodruff, J.M.; Lewis, J.L. Malignant Melanoma of the Vulva. Obstet. Gynecol. 1975, 45, 638–646. [Google Scholar] [CrossRef]
- Lotem, M.; Anteby, S.; Peretz, T.; Ingber, A.; Avinoach, I.; Prus, D. Mucosal Melanoma of the Female Genital Tract Is a Multifocal Disorder. Gynecol. Oncol. 2003, 88, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Piura, B. Management of primary melanoma of the female urogenital tract. Lancet Oncol. 2008, 9, 973–981. [Google Scholar] [CrossRef]
- Podratz, K.C.; Gaffey, T.A.; Symmonds, R.E.; Johansen, K.L.; O’Brien, P.C. Melanoma of the vulva: An update. Gynecol. Oncol. 1983, 16, 153–168. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Thompson, J.F.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Atkins, M.B.; Balch, C.M.; Barnhill, R.L.; et al. Melanoma of the Skin. AJCC Cancer Staging Man. 2016, 2017, 563–586. [Google Scholar] [CrossRef]
- Baiocchi, G.; Duprat, J.P.; Neves, R.I.; Fukazawa, E.M.; Landman, G.; Guimarães, G.C.; Valadares, L.J. Vulvar melanoma: Report on eleven cases and review of the literature. Sao Paulo Med. J. 2010, 128, 38–41. [Google Scholar] [CrossRef]
- Pusceddu, S.; Bajetta, E.; Carcangiu, M.L.; Formisano, B.; Ducceschi, M.; Buzzoni, R. A literature overview of primary cervical malignant melanoma: An exceedingly rare cancer. Crit. Rev. Oncol. 2012, 81, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Malpica, A.; Deavers, M.T.; Silva, E.G. Vaginal Melanoma. Am. J. Surg. Pathol. 2002, 26, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.-N.; Yu, G.-P.; McCormick, S.A. Population-based incidence of vulvar and vaginal melanoma in various races and ethnic groups with comparisons to other site-specific melanomas. Melanoma Res. 2010, 20, 153–158. [Google Scholar] [CrossRef]
- Irvin, W.P.; Bliss, S.A.; Rice, L.W.; Taylor, P.T.; Andersen, W.A. Malignant Melanoma of the Vagina and Locoregional Control: Radical Surgery Revisited. Gynecol. Oncol. 1998, 71, 476–480. [Google Scholar] [CrossRef]
- Nigogosyan, G.; La De Pava, S.; Pickren, J.W. Melanoblasts in vaginal mucosa.Origin for primary malignant melanoma. Cancer 1964, 17, 912–913. [Google Scholar] [CrossRef]
- Allen, A.C.; Spitz, S. Malignant melanoma. A clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer 1953, 6, 1–45. [Google Scholar] [CrossRef]
- Reid, G.C.; Schmidt, R.W.; Roberts, J.A.; Hopkins, M.P.; Barrett, R.J.; Morley, G.W. Primary melanoma of the vagina: A clinicopatholog-ic analysis. Obstet. Gynecol. 1989, 74, 190–199. [Google Scholar] [PubMed]
- Borazjani, G.; Prem, K.A.; Okagaki, T.; Twiggs, L.B.; Adcock, L.L. Primary malignant melanoma of the vagina: A clinicopathological analysis of 10 cases. Gynecol. Oncol. 1990, 37, 264–267. [Google Scholar] [CrossRef]
- Miner, T.J.; Delgado, R.; Zeisler, J.; Busam, K.; Alektiar, K.; Barakat, R.; Poynor, E. Primary vaginal melanoma: A critical analysis of therapy. Ann. Surg. Oncol. 2004, 11, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Bhavani, M.; Deshpande, A. Trends of vulvar cancer. J. Obstet. Gynaecol. 2014, 34, 165–168. [Google Scholar] [CrossRef]
- Stroup, A.; Harlan, L.; Trimble, E. Demographic, clinical, and treatment trends among women diagnosed with vulvar cancer in the United States. Gynecol. Oncol. 2008, 108, 577–583. [Google Scholar] [CrossRef]
- Trone, J.-C.; Guy, J.-B.; Mery, B.; Escure, J.L.; Lahmar, R.; Moncharmont, C.; Rivoirard, R.; Semay, T.; Chauleur, C.; Collard, O.; et al. Mélanomes du tractus génital féminin: État des lieux. Bull Cancer 2014, 101, 102–106. [Google Scholar] [CrossRef]
