Effects of Alirocumab on Triglyceride Metabolism: A Fat-Tolerance Test and Nuclear Magnetic Resonance Spectroscopy Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Clinical Characterization
2.3. Fat-Tolerance Test
2.4. Lipoprotein Analysis by Ultracentrifugation and Precipitation
2.5. Enzyme-Linked Immunosorbent Assays
2.6. Nuclear Magnetic Resonance Spectroscopy
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Characteristics of Trial Participants
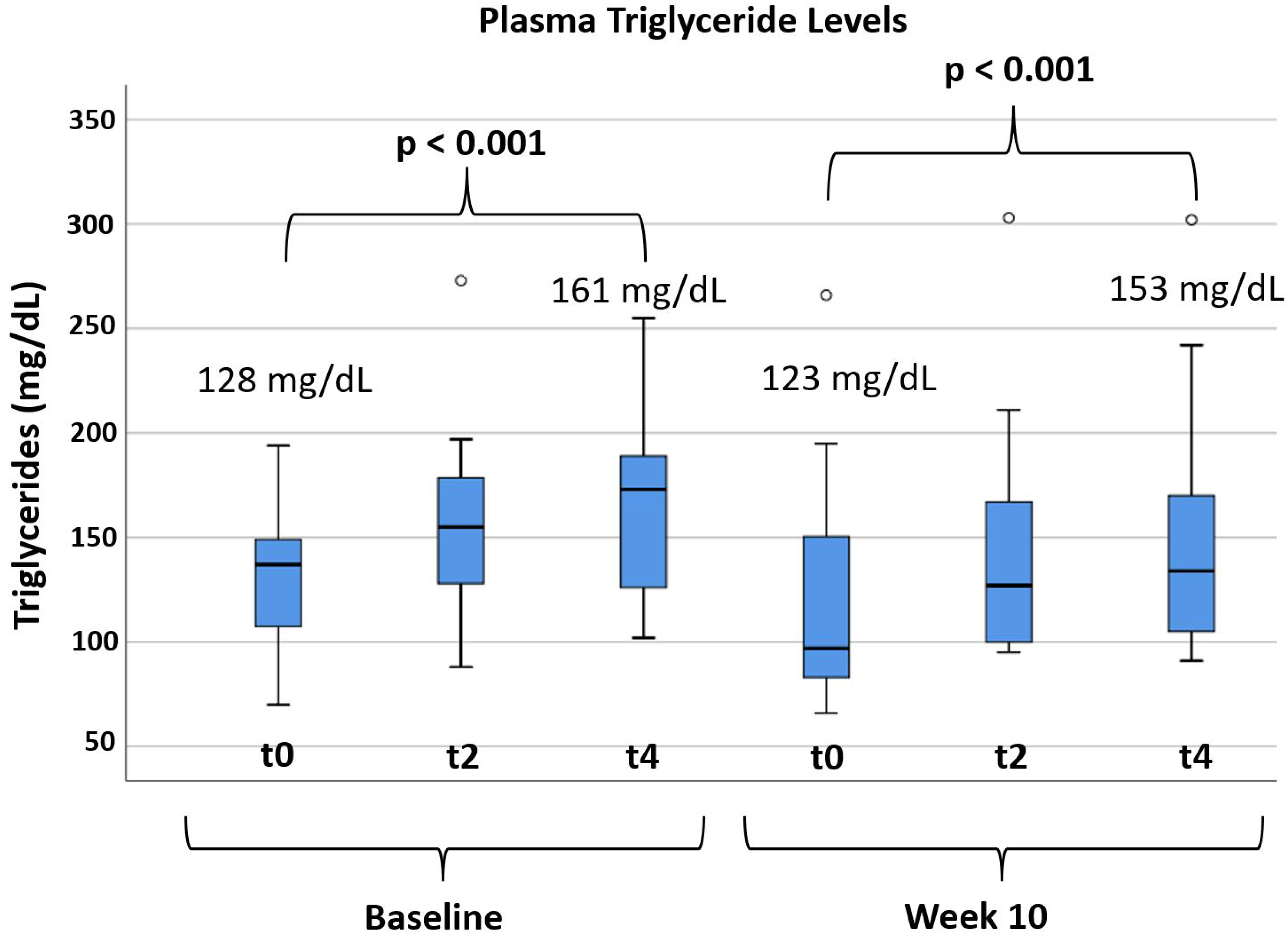
3.3. Effects of Alirocumab on Post-Prandial Lipemia
3.4. Effects of Alirocumab on Lipids and Lipoprotein Particles
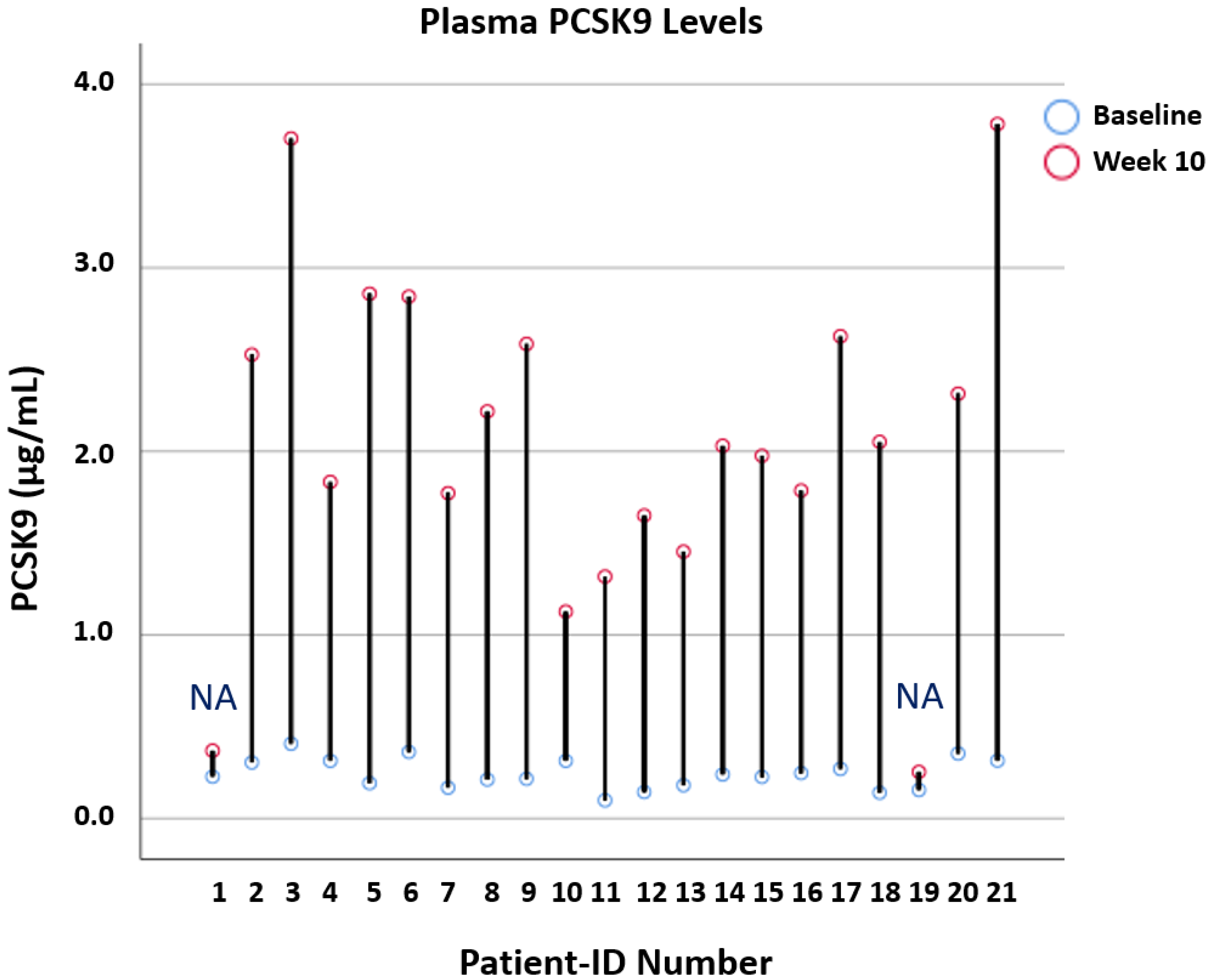
3.5. Effects of Alirocumab on Lipoprotein Lipase Regulators and Circulating PCSK9
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ference, B.A.; Graham, I.; Tokgozoglu, L.; Catapano, A.L. Impact of Lipids on Cardiovascular Health: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Sampson, U.K.; Fazio, S.; Linton, M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: The evidence, etiology, and therapeutic challenges. Curr. Atheroscler. Rep. 2012, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights from Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Ference, B.A.; Kastelein, J.J.P.; Catapano, A.L. Lipids and Lipoproteins in 2020. JAMA 2020, 324, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J.; Boren, J.; Taskinen, M.R. Causes and Consequences of Hypertriglyceridemia. Front. Endocrinol. 2020, 11, 252. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, S.G. Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes Metab. J. 2020, 44, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Zimetti, F.; Adorni, M.P.; Sirtori, C.R.; Lupo, M.G.; Ferri, N. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: New therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacol. Res. 2020, 153, 104653. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.A.; East, C.; Zhang, J.; McCullough, P.A. ApoCIII as a Cardiovascular Risk Factor and Modulation by the Novel Lipid-Lowering Agent Volanesorsen. Curr. Atheroscler. Rep. 2017, 19, 62. [Google Scholar] [CrossRef]
- Chait, A.; Eckel, R.H. The Chylomicronemia Syndrome Is Most Often Multifactorial: A Narrative Review of Causes and Treatment. Ann. Intern. Med. 2019, 170, 626–634. [Google Scholar] [CrossRef]
