Discovery Potent of Thiazolidinedione Derivatives as Antioxidant, α-Amylase Inhibitor, and Antidiabetic Agent
Abstract
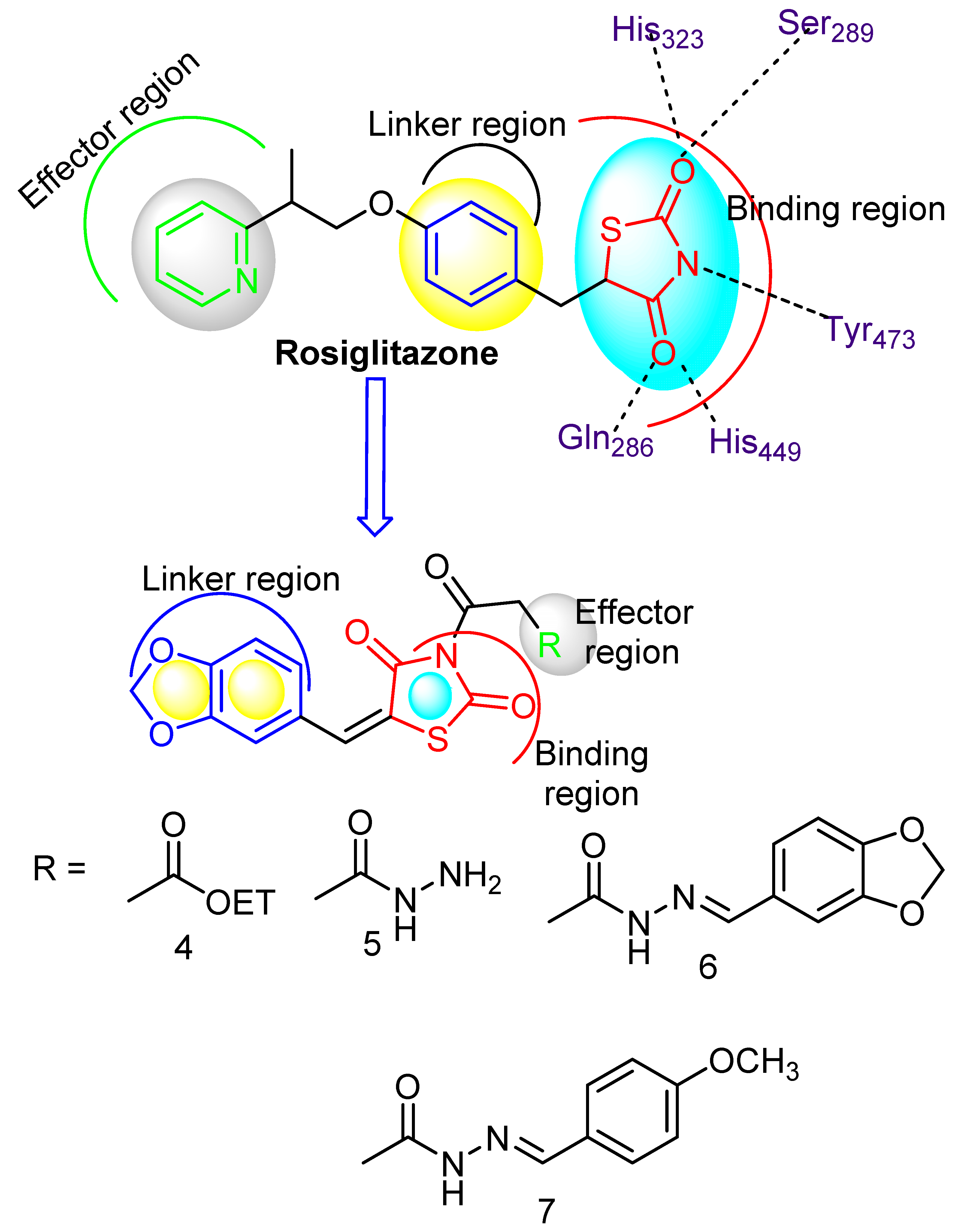
1. Introduction
2. Materials and Methods
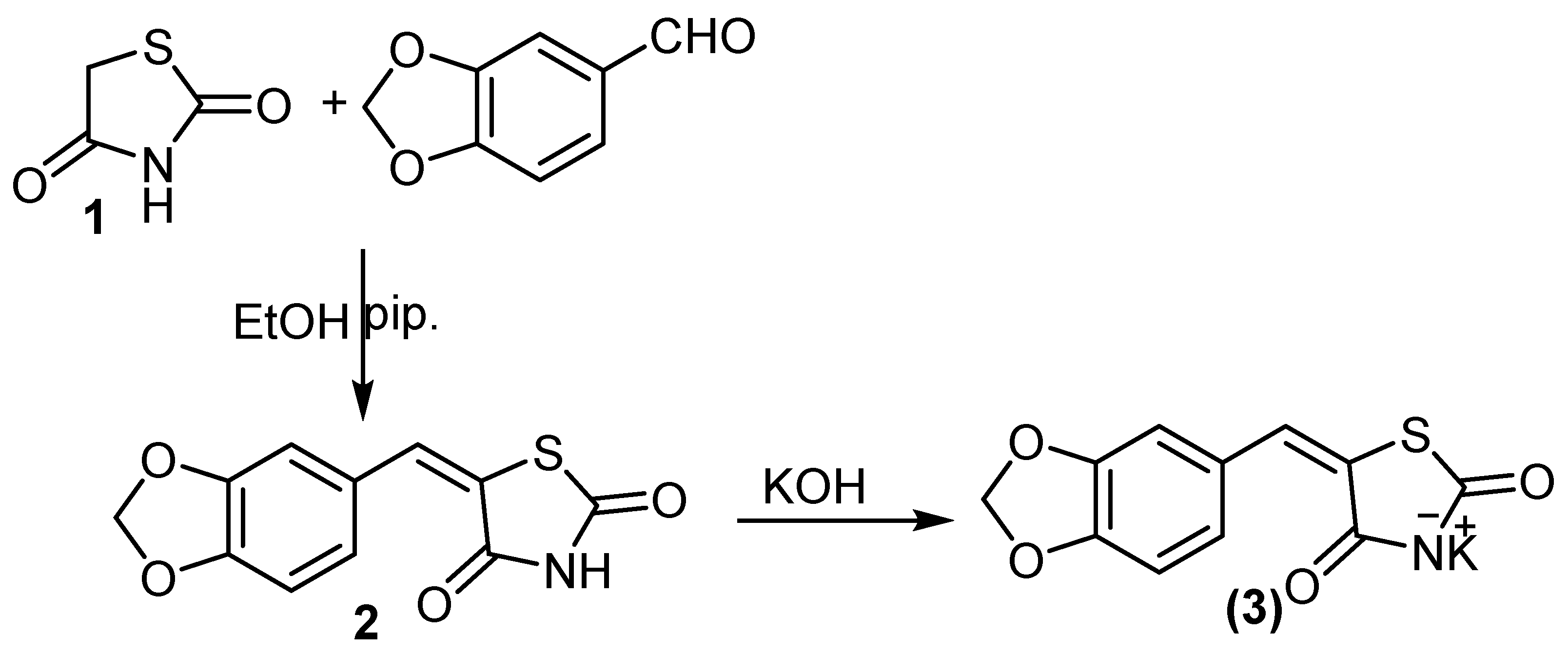
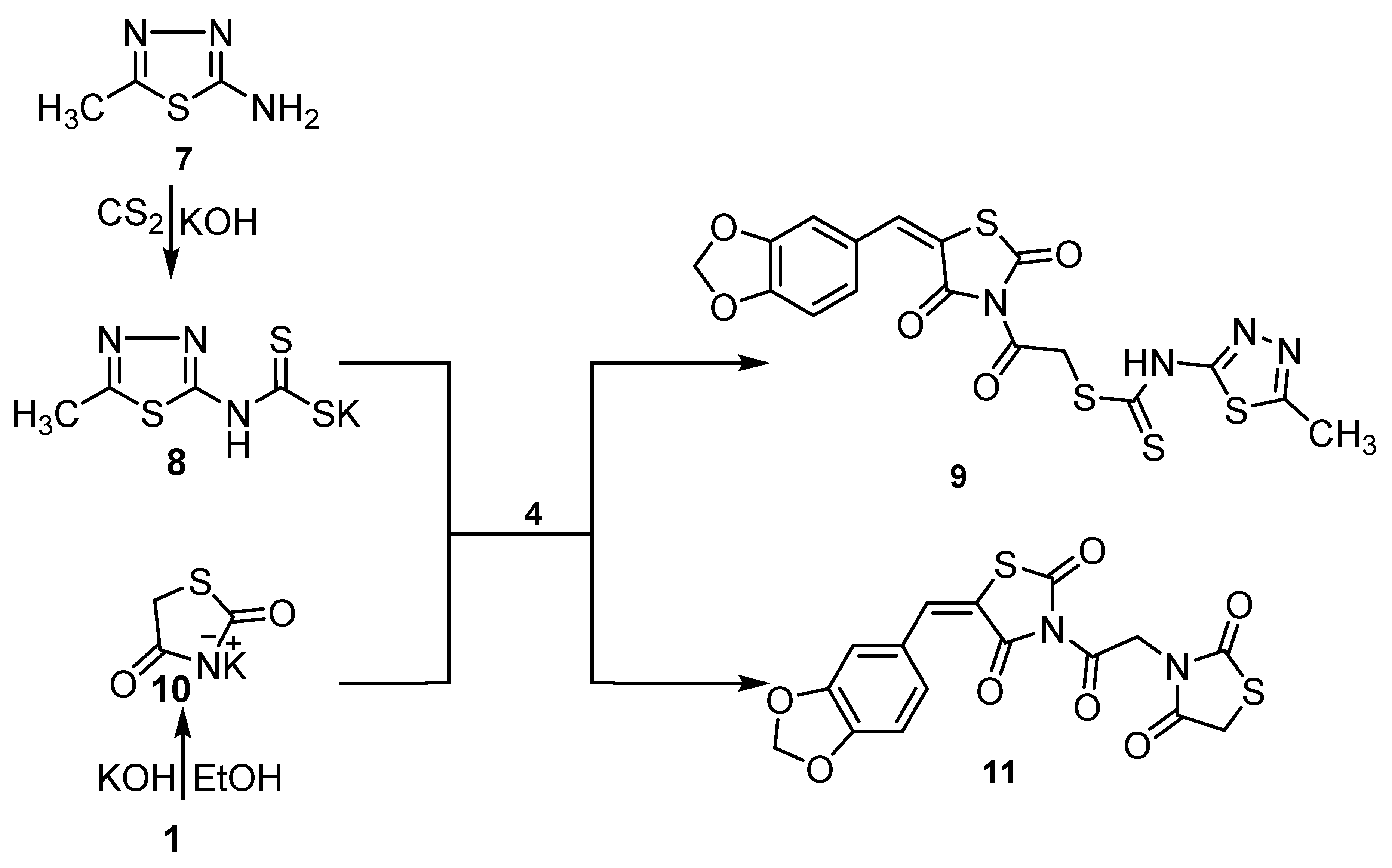
2.1. Chemistry
2.1.1. Interaction of the Potassium Salt 3 with Halogenated Compounds
2.1.2. 2-(5-(Benzo[d][1,3]dioxol-5-ylmethylene)-2,4-dioxothiazolidin-3-yl)-2-oxoethyl (5-Methyl-1,3,4-thiadiazol-2-yl) Carbamodithioate (9)
2.1.3. 5-(Benzo[d][1,3]dioxol-5-ylmethylene)-3-(2-(2,4-dioxothiazolidin-3-yl)acetyl) Thiazolidine-2,4-dione (11)
2.2. Computational Study
2.2.1. Preparation of the Small Molecule
2.2.2. Protein Selection and Molecular Docking
2.3. Biological Study
2.3.1. Animals
2.3.2. Induction of Diabetes
2.3.3. Determination of the Blood Glucose Levels
2.3.4. Biochemical Analysis
2.3.5. The In Vitro Inhibition of α-Amylase Assay
2.3.6. DPPH Radical Scavenging Activity
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Chemistry
3.2. Molecular Modeling Studies
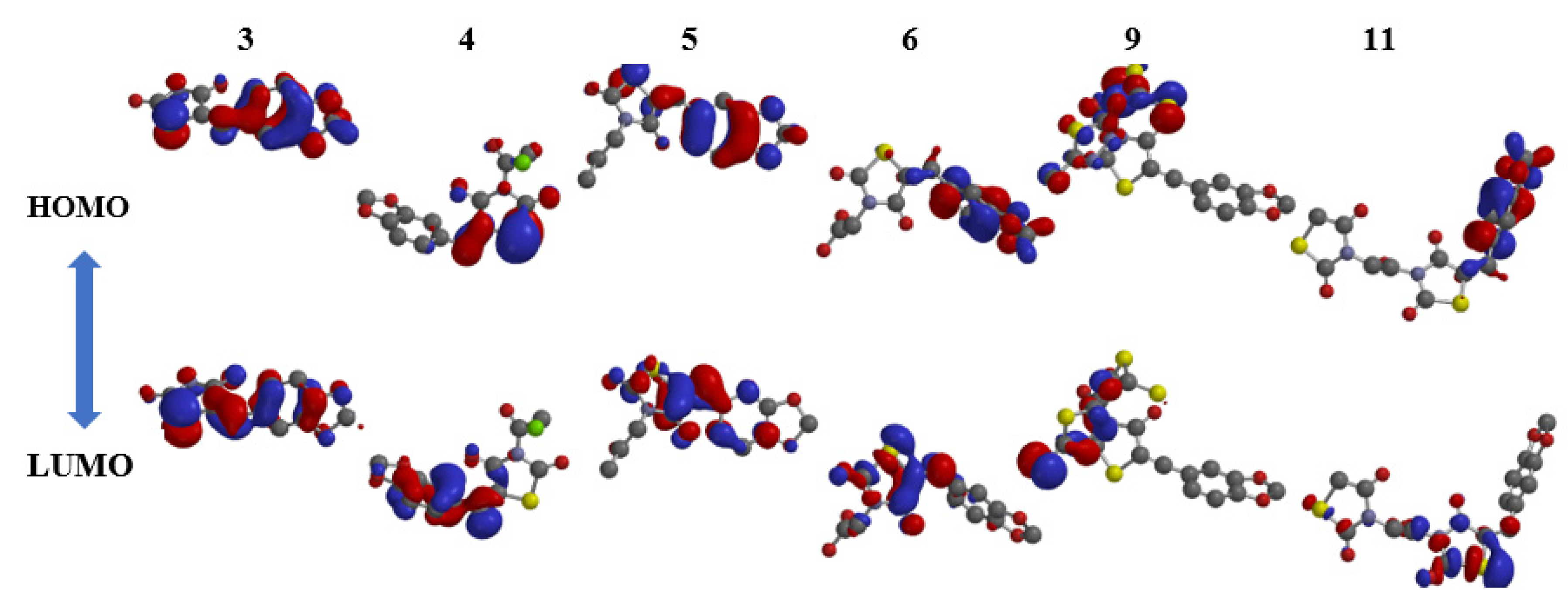
3.2.1. Kinase Inter-/Intramolecular Interaction Stability Based on FMO Analysis
3.2.2. Molecular Electrostatic Potential (MEP)
3.3. Docking Study
3.4. Biological Study
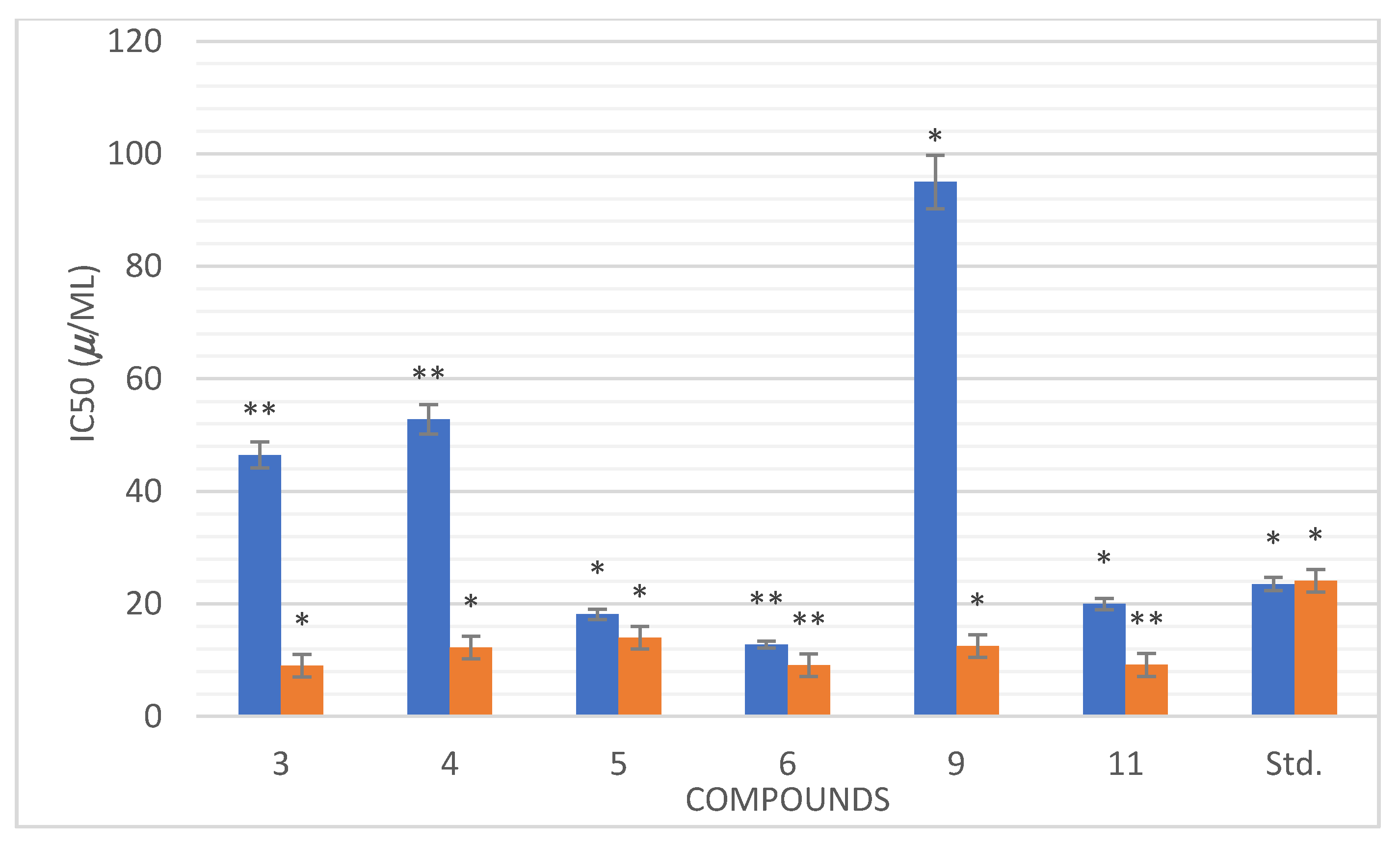
3.4.1. The In Vitro α-Amylase Inhibitory Profile
3.4.2. In Vitro Anti-Oxidant Activity
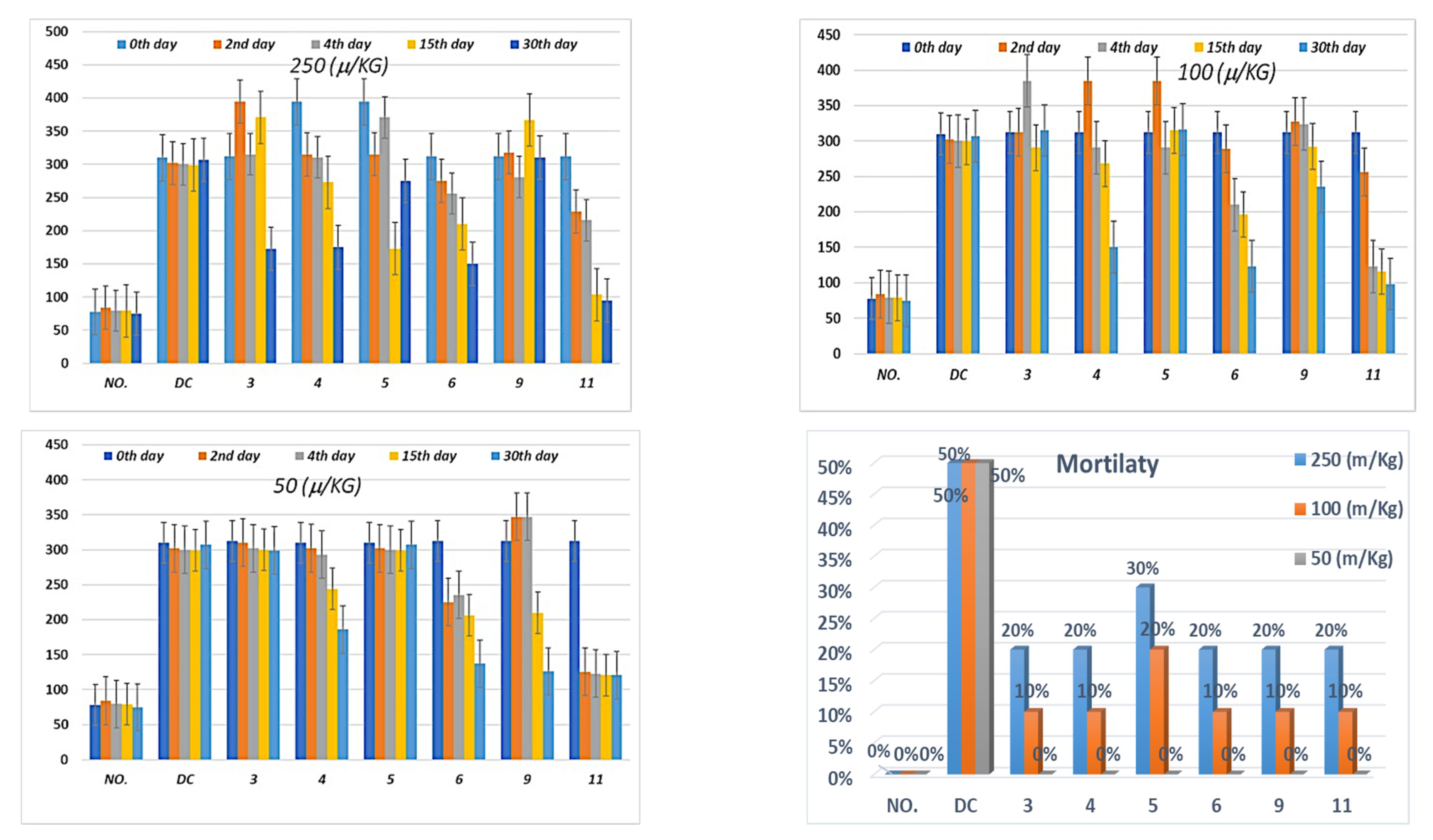
3.4.3. The In Vivo Regulation of Blood Glucose Level
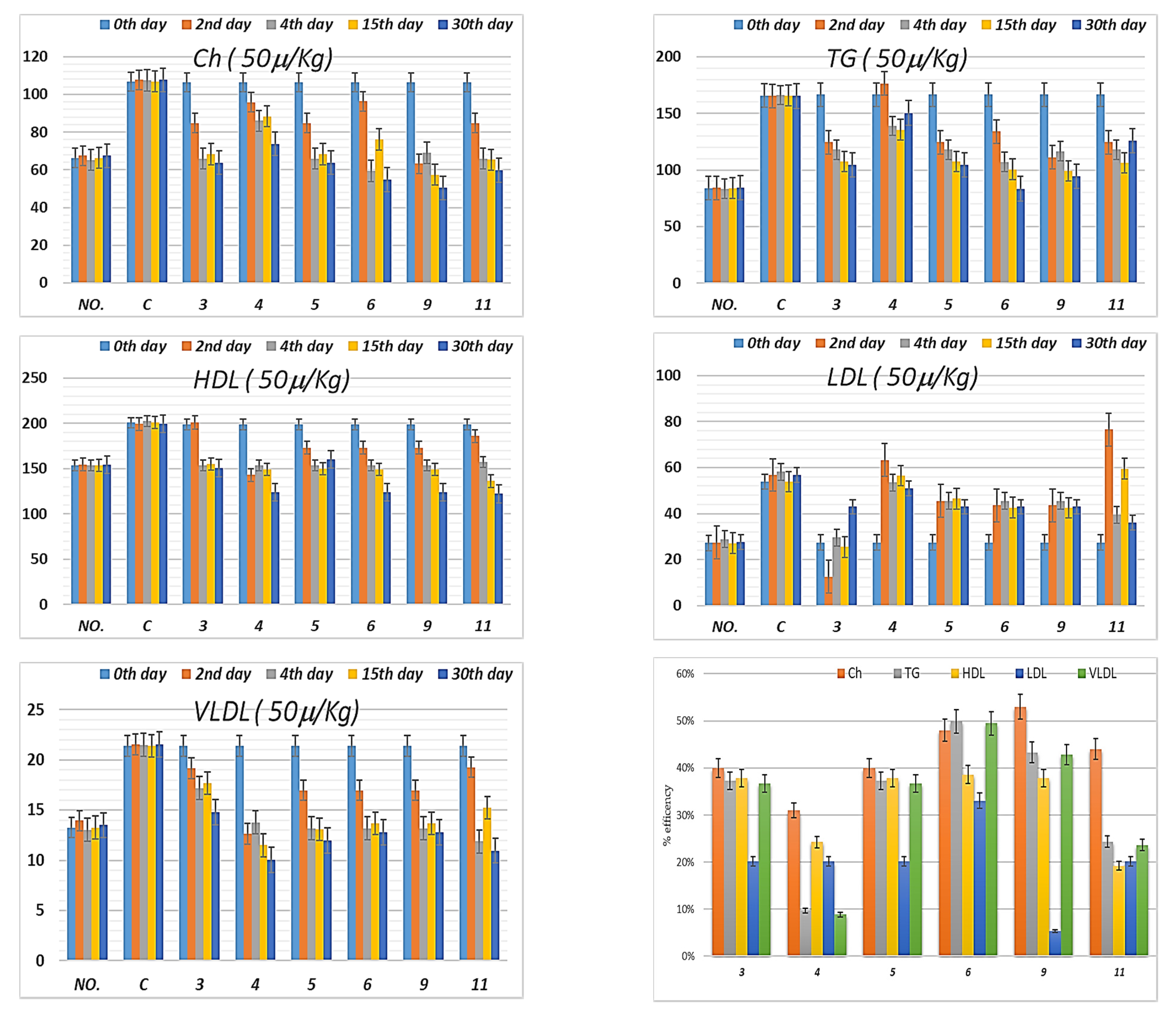
3.4.4. Lipid Profile
3.5. In Silico Pharmacokinetic Profile
3.6. SAR Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.H.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030: Response to Rathman and Giani. Diabetes Care 2004, 27, 2569. [Google Scholar] [CrossRef]
- Bhutani, R.; Pathak, D.P.; Kapoor, G.; Husain, A.; Kant, R.; Iqbal, M.A. Synthesis, Molecular modelling studies and ADME prediction of benzothiazole clubbed oxadiazole-Mannich bases, and evaluation of their Anti-diabetic activity through in-vivo model. Bioorganic Chem. 2018, 77, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Diamond, J. The double puzzle of diabetes. Nature 2003, 423, 599–602. [Google Scholar] [CrossRef] [PubMed]