- Regan, K.; Breen, M.; Ramaiya, N.; Jagannathan, J.; DiPiro, P.J.; Hodi, F.S.; Abbeele, A.D.V.D. Metastatic mucosal melanoma: Imaging patterns of metastasis and recurrence. Cancer Imaging 2013, 13, 626–632. [Google Scholar] [CrossRef]
- Vitale, S.G.; Valenti, G.; Biondi, A.; Rossetti, D.; Frigerio, L. Recent trends in surgical and reconstructive management of vulvar cancer: Review of literature. Updat. Surg. 2015, 67, 367–371. [Google Scholar] [CrossRef]
- Mert, I.; Semaan, A.; Winer, I.; Morris, R.T.; Ali-Fehmi, R. Vulvar/Vaginal Melanoma. Int. J. Gynecol. Cancer 2013, 23, 1118–1126. [Google Scholar] [CrossRef]
- Alkatout, I.; Schubert, M.; Garbrecht, N.; Weigel, M.T.; Jonat, W.; Mundhenke, C.; Günther, V. Vulvar cancer: Epidemiology, clinical presentation, and management options. Int. J. Women’s Health 2015, 7, 305–313. [Google Scholar] [CrossRef]
- Ditto, A.; Bogani, G.; Martinelli, F.; Di Donato, V.; Laufer, J.; Scasso, S.; Chiappa, V.; Signorelli, M.; Indini, A.; Lorusso, D.; et al. Surgical Management and Prognostic Factors of Vulvovaginal Melanoma. J. Low. Genit. Tract Dis. 2016, 20, e24–e29. [Google Scholar] [CrossRef]
- Moxley, K.; Fader, A.; Rose, P.; Case, A.; Mutch, D.; Berry, E.; Schink, J.; Kim, C.; Chi, D.; Moore, K. Malignant melanoma of the vulva: An extension of cutaneous melanoma? Gynecol. Oncol. 2011, 122, 612–617. [Google Scholar] [CrossRef]
- Trimble, E.L.; Lewis, J.L.; Williams, L.L.; Curtin, J.P.; Chapman, D.; Woodruff, J.M.; Rubin, S.C.; Hoskins, W.J. Management of vulvar melanoma. Gynecol. Oncol. 1992, 45, 254–258. [Google Scholar] [CrossRef]
- De Simone, P.; Silipo, V.; Buccini, P.; Mariani, G.; Marenda, S.; Eibenschutz, L.; Ferrari, A.; Catricalà, C. Vulvar melanoma: A report of 10 cases and review of the literature. Melanoma Res. 2008, 18, 127–133. [Google Scholar] [CrossRef] [PubMed]
- DeMatos, P.; Tyler, D.; Seigler, H.F. Mucosal melanoma of the female genitalia: A clinicopathologic study of forty-three cases at Duke University Medical Center. Surgery 1998, 124, 38–48. [Google Scholar] [CrossRef]
- Phillips, G.L.; Bundy, B.N.; Okagaki, T.; Kucera, P.R.; Stehman, F.B. Malignant melanoma of the vulva treated by radical hemivul-vectomy. A prospective study of the Gynecologic Oncology Group. Cancer 1994, 73, 2626–2632. [Google Scholar] [CrossRef]
- de Hullu, J.A.; Hollema, H.; Hoekstra, H.J.; Piers, D.A.; Mourits, M.J.E.; Aalders, J.G.; van der Zee, A.G.J. Vulvar melanoma. Cancer 2002, 94, 486–491. [Google Scholar] [CrossRef]
- Iacoponi, S.; Rubio, P.; Garcia, E.; Oehler, M.K.; Diez, J.; De-La-Noval, B.D.; Mora, P.; Gardella, B.; Gomez, I.; Kotsopoulos, I.C.; et al. Prognostic Factors of Recurrence and Survival in Vulvar Melanoma: Subgroup Analysis of the VULvar CANcer Study. Int. J. Gynecol. Cancer 2016, 26, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Ballo, M.T.; Ang, K. Radiation therapy for malignant melanoma. Surg. Clin. N. Am. 2003, 83, 323–342. [Google Scholar] [CrossRef]
- Samstein, R.M.; Carvajal, R.D.; Postow, M.A.; Callahan, M.K.; Shoushtari, A.N.; Patel, S.G.; Lee, N.Y.; Barker, C.A. Localized sinonasal mucosal melanoma: Outcomes and associations with stage, radiotherapy, and positron emission tomography response. Head Neck 2016, 38, 1310–1317. [Google Scholar] [CrossRef]
- Vongtama, R.; Safa, A.; Gallardo, D.; Calcaterra, T.; Juillard, G. Efficacy of radiation therapy in the local control of desmoplastic malignant melanoma. Head Neck 2003, 25, 423–428. [Google Scholar] [CrossRef]