- Wolska, A.; Reimund, M.; Remaley, A.T. Apolipoprotein C-II: The re-emergence of a forgotten factor. Curr. Opin. Lipidol. 2020, 31, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Ference, B.; Staley, J.; Freitag, D.F.; Mason, A.; Nielsen, S.F.; Willeit, P.; Young, R.; Surendran, P.; Karthikeyan, S.; et al. Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis. JAMA Cardiol. 2018, 3, 619–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langsted, A.; Nordestgaard, B.G. Genetics of Lipoprotein(a): Cardiovascular Disease and Future Therapy. Curr. Atheroscler. Rep. 2021, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Langsted, A. Lipoprotein(a) as a cause of cardiovascular disease: Insights from epidemiology, genetics, and biology. J. Lipid Res. 2016, 57, 1953–1975. [Google Scholar] [CrossRef] [Green Version]
- Koba, S.; Hirano, T.; Kondo, T.; Shibata, M.; Suzuki, H.; Murakami, M.; Geshi, E.; Katagiri, T. Significance of small dense low-density lipoproteins and other risk factors in patients with various types of coronary heart disease. Am. Heart J. 2002, 144, 1026–1035. [Google Scholar] [CrossRef]
- Meeusen, J.W. Is Small Dense LDL a Highly Atherogenic Lipid or a Biomarker of Pro-Atherogenic Phenotype? Clin. Chem. 2021, 67, 927–928. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.; Crosby, J.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Krauss, R.M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004, 27, 1496–1504. [Google Scholar] [CrossRef] [Green Version]
- März, W.; Kleber, M.E.; Scharnagl, H.; Speer, T.; Zewinger, S.; Ritsch, A.; Parhofer, K.G.; Von Eckardstein, A.; Landmesser, U.; Laufs, U. HDL cholesterol: Reappraisal of its clinical relevance. Clin. Res. Cardiol. 2017, 106, 663–675. [Google Scholar] [CrossRef] [Green Version]
- Silbernagel, G.; Pagel, P.; Pfahlert, V.; Genser, B.; Scharnagl, H.; Kleber, M.E.; Delgado, G.; Ohrui, H.; Ritsch, A.; Grammer, T.B.; et al. High-Density Lipoprotein Subclasses, Coronary Artery Disease, and Cardiovascular Mortality. Clin. Chem. 2017, 63, 1886–1896. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Tentolouris, N.; Kanellos, P.T.; Siami, E.; Athanasopoulou, E.; Chaviaras, N.; Kolovou, G.; Sfikakis, P.P.; Katsilambros, N. Assessment of the Validity and Reproducibility of a Novel Standardized Test Meal for the Study of Postprandial Triacylglycerol Concentrations. Lipids 2017, 52, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Horl, W.H.; Luley, C.H.; Wieland, H. Effects of HMG-CoA reductase inhibitors in hypercholesterolemic patients on