The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assessment of Gestational Weight Gain
2.3. Covariate Ascertainment
2.4. DNA Extraction
2.5. Relative Telomere Length
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Relationship of Telomere Length with Maternal Characteristics
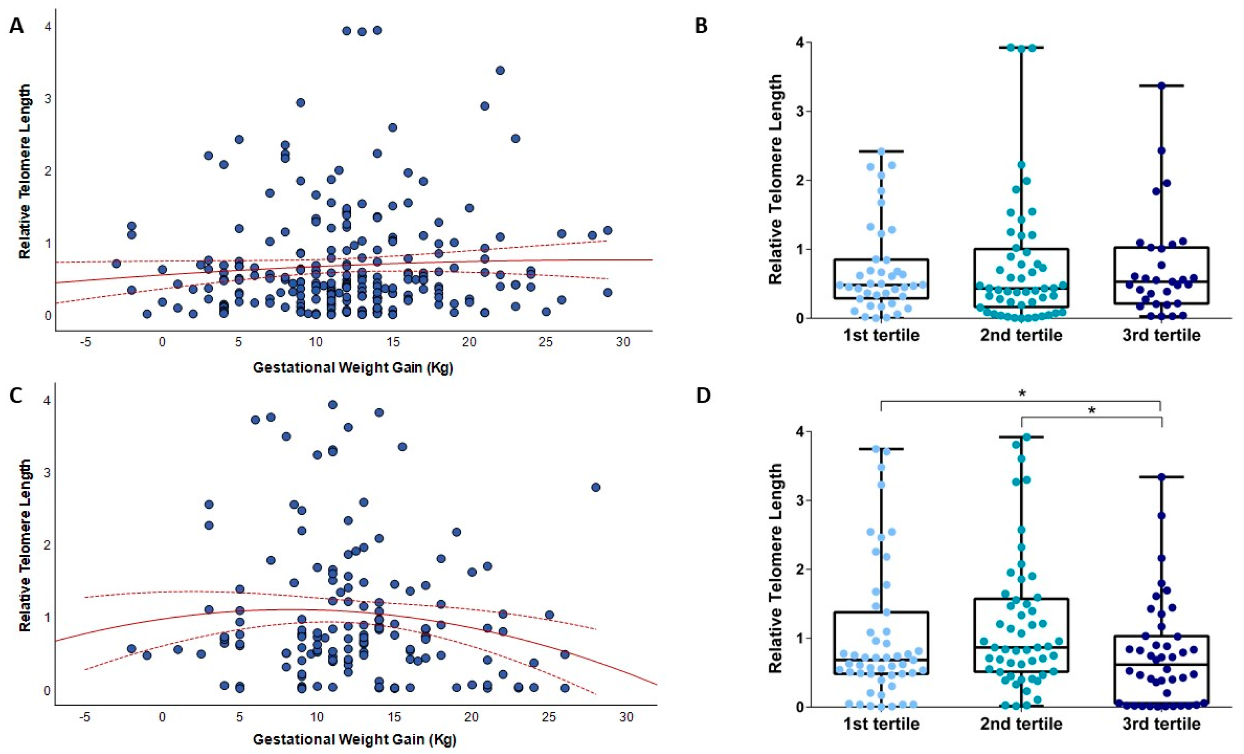
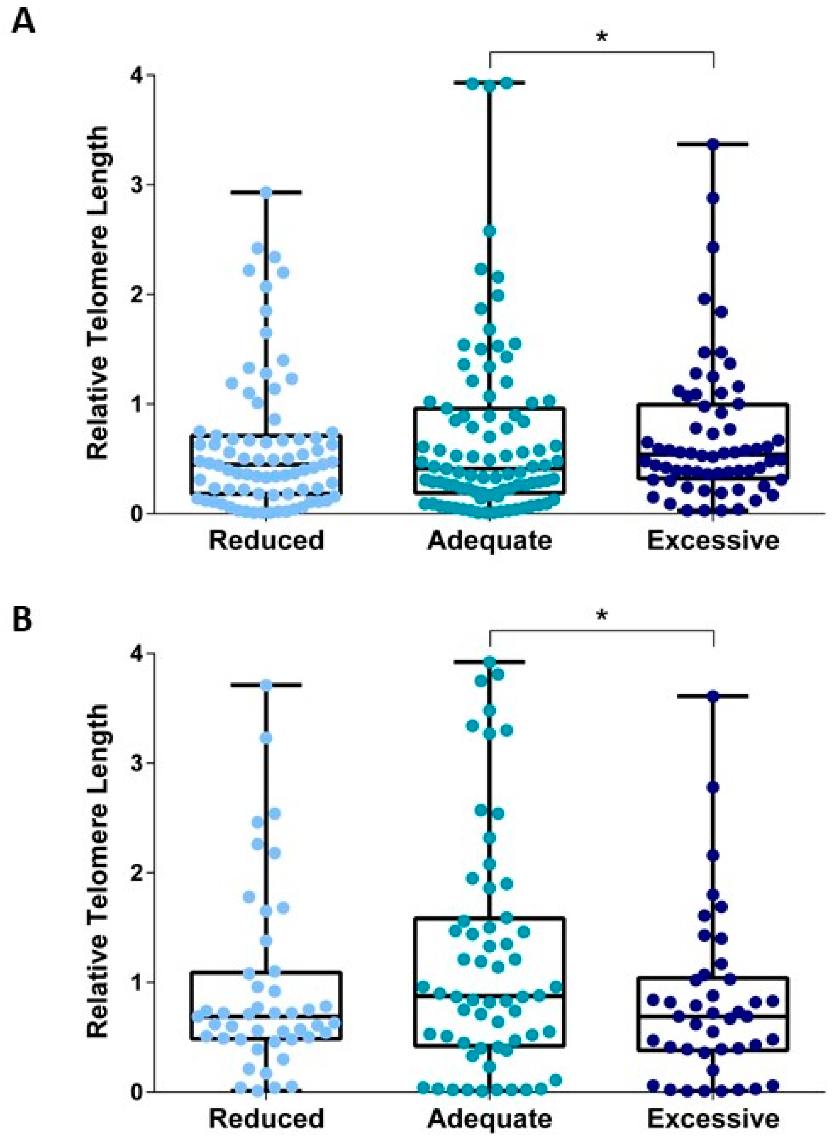
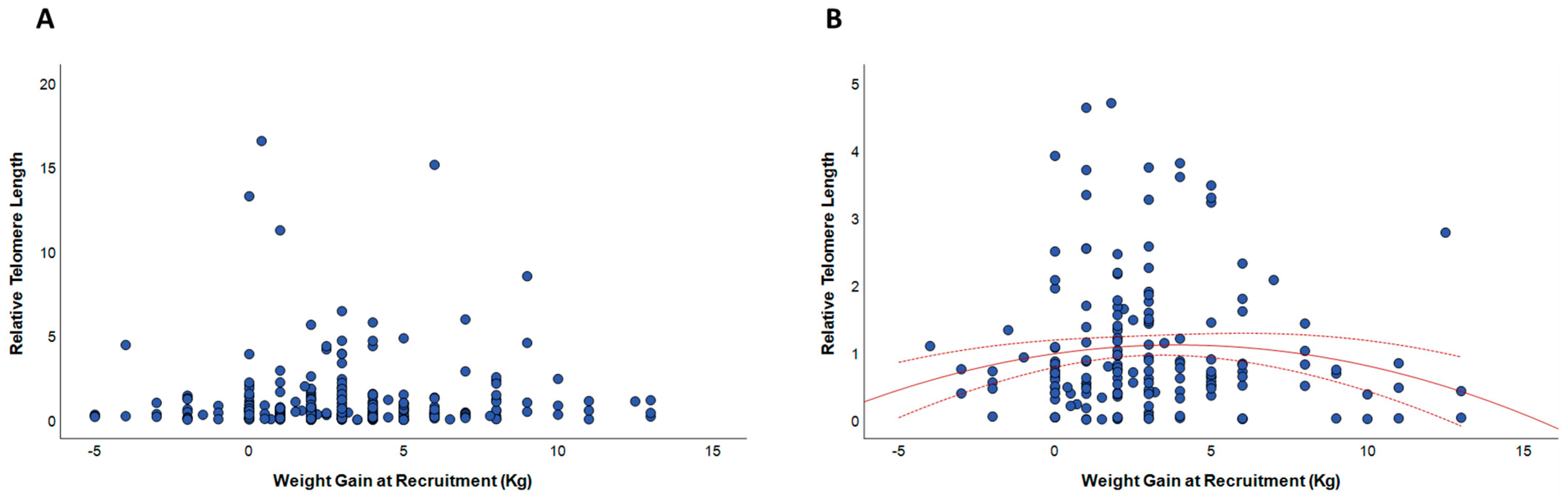