- Testori, A.; Rutkowski, P.; Marsden, J.; Bastholt, L.; Chiarion-Sileni, V.; Hauschild, A.; Eggermont, A.M.M. Surgery and radiotherapy in the treatment of cutaneous melanoma. Ann. Oncol. 2009, 20, vi22–vi29. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Accorona, R.; Botti, G.; Farina, D.; Fossati, P.; Gatta, G.; Gogas, H.; Lombardi, D.; Maroldi, R.; Nicolai, P.; et al. Mucosal melanoma of the head and neck. Crit. Rev. Oncol. 2017, 112, 136–152. [Google Scholar] [CrossRef]
- Schiavone, M.B.; Broach, V.; Shoushtari, A.N.; Carvajal, R.D.; Alektiar, K.; Kollmeier, M.A.; Abu-Rustum, N.R.; Leitao, M.M. Combined immunotherapy and radiation for treatment of mucosal melanomas of the lower genital tract. Gynecol. Oncol. Rep. 2016, 16, 42–46. [Google Scholar] [CrossRef]
- Strojan, P. Role of radiotherapy in melanoma management. Radiol. Oncol. 2010, 44, 1. [Google Scholar] [CrossRef]
- Janco, J.M.T.; Markovic, S.N.; Weaver, A.L.; Cliby, W.A. Vulvar and vaginal melanoma: Case series and review of current management options including neoadjuvant chemotherapy. Gynecol. Oncol. 2013, 129, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Tcheung, W.J.; Selim, M.A.; Herndon, J.E.; Abernethy, A.; Nelson, K. Clinicopathologic study of 85 cases of melanoma of the female genitalia. J. Am. Acad. Dermatol. 2012, 67, 598–605. [Google Scholar] [CrossRef] [PubMed]
- van Geel, A.N.; Bakker, M.A.D.; Kirkels, W.; Horenblas, S.; Kroon, B.B.; de Wilt, J.H.; Eggermont, A.M.; Mooi, W.J.; van der Aa, M.N. Prognosis of Primary Mucosal Penile Melanoma: A Series of 19 Dutch Patients and 47 Patients from the Literature. Urology 2007, 70, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortiz, R.; Huang, S.F.; Tamboli, P.; Prieto, V.G.; Hester, G.; Pettaway, C.A. Melanoma of the penis, scrotum and male urethra: A 40-year single institution experience. J. Urol. 2005, 173, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, T.J.; Zincke, H.; Gaffey, T.A.; Woods, J.E. Malignant Melanoma of the Penis. J. Urol. 1988, 140, 72–75. [Google Scholar] [CrossRef]
- Oliva, E.; Quinn, T.R.; Amin, M.B.; Eble, J.N.; Epstein, J.I.; Srigley, J.R.; Young, R.H. Primary Malignant Melanoma of the Urethra. Am. J. Surg. Pathol. 2000, 24, 785–796. [Google Scholar] [CrossRef]
- Oldbring, J.; Mikulowski, P. Malignant melanoma of the penis and male urethra. Report of nine cases and review of the litera-ture. Cancer. 1987, 59, 581–587. [Google Scholar] [CrossRef]
- Jabiles, A.G.; Del Mar, E.Y.; Perez, G.A.D.; Vera, F.Q.; Montoya, L.M.; Deza, C.M.M. Penile melanoma: A 20-Year analysis of six patients at the National Cancer Institute of Peru, Lima. Ecancermedicalscience 2017, 11, 731. [Google Scholar] [CrossRef]
- Tacastacas, J.D.; Bray, J.; Cohen, Y.K.; Arbesman, J.; Kim, J.; Koon, H.B.; Honda, K.; Cooper, K.; Gerstenblith, M.R. Update on primary mucosal melanoma. J. Am. Acad. Dermatol. 2014, 71, 366–375. [Google Scholar] [CrossRef]
- Rambhia, P.H.; Scott, J.F.; Vyas, R.; Gerstenblith, M.R. Genitourinary Melanoma. In Noncutaneous Melanoma; Scott, J.F., Ger-stenblith, M.R., Eds.; Codon Publications: Brisbane, Australia, 2018; Chapter 5. [Google Scholar]
- Bechara, G.R.; Schwindt, A.B.D.S.; Ornellas, A.A.; Da Silva, D.E.A.; Lott, F.; De Campos, F.S. Penile primary melanoma: Analysis of 6 patients treated at Brazilian national cancer institute in the last eight years. Int. Braz. J. Urol. 2013, 39, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Raskyn, Y.; Vos, G.; Albersen, M. Penile Mucosal Melanoma: A case report and review of literature. Belg. J. Med. Oncol. 2020, 14, 74–79. [Google Scholar]
