Muscular and Tendon Degeneration after Achilles Rupture: New Insights into Future Repair Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model of Injury
2.2. Immunostaining
2.3. Multiphoton Image Acquisition
2.4. Digital Image Acquisition and Quantification
2.5. Morphometric Analysis
2.6. Muscular Mechanical Characterization
2.7. Statistical Analysis
3. Results
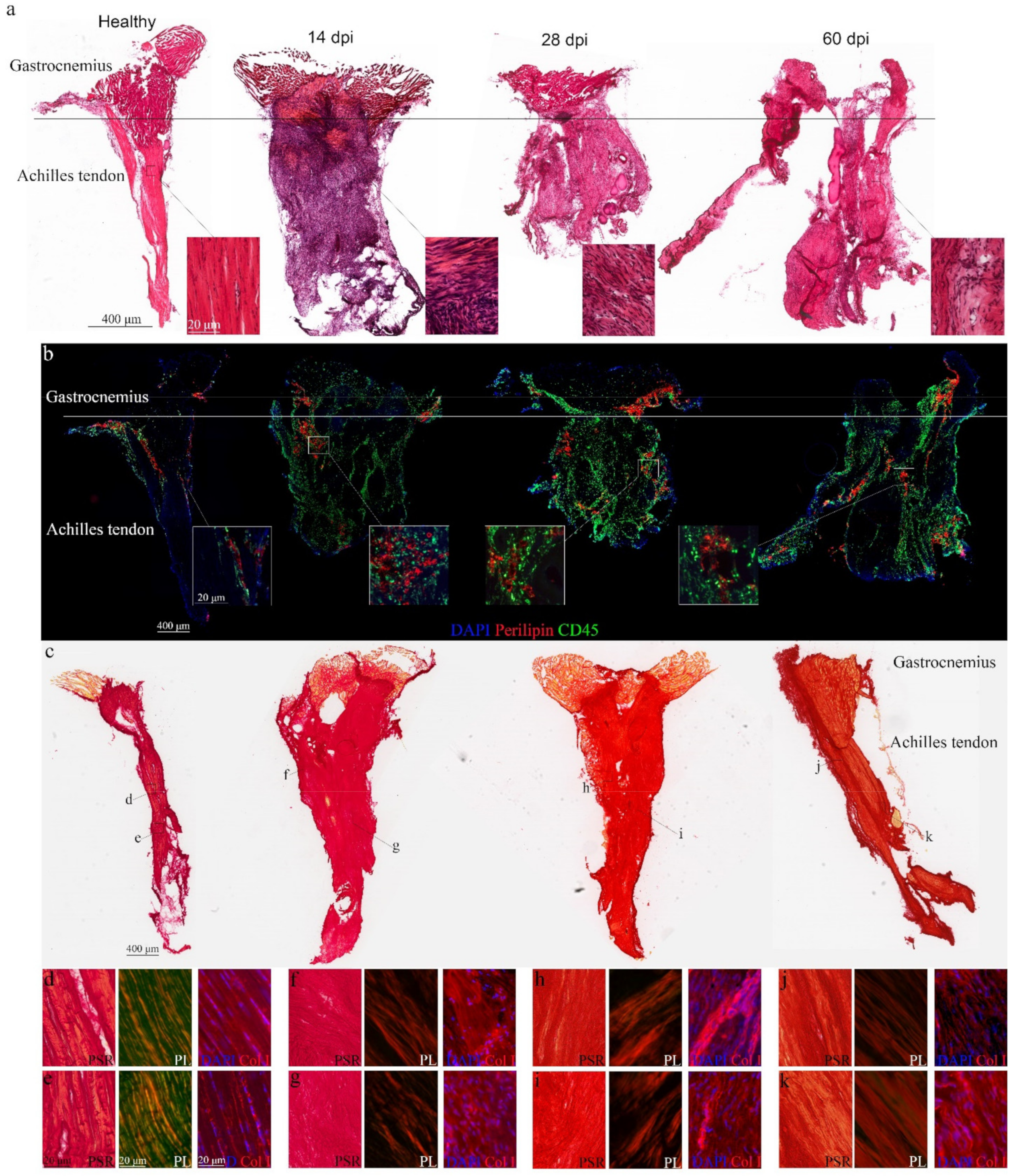
3.1. Morphological Changes in the Achilles Tendon after Complete Rupture
3.2. Pathological Progression in the Gastrocnemius after Achilles Tendon Complete Rupture
3.3. Loss of Self-Renewed Satellite Cells after Achilles Tendon Rupture
3.4. Muscular Function Decline after Achilles Tendon Rupture
3.5. Tendon-Skeletal Muscle Structure after Achilles Tendon Complete Rupture
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hopkins, C.; Fu, S.C.; Chua, E.; Hu, X.; Rolf, C.; Mattila, V.M.; Qin, L.; Yung, P.S.-H.; Chan, K.-M. Critical review on the socio-economic impact of tendinopathy. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2016, 4, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Järvinen, T.A.; Kannus, P.; Maffulli, N.; Khan, K.M. Achilles Tendon Disorders: Etiology and Epidemiology. Foot Ankle Clin. 2005, 10, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Kjaer, M.; Eliasson, P. Achilles tendon rupture—Treatment and complications: A systematic review. Scand. J. Med. Sci. Sports 2014, 25, e1–e10. [Google Scholar] [CrossRef]
- Egger, A.C.; Berkowitz, M.J. Achilles tendon injuries. Curr. Rev. Musculoskelet. Med. 2017, 10, 72–80. [Google Scholar] [CrossRef]
- Lemme, N.J.; Li, N.Y.; DeFroda, S.F.; Kleiner, J.; Owens, B.D. Epidemiology of Achilles Tendon Ruptures in the United States: Athletic and Nonathletic Injuries From 2012 to 2016. Orthop. J. Sports Med. 2018, 6, 2325967118808238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, N.; Helander, K.N.; Senorski, E.H.; Holm, A.; Karlsson, J.; Svensson, M.; Westin, O. The economic cost and patient-reported outcomes of chronic Achilles tendon ruptures. J. Exp. Orthop. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.P.; Langberg, H.; Kjaer, M. The pathogenesis of tendinopathy: Balancing the response to loading. Nat. Rev. Rheumatol. 2010, 6, 262–268. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Screen, H.R.C. Tendon Structure and Composition. Metab. Influ. Risk Tendon Disord. 2016, 920, 3–10. [Google Scholar] [CrossRef]
- Kirkendall, D.T.; Garrett, W.E. Function and biomechanics of tendons. Scand. J. Med. Sci. Sports 2007, 7, 62–66. [Google Scholar] [CrossRef]
- Heikkinen, J.; Lantto, I.; Flinkkila, T.; Ohtonen, P.; Niinimaki, J.; Siira, P.; Laine, V.; Leppilahti, J. Soleus atrophy is common after the nonsurgical treatment of acute achilles tendon ruptures: A randomized clinical trial comparing surgical and non-surgical functional treatments. Am. J. Sports Med. 2017, 45, 1395–1404. [Google Scholar] [CrossRef]
- Hoffmann, A.; Mamisch, N.; Buck, F.M.; Espinosa, N.; Pfirrmann, C.W.A.; Zanetti, M. Oedema and fatty degeneration of the soleus and gastrocnemius muscles on MR images in patients with achilles tendon abnormalities. Eur. Radiol. 2011, 21, 1996–2003. [Google Scholar] [CrossRef] [Green Version]
- Leppilahti, J.; Lãhde, S.L.; Forsman, K.; Kangas, J.; Kauranen, K.; Orava, S. Relationship between calf muscle size and strength after achilles rupture repair. Foot Ankle Int. 2000, 21, 330–335. [Google Scholar] [CrossRef]