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef]
- Del Prato, S.; Chilton, R. Practical strategies for improving outcomes in T2DM: The potential role of pioglitazone and DPP4 inhibitors. Diabetes Obes. Metab. 2018, 20, 786–799. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. S1), S90–S102. [Google Scholar] [CrossRef]
- Tangphatsornruang, S.; Naconsie, M.; Thammarongtham, C.; Narangajavana, J. Isolation and characterization of an α-amylase gene in cassava (Manihot esculenta). Plant Physiol. Biochem. 2005, 43, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Feng, J.; Lu, Y.; Cai, Z.F.; Zhang, S.P.; Guo, Z.R. Design, synthesis and in vitro evaluation of a series thiazolidinediones analogs as PPAR modulators. Chin. Chem. Lett. 2007, 18, 45–47. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M.; Hirsch, I.B. Glycemic variability: A hemoglobin A1c–independent risk factor for diabetic complications. JAMA 2006, 295, 1707–1708. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.F.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for the prevention of Type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: Facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Diabetologia 2004, 47, 969–975. [Google Scholar] [CrossRef]
- Yokoyama, H.; Katakami, N.; Yamasaki, Y. Recent advances of intervention to inhibit progression of carotid intima-media thickness in patients with type 2 diabetes mellitus. Stroke 2006, 37, 2420–2427. [Google Scholar] [CrossRef]
- Davidson, M.A.; Mattison, D.R.; Azoulay, L.; Krewski, D. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: Past, present and future. Crit. Rev. Toxicol. 2018, 48, 52–108. [Google Scholar] [CrossRef]
- Xiao, B.; Xiao, Y.; Ning, H.; Han, X.; Li, W.; Ma, Y.; Zhao, N.; Du, G.; Dong, Y.; Jung, J.H. In vitro dual-target activities and in vivo antidiabetic effect of 3-hydroxy-N-(p-hydroxy-phenethyl) phthalimide in high-fat diet and streptozotocin-induced diabetic golden hamsters. Med. Chem. Res. 2020, 29, 2077–2088. [Google Scholar] [CrossRef]
- Khatoon, H.; Najam, R. Effects of Rosiglitazone and Acarbose (with and without Cornstarch Diet) on serum electrolytes in diabetic rats. J. Appl. Pharm. Sci. 2012, 2, 50. [Google Scholar] [CrossRef][Green Version]