- Bracken, R.; Diokno, A. Melanoma of the Penis and the Urethra: 2 Case Reports and Review of the Literature. J. Urol. 1974, 111, 198–200. [Google Scholar] [CrossRef]
- Franceschelli, A.; Palmisano, F.; Gentile, G.; Vagnoni, V.; Zannetti, G.; Cipriani, R.; Colombo, F. Melanoma of glans penis and urethra: A case report and systematic review of the literature of a rare and complex neoplasm. Urol. J. 2021, 3915603211046471. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Si, L.; Cui, C.; Chi, Z.; Sheng, X.; Mao, L.; Li, S.; Kong, Y.; Tang, B.; Guo, J. Phase II randomized trial comparing high-dose IFN-α2b with temozolomide plus cisplatin as sys-temic adjuvant therapy for resected mucosal melanoma. Clin. Cancer Res. 2013, 15, 4488–4498. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Ha, H.K.; Kim, A.Y.; Kim, T.K.; Kim, J.-S.; Yu, C.S.; Park, S.W.; Park, M.-S.; Kim, H.J.; Kim, P.-N.; et al. Primary Malignant Melanoma of the Rectum: CT Findings in Eight Patients. Radiol. 2004, 232, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Sanguino, A.M.; Hodges, C.; Papadopoulos, N.E.; Eton, O.; Camacho, L.H.; Broemeling, L.D.; Johnson, M.M.; Ballo, M.T.; Ross, M.I.; et al. Biochemotherapy in patients with metastatic anorectal mucosal melanoma. Cancer 2004, 100, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.; Liong, J.; Kumar, D.; Glees, J. A striking response of anorectal melanoma to radiotherapy (locoregional disease confined to perineum and anal canal). Ann. R. Coll. Surg. Engl. 2010, 92, e10–e12. [Google Scholar] [CrossRef][Green Version]
- Harting, M.S.; Kim, K.B. Biochemotherapy in patients with advanced vulvovaginal mucosal melanoma. Melanoma Res. 2004, 14, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Tokita, T.; Kawahara, T.; Ito, Y.; Tsutsumi, S.; Abe, K.; Namura, K.; Sano, F.; Shioi, K.; Takamoto, D.; Yumura, Y.; et al. Primary amelanotic malignant melanoma of the male urethra with inguinal lymph node metastasis successfully controlled by nivolumab: A case report. Urol. Case Rep. 2018, 18, 54–56. [Google Scholar] [CrossRef]
- Tosev, G.; Kuru, T.H.; Huber, J.C.; Freier, G.; Bergmann, F.; Hassel, J.C.; Pahernik, S.A.; Hohenfellner, M.; Hadaschik, B.A. Primary melanoma of the prostate: Case report and review of the literature. BMC Urol. 2015, 15, 68. [Google Scholar] [CrossRef]
- Ottaviano, M.; De Placido, S.; Ascierto, P.A. Recent success and limitations of immune checkpoint inhibitors for cancer: A lesson from melanoma. Virchows Arch. 2019, 474, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kan, H.; Zhao, L.; Sun, Z.; Bai, C. Immune checkpoint inhibitors in advanced or metastatic mucosal melanoma: A systematic review. Ther. Adv. Med. Oncol. 2020, 12, 1758835920922028. [Google Scholar] [CrossRef] [PubMed]
- Mignard, C.; Huvier, A.D.; Gillibert, A.; Modeste, A.B.D.; Dutriaux, C.; Khammari, A.; Avril, M.-F.; Kramkimel, N.; Mortier, L.; Marcant, P.; et al. Efficacy of Immunotherapy in Patients with Metastatic Mucosal or Uveal Melanoma. J. Oncol. 2018, 2018, 1908065. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination With Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Munhoz, R.R.; Kuk, D.; Ott, P.A.; Johnson, D.B.; Tsai, K.K.; Rapisuwon, S.; Eroglu, Z.; Sullivan, R.J.; Luke, J.J.; et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer 2016, 122, 3354–3362. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Ribas, A.; Hodi, F.S.; Walpole, E.; Daud, A.; Arance, A.S.; Brown, E.; Hoeller, C.; Mortier, L.; et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: A post-hoc analysis of KEYNOTE-001, 002, 006. Br. J. Cancer 2018, 119, 670–674. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, J.S.; Roh, M.R.; Oh, B.H.; Chung, K.Y.; Shin, S.J.; Koom, W.S. Effect of Radiotherapy Combined With Pembrolizumab on Local Tumor Control in Mucosal Melanoma Patients. Front. Oncol. 2019, 9, 835. [Google Scholar] [CrossRef]
- van Zeijl, M.C.; Boer, F.L.; van Poelgeest, M.I.; Eertwegh, A.J.V.D.; Wouters, M.W.; de Wreede, L.C.; Aarts, M.J.; Berkmortel, F.W.V.D.; de Groot, J.W.B.; Hospers, G.A.; et al. Survival outcomes of patients with advanced mucosal melanoma diagnosed from 2013 to 2017 in the Netherlands—A nationwide population-based study. Eur. J. Cancer 2020, 137, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Lorusso, D.; Raspagliesi, F.; Del Vecchio, M. Investigating the role of immunotherapy in advanced/recurrent female genital tract melanoma: A preliminary experience. J. Gynecol. Oncol. 2019, 30, e94. [Google Scholar] [CrossRef] [PubMed]
- Quéreux, G.; Wylomanski, S.; Bouquin, R.; Saint-Jean, M.; Peuvrel, L.; Knol, A.C.; Hanf, M.; Dréno, B. Are checkpoint inhibitors a valuable option for metastatic or unresectable vulvar and vaginal melanomas? J. Eur. Acad. Dermatol. Venereol. 2017, 32. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Wagstaff, J.; Ascierto, P.A.; Butler, M.O.; Lao, C.D.; Marquez-Rodas, I.; Chiarion-Sileni, V.; Dummer, R.; Ferrucci, P.F.; Lorigan, P.; et al. CheckMate 067: Long-term outcomes in patients with mucosal melanoma. J. Clin. Oncol. 2020, 38, 10019. [Google Scholar] [CrossRef]
- Seban, R.-D.; Moya-Plana, A.; Antonios, L.; Yeh, R.; Marabelle, A.; Deutsch, E.; Schwartz, L.H.; Gómez, R.G.H.; Saenger, Y.; Robert, C.; et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur. J. Pediatr. 2020, 47, 2301–2312. [Google Scholar] [CrossRef]
- Postow, M.A.; Hamid, O.; Carvajal, R.D. Mucosal Melanoma: Pathogenesis, Clinical Behavior, and Management. Curr. Oncol. Rep. 2012, 14, 441–448. [Google Scholar] [CrossRef]
- Long, G.; Eroglu, Z.; Infante, J.; Patel, S.; Daud, A.; Johnson, D.B.; Gonzalez, R.; Kefford, R.; Hamid, O.; Schuchter, L.; et al. Long-Term Outcomes in Patients With BRAF V600–Mutant Metastatic Melanoma Who Received Dabrafenib Combined With Trametinib. J. Clin. Oncol. 2018, 36, 667–673. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Ito, T.; Kato, H.; Irie, H.; Kaji, T.; Maekawa, T.; Asai, J.; Yamamoto, Y.; Fujimura, T.; Nakai, Y.; et al. Outcome of combination therapy using BRAF and MEK inhibitors among Asian patients with advanced melanoma: An analysis of 112 cases. Eur. J. Cancer 2021, 145, 210–220. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Schadendorf, D.; Berking, C.; Agarwala, S.S.; van Herpen, C.M.; Queirolo, P.; Blank, C.U.; Hauschild, A.; Beck, J.T.; St-Pierre, A.; et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: A non-randomised, open-label phase 2 study. Lancet Oncol. 2013, 14, 249–256. [Google Scholar] [CrossRef]
- Trunzer, K.; Pavlick, A.C.; Schuchter, L.; Gonzalez, R.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; Kim, K.B.; Weber, J.S.; et al. Pharmacodynamic Effects and Mechanisms of Resistance to Vemurafenib in Patients With Metastatic Melanoma. J. Clin. Oncol. 2013, 31, 1767–1774. [Google Scholar] [CrossRef]
