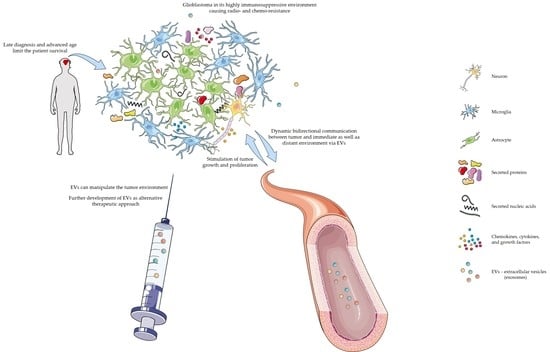
Dynamic Intercell Communication between Glioblastoma and Microenvironment through Extracellular Vesicles
Abstract
:1. The Genetic Component of Glioblastoma
2. Extracellular Vesicles in Glioblastoma
3. Inflammation
3.1. Inflammation and Cancer
3.2. Inflammation and Extracellular Vesicles
4. Glioblastoma Interactions with Its Microenvironment
5. Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current Opinion on Molecular Characterization for GBM Classification in Guiding Clinical Diagnosis, Prognosis, and Therapy. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef]
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, L.M.; Mellinghoff, I.K. Virchow 2011 or How to ID(H) Human Glioblastoma. J. Clin. Oncol. 2011, 29, 4473–4474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoyanov, G.S.; Dzhenkov, D.L. On the Concepts and History of Glioblastoma Multiforme-Morphology, Genetics and Epigenetics. Folia Med. 2018, 60, 48–66. [Google Scholar] [CrossRef] [Green Version]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Tiek, D.M.; Rone, J.D.; Graham, G.T.; Pannkuk, E.L.; Haddad, B.R.; Riggins, R.B. Alterations in Cell Motility, Proliferation, and Metabolism in Novel Models of Acquired Temozolomide Resistant Glioblastoma. Sci. Rep. 2018, 8, 7222. [Google Scholar] [CrossRef]
- Alifieris, K.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Noch, E.K.; Ramakrishna, R.; Magge, R. Challenges in the Treatment of Glioblastoma: Multisystem Mechanisms of Therapeutic Resistance. World Neurosurg. 2018, 116, 505–517. [Google Scholar] [CrossRef]
- Lah, T.T.; Novak, M.; Breznik, B. Brain malignancies: Glioblastoma and brain metastases. Semin. Cancer Biol. 2020, 60, 262–273. [Google Scholar] [CrossRef]
- Incekara, F.; Koene, S.; Vincent, A.J.P.E.; van den Bent, M.J.; Smits, M. Association Between Supratotal Glioblastoma Resection and Patient Survival: A Systematic Review and Meta-Analysis. World Neurosurg. 2019, 127, 617–624.e612. [Google Scholar] [CrossRef]
- Chen, B.; Chen, C.; Zhang, Y.; Xu, J. Recent incidence trend of elderly patients with glioblastoma in the United States, 2000–2017. BMC Cancer 2021, 21, 54. [Google Scholar] [CrossRef]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.V.; Davis, F.G. Malignant primary brain and other central nervous system tumors diagnosed in Canada from 2009 to 2013. Neuro-Oncology 2019, 21, 360–369. [Google Scholar] [CrossRef]
- Yang, W.; Warrington, N.M.; Taylor, S.J.; Whitmire, P.; Carrasco, E.; Singleton, K.W.; Wu, N.; Lathia, J.D.; Berens, M.E.; Kim, A.H.; et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Onizuka, H.; Masui, K.; Komori, T. Diffuse gliomas to date and beyond 2016 WHO Classification of Tumours of the Central Nervous System. Int. J. Clin. Oncol. 2020, 25, 997–1003. [Google Scholar] [CrossRef]
- Hartmann, C.; Hentschel, B.; Simon, M.; Westphal, M.; Schackert, G.; Tonn, J.C.; Loeffler, M.; Reifenberger, G.; Pietsch, T.; von Deimling, A.; et al. Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin. Cancer Res. 2013, 19, 5146–5157. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Grading of adult diffuse gliomas according to the 2021 WHO Classification of Tumors of the Central Nervous System. Lab. Investig. 2021, 1–8. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; de Carvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.; Yong, R.L.; Paddison, P.; Zhu, J. Comparison of glioblastoma (GBM) molecular classification methods. Semin. Cancer Biol. 2018, 53, 201–211. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, e1535750. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Nikoobakht, M.; Shamshiripour, P.; Shahin, M.; Bouzari, B.; Razavi-Hashemi, M.; Ahmadvand, D.; Akbarpour, M. A systematic update to circulating extracellular vesicles proteome; transcriptome and small RNA-ome as glioma diagnostic, prognostic and treatment-response biomarkers. Cancer Treat. Res. Commun. 2021, 30, 100490. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2018, 39, 501–513. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pillai, P.P. Current insights on extracellular vesicle-mediated glioblastoma progression: Implications in drug resistance and epithelial-mesenchymal transition. Biochim. Biophys. Acta Gen. Subj. 2021, 1866, 130065. [Google Scholar] [CrossRef]
- Lee, Y.; El Andaloussi, S.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef] [Green Version]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Wang, J.; Hao, Y.; Liu, F.; Wang, X.; Yang, L.; Lu, Z. The Roles of Exosomes as Future Therapeutic Agents and Diagnostic Tools for Glioma. Front. Oncol. 2021, 11, 733529. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Zhu, A.; Gong, L. Exosomes of glioma cells deliver miR-148a to promote proliferation and metastasis of glioblastoma via targeting CADM1. Bull. Cancer 2018, 105, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cai, Y.; Peng, Y.; Xu, B.; Hui, W.; Jiang, Y. Exosomal LGALS9 in the cerebrospinal fluid of glioblastoma patients suppressed dendritic cell antigen presentation and cytotoxic T-cell immunity. Cell Death Dis. 2020, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jia, J.; Cui, Y.; Peng, Y.; Jiang, Y. CD73-positive extracellular vesicles promote glioblastoma immunosuppression by inhibiting T-cell clonal expansion. Cell Death Dis. 2021, 12, 1065. [Google Scholar] [CrossRef]
- Verderio, C.; Muzio, L.; Turola, E.; Bergami, A.; Novellino, L.; Ruffini, F.; Riganti, L.; Corradini, I.; Francolini, M.; Garzetti, L.; et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 2012, 72, 610–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.L.; Johnson, K.M.; Conrad, C.; Patterson, J.T.; Sowers, L.C. The role of inflammation in brain cancer. Adv. Exp. Med. Biol. 2014, 816, 75–105. [Google Scholar] [CrossRef] [PubMed]
- Broekman, M.L.; Maas, S.L.N.; Abels, E.R.; Mempel, T.R.; Krichevsky, A.M.; Breakefield, X.O. Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol. 2018, 14, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Haidich, A.B. Meta-analysis in medical research. Hippokratia 2010, 14, 29–37. [Google Scholar]
- Yang, C.; Wen, H.B.; Zhao, Y.H.; Huang, W.H.; Wang, Z.F.; Li, Z.Q. Systemic Inflammatory Indicators as Prognosticators in Glioblastoma Patients: A Comprehensive Meta-Analysis. Front. Neurol. 2020, 11, 580101. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Pinioti, S.; Schellenberger, P.; Rajeeve, V.; Wendler, F.; Cutillas, P.R.; King, A.; Stebbing, J.; Giamas, G. Shedding of bevacizumab in tumour cells-derived extracellular vesicles as a new therapeutic escape mechanism in glioblastoma. Mol. Cancer 2018, 17, 132. [Google Scholar] [CrossRef]