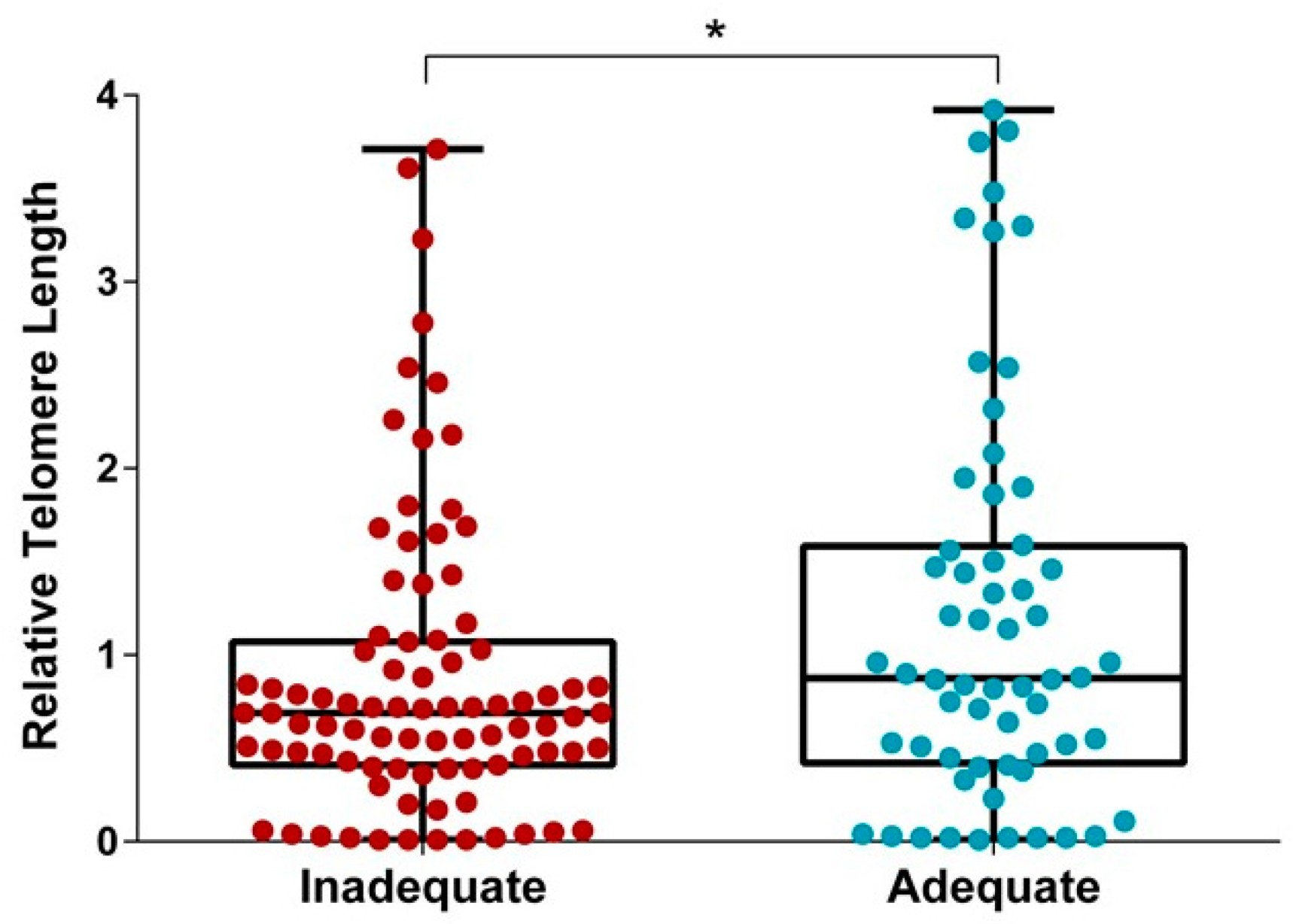
3.3. Relationships between Gestational Weight Gain and Telomere Length
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines; Institute of Medicine Guidelines: Washington, DC, USA, 2009.