- Algazi, A.P.; Muthukumar, A.H.; O’Brien, K.; Lencioni, A.; Tsai, K.K.; Kadafour, M.; Chapman, P.B.; Daud, A. Phase II trial of trametinib in combination with the AKT inhibitor GSK 2141795 in BRAF wild-type melanoma. J. Clin. Oncol. 2015, 33, 9068. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kittaneh, M.; Lolkema, M.P.J.K.; Postow, M.A.; Schwartz, G.; Franklin, C.; Matano, A.; Bhansali, S.; Parasuraman, S.; Kim, K. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity. J. Clin. Oncol. 2014, 32, 9009. [Google Scholar] [CrossRef]
- Wei, B.-R.; Hoover, S.B.; Peer, C.J.; Dwyer, J.E.; Adissu, H.A.; Shankarappa, P.; Yang, H.; Lee, M.; Peat, T.J.; Figg, W.D.; et al. Efficacy, Tolerability, and Pharmacokinetics of Combined Targeted MEK and Dual mTORC1/2 Inhibition in a Preclinical Model of Mucosal Melanoma. Mol. Cancer Ther. 2020, 19, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Montella, L.; Ottaviano, M.; Riccio, V.; Picozzi, F.; Facchini, G.; Insabato, L.; Giuliano, M.; Palmieri, G. Results of TETimaX Trial of Langerhans Cell Histiocytosis Treatment and Perspectives on the Role of Imatinib Mesylate in the Era of MAPK Signaling. Biomedicines 2021, 9, 1759. [Google Scholar] [CrossRef] [PubMed]
- Minor, D.R.; Kashani-Sabet, M.; Garrido, M.; O’Day, S.J.; Hamid, O.; Bastian, B.C. Sunitinib Therapy for Melanoma Patients with KIT Mutations. Clin. Cancer Res. 2012, 18, 1457–1463. [Google Scholar] [CrossRef]
- Kluger, H.M.; Dudek, A.Z.; McCann, C.; Ritacco, J.; Southard, N.; Jilaveanu, L.B.; Molinaro, A.; Sznol, M. A phase II trial of dasatinib in advanced melanoma. Cancer 2011, 117, 2202–2208. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, K.M.; Kwon, M.; Kim, J.H.; Lee, J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Investig. New Drugs 2012, 30, 2008–2014. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Lawrence, D.P.; Weber, J.S.; Gajewski, T.F.; Gonzalez, R.; Lutzky, J.; O’Day, S.J.; Hamid, O.; Wolchok, J.D.; Chapman, P.B.; et al. Phase II Study of Nilotinib in Melanoma Harboring KIT Alterations Following Progression to Prior KIT Inhibition. Clin. Cancer Res. 2015, 21, 2289–2296. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.-A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a Therapeutic Target in Metastatic Melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Carvajal, R.D.; Dummer, R.; Hauschild, A.; Daud, A.; Bastian, B.C.; Markovic, S.N.; Queirolo, P.; Arance, A.; Berking, C.; et al. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: Final results from the global, single-arm, phase II TEAM trial. Ann. Oncol. 2017, 28, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Friedlander, P.; Gonzalez, R.; Weber, J.S.; Gajewski, T.F.; et al. Imatinib for Melanomas Harboring Mutationally Activated or Amplified KIT Arising on Mucosal, Acral, and Chronically Sun-Damaged Skin. J. Clin. Oncol. 2013, 31, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Zukotynski, K.; Yap, J.T.; Giobbie-Hurder, A.; Weber, J.; Gonzalez, R.; Gajewski, T.F.; O’Day, S.; Kim, K.; Hodi, F.S.; Abbeele, A.D.V.D. Metabolic response by FDG-PET to imatinib correlates with exon 11 KIT mutation and predicts outcome in patients with mucosal melanoma. Cancer Imaging 2014, 14, 30. [Google Scholar] [CrossRef]
- Wei, X.; Mao, L.; Chi, Z.; Sheng, X.; Cui, C.; Kong, Y.; Dai, J.; Wang, X.; Li, S.; Tang, B.; et al. Efficacy Evaluation of Imatinib for the Treatment of Melanoma: Evidence From a Retrospective Study. Oncol. Res. 2019, 27, 495–501. [Google Scholar] [CrossRef]