- Bennett, W.L.; Maruthur, N.M.; Singh, S.; Segal, J.B.; Wilson, L.M.; Chatterjee, R.; Marinopoulos, S.S.; Puhan, M.A.; Ranasinghe, P.; Block, L. Comparative effectiveness and safety of medications for type 2 diabetes: An update including new drugs and 2-drug combinations. Ann. Intern. Med. 2011, 154, 602–613. [Google Scholar] [CrossRef]
- Sims, H.; Smith, K.H.; Bramlage, P.; Minguet, J. Sotagliflozin: A dual sodium-glucose co-transporter-1 and-2 inhibitor for the management of Type 1 and Type 2 diabetes mellitus. Diabet. Med. 2018, 35, 1037–1048. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Garcia-Vallvé, S.; Guasch, L.; Tomas-Hernández, S.; del Bas, J.M.; Ollendorff, V.; Arola, L.; Pujadas, G.; Mulero, M. Peroxisome Proliferator-Activated Receptor γ (PPARγ) and Ligand Choreography: Newcomers Take the Stage: Miniperspective. J. Med. Chem. 2015, 58, 5381–5394. [Google Scholar] [CrossRef] [PubMed]
- May, L.D.; Lefkowitch, J.H.; Kram, M.T.; Rubin, D.E. Mixed hepatocellular–cholestatic liver injury after pioglitazone therapy. Ann. Intern. Med. 2002, 136, 449–452. [Google Scholar] [CrossRef]
- Okumura, T. Mechanisms by which thiazolidinediones induce anti-cancer effects in cancers in digestive organs. J. Gastroenterol. 2010, 45, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Devji, T.; Reddy, C.; Woo, C.; Awale, S.; Kadota, S.; Carrico-Moniz, D. Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorg. Med. Chem. Lett. 2011, 21, 5770–5773. [Google Scholar] [CrossRef] [PubMed]
- Naim, M.J.; Alam, M.J.; Ahmad, S.; Nawaz, F.; Shrivastava, N.; Sahu, M.; Alam, O. Therapeutic journey of 2,4-thiazolidinediones as a versatile scaffold: An insight into structure activity relationship. Eur. J. Med. Chem. 2017, 129, 218–250. [Google Scholar] [CrossRef]
- S Bahare, R.; Ganguly, S.; Agrawal, R.; N Dikshit, S. Thiazolidine: A Potent Candidate for Central Nervous System Diseases. Cent. Nerv. Syst. Agents Med. Chem. Former. Curr. Med. Chem.-Cent. Nerv. Syst. Agents 2017, 17, 26–29. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.P.; Lagishetty, C.V.; Panda, V.S.; Naik, S.R. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Complementary Altern. Med. 2007, 7, 29. [Google Scholar] [CrossRef]