- Cedergren, M.I. Optimal gestational weight gain for body mass index categories. Obstet. Gynecol. 2007, 110, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Beyerlein, A.; Schiessl, B.; Lack, N.; von Kries, R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: A novel approach. Am. J. Clin. Nutr. 2009, 90, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Cheikh Ismail, L.; Bishop, D.C.; Pang, R.; Ohuma, E.O.; Kac, G.; Abrams, B.; Rasmussen, K.; Barros, F.C.; Hirst, J.E.; Lambert, A.; et al. Gestational weight gain standards based on women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: A prospective longitudinal cohort study. BMJ 2016, 352, i555. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Catalano, P.M.; Yaktine, A.L. New guidelines for weight gain during pregnancy: What obstetrician/gynecologists should know. Curr. Opin. Obstet. Gynecol. 2009, 21, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.G.; Taylor, R.S.; Thompson, J.M.; Anderson, N.H.; Dekker, G.A.; Kenny, L.C.; McCowan, L.M.; Consortium, S. Gestational weight gain and adverse pregnancy outcomes in a nulliparous cohort. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Durie, D.E.; Thornburg, L.L.; Glantz, J.C. Effect of second-trimester and third-trimester rate of gestational weight gain on maternal and neonatal outcomes. Obstet. Gynecol. 2011, 118, 569–575. [Google Scholar] [CrossRef]
- Simas, T.A.; Liao, X.; Garrison, A.; Sullivan, G.M.; Howard, A.E.; Hardy, J.R. Impact of updated Institute of Medicine guidelines on prepregnancy body mass index categorization, gestational weight gain recommendations, and needed counseling. J. Womens Health (Larchmt) 2011, 20, 837–844. [Google Scholar] [CrossRef]
- Cedergren, M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int. J. Gynaecol. Obstet. 2006, 93, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.C.; McNeil, D.A.; Best, M.; MacLeod, C. A growth status measurement pilot in four Calgary area schools: Perceptions of grade 5 students and their parents. J. Sch. Nurs. 2011, 27, 61–69. [Google Scholar] [CrossRef]
- Nohr, E.A.; Vaeth, M.; Baker, J.L.; Sørensen, T.I.; Olsen, J.; Rasmussen, K.M. Pregnancy outcomes related to gestational weight gain in women defined by their body mass index, parity, height, and smoking status. Am. J. Clin. Nutr. 2009, 90, 1288–1294. [Google Scholar] [CrossRef]
- Rooney, B.L.; Schauberger, C.W.; Mathiason, M.A. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet. Gynecol. 2005, 106, 1349–1356. [Google Scholar] [CrossRef]
- Rooney, B.L.; Schauberger, C.W. Excess pregnancy weight gain and long-term obesity: One decade later. Obstet. Gynecol. 2002, 100, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, Z.M.; Contador, F.; Tawfiq, A.; Adamo, K.B.; Gaudet, L. Gestational weight gain and medical outcomes of pregnancy. Obstet. Med. 2015, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- DeVader, S.R.; Neeley, H.L.; Myles, T.D.; Leet, T.L. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet. Gynecol. 2007, 110, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Margerison Zilko, C.E.; Rehkopf, D.; Abrams, B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am. J. Obstet. Gynecol. 2010, 202, 574.e1–574.e8. [Google Scholar] [CrossRef]
- de la Torre, L.; Flick, A.A.; Istwan, N.; Rhea, D.; Cordova, Y.; Dieguez, C.; Desch, C.; González-Quintero, V.H. The effect of new antepartum weight gain guidelines and prepregnancy body mass index on the development of pregnancy-related hypertension. Am. J. Perinatol. 2011, 28, 285–292. [Google Scholar] [CrossRef]
- Mamun, A.A.; Kinarivala, M.; O’Callaghan, M.J.; Williams, G.M.; Najman, J.M.; Callaway, L.K. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: Evidence from 21 y postpartum follow-up. Am. J. Clin. Nutr. 2010, 91, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; White, J.; Murphy, P.; Burrage, L.; Hutchens, D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. J. Obstet. Gynaecol. Can. 2009, 31, 28–35. [Google Scholar] [CrossRef]