hemodialysis. Kidney Int. 1991, 39, 754–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachorik, P.S.; Ross, J.W. National Cholesterol Education Program recommendations for measurement of low-density lipoprotein cholesterol: Executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin. Chem. 1995, 41, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, G.; Scharnagl, H.; Kleber, M.E.; Delgado, G.; Stojakovic, T.; Laaksonen, R.; Erdmann, J.; Rankinen, T.; Bouchard, C.; Landmesser, U.; et al. LDL triglycerides, hepatic lipase activity, and coronary artery disease: An epidemiologic and Mendelian randomization study. Atherosclerosis 2019, 282, 37–44. [Google Scholar] [CrossRef]
- Hollstein, T.; Vogt, A.; Grenkowitz, T.; Stojakovic, T.; März, W.; Laufs, U.; Bölükbasi, B.; Steinhagen-Thiessen, E.; Scharnagl, H.; Kassner, U. Treatment with PCSK9 inhibitors reduces atherogenic VLDL remnants in a real-world study. Vasc. Pharmacol. 2019, 116, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Needham, L.L.; Smy, L.; Lee, M.A.; Kunzler, T.M.; Genzen, J.R. Phlebotomy tube interference with nuclear magnetic resonance (NMR) lipoprotein subclass analysis. Clin. Chim. Acta 2018, 488, 235–241. [Google Scholar] [CrossRef]
- Henry, R.R.; Müller-Wieland, D.; Taub, P.R.; Bujas-Bobanovic, M.; Louie, M.J.; Letierce, A.; Ginsberg, H.N. Effect of alirocumab on lipids and lipoproteins in individuals with metabolic syndrome without diabetes: Pooled data from 10 phase 3 trials. Diabetes Obes. Metab. 2018, 20, 1632–1641. [Google Scholar] [CrossRef] [Green Version]
- Leitner, D.R.; Toplak, H.; Kedenko, L.; Steinmaurer, T.; Gräff, V.; Metzner, T.; Schwaiger, E.M.; Prager, R. Efficacy and tolerability of alirocumab in Austrian clinical practice—Results of the non-interventional PEARL-AT study. Curr. Med Res. Opin. 2020, 36, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Del Prato, S.; Müller-Wieland, D.; Cariou, B.; Colhoun, H.M.; Tinahones, F.J.; Domenger, C.; Letierce, A.; Mandel, J.; Samuel, R.; et al. Alirocumab therapy in individuals with type 2 diabetes mellitus and atherosclerotic cardiovascular disease: Analysis of the ODYSSEY DM-DYSLIPIDEMIA and DM-INSULIN studies. Cardiovasc. Diabetol. 2019, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Burggraaf, B.; Pouw, N.M.; Fernández-Arroyo, S.; Zee, L.C.V.V.