- Bodade, S.S.; Shaikh, K.S.; Kamble, M.S.; Chaudhari, P.D. A study on ethosomes as mode for transdermal delivery of an antidiabetic drug. Drug Deliv. 2013, 20, 40–46. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Sasikumar, P. Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, 281–286. [Google Scholar] [CrossRef]
- Pareek, H.; Sharma, S.; Khajja, B.S.; Jain, K.; Jain, G. Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.). BMC Complementary Altern. Med. 2009, 9, 48. [Google Scholar] [CrossRef]
- Gadadare, R.; Mandpe, L.; Pokharkar, V. Ultra rapidly dissolving repaglinide nanosized crystals prepared via bottom-up and top-down approach: Influence of food on pharmacokinetics behavior. AAPS PharmSciTech 2015, 16, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kohli, S.; Dinda, A. In-vitro and in-vivo evaluation of repaglinide loaded floating microspheres prepared from different viscosity grades of HPMC polymer. Saudi Pharm. J. 2015, 23, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Naeem, F.; Nadeem, H.; Muhammad, A.; Zahid, M.A.; Saeed, A. Synthesis, α-Amylase Inhibitory Activity and Molecular Docking Studies of 2,4-Thiazolidinedione Derivatives. Open Chem. J. 2018, 5, 134–144. [Google Scholar] [CrossRef]
- Elhenawy, A.; Salama, A.A.; All, M.M.A.; Alomri, A.A.; Nassar, H. Synthesis, characterization and discovery novel anti-diabetic and anti-hyperlipidemic thiazolidinedione derivatives. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 23–30. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Hofmann, P. Arvi Rauk: “Orbital Interaction Theory of Organic Chemistry”; Wiley & Sons: New York, NY, USA, 1994. ISBN 0-471-59389-3. 307 Seiten, mit HMO—Programmdiskette, Preis: $45.50. Ber. Der Bunsenges. Für Phys. Chem. 1995, 99, 997–999. [Google Scholar]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef]
- Wildman, S.A.; Crippen, G.M. Prediction of physicochemical parameters by atomic contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Parr, R.G.; Chattaraj, P.K. Principle of maximum hardness. J. Am. Chem. Soc. 1991, 113, 1854–1855. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Zheludkevich, M.L.; Yasakau, K.A.; Serra, R.; Poznyak, S.; Ferreira, M. Nanoporous titania interlayer as reservoir of corrosion inhibitors for coatings with self-healing ability. Prog. Org. Coat. 2007, 58, 127–135. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Elhenawy, A.A.; Al-Harbi, L.M.; El-Gazzar, M.A.; Khowdiary, M.M.; Moustfa, A. Synthesis, molecular properties and comparative docking and QSAR of new 2-(7-hydroxy-2-oxo-2H-chromen-4-yl)acetic acid derivatives as possible anticancer agents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 218, 248–262. [Google Scholar] [CrossRef]
- Elgendy, A.; Nady, H.; El-Rabiei, M.M.; Elhenawy, A.A. Understanding the adsorption performance of two glycine derivatives as novel and environmentally safe anti-corrosion agents for copper in chloride solutions: Experimental, DFT, and MC studies. RSC Adv. 2019, 9, 42120–42131. [Google Scholar] [CrossRef]
- El Gaafary, M.; Syrovets, T.; Mohamed, H.M.; Elhenawy, A.A.; El-Agrody, A.M.; Amr, A.E.-G.E.; Ghabbour, H.A.; Almehizia, A.A. Synthesis, Cytotoxic Activity, Crystal Structure, DFT Studies and Molecular Docking of 3-Amino-1-(2, 5-dichlorophenyl)-8-methoxy-1H-benzo [f] chromene-2-carbonitrile. Crystals 2021, 11, 184. [Google Scholar] [CrossRef]
- Al-Harbi, L.M.; Nassar, H.S.; Moustfa, A.; Alosaimi, A.M.; Mohamed, H.M.; Khowdiary, M.M.; El-Gazzar, M.A.; Elhenawy, A.A. Novel coumarin amino acid derivatives: Design, synthesis, docking, absorption, distribution, metabolism, elimination, toxicity (ADMET), quantitative structure–activity relationship (QSAR) and anticancer studies. Mater. Express 2020, 10, 1375–1394. [Google Scholar] [CrossRef]
- Elhenawy, A.A.; Al-Harbi, L.M.; El-Gazzar, M.A.; Khowdiary, M.M.; Ouidate, A.; Alosaimi, A.M.; Elhamid Salim, A. Naproxenylamino acid derivatives: Design, synthesis, docking, QSAR and anti-inflammatory and analgesic activity. Biomed. Pharm. 2019, 116, 109024. [Google Scholar] [CrossRef]
- Elhenawy, A.A.; Al-Harbi, L.M.; Moustafa, G.O.; El-Gazzar, M.A.; Abdel-Rahman, R.F.; Salim Abd Elhamid Drug Design. Development and Therapy, Synthesis, comparative docking, and pharmacological activity of naproxen amino acid derivatives as possible anti-inflammatory and analgesic agents. Drug Des. Devel. 2019, 13, 1773–1790. [Google Scholar] [CrossRef]