- Mannan, M.; Doi, S.A.; Mamun, A.A. Association between weight gain during pregnancy and postpartum weight retention and obesity: A bias-adjusted meta-analysis. Nutr. Rev. 2013, 71, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Herring, S.J.; Rose, M.Z.; Skouteris, H.; Oken, E. Optimizing weight gain in pregnancy to prevent obesity in women and children. Diabetes Obes. Metab. 2012, 14, 195–203. [Google Scholar] [CrossRef]
- Lucia Bergmann, R.; Bergmann, K.E.; Haschke-Becher, E.; Richter, R.; Dudenhausen, J.W.; Barclay, D.; Haschke, F. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? J. Perinat. Med. 2007, 35, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Poston, L. Gestational weight gain: Influences on the long-term health of the child. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 252–257. [Google Scholar] [CrossRef]
- Sridhar, S.B.; Darbinian, J.; Ehrlich, S.F.; Markman, M.A.; Gunderson, E.P.; Ferrara, A.; Hedderson, M.M. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am. J. Obstet. Gynecol. 2014, 211, 259.e1–259.e8. [Google Scholar] [CrossRef]
- Han, Z.; Lutsiv, O.; Mulla, S.; Rosen, A.; Beyene, J.; McDonald, S.D.; Group, K.S. Low gestational weight gain and the risk of preterm birth and low birthweight: A systematic review and meta-analyses. Acta Obstet. Gynecol. Scand. 2011, 90, 935–954. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Collins, K. The biogenesis and regulation of telomerase holoenzymes. Nat. Rev. Mol. Cell Biol. 2006, 7, 484–494. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight 2012; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Mundstock, E.; Sarria, E.E.; Zatti, H.; Mattos Louzada, F.; Kich Grun, L.; Herbert Jones, M.; Guma, F.T.; Mazzola In Memoriam, J.; Epifanio, M.; Stein, R.T.; et al. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity (Silver Spring) 2015, 23, 2165–2174. [Google Scholar] [CrossRef]
- Njajou, O.T.; Cawthon, R.M.; Blackburn, E.H.; Harris, T.B.; Li, R.; Sanders, J.L.; Newman, A.B.; Nalls, M.; Cummings, S.R.; Hsueh, W.C. Shorter telomeres are associated with obesity and weight gain in the elderly. Int. J. Obes. (Lond.) 2012, 36, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Gorenjak, V.; Petrelis, A.M.; Stathopoulou, M.G.; Visvikis-Siest, S. Telomere length determinants in childhood. Clin. Chem. Lab. Med. 2020, 58, 162–177. [Google Scholar] [CrossRef]
- Liu, B.; Song, L.; Zhang, L.; Wu, M.; Wang, L.; Cao, Z.; Xiong, C.; Zhang, B.; Li, Y.; Xia, W.; et al. Prenatal second-hand smoke exposure and newborn telomere length. Pediatr. Res. 2020, 87, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Salihu, H.M.; King, L.M.; Nwoga, C.; Paothong, A.; Pradhan, A.; Marty, P.J.; Daas, R.; Whiteman, V.E. Association Between Maternal-Perceived Psychological Stress and Fetal Telomere Length. South. Med. J. 2016, 109, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.S.; Cox, B.; Janssen, B.G.; Clemente, D.B.P.; Gasparrini, A.; Vanpoucke, C.; Lefebvre, W.; Roels, H.A.; Plusquin, M.; Nawrot, T.S. Prenatal Air Pollution and Newborns’ Predisposition to Accelerated Biological Aging. JAMA Pediatr. 2017, 171, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Isaevska, E.; Moccia, C.; Asta, F.; Cibella, F.; Gagliardi, L.; Ronfani, L.; Rusconi, F.; Stazi, M.A.; Richiardi, L. Exposure to ambient air pollution in the first 1000 days of life and alterations in the DNA methylome and telomere length in children: A systematic review. Environ. Res. 2021, 193, 110504. [Google Scholar] [CrossRef]
- Martens, D.S.; Plusquin, M.; Gyselaers, W.; De Vivo, I.; Nawrot, T.S. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016, 14, 148. [Google Scholar] [CrossRef]
- Vasu, V.; Turner, K.J.; George, S.; Greenall, J.; Slijepcevic, P.; Griffin, D.K. Preterm infants have significantly longer telomeres than their term born counterparts. PLoS ONE 2017, 12, e0180082. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Hande, P.; Yeo, G.S.; Tan, E.C. Correlation of cord blood telomere length with birth weight. BMC Res. Notes 2017, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Sibert, N.T.; Ventura Ferreira, M.S.; Wagner, W.; Eipel, M.; Dreschers, S.; Brümmendorf, T.H.; Orlikowsky, T.; Beier, F. Cord blood telomere shortening associates with increased gestational age and birth weight in preterm neonates. Exp. Ther. Med. 2021, 21, 344. [Google Scholar] [CrossRef]