- Panzarini, E.; Tacconi, S.; Carata, E.; Mariano, S.; Tata, A.M.; Dini, L. Molecular Characterization of Temozolomide-Treated and Non Temozolomide-Treated Glioblastoma Cells Released Extracellular Vesicles and Their Role in the Macrophage Response. Int. J. Mol. Sci. 2020, 21, 8353. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Gaikwad, M.; Bhattacharrya, D.K.; Barah, P. Identification of Systems Level Molecular Signatures from Glioblastoma Multiforme Derived Extracellular Vesicles. J. Mol. Neurosci. 2021, 71, 1156–1167. [Google Scholar] [CrossRef]
- Abels, E.R.; Maas, S.L.N.; Nieland, L.; Wei, Z.Y.; Cheah, P.S.; Tai, E.; Kolsteeg, C.J.; Dusoswa, S.A.; Ting, D.T.; Hickman, S.; et al. Glioblastoma-Associated Microglia Reprogramming Is Mediated by Functional Transfer of Extracellular miR-21. Cell Rep. 2019, 28, 3105–3119.e7. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Qiu, W.; Liu, Q.; Qian, M.; Wang, S.; Zhang, Z.; Gao, X.; Chen, Z.; Xue, H.; Li, G. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene 2018, 37, 4239–4259. [Google Scholar] [CrossRef]
- van der Vos, K.E.; Abels, E.R.; Zhang, X.; Lai, C.; Carrizosa, E.; Oakley, D.; Prabhakar, S.; Mardini, O.; Crommentuijn, M.H.; Skog, J.; et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro-Oncology 2016, 18, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, Y.; Dai, L.; Shi, G.; Deng, J.; Luo, Q.; Xie, Q.; Cheng, L.; Li, C.L.; Lin, Y.; et al. BATF2 prevents glioblastoma multiforme progression by inhibiting recruitment of myeloid-derived suppressor cells. Oncogene 2021, 40, 1516–1530. [Google Scholar] [CrossRef]
- Palumbo, P.; Lombardi, F.; Augello, F.R.; Giusti, I.; Dolo, V.; Leocata, P.; Cifone, M.G.; Cinque, B. Biological effects of selective COX-2 inhibitor NS398 on human glioblastoma cell lines. Cancer Cell Int. 2020, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Hallal, S.; Mallawaaratchy, D.M.; Wei, H.; Ebrahimkhani, S.; Stringer, B.W.; Day, B.W.; Boyd, A.W.; Guillemin, G.J.; Buckland, M.E.; Kaufman, K.L. Extracellular Vesicles Released by Glioblastoma Cells Stimulate Normal Astrocytes to Acquire a Tumor-Supportive Phenotype Via p53 and MYC Signaling Pathways. Mol. Neurobiol. 2019, 56, 4566–4581. [Google Scholar] [CrossRef] [Green Version]
- Gabrusiewicz, K.; Li, X.; Wei, J.; Hashimoto, Y.; Marisetty, A.L.; Ott, M.; Wang, F.; Hawke, D.; Yu, J.; Healy, L.M.; et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 2018, 7, e1412909. [Google Scholar] [CrossRef] [PubMed]
- Kore, R.A.; Abraham, E.C. Inflammatory cytokines, interleukin-1 beta and tumor necrosis factor-alpha, upregulated in glioblastoma multiforme, raise the levels of CRYAB in exosomes secreted by U373 glioma cells. Biochem. Biophys. Res. Commun. 2014, 453, 326–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, D.; Li, S.; Li, D.; Xue, H.; Yang, D.; Liu, Y. Mining TCGA database for genes of prognostic value in glioblastoma microenvironment. Aging 2018, 10, 592–605. [Google Scholar] [CrossRef] [Green Version]
- Benecke, L.; Coray, M.; Umbricht, S.; Chiang, D.; Figueiro, F.; Muller, L. Exosomes: Small EVs with Large Immunomodulatory Effect in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 3600. [Google Scholar] [CrossRef]
- Brandao, M.; Simon, T.; Critchley, G.; Giamas, G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 2019, 67, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, M.; De Gregorio, V.; Iorio, A.L.; Giunti, L.; Guidi, M.; de Martino, M.; Genitori, L.; Sardi, I. Glioblastoma Chemoresistance: The Double Play by Microenvironment and Blood-Brain Barrier. Int. J. Mol. Sci. 2018, 19, 2879. [Google Scholar] [CrossRef] [Green Version]