- Elsisi, D.M.; Ragab, A.; Elhenawy, A.A.; Farag, A.A.; Ali, A.M.; Ammar, Y.A. Experimental and theoretical investigation for 6-Morpholinosulfonylquinoxalin-2 (1H)-one and its haydrazone derivate: Synthesis, characterization, tautomerization and antimicrobial evaluation. J. Mol. Struct. 2022, 1247, 131314. [Google Scholar] [CrossRef]
- Luque, F.J.; López, J.M.; Orozco, M. Perspective on “Electrostatic interactions of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects”. In Theoretical Chemistry Accounts; Springer: Berlin/Heidelberg, Germany, 2000; pp. 343–345. [Google Scholar]
- Molecular Operating Environment (MOE). C.C.G.U., 1010 Handbook St. West, Suite 910; Montreal, QC, Canada, 2017. Available online: https://www.chemcomp.com/Products.htm (accessed on 25 October 2021).
- Willson, T.M.; Brown, P.J.; Sternbach, D.D.; Henke, B.R. The PPARs: From orphan receptors to drug discovery. J. Med. Chem. 2000, 43, 527–550. [Google Scholar] [CrossRef]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative Catalytic Anions Differentially Modulate Human α-Amylase Activity and Specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef]
- Bohacek, R.S.; McMartin, C.; Guida, W.C. The art and practice of structure—Based drug design: A molecular modeling perspective. Med. Res. Rev. 1996, 16, 3–50. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Keserü, G.M.; Leeson, P.D.; Rees, D.C.; Reynolds, C.H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 2014, 13, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Nguyet, T.T.; Dat, D.T.; Ha, N.V. Stereoelectronic Properties of 1,2,4-Triazole-Derived N-heterocyclic Carbenes—A Theoretical Study. VNU J. Sci. Nat. Sci. Technol. 2019, 35. [Google Scholar] [CrossRef]
- Saravanan, R.; Pari, L. Antihyperlipidemic and antiperoxidative effect of Diasulin, a polyherbal formulation in alloxan induced hyperglycemic rats. BMC Complementary Altern. Med. 2005, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.H.; Giribabu, N.; Rao, P.V.; Sayem, A.S.M.; Arya, A.; Panichayupakaranant, P.; Korla, P.K.; Salleh, N. Rhinacanthin C ameliorates hyperglycaemia, hyperlipidemia and pancreatic destruction in streptozotocin–nicotinamide induced adult male diabetic rats. Eur. J. Pharmacol. 