- Heikkinen, J.; Lantto, I.; Piilonen, J.; Flinkkila, T.; Ohtonen, P.; Siira, P.; Laine, V.; Niinimäki, J.; Pajala, A.; Leppilahti, J. Tendon length, calf muscle atrophy, and strength deficit after acute achilles tendon rupture: Long-term follow-up of patients in a previous study. J. Bone Jt. Surg. Am. 2017, 99, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Vavken, P.; Polzer, C.; Buckland, D.M.; Studler, U.; Weisskopf, L.; Lottenbach, M.; Müller, A.M.; Valderrabano, V. Long-term outcomes of muscle volume and achilles tendon length after achilles tendon ruptures. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 21–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, B.A.; Mistovich, R.J.; Janout, M.; Stills, H.F.; Laughlin, R.T. Fatty infiltration of the gastrocsoleus after tendo-achilles lengthening and gastrocnemius recession in a rabbit model. Foot Ankle Int. 2009, 30, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Gordon, J.A.; Soslowsky, L.J. The Achilles tendon: Fundamental properties and mechanisms governing healing. Muscle Ligaments Tendons J. 2014, 4, 245–255. [Google Scholar] [CrossRef]
- Diniz, P.; Pacheco, J.; Guerra-Pinto, F.; Pereira, H.; Ferreira, F.C.; Kerkhoffs, G. Achilles tendon elongation after acute rupture: Is it a problem? a systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2000, 28, 4011–4030. [Google Scholar] [CrossRef]
- Wu, Y.; Mu, Y.; Yin, L.; Wang, Z.; Liu, W.; Wan, H. Complications in the Management of Acute Achilles Tendon Rupture: A Systematic Review and Network Meta-analysis of 2060 Patients. Am. J. Sports Med. 2019, 47, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Eken, G.; Misir, A.; Tangay, C.; Atici, T.; Demirhan, N.; Sener, N. Effect of muscle atrophy and fatty infiltration on midterm clinical, and functional outcomes after Achilles tendon repair. Foot Ankle Surg. 2020, 27, 730–735. [Google Scholar] [CrossRef]
- Zantop, T.; Gilbert, T.W.; Yoder, M.C.; Badylak, S.F. Extracellular matrix scaffolds are repopu- lated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. J. Orthop. Res. 2006, 24, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Beattie, A.J.; Gilbert, T.W.; Guyot, J.P.; Yates, A.J.; Badylak, S.F. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng. Part A 2009, 15, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Beason, D.P.; Kuntz, A.; Hsu, J.E.; Miller, K.S.; Soslowsky, L.J. Development and evaluation of multiple tendon injury models in the mouse. J. Biomech. 2012, 45, 1550–1553. [Google Scholar] [CrossRef] [Green Version]
- Terrón, V.M.; López, E.M.; Granero-Moltó, F.; Esteban, M.A.; Prosper, F.; Pérez-Ruiz, A.; Pons-Villanueva, J. Muscular injuries after tendon rupture in the rotator cuff of animal models. systematic review. Muscle Ligaments Tendon J. 2018, 8, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Ohzono, H.; Gotoh, M.; Nakamura, H.; Honda, H.; Mitsui, Y.; Kakuma, T.; Okawa, T.; Shiba, N. Effect of Preoperative Fatty Degeneration of the Rotator Cuff Muscles on the Clinical Outcome of Patients with Intact Tendons After Arthroscopic Rotator Cuff Repair of Large/Massive Cuff Tears. Am. J. Sports Med. 2017, 45, 2975–2981. [Google Scholar] [CrossRef]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon Healing: Repair and Regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef]
