Inhibition of DUSP6 Activates Autophagy and Rescues the Retinal Pigment Epithelium in Sodium Iodate-Induced Retinal Degeneration Models In Vivo and In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Cell Culture
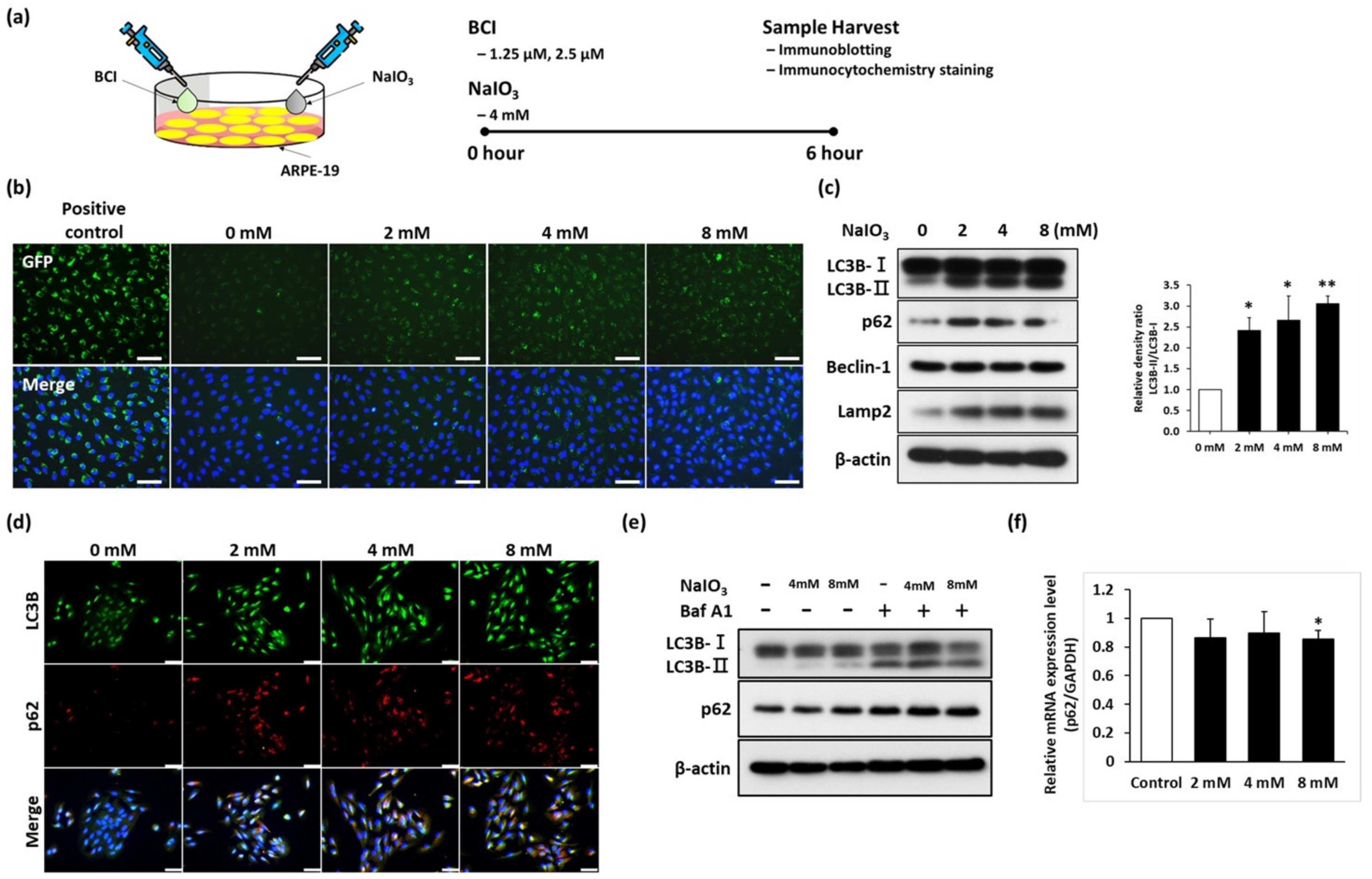
2.2. Sodium Iodate and Protein Regulators Treatment in ARPE-19
2.3. Immunoblotting Analysis
2.4. Autophagic Flux Assay
2.5. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.6. 3(4,5. Dimethyl 2 Thiazolyl) 2,5 Diphenyl 2 H Tetrazolium Bromide (MTT) Assay
2.7. ROS Detection Assay (DHR-123 Assay)
2.8. Animal
2.9. Retinal Degeneration Model Established with Sodium Iodate Treatment by Intraperitoneal Injection
2.10. Histological Assessment of the Retina
2.11. Fundoscopy & Optical Coherence Tomography
2.12. Immunofluorescence Staining
2.13. DUSP6 Inhibitor Treatment in Mice
2.14. Statistical Analysis
3. Results
3.1. Sodium Iodate Disrupted the Autophagy Flux in ARPE-19 Cells
3.2. NaIO3 Treatment Induces DUSP6 and MAPK Upregulation
3.3. BCI Promotes the Autophagic Flux via ERK Pathway Activity in ARPE-19 Cells
3.4. DUSP6/p-ERK Axis Are Upregulated in NaIO3-Induced Retinal Degeneration In Vivo
3.5. The Expression of Autophagic Markers Are Increased in Retinal Degeneration Model
3.6. BCI Activated the Autophagic Flux via ERK Pathway Upregulation In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rim, T.H.; Kawasaki, R.; Tham, Y.C.; Kang, S.W.; Ruamviboonsuk, P.; Bikbov, M.M.; Miyake, M.; Hao, J.; Fletcher, A.; Sasaki, M.; et al. Prevalence and Pattern of Geographic Atrophy in Asia: The Asian Eye Epidemiology Consortium. Ophthalmology 2020, 127, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of Autophagy in Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef]
- Intartaglia, D.; Giamundo, G.; Conte, I. Autophagy in the retinal pigment epithelium: A new vision and future challenges. FEBS J. 2021. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef]
- Zhuang, X.X.; Wang, S.F.; Tan, Y.; Song, J.X.; Zhu, Z.; Wang, Z.Y.; Wu, M.Y.; Cai, C.Z.; Huang, Z.J.; Tan, J.Q.; et al. Pharmacological enhancement of TFEB-mediated autophagy alleviated neuronal death in oxidative stress-induced Parkinson’s disease models. Cell Death Dis. 2020, 11, 128. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.L.; Merida, S.; Bosch-Morell, F.; Miranda, M.; Villar, V.M. Autophagy Dysfunction and Oxidative Stress, Two Related Mechanisms Implicated in Retinitis Pigmentosa. Front. Physiol. 2018, 9, 1008. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [Green Version]