- Fragkiadaki, P.; Tsoukalas, D.; Fragkiadoulaki, I.; Psycharakis, C.; Nikitovic, D.; Spandidos, D.A.; Tsatsakis, A.M. Telomerase activity in pregnancy complications (Review). Mol. Med. Rep. 2016, 14, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.W. Circulating fetal DNA: Its origin and diagnostic potential-a review. Placenta 2004, 25 (Suppl. A), S93–S101. [Google Scholar] [CrossRef]
- Lo, Y.M. Recent advances in fetal nucleic acids in maternal plasma. J. Histochem. Cytochem. 2005, 53, 293–296. [Google Scholar] [CrossRef]
- Taback, B.; Hoon, D.S. Circulating nucleic acids in plasma and serum: Past, present and future. Curr. Opin. Mol. Ther. 2004, 6, 273–278. [Google Scholar] [PubMed]
- Deligezer, U.; Erten, N.; Akisik, E.E.; Dalay, N. Circulating fragmented nucleosomal DNA and caspase-3 mRNA in patients with lymphoma and myeloma. Exp. Mol. Pathol. 2006, 80, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Weikert, S.; Christoph, F.; Schrader, M.; Krause, H.; Miller, K.; Müller, M. Quantitative analysis of survivin mRNA expression in urine and tumor tissue of bladder cancer patients and its potential relevance for disease detection and prognosis. Int. J. Cancer 2005, 116, 100–104. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; St John, M.A.; Wong, D.T. RNA profiling of cell-free saliva using microarray technology. J. Dent. Res. 2004, 83, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Larrabee, P.B.; Johnson, K.L.; Pestova, E.; Lucas, M.; Wilber, K.; LeShane, E.S.; Tantravahi, U.; Cowan, J.M.; Bianchi, D.W. Microarray analysis of cell-free fetal DNA in amniotic fluid: A prenatal molecular karyotype. Am. J. Hum. Genet. 2004, 75, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Nemati, M.; Maralani, M.; Estiar, M.A.; Andalib, S.; Fardiazar, Z.; Sakhinia, E. Cell-free fetal DNA in amniotic fluid supernatant for prenatal diagnosis. Cell. Mol. Biol. (Noisy-le-grand) 2016, 62, 14–17. [Google Scholar]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic fluid: Not just fetal urine anymore. J. Perinatol. 2005, 25, 341–348. [Google Scholar] [CrossRef]
- Larrabee, P.B.; Johnson, K.L.; Lai, C.; Ordovas, J.; Cowan, J.M.; Tantravahi, U.; Bianchi, D.W. Global gene expression analysis of the living human fetus using cell-free messenger RNA in amniotic fluid. JAMA 2005, 293, 836–842. [Google Scholar] [CrossRef]
- Zwemer, L.M.; Bianchi, D.W. The amniotic fluid transcriptome as a guide to understanding fetal disease. Cold Spring Harb. Perspect. Med. 2015, 5, a023101. [Google Scholar] [CrossRef]
- Slonim, D.K.; Koide, K.; Johnson, K.L.; Tantravahi, U.; Cowan, J.M.; Jarrah, Z.; Bianchi, D.W. Functional genomic analysis of amniotic fluid cell-free mRNA suggests that oxidative stress is significant in Down syndrome fetuses. Proc. Natl. Acad. Sci. USA 2009, 106, 9425–9429. [Google Scholar] [CrossRef]
- Koide, K.; Slonim, D.K.; Johnson, K.L.; Tantravahi, U.; Cowan, J.M.; Bianchi, D.W. Transcriptomic analysis of cell-free fetal RNA suggests a specific molecular phenotype in trisomy 18. Hum. Genet. 2011, 129, 295–305. [Google Scholar] [CrossRef]
- Edlow, A.G.; Vora, N.L.; Hui, L.; Wick, H.C.; Cowan, J.M.; Bianchi, D.W. Maternal obesity affects fetal neurodevelopmental and metabolic gene expression: A pilot study. PLoS ONE 2014, 9, e88661. [Google Scholar] [CrossRef]
- Massingham, L.J.; Johnson, K.L.; Scholl, T.M.; Slonim, D.K.; Wick, H.C.; Bianchi, D.W. Amniotic fluid RNA gene expression profiling provides insights into the phenotype of Turner syndrome. Hum. Genet. 2014, 133, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Kamath-Rayne, B.D.; Du, Y.; Hughes, M.; Wagner, E.A.; Muglia, L.J.; DeFranco, E.A.; Whitsett, J.A.; Salomonis, N.; Xu, Y. Systems biology evaluation of cell-free amniotic fluid transcriptome of term and preterm infants to detect fetal maturity. BMC Med. Genom. 2015, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Cho, Y.; Shin, Y.J.; Park, J.; Shim, S.; Jung, Y.; Cha, D. Functional analysis of cell-free RNA using mid-trimester amniotic fluid supernatant in pregnancy with the fetal growth restriction. Medicine (Baltimore) 2018, 97, e9572. [Google Scholar] [CrossRef]