- Bobadilla, M.; Sainz, N.; Abizanda, G.; Orbe, J.; Rodriguez, J.; Páramo, J.; Prósper, F.; Pérez-Ruiz, A. The cxcr4/sdf1 axis improves muscle regeneration through mmp-10 activity. Stem Cells Dev. 2014, 23, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Grasa, J.; Sierra, M.; Lauzeral, N.; Muñoz, M.; Mena, F.J.M.; Calvo, B. Active behavior of abdominal wall muscles: Experimental results and numerical model formulation. J. Mech. Behav. Biomed. Mater. 2016, 61, 444–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forcina, L.; Miano, C.; Pelosi, L.; Musarò, A. An Overview About the Biology of Skeletal Muscle Satellite Cells. Curr. Genom. 2019, 20, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Liu, Y.; Zhu, X.; Huang, Z.; Cai, J.; Chen, R.; Xiong, S.; Zeng, H. Phase and texture characterizations of scar collagen second-harmonic generation images varied with scar duration. Microsc. Microanal. 2015, 21, 855–862. [Google Scholar] [CrossRef]
- Blake, D.J.; Weir, A.; Newey, S.E.; Davies, K.E. Function and Genetics of Dystrophin and Dystrophin-Related Proteins in Muscle. Physiol. Rev. 2002, 82, 291–329. [Google Scholar] [CrossRef] [Green Version]
- Débarre, D.; Supatto, W.; Pena, A.-M.; Fabre, A.; Tordjmann, T.; Combettes, L.; Schanne-Klein, M.-C.; Beaurepaire, E. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat. Methods 2006, 3, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bottagisio, M.; Lovati, A.B. A review on animal models and treatments for the reconstruction of achilles and flexor tendons. J. Mater. Sci. Mater. Med. 2017, 28, 45. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Messina, S.; Mileto, A.; Vita, G.L.; Ascenti, G.; Vinci, S.; Bottari, A.; Vita, G.; Settineri, N.; Bruschetta, D.; et al. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: Evaluation of disease distribution and correlation with clinical assessments. Skelet. Radiol. 2011, 41, 955–961. [Google Scholar] [CrossRef]
- Joe, A.W.B.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.; Rossi, F.M.V. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature 2010, 12, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Farup, J.; Madaro, L.; Puri, P.L.; Mikkelsen, U.R. Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis. 2015, 6, e1830. [Google Scholar] [CrossRef] [Green Version]
- Uezumi, A.; Fukada, S.-I.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Rowshan, K.; Hadley, S.; Pham, K.; Caiozzo, V.; Lee, T.Q.; Gupta, R. Development of Fatty Atrophy After Neurologic and Rotator Cuff Injuries in an Animal Model of Rotator Cuff Pathology. J. Bone Jt. Surg. Am. Vol. 2010, 92, 2270–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uezumi, A.; Fukada, S.-I.; Yamamoto, N.; Ikemoto-Uezumi, M.; Nakatani, M.; Morita, M.; Yamaguchi, A.; Yamada, H.; Nishino, I.; Hamada, Y.; et al. Identification and characterization of PDGFRα+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014, 5, e1186. [Google Scholar] [CrossRef] [Green Version]
- Harvey, T.; Flamenco, S.; Fan, C.-M. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nature 2019, 21, 1490–1503. [Google Scholar] [CrossRef]