2016, 771, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Ravi, K.; Sivagnanam, K.; Subramanian, S. Beneficial effects of Aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin. Exp. Pharmacol. Physiol. 2006, 33, 232–237. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Clark, D.E.; Pickett, S.D. Computational methods for the prediction of ‘drug-likeness’. Drug Discov. Today 2000, 5, 49–58. [Google Scholar] [CrossRef]




| 3 | 4 | 5 | 6 | 9 | 11 | 3 | 4 | 5 | 6 | 9 | 11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HOMO | −8.18 | −6.839 | −8.56 | −8.93 | −8.81 | −9.23 | µ+ | −2.99 | −1.79 | −2.972 | −3.345 | −3.245 | −3.35 |
| LUMO | −1.26 | −0.108 | −1.11 | −1.35 | −1.39 | −1.39 | µ− | −6.45 | −5.16 | −6.697 | −7.035 | −6.955 | −7.027 |
| ΔG | −6.92 | −6.731 | −7.45 | −7.58 | −7.42 | −7.84 | ω− | 6.11 | 3.95 | 6.21 | 6.529 | 6.519 | 6.741 |
| IP | 8.18 | 6.839 | 9.23 | 8.93 | 8.81 | 8.56 | ω+ | 2.786 | 1.37 | 2.672 | 3.011 | 3.041 | 3.106 |
| A | 1.26 | 0.108 | 1.11 | 1.35 | 1.39 | 1.39 | ω± | 8.798 | 5.32 | 8.693 | 9.58 | 9.56 | 9.847 |
| η | 3.46 | 3.365 | 3.725 | 3.79 | 3.71 | 3.92 | ΔNmax | −0.682 | 1.03 | −0.648 | −0.678 | −0.687 | −0.677 |
| S | 0.289 | 0.297 | 0.268 | 0.263 | 0.269 | 0.255 | 2.728 | −1.53 | 2.595 | 2.712 | 2.06 | 2.709 | |
| χ | −4.72 | −3.473 | −4.835 | −5.14 | −5.1 | −5.31 | −0.682 | −0.84 | −0.648 | −0.678 | −0.687 | −0.677 | |
| ωi | 3.219 | 3.453 | 3.137 | 3.485 | 3.505 | 3.596 |
| Cpd. | Ref.Inhibtor | 3 | 4 | 5 | 6 | 9 | 11 |
|---|---|---|---|---|---|---|---|
| PPAR-γ(PDB: 2PRG) | |||||||
| ΔΕ | −7.86 | −9.80 | −6.48 | −11.85 | −9.14 | −11.40 | −9.01 |
| RMSD | 1.95 | 1.60 | 1.50 | 1.33 | 1.79 | 1.77 | 1.89 |
| E_place | −47.36 | −112.41 | −39.25 | −37.56 | −59.86 | −89.46 | −118.45 |
| E.Int. | −35.63 | −30.39 | −33.70 | −32.85 | −34.63 | −38.27 | −33.03 |
| E.H.B. | −0.25 | −1.33 | 0.29 | −0.65 | −0.12 | 0.37 | −0.23 |
| L.E | 4.03 | 6.76 | 4.34 | 8.88 | 5.11 | 6.46 | 4.76 |
| Ki(μM) | 1.39 | 1.70 | 2.21 | 1.61 | 1.87 | 1.65 | 1.88 |
| LEscale | 2.67 | 2.84 | 2.90 | 3.00 | 2.74 | 2.75 | 2.69 |
| FQ | 1.36 | 3.92 | 1.44 | 5.88 | 2.37 | 3.70 | 2.07 |
| α-amylase, (PDB:2QV4) | |||||||
| ΔΕ | −5.59 | −6.23 | −6.50 | −6.74 | −6.14 | −7.66 | −6.33 |
| RMSD | 1.79 | 0.93 | 1.33 | 1.95 | 1.95 | 1.19 | 1.37 |