- Jung, Y.W.; Shim, J.I.; Shim, S.H.; Shin, Y.J.; Chang, S.W.; Cha, D.H. Global gene expression analysis of cell-free RNA in amniotic fluid from women destined to develop preeclampsia. Medicine (Baltimore) 2019, 98, e13971. [Google Scholar] [CrossRef] [PubMed]
- Tarca, A.L.; Romero, R.; Pique-Regi, R.; Pacora, P.; Done, B.; Kacerovsky, M.; Bhatti, G.; Jaiman, S.; Hassan, S.S.; Hsu, C.D.; et al. Amniotic fluid cell-free transcriptome: A glimpse into fetal development and placental cellular dynamics during normal pregnancy. BMC Med. Genom. 2020, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Grisaru-Granovsky, S.; Reichman, B.; Lerner-Geva, L.; Boyko, V.; Hammerman, C.; Samueloff, A.; Schimmel, M.S.; Network, I.N. Population-based trends in mortality and neonatal morbidities among singleton, very preterm, very low birth weight infants over 16 years. Early Hum. Dev. 2014, 90, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Hug, L.; Alexander, M.; You, D.; Alkema, L.; Estimation, U.I.-a.G.f.C.M. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: A systematic analysis. Lancet Glob. Health 2019, 7, e710–e720. [Google Scholar] [CrossRef]
- Moutquin, J.M. Classification and heterogeneity of preterm birth. BJOG 2003, 110 (Suppl. 20), 30–33. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Agodi, A. Dietary Folate Intake and Folic Acid Supplements among Pregnant Women from Southern Italy: Evidence from the “Mamma & Bambino” Cohort. Int. J. Environ. Res. Public Health 2020, 17, 638. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Agrifoglio, O.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Magnano San Lio, R.; Panella, M.; Cianci, A.; Agodi, A. The impact of social determinants and lifestyles on dietary patterns during pregnancy: Evidence from the “Mamma & Bambino” study. Ann. Ig. 2019, 31, 81–89. [Google Scholar] [PubMed]
- Maugeri, A.; Barchitta, M.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Magnano San Lio, R.; Agodi, A. Maternal Dietary Patterns Are Associated with Pre-Pregnancy Body Mass Index and Gestational Weight Gain: Results from the “Mamma & Bambino” Cohort. Nutrients 2019, 11, 1308. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; La Rosa, M.C.; Magnano San Lio, R.; Favara, G.; Panella, M.; Cianci, A.; Agodi, A. Single Nucleotide Polymorphisms in Vitamin D Receptor Gene Affect Birth Weight and the Risk of Preterm Birth: Results From the “Mamma & Bambino” Cohort and A Meta-Analysis. Nutrients 2018, 10, 1172. [Google Scholar] [CrossRef]
- Eveleth, P.B.; Andres, R.; Chumlea, W.C.; Eiben, O.; Ge, K.; Harris, T.; Heymsfield, S.B.; Launer, L.J.; Rosenberg, I.H.; Solomons, N.W.; et al. Uses and interpretation of anthropometry in the elderly for the assessment of physical status. Report to the Nutrition Unit of the World Health Organization: The Expert Subcommittee on the Use and Interpretation of Anthropometry in the Elderly. J. Nutr. Health Aging 1998, 2, 5–17. [Google Scholar]
- Magnano San Lio, R.; Maugeri, A.; La Rosa, M.C.; Cianci, A.; Panella, M.; Giunta, G.; Agodi, A.; Barchitta, M. The Impact of Socio-Demographic Factors on Breastfeeding: Findings from the “Mamma & Bambino” Cohort. Medicina (Kaunas) 2021, 57, 103. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; La Mastra, C.; Favara, G.; Giunta, G.; Cianci, A.; Agodi, A. Vaccination Status of Mothers and Children from the ‘Mamma & Bambino’ Cohort. Vaccines 2021, 9, 168. [Google Scholar]
- Maugeri, A.; Barchitta, M.; Magnano San Lio, R.; La Rosa, M.C.; La Mastra, C.; Favara, G.; Ferlito, M.; Giunta, G.; Panella, M.; Cianci, A.; et al. The Effect of Alcohol on Telomere Length: A Systematic Review of Epidemiological Evidence and a Pilot Study during Pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 5038. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Barone, G.; Mazzoleni, P.; Catalfo, A.; De Guidi, G.; Iemmolo, M.G.; Crimi, N.; Agodi, A. Mediterranean Diet and Particulate Matter Exposure Are Associated With LINE-1 Methylation: Results From a Cross-Sectional Study in Women. Front. Genet. 2018, 9, 514. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Fiore, V.; Rosta, G.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Magnano San Lio, R.; Agodi, A. Determinants of Adherence to the Mediterranean Diet: Findings from a Cross-Sectional Study in Women from Southern Italy. Int. J. Environ. Res. Public Health 2019, 16, 2963. [Google Scholar] [CrossRef]