- Misir, A.; Kizkapan, T.B.; Arikan, Y.; Akbulut, D.; Onder, M.; Yildiz, K.I.; Ozkoçer, S.E. Repair within the first 48 h in the treatment of acute Achilles tendon ruptures achieves the best biomechanical and histological outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2019, 28, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Meulenkamp, B.; Woolnough, T.; Cheng, W.; Shorr, R.; Stacey, D.; Richards, M.; Gupta, A.; Fergusson, D.; Graham, I.D. What is the best evidence to guide management of acute achilles tendon ruptures? a systematic review and network meta-analysis of randomized controlled trials. Clin. Orthop. Relat. Res. 2021, 479, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bozec, L.; Horton, M. Topography and Mechanical Properties of Single Molecules of Type I Collagen Using Atomic Force Microscopy. Biophys. J. 2005, 88, 4223–4231. [Google Scholar] [CrossRef] [Green Version]
- Pawley, J.B. Handbook of Biological Confocal Microscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Podoleanu, A.G. Optical coherence tomography. J. Microsc. 2012, 247, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund, I.; Deutsch, M. Second-harmonic microscopy of biological tissue. Opt. Lett. 1986, 11, 94–96. [Google Scholar] [CrossRef]
- Zipfel, W.R.; Williams, R.M.; Christie, R.; Nikitin, A.Y.; Hyman, B.T.; Webb, W.W. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 2003, 100, 7075–7080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.; McKee, T.; diTomaso, E.; Pluen, A.; Seed, B.; Boucher, Y.; Jain, R.K. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 2003, 9, 796–800. [Google Scholar] [CrossRef]
- Strupler, M.; Pena, A.-M.; Hernest, M.; Tharaux, P.-L.; Martin, J.-L.; Beaurepaire, E.; Schanne-Klein, M.-C. Second harmonic imaging and scoring of collagen in fibrotic tissues. Opt. Express 2007, 15, 4054–4065. [Google Scholar] [CrossRef] [PubMed]
- Zoumi, A.; Lu, X.; Kassab, G.S.; Tromberg, B.J. Imaging coronary artery microstructure using second harmonic and two-photon fluorescence microscopy. Biophys. J. 2004, 87, 2778–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Melgosa, L.; Grasa, J.; Urbiola, A.; Llombart, R.; Susaeta Ruiz, M.; Montiel, V.; Ederra, C.; Calvo, B.; Ariz, M.; Ripalda-Cemborain, P.; et al. Muscular and Tendon Degeneration after Achilles Rupture: New Insights into Future Repair Strategies. Biomedicines 2022, 10, 19. https://doi.org/10.3390/biomedicines10010019
Gil-Melgosa L, Grasa J, Urbiola A, Llombart R, Susaeta Ruiz M, Montiel V, Ederra C, Calvo B, Ariz M, Ripalda-Cemborain P, et al. Muscular and Tendon Degeneration after Achilles Rupture: New Insights into Future Repair Strategies. Biomedicines. 2022; 10(1):19. https://doi.org/10.3390/biomedicines10010019
Chicago/Turabian StyleGil-Melgosa, Lara, Jorge Grasa, Ainhoa Urbiola, Rafael Llombart, Miguel Susaeta Ruiz, Verónica Montiel, Cristina Ederra, Begoña Calvo, Mikel Ariz, Purificación Ripalda-Cemborain, and et al. 2022. "Muscular and Tendon Degeneration after Achilles Rupture: New Insights into Future Repair Strategies" Biomedicines 10, no. 1: 19. https://doi.org/10.3390/biomedicines10010019
APA StyleGil-Melgosa, L., Grasa, J., Urbiola, A., Llombart, R., Susaeta Ruiz, M., Montiel, V., Ederra, C., Calvo, B., Ariz, M., Ripalda-Cemborain, P., Prosper, F., Ortiz-de-Solórzano, C., Pons-Villanueva, J., & Pérez Ruiz, A. (2022). Muscular and Tendon Degeneration after Achilles Rupture: New Insights into Future Repair Strategies. Biomedicines, 10(1), 19. https://doi.org/10.3390/biomedicines10010019