- Trachsel-Moncho, L.; Benlloch-Navarro, S.; Fernandez-Carbonell, A.; Ramirez-Lamelas, D.T.; Olivar, T.; Silvestre, D.; Poch, E.; Miranda, M. Oxidative stress and autophagy-related changes during retinal degeneration and development. Cell Death Dis. 2018, 9, 812. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Y.; Ng, T.K.; Brelen, M.E.; Wu, D.; Wang, J.X.; Chan, K.P.; Yung, J.S.Y.; Cao, D.; Wang, Y.; Zhang, S.; et al. Continuous exposure to non-lethal doses of sodium iodate induces retinal pigment epithelial cell dysfunction. Sci. Rep. 2016, 6, 37279. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Horng, L.Y.; Sung, H.C.; Wu, R.T. Sodium Iodate Disrupted the Mitochondrial-Lysosomal Axis in Cultured Retinal Pigment Epithelial Cells. J. Ocul. Pharmacol. Ther. 2018, 34, 500–511. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 40. [Google Scholar] [CrossRef]
- Kochetkova, E.Y.; Blinova, G.I.; Zubova, S.G.; Bykova, T.V.; Pospelov, V.A.; Pospelova, T.V. Mek/Erk-Pathway is Required to Maintain Cytoprotective Autophagy Process in Irradiated E1a+Cha-Ras Transformants. Tsitologiia 2016, 58, 947–954. [Google Scholar]
- Dagda, R.K.; Zhu, J.; Kulich, S.M.; Chu, C.T. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: Implications for Parkinson’s disease. Autophagy 2008, 4, 770–782. [Google Scholar] [CrossRef] [Green Version]
- Theodosiou, A.; Ashworth, A. MAP kinase phosphatases. Genome Biol. 2002, 3, reviews3009. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.K.; Abdollah, N.A.; Shafie, N.H.; Yusof, N.M.; Razak, S.R.A. Dual-specificity phosphatase 6 (DUSP6): A review of its molecular characteristics and clinical relevance in cancer. Cancer Biol. Med. 2018, 15, 14–28. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.N.; Liao, Y.F.; Lu, Y.X.; Wang, Y.; Lu, J.H.; Zeng, Z.L.; Huang, Q.T.; Sheng, H.; Yun, J.P.; Xie, D.; et al. Pharmacological inhibition of DUSP6 suppresses gastric cancer growth and metastasis and overcomes cisplatin resistance. Cancer Lett. 2018, 412, 243–255. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Athonvarangkul, D.; Mishall, P.; Sahu, S.; Singh, R. Autophagy proteins regulate ERK phosphorylation. Nat. Commun. 2013, 4, 2799. [Google Scholar] [CrossRef]
- Syu, J.P.; Buddhakosai, W.; Chen, S.J.; Ke, C.C.; Chiou, S.H.; Kuo, W.C. Supercontinuum source-based multi-contrast optical coherence tomography for rat retina imaging. Biomed. Opt. Express 2018, 9, 6132–6144. [Google Scholar] [CrossRef]