| E_place | −26.39 | −43.10 | −62.93 | −23.29 | 15.12 | −113.81 | −37.51 |
| E.Int. | −32.26 | −24.52 | −19.04 | −24.48 | −15.75 | −26.46 | −18.00 |
| E.H.B. | −17.58 | −15.46 | −10.35 | −11.87 | −10.07 | −11.36 | −10.01 |
| L.E | 3.12 | 6.67 | 4.89 | 3.46 | 3.15 | 6.44 | 4.61 |
| Ki(μM) | 1.14 | 2.25 | 2.21 | 2.17 | 2.27 | 2.05 | 2.24 |
| LEscale | −2.74 | −3.31 | −3.00 | −2.67 | −2.67 | −3.10 | −2.97 |
| FQ | 0.38 | 3.36 | 1.89 | 0.79 | 0.48 | 3.34 | 1.64 |
| Cpd. | 3 | 5 | 6 | 9 | 11 |
|---|---|---|---|---|---|
| HBD | 1 | 2 | 4 | 3 | 2 |
| HBA | 8 | 9 | 8 | 7 | 8 |
| Log.P | 2.83 | 3.21 | 2.25 | 2.42 | 2.74 |
| Log.S | −5.53 | −6.13 | −5.73 | −4.26 | −5.11 |
| V | 0 | 0 | 0 | 0 | 0 |
| TPSA | 105.5 | 132.48 | 157.05 | 112.93 | 116.5 |
| %ABS | 72.6 | 73.83 | 66.7 | 70.03 | 68.8 |
| Mutagenic | none | none | none | low | none |
| Tumorigenic | none | none | none | high | none |
| Reproductive Effective | none | none | none | high | none |
| Irritant | none | none | none | high | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sameeh, M.Y.; Khowdiary, M.M.; Nassar, H.S.; Abdelall, M.M.; Alderhami, S.A.; Elhenawy, A.A. Discovery Potent of Thiazolidinedione Derivatives as Antioxidant, α-Amylase Inhibitor, and Antidiabetic Agent. Biomedicines 2022, 10, 24. https://doi.org/10.3390/biomedicines10010024
Sameeh MY, Khowdiary MM, Nassar HS, Abdelall MM, Alderhami SA, Elhenawy AA. Discovery Potent of Thiazolidinedione Derivatives as Antioxidant, α-Amylase Inhibitor, and Antidiabetic Agent. Biomedicines. 2022; 10(1):24. https://doi.org/10.3390/biomedicines10010024
Chicago/Turabian StyleSameeh, Manal Y., Manal M. Khowdiary, Hisham S. Nassar, Mahmoud M. Abdelall, Suliman A. Alderhami, and Ahmed A. Elhenawy. 2022. "Discovery Potent of Thiazolidinedione Derivatives as Antioxidant, α-Amylase Inhibitor, and Antidiabetic Agent" Biomedicines 10, no. 1: 24. https://doi.org/10.3390/biomedicines10010024
APA StyleSameeh, M. Y., Khowdiary, M. M., Nassar, H. S., Abdelall, M. M., Alderhami, S. A., & Elhenawy, A. A. (2022). Discovery Potent of Thiazolidinedione Derivatives as Antioxidant, α-Amylase Inhibitor, and Antidiabetic Agent. Biomedicines, 10(1), 24. https://doi.org/10.3390/biomedicines10010024