- Azambuja, J.H.; Ludwig, N.; Yerneni, S.; Rao, A.; Braganhol, E.; Whiteside, T.L. Molecular profiles and immunomodulatory activities of glioblastoma-derived exosomes. Neuro-Oncol. Adv. 2020, 2, vdaa056. [Google Scholar] [CrossRef]
- Tankov, S.; Walker, P.R. Glioma-Derived Extracellular Vesicles–Far More Than Local Mediators. Front. Immunol. 2021, 12, 679954. [Google Scholar] [CrossRef]
- Bergamin, L.S.; Braganhol, E.; Figueiro, F.; Casali, E.A.; Zanin, R.F.; Sevigny, J.; Battastini, A.M. Involvement of purinergic system in the release of cytokines by macrophages exposed to glioma-conditioned medium. J. Cell. Biochem. 2015, 116, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.S.; Roy, A.; Rajpoot, S.; Liu, D.; Savai, R.; Banerjee, S.; Kawada, M.; Faisal, S.M.; Saluja, R.; Saqib, U.; et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 2020, 69, 435–451. [Google Scholar] [CrossRef]
- Qian, M.; Wang, S.; Guo, X.; Wang, J.; Zhang, Z.; Qiu, W.; Gao, X.; Chen, Z.; Xu, J.; Zhao, R.; et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-kappaB pathways. Oncogene 2020, 39, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Scholl, J.N.; de Fraga Dias, A.; Pizzato, P.R.; Lopes, D.V.; Moritz, C.E.J.; Jandrey, E.H.F.; Souto, G.D.; Colombo, M.; Rohden, F.; Sevigny, J.; et al. Characterization and antiproliferative activity of glioma-derived extracellular vesicles. Nanomedicine 2020, 15, 1001–1018. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azambuja, J.H.; Ludwig, N.; Yerneni, S.S.; Braganhol, E.; Whiteside, T.L. Arginase-1+ Exosomes from Reprogrammed Macrophages Promote Glioblastoma Progression. Int. J. Mol. Sci. 2020, 21, 3990. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, J.H.; da Silveira, E.F.; de Carvalho, T.R.; Oliveira, P.S.; Pacheco, S.; do Couto, C.T.; Beira, F.T.; Stefanello, F.M.; Spanevello, R.M.; Braganhol, E. Glioma sensitive or chemoresistant to temozolomide differentially modulate macrophage protumor activities. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2652–2662. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.J.; Kucharzewska, P.; Christianson, H.C.; Skold, S.; Lofstedt, T.; Johansson, M.C.; Morgelin, M.; Bengzon, J.; Ruf, W.; Belting, M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13147–13152. [Google Scholar] [CrossRef] [Green Version]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringner, M.; Morgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Kumar, U.S.; Sadeghipour, N.; Massoud, T.F.; Paulmurugan, R. A Microfluidics-Based Scalable Approach to Generate Extracellular Vesicles with Enhanced Therapeutic MicroRNA Loading for Intranasal Delivery to Mouse Glioblastomas. ACS Nano 2021, 15, 18327–18346. [Google Scholar] [CrossRef]
- Jiang, X.C.; Gao, J.Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zottel, A.; Samec, N.; Paska, A.V.; Jovcevska, I. Coding of Glioblastoma Progression and Therapy Resistance through Long Noncoding RNAs. Cancers 2020, 12, 1842. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yang, Z.; Li, W.; Wu, A.; Su, Z.; Jiang, B. Exosome-Mediated Transfer of Long Noncoding RNA HOTAIR Regulates Temozolomide Resistance by miR-519a-3p/RRM1 Axis in Glioblastoma. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yin, J.; Lu, C.; Wei, Y.; Zeng, A.; You, Y. Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 166. [Google Scholar] [CrossRef]