-V.D.; Van De Geijn, G.M.; Birnie, E.; Msc, J.H.; Van Der Zwan, E.M.; Mulder, M.T.; Rensen, P.C.; et al. A placebo-controlled proof-of-concept study of alirocumab on postprandial lipids and vascular elasticity in insulin-treated patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2020, 22, 807–816. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Björnson, E.; Andersson, L.; Kahri, J.; Porthan, K.; Matikainen, N.; Söderlund, S.; Pietiläinen, K.; Hakkarainen, A.; Lundbom, N.; et al. Impact of proprotein convertase subtilisin/kexin type 9 inhibition with evolocumab on the postprandial responses of triglyceride-rich lipoproteins in type II diabetic subjects. J. Clin. Lipidol. 2020, 14, 77–87. [Google Scholar] [CrossRef]
- Williams, P.T.; Zhao, X.-Q.; Marcovina, S.M.; Otvos, J.D.; Brown, B.G.; Krauss, R.M. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis 2014, 233, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Koren, M.J.; Kereiakes, D.; Pourfarzib, R.; Winegar, D.; Banerjee, P.; Hamon, S.; Hanotin, C.; McKenney, J.M. Effect of PCSK9 Inhibition by Alirocumab on Lipoprotein Particle Concentrations Determined by Nuclear Magnetic Resonance Spectroscopy. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Bittner, V.A.; Szarek, M.; Aylward, P.E.; Bhatt, D.L.; Diaz, R.; Edelberg, J.M.; Fras, Z.; Goodman, S.G.; Halvorsen, S.; Hanotin, C.; et al. Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk after Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 75, 133–144. [Google Scholar] [CrossRef]
- Tsimikas, S.; Moriarty, P.M.; Stroes, E.S. Emerging RNA Therapeutics to Lower Blood Levels of Lp(a): JACC Focus Seminar 2/4. J. Am. Coll. Cardiol. 2021, 77, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, G.; Steiner, L.K.; Hollstein, T.; Fauler, G.; Scharnagl, H.; Stojakovic, T.; Schumann, F.; Bölükbasi, B.; März, W.; Steinhagen-Thiessen, E.; et al. The interrelations between PCSK9 metabolism and cholesterol synthesis and absorption. J. Lipid Res. 2019, 60, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drexel, H.; Coats, A.J.S.; Spoletini, I.; Bilato, C.; Mollace, V.; Perrone-Filardi, P.; Rosano, G.M.C. ESC position paper on statins adherence and implementation of new lipid-lowering medications: Barriers to be overcome. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 115–121. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Alirocumab (N = 19) |
|---|---|
| Age—year | 66 (9) |
| Female sex—n (%) | 9 (47.4) |
| Male sex—n (%) | 10 (52.6) |
| Smoker a—n (%) | 9 (47.4) |
| Current Smoker b—n (%) | 4 (21.1) |
| Concomitant Diseases—n (%) | |