- Gielen, M.; Hageman, G.J.; Antoniou, E.E.; Nordfjall, K.; Mangino, M.; Balasubramanyam, M.; de Meyer, T.; Hendricks, A.E.; Giltay, E.J.; Hunt, S.C.; et al. Body mass index is negatively associated with telomere length: A collaborative cross-sectional meta-analysis of 87 observational studies. Am. J. Clin. Nutr. 2018, 108, 453–475. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2020, 11, 630186. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Mahdi, F. Telomere length variations in aging and age-related diseases. Curr. Aging Sci. 2014, 7, 161–167. [Google Scholar] [CrossRef]
- Giller, A.; Andrawus, M.; Gutman, D.; Atzmon, G. Pregnancy as a model for aging. Ageing Res. Rev. 2020, 62, 101093. [Google Scholar] [CrossRef]
- Gielen, M.; Hageman, G.; Pachen, D.; Derom, C.; Vlietinck, R.; Zeegers, M.P. Placental telomere length decreases with gestational age and is influenced by parity: A study of third trimester live-born twins. Placenta 2014, 35, 791–796. [Google Scholar] [CrossRef]
- Clemente, D.B.P.; Maitre, L.; Bustamante, M.; Chatzi, L.; Roumeliotaki, T.; Fossati, S.; Grazuleviciene, R.; Gützkow, K.B.; Lepeule, J.; Martens, D.S.; et al. Obesity is associated with shorter telomeres in 8 year-old children. Sci. Rep. 2019, 9, 18739. [Google Scholar] [CrossRef]
- Epel, E.S.; Lin, J.; Wilhelm, F.H.; Wolkowitz, O.M.; Cawthon, R.; Adler, N.E.; Dolbier, C.; Mendes, W.B.; Blackburn, E.H. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology 2006, 31, 277–287. [Google Scholar] [CrossRef]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Wang, C.; Nawrot, T.S.; Van Der Stukken, C.; Tylus, D.; Sleurs, H.; Peusens, M.; Alfano, R.; Langie, S.A.S.; Plusquin, M.; Martens, D.S. Different epigenetic signatures of newborn telomere length and telomere attrition rate in early life. Aging (Albany N. Y.) 2021, 13, 14630–14650. [Google Scholar] [CrossRef]
- Skordalakes, E. Telomerase and the benefits of healthy living. Lancet Oncol. 2008, 9, 1023–1024. [Google Scholar] [CrossRef]
- Kamath-Rayne, B.D.; Smith, H.C.; Muglia, L.J.; Morrow, A.L. Amniotic fluid: The use of high-dimensional biology to understand fetal well-being. Reprod. Sci. 2014, 21, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.W.; LeShane, E.S.; Cowan, J.M. Large amounts of cell-free fetal DNA are present in amniotic fluid. Clin. Chem. 2001, 47, 1867–1869. [Google Scholar] [CrossRef]
- Hui, L.; Bianchi, D.W. Cell-free fetal nucleic acids in amniotic fluid. Hum. Reprod. Update 2011, 17, 362–371. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.E.; Ellison, G.T. Practical approaches for estimating prepregnant body weight. J. Nurse Midwifery 1998, 43, 97–101. [Google Scholar] [CrossRef]
- Oken, E.; Taveras, E.M.; Kleinman, K.P.; Rich-Edwards, J.W.; Gillman, M.W. Gestational weight gain and child adiposity at age 3 years. Am. J. Obstet. Gynecol. 2007, 196, 322.e1–322.e8. [Google Scholar] [CrossRef]
- Lun, F.M.; Chiu, R.W.; Leung, T.Y.; Leung, T.N.; Lau, T.K.; Lo, Y.M. Epigenetic analysis of RASSF1A gene in cell-free DNA in amniotic fluid. Clin. Chem. 2007, 53, 796–798. [Google Scholar] [CrossRef]
- Aviv, A.; Hunt, S.C.; Lin, J.; Cao, X.; Kimura, M.; Blackburn, E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011, 39, e134. [Google Scholar] [CrossRef]
- Soubry, A.; Murphy, S.K.; Wang, F.; Huang, Z.; Vidal, A.C.; Fuemmeler, B.F.; Kurtzberg, J.; Murtha, A.; Jirtle, R.L.; Schildkraut, J.M.; et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int. J. Obes. (Lond.) 2015, 39, 650–657. [Google Scholar] [CrossRef]
- Soubry, A.; Schildkraut, J.M.; Murtha, A.; Wang, F.; Huang, Z.; Bernal, A.; Kurtzberg, J.; Jirtle, R.L.; Murphy, S.K.; Hoyo, C. Paternal obesity is associated with IGF2 hypomethylation in newborns: Results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 2013, 11, 29. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall (n = 270) | Reduced GWG (n = 91) | Adequate GWG (n = 101) | Excessive GWG (n = 78) | p-Value a |
|---|---|---|---|---|---|
| Age b | 37.0 (4.0) | 37.0 (4.0) | 38.0 (4.0) | 37.0 (4.0) | 0.