- Yin, J.; Zeng, A.; Zhang, Z.; Shi, Z.; Yan, W.; You, Y. Exosomal transfer of miR-1238 contributes to temozolomide-resistance in glioblastoma. EBioMedicine 2019, 42, 238–251. [Google Scholar] [CrossRef] [Green Version]
- Munoz, J.L.; Walker, N.D.; Mareedu, S.; Pamarthi, S.H.; Sinha, G.; Greco, S.J.; Rameshwar, P. Cycling Quiescence in Temozolomide Resistant Glioblastoma Cells Is Partly Explained by microRNA-93 and -193-Mediated Decrease of Cyclin D. Front. Pharmacol. 2019, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Zeng, A.; Wei, Z.; Yan, W.; Yin, J.; Huang, X.; Zhou, X.; Li, R.; Shen, F.; Wu, W.; Wang, X.; et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018, 436, 10–21. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther. Nucleic Acids 2013, 2, e126. [Google Scholar] [CrossRef] [PubMed]

| Type of Extracellular Vesicle | Cargo Type | Tissue/Cell Type | Main Outcome | Author |
|---|---|---|---|---|
| EVs | RNA | Plasma and serum samples of patients | Difference in EVs cargo from plasma and EVs cargo from serum (269 and 636 differentially expressed genes in plasma and serum). Changes in plasma EVs associated with inflammation, changes in serum EVs associated with ubiquitinylation and cytokine signaling. | Roy et al., 2021 [49] |
| Exosomes and microvesicles | miRNA | GL261 mouse glioma cell line | Glioma-derived vesicles can be transported to microglia which was demonstrated using miR-21. In microglia there was a down-regulation of miR-21 target genes, resulting in increased microglia proliferation. | Abels et al., 2019 [50] |
| Exosomes | miRNA | U87 and P3 human glioma cell lines U87 and P3 mouse glioma cell lines | Increased formation of exosomes under hypoxic conditions compared to normal. Hypoxia-derived exosomes induced more myeloid-derived suppressor cells. Exosomal miR-10a and miR-21 induced the expansion and activation of myeloid-derived suppressor cells via RORA and PTEN pathway. | Guo et al., 2018 [51] |
| EVs (majority 100–200 nm) | miRNAs | Primary human glioblastoma cells mouse microglia | Glioblastoma-isolated EVs were taken up by microglia, resulting in increased proliferation. Cytokine profile trend toward immunosuppression. Most abundant miRNAs in vesicles were miR-451 and miR-21. Both miRNA target c-Myc mRNA, which decreased in microglia that uptook EVs. | van der Vos et al., 2016 [52] |
| Exosomes | Protein | BATF2-overexpressing glioma cell lines GBM patient plasma | BATF2 is involved in inflammatory antitumor response. It inhibits the recruitment of myeloid-derived suppressor cells.BATF2 positive exosomes as a potential biomarker (distinction between stage III-IV vs. stage I-II vs. healthy subjects). | Zhang et al., 2021 [53] |
| Exosomes | Protein | U87MG and T98G Glioma stem cells | Addition of a selective COX-2 inhibitor leads to a change in function of secreted exosomes from glioma stem cells (decreased adherent cell migration of U87MG and T98G). | Palumbo et al., 2020 [54] |
| Microvesicles | Protein | Human and mouse tissue | Myeloid-derived suppressor cells can promote regulatory B-cell function via microvesicles. Microvesicles contained PD-L1, resulting in the ability of regulatory B-cell to suppress the CD8þ T-cell activation. | Lee-Chang et al., 2019 [51] |