| Cardiovascular Disease | 19 (100) |
| a. Coronary Heart Disease | 19 (100) |
| Coronary Intervention or Surgery | 16 (84.2) |
| Documentation of Coronary Stenosis c | 3 (15.8) |
| b. Peripheral Artery Disease | 3 (15.8) |
| c. Cerebral Artery Disease | 6 (31.6) |
| Chronic Kidney Disease | 4 (21.1) |
| Familial Hypercholesterolemia d | 2 (10.5) |
| Adiposity | 4 (21.1) |
| Type-2 Diabetes Mellitus | 4 (21.1) |
| Type-1 Diabetes Mellitus | 0 (0) |
| Hypertension | 15 (78.9) |
| Number of prior Cardiovascular Events e—n (%) | |
| Three | 2 (10.5) |
| Two | 5 (26.3) |
| One | 9 (47.4) |
| Zero | 3 (15.8) |
| Concomitant Lipid Medication—n (%) | |
| High-Intensity Statins f | 3 (15.8) |
| Statins | 5 (26.3) |
| Ezetimibe | 13 (68.4) |
| Dietary Supplements g | 5 (26.3) |
| Statin Intolerance h | 16 (84.2) |
| Parameters | Baseline | Week 10 | Absolute Change (mg/dL) | ±SD | Relative Change (%) | p-Value |
|---|---|---|---|---|---|---|
| Cholesterol (mg/dL) | 236 | 162 | −74 | 42 | −31 | <0.001 |
| Triglycerides (mg/dL) | 144 | 130 | −15 | 69 | −10 | 0.362 |
| Lp(a) (mg/dL) | 47 | 41 | −7 | 8 | −14 | 0.002 |
| ApoAI (mg/dL) | 154 | 164 | +10 | 13 | +7 | 0.002 |
| ApoAII (mg/dL) | 34 | 35 | +1 | 4 | +2 | 0.384 |
| ApoB (mg/dL) | 110 | 66 | −44 | 23 | −40 | <0.001 |
| ApoCII (mg/dL) | 6 | 5 | −1 | 1 | −10 | 0.069 |
| ApoCIII (mg/dL) | 14 | 14 | −1 | 3 | −5 | 0.411 |
| ApoE (mg/dL) | 12 | 9 | −3 | 3 | −21 | 0.001 |
| VLDL Cholesterol (mg/dL) | 35 | 23 | −12 | 41 | −34 | 0.219 |
| VLDL Triglycerides (mg/dL) | 96 | 91 | −5 | 67 | −5 | 0.772 |
| VLDL ApoB (mg/dL) | 15 | 10 | −5 | 10 | −32 | 0.046 |
| LDL Cholesterol (mg/dL) | 158 | 89 | −69 | 63 | −44 | <0.001 |
| LDL Triglycerides (mg/dL) | 36 | 24 | −11 | 7 | −32 | <0.001 |
| LDL ApoB (mg/dL) | 95 | 56 | −39 | 27 | −41 | <0.001 |
| HDL Cholesterol (mg/dL) | 42 | 49 | +7 | 7 | +17 | <0.001 |
| HDL Triglycerides (mg/dL) | 13 | 14 | +1 | 2 | +9 | 0.055 |
| Parameters | Baseline | Week 10 | Absolute Change | ±SD | Relative Change (%) | p-Value |
|---|---|---|---|---|---|---|
| Lipoprotein Particle Number a | ||||||
| L-VLDLp (nmol/L) | 6.5 | 6.9 | +0.4 | 5.6 | +6 | 0.752 |
| LDLp (nmol/L) | 1573 | 930 | −643 | 317 | −41 | <0.001 |
| L-LDLp (nmol/L) | 832 | 498 | −335 | 454 | −40 | 0.005 |
| S-LDLp (nmol/L) | 741 | 448 | −293 | 301 | −40 | <0.001 |
| HDLp (nmol/L) | 32,391 | 34,585 | +2194 | 2294 | +7 | 0.001 |
| L-HDLp (nmol/L) | 4758 | 6083 | +1325 | 1382 | +28 | 0.001 |