- Lamark, T.; Svenning, S.; Johansen, T. Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays Biochem. 2017, 61, 609–624. [Google Scholar] [CrossRef]
- Yamada, Y.; Tian, J.; Yang, Y.; Cutler, R.G.; Wu, T.; Telljohann, R.S.; Mattson, M.P.; Handa, J.T. Oxidized low density lipoproteins induce a pathologic response by retinal pigmented epithelial cells. J. Neurochem. 2008, 105, 1187–1197. [Google Scholar] [CrossRef]
- Chowers, G.; Cohen, M.; Marks-Ohana, D.; Stika, S.; Eijzenberg, A.; Banin, E.; Obolensky, A. Course of Sodium Iodate-Induced Retinal Degeneration in Albino and Pigmented Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2239–2249. [Google Scholar] [CrossRef]
- Wang, J.; Iacovelli, J.; Spencer, C.; Saint-Geniez, M. Direct effect of sodium iodate on neurosensory retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Moriguchi, M.; Nakamura, S.; Inoue, Y.; Nishinaka, A.; Nakamura, M.; Shimazawa, M.; Hara, H. Irreversible Photoreceptors and RPE Cells Damage by Intravenous Sodium Iodate in Mice Is Related to Macrophage Accumulation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3476–3487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Onrubia, L.; Valentin-Bravo, F.J.; Coco-Martin, R.M.; Gonzalez-Sarmiento, R.; Pastor, J.C.; Usategui-Martin, R.; Pastor-Idoate, S. Matrix Metalloproteinases in Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2020, 21, 5934. [Google Scholar] [CrossRef] [PubMed]
- Bhore, N.; Wang, B.J.; Chen, Y.W.; Liao, Y.F. Critical Roles of Dual-Specificity Phosphatases in Neuronal Proteostasis and Neurological Diseases. Int. J. Mol. Sci. 2017, 18, 1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, E.; Bejarano, E.; Rakshit, M.; Lee, K.; Hanson, H.H.; Zaarur, N.; Phillips, G.R.; Sherman, M.Y.; Cuervo, A.M. Molecular determinants of selective clearance of protein inclusions by autophagy. Nat. Commun. 2012, 3, 1240. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. The Function of Autophagy in Neurodegenerative Diseases. Int. J. Mol. Sci. 2015, 16, 26797–26812. [Google Scholar] [CrossRef] [Green Version]
- Kyosseva, S.V. Targeting MAPK Signaling in Age-Related Macular Degeneration. Ophthalmol. Eye Dis. 2016, 8, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Lei, T.; Guo, P.; Yu, J.; Xu, Q.; Luo, Y.; Ke, R.; Huang, D. Mechanisms shaping the role of ERK1/2 in cellular senescence (Review). Mol. Med. Rep. 2019, 19, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Aung, K.H.; Liu, H.; Ke, Z.; Jiang, S.; Huang, J. Glabridin Attenuates the Retinal Degeneration Induced by Sodium Iodate In Vitro and In Vivo. Front. Pharmacol. 2020, 11, 566699. [Google Scholar] [CrossRef]
- Ferreira-Marques, M.; Carvalho, A.; Cavadas, C.; Aveleira, C.A. PI3K/AKT/MTOR and ERK1/2-MAPK signaling pathways are involved in autophagy stimulation induced by caloric restriction or caloric restriction mimetics in cortical neurons. Aging 2021, 13, 7872–7882. [Google Scholar] [CrossRef]
- Kauppinen, A.; Kaarniranta, K.; Salminen, A. Potential Role of Myeloid-Derived Suppressor Cells (MDSCs) in Age-Related Macular Degeneration (AMD). Front. Immunol. 2020, 11, 384. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Tang, B.; Zhang, Z.; Xu, D.; Ma, G. DUSP6 Inhibitor (E/Z)-BCI Hydrochloride Attenuates Lipopolysaccharide-Induced Inflammatory Responses in Murine Macrophage Cells via Activating the Nrf2 Signaling Axis and Inhibiting the NF-kappaB Pathway. Inflammation 2019, 42, 672–681. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Chaney, K.E.; Milewski, D.; Williams, K.B.; Williams, R.L.; Choi, K.; Miller, A.; Kalin, T.V.; Pressey, J.G.; Szabo, S.; et al. Targeted Inhibition of the Dual Specificity Phosphatases DUSP1 and DUSP6 Suppress MPNST Growth via JNK. Clin. Cancer Res. 2019, 25, 4117–4127. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-Y.; Lai, H.I.A.M.; Chen, Z.-Y.; Lin, T.-C.; Khor, W.; Kuo, W.-C.; Syu, J.-P.; Tsai, P.-H.; Yang, Y.-P.; Chien, Y.; et al. Inhibition of DUSP6 Activates Autophagy and Rescues the Retinal Pigment Epithelium in Sodium Iodate-Induced Retinal Degeneration Models In Vivo and In Vitro. Biomedicines 2022, 10, 159. https://doi.org/10.3390/biomedicines10010159
Tsai H-Y, Lai HIAM, Chen Z-Y, Lin T-C, Khor W, Kuo W-C, Syu J-P, Tsai P-H, Yang Y-P, Chien Y, et al. Inhibition of DUSP6 Activates Autophagy and Rescues the Retinal Pigment Epithelium in Sodium Iodate-Induced Retinal Degeneration Models In Vivo and In Vitro. Biomedicines. 2022; 10(1):159. https://doi.org/10.3390/biomedicines10010159
Chicago/Turabian StyleTsai, Hao-Yu, Henkie Isahwan Ahmad Mulyadi Lai, Zhang-Yuan Chen, Tai-Chi Lin, Winnie Khor, Wen-Chuan Kuo, Jia-Pu Syu, Ping-Hsing Tsai, Yi-Ping Yang, Yueh Chien, and et al. 2022. "Inhibition of DUSP6 Activates Autophagy and Rescues the Retinal Pigment Epithelium in Sodium Iodate-Induced Retinal Degeneration Models In Vivo and In Vitro" Biomedicines 10, no. 1: 159. https://doi.org/10.3390/biomedicines10010159