- Woodman, S.E.; Davies, M.A. Targeting KIT in melanoma: A paradigm of molecular medicine and targeted therapeutics. Biochem. Pharmacol. 2010, 80, 568–574. [Google Scholar] [CrossRef]
- Todd, J.R.; Becker, T.M.; Kefford, R.F.; Rizos, H. Secondary c-Kit mutations confer acquired resistance to RTK inhibitors in c-Kit mutant melanoma cells. Pigment. Cell Melanoma Res. 2013, 26, 518–526. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Palumbo, G.; Di Lorenzo, G.; Ottaviano, M.; Damiano, V. The future of melanoma therapy: Developing new drugs and improving the use of old ones. Futur. Oncol. 2016, 12, 2531–2534. [Google Scholar] [CrossRef] [PubMed]
- Trotter, S.C.; Sroa, N.; Winkelmann, R.R.; Olencki, T.; Bechtel, M. A Global Review of Melanoma Follow-up Guidelines. J. Clin. aesthetic Dermatol. 2013, 6, 18–26. [Google Scholar]
- Irvin, W.P.; Legallo, R.L.; Stoler, M.H.; Rice, L.W.; Taylor, P.T.; Andersen, W.A. Vulvar Melanoma: A Retrospective Analysis and Literature Review. Gynecol. Oncol. 2001, 83, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Mehra, T.; Grözinger, G.; Mann, S.; Guenova, E.; Moos, R.; Röcken, M.; Claussen, C.D.; Dummer, R.; Clasen, S.; Naumann, A.; et al. Primary Localization and Tumor Thickness as Prognostic Factors of Survival in Patients with Mucosal Melanoma. PLoS ONE 2014, 9, e112535. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Obermair, A.; Cella, D.; Crandon, A.J.; Trimmel, M. Vulvar cancer patients’ quality of life: A qualitative assessment. Int. J. Gynecol. Cancer 2004, 14, 875–881. [Google Scholar] [CrossRef] [PubMed]
- DiSaia, P.J.; Creasman, W.T.; Rich, W.M. An alternate approach to early cancer of the vulva. Am. J. Obstet. Gynecol. 1979, 133, 825–832. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottaviano, M.; Giunta, E.F.; Marandino, L.; Tortora, M.; Attademo, L.; Bosso, D.; Cardalesi, C.; Fabbrocini, A.; Rosanova, M.; Silvestri, A.; et al. Anorectal and Genital Mucosal Melanoma: Diagnostic Challenges, Current Knowledge and Therapeutic Opportunities of Rare Melanomas. Biomedicines 2022, 10, 150. https://doi.org/10.3390/biomedicines10010150
Ottaviano M, Giunta EF, Marandino L, Tortora M, Attademo L, Bosso D, Cardalesi C, Fabbrocini A, Rosanova M, Silvestri A, et al. Anorectal and Genital Mucosal Melanoma: Diagnostic Challenges, Current Knowledge and Therapeutic Opportunities of Rare Melanomas. Biomedicines. 2022; 10(1):150. https://doi.org/10.3390/biomedicines10010150
Chicago/Turabian StyleOttaviano, Margaret, Emilio Francesco Giunta, Laura Marandino, Marianna Tortora, Laura Attademo, Davide Bosso, Cinzia Cardalesi, Antonietta Fabbrocini, Mario Rosanova, Antonia Silvestri, and et al. 2022. "Anorectal and Genital Mucosal Melanoma: Diagnostic Challenges, Current Knowledge and Therapeutic Opportunities of Rare Melanomas" Biomedicines 10, no. 1: 150. https://doi.org/10.3390/biomedicines10010150
APA StyleOttaviano, M., Giunta, E. F., Marandino, L., Tortora, M., Attademo, L., Bosso, D., Cardalesi, C., Fabbrocini, A., Rosanova, M., Silvestri, A., Montella, L., Tammaro, P., Marra, E., Trojaniello, C., Vitale, M. G., Simeone, E., Troiani, T., Daniele, B., & Ascierto, P. A. (2022). Anorectal and Genital Mucosal Melanoma: Diagnostic Challenges, Current Knowledge and Therapeutic Opportunities of Rare Melanomas. Biomedicines, 10(1), 150. https://doi.org/10.3390/biomedicines10010150