| S-HDLp (nmol/L) | 28,436 | 28,940 | −593 | 2122 | +2 | 0.252 |
| Lipoprotein Particle Size b | ||||||
| VLDL size (nm) | 49 | 52 | +3 | 5 | +6 | 0.031 |
| LDL size (nm) | 21 | 21 | 0 | 0.5 | 0 | 0.159 |
| HDL size (nm) | 8.8 | 9.0 | +0.2 | 0.2 | +2.3 | <0.001 |
| Ratios of Lipoprotein Particles c | ||||||
| Triglycerides/ApoB | 1.3 | 2.2 | +0.9 | 0.8 | +69 | <0.001 |
| VLDL Triglycerides/ApoB | 0.9 | 1.6 | +0.7 | 0.8 | +78 | 0.001 |
| VLDL Triglycerides/VLDL-ApoB | 6.9 | 9.7 | +2.8 | 2.8 | +41 | <0.001 |
| Cholesterol/ApoB | 2.2 | 2.6 | +0.5 | 0.3 | +18 | <0.001 |
| Lipoprotein Lipase Regulators d | ||||||
| ANGPTL-3 (ng/mL) | 66.5 | 66.3 | −0.2 | 4.3 | −0.3 | 0.835 |
| ANGPTL-4 (pg/mL) | 138.3 | 140.6 | −2.2 | 43.1 | +1.7 | 0.825 |
| GPIHBP-1 (pg/mL) | 894.3 | 913.7 | +19.4 | 118.3 | +2.2 | 0.484 |
| Responder | Non-Responder | Non-Adherent | |
|---|---|---|---|
| LDL Cholesterol ↓ | Yes | No | No |
| PCSK9 ↑ (x3) | N.A. | Yes | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metzner, T.; Leitner, D.R.; Mellitzer, K.; Beck, A.; Sourij, H.; Stojakovic, T.; Reishofer, G.; März, W.; Landmesser, U.; Scharnagl, H.; et al. Effects of Alirocumab on Triglyceride Metabolism: A Fat-Tolerance Test and Nuclear Magnetic Resonance Spectroscopy Study. Biomedicines 2022, 10, 193. https://doi.org/10.3390/biomedicines10010193
Metzner T, Leitner DR, Mellitzer K, Beck A, Sourij H, Stojakovic T, Reishofer G, März W, Landmesser U, Scharnagl H, et al. Effects of Alirocumab on Triglyceride Metabolism: A Fat-Tolerance Test and Nuclear Magnetic Resonance Spectroscopy Study. Biomedicines. 2022; 10(1):193. https://doi.org/10.3390/biomedicines10010193
Chicago/Turabian StyleMetzner, Thomas, Deborah R. Leitner, Karin Mellitzer, Andrea Beck, Harald Sourij, Tatjana Stojakovic, Gernot Reishofer, Winfried März, Ulf Landmesser, Hubert Scharnagl, and et al. 2022. "Effects of Alirocumab on Triglyceride Metabolism: A Fat-Tolerance Test and Nuclear Magnetic Resonance Spectroscopy Study" Biomedicines 10, no. 1: 193. https://doi.org/10.3390/biomedicines10010193
APA StyleMetzner, T., Leitner, D. R., Mellitzer, K., Beck, A., Sourij, H., Stojakovic, T., Reishofer, G., März, W., Landmesser, U., Scharnagl, H., Toplak, H., & Silbernagel, G. (2022). Effects of Alirocumab on Triglyceride Metabolism: A Fat-Tolerance Test and Nuclear Magnetic Resonance Spectroscopy Study. Biomedicines, 10(1), 193. https://doi.org/10.3390/biomedicines10010193