| EVs | Protein | Patient-derived glioblastoma stem cells | Glioblastoma-derived EVs were associated with changes in astrocyte proteome. In-silico prediction of MYC, NFE2L2, FN1, and TGFβ1 activation, and p53 inhibition, leading to a tumor-favoring phenotype of astrocytes. | Hallal et al., 2018 [55] |
| Exosomes | Protein | Glioblastoma-derived stem cells | Secreted exosomes are taken up by monocytes, which results in phenotypic change to immunosuppressive M2 macrophages. | Gabrusiewicz et al., 2018 [56] |
| Exosomes | Protein | U373 glioma cells | Increased levels of CRYAB when stimulated with IL-1b and TNF. Changes in composition of the secreted exosomal proteome when stimulated with cytokines. | Kore et al., 2014 [57] |
| Clinical Trial Status | Study Title | Conditions or Disease/Biological Sample | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Recruiting | Blood Biomarker Signature in Glioma | Glioma/Serum | NCT03698201 |
| Recruiting | Evaluating the Expression Levels of MicroRNA-10b in Patients with Gliomas | Astrocytoma Oligodendroglioma Oligoastrocytoma Anaplastic Astrocytoma Anaplastic Oligodendroglioma Anaplastic Oligoastrocytoma Glioblastoma Brain Tumors Brain Cancer/Blood Tumor tissue Cerebrospinal fluid | NCT01849952 |
| Not yet recruiting | LIQUID BIOPSY IN Low-grade Glioma Patients (GLIOLIPSY) | Glioma/Blood samples | NCT05133154 |
| Recruiting | Multicenter Safety Trial Assessing an Innovative Tumor Molecular and Cellular Print Medical Device in Glioma (ProTool) | Oligodendroglioma Astrocytoma/Brain tissue | NCT02077543 |
| Recruiting | Research on Precise Immune Prevention and Treatment of Glioma Based on Multi-omics Sequencing Data | Glioma/Peripheral blood Urine Feces Glioma tissue Brain tissue Meninges Cerebrospinal fluid | NCT04792437 |
| Recruiting | Determination of Immune Phenotype in Glioblastoma Patients | Glioblastoma Multiforme/Peripheral Blood Mononuclear Cell Plasma Tumor tissue | NCT02751138 |
| Recruiting | A Phase II/III Study of High-dose, Intermittent Sunitinib in Patients with Recurrent Glioblastoma Multiforme (STELLAR) | Glioblastoma Multiforme/Blood | NCT03025893 |
| Recruiting | Molecular Genetic, Host-derived, and Clinical Determinants of Long-term Survival in Glioblastoma | Glioblastoma/Blood | NCT03770468 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krapež, G.; Kouter, K.; Jovčevska, I.; Videtič Paska, A. Dynamic Intercell Communication between Glioblastoma and Microenvironment through Extracellular Vesicles. Biomedicines 2022, 10, 151. https://doi.org/10.3390/biomedicines10010151
Krapež G, Kouter K, Jovčevska I, Videtič Paska A. Dynamic Intercell Communication between Glioblastoma and Microenvironment through Extracellular Vesicles. Biomedicines. 2022; 10(1):151. https://doi.org/10.3390/biomedicines10010151
Chicago/Turabian StyleKrapež, Gloria, Katarina Kouter, Ivana Jovčevska, and Alja Videtič Paska. 2022. "Dynamic Intercell Communication between Glioblastoma and Microenvironment through Extracellular Vesicles" Biomedicines 10, no. 1: 151. https://doi.org/10.3390/biomedicines10010151
APA StyleKrapež, G., Kouter, K., Jovčevska, I., & Videtič Paska, A. (2022). Dynamic Intercell Communication between Glioblastoma and Microenvironment through Extracellular Vesicles. Biomedicines, 10(1), 151. https://doi.org/10.3390/biomedicines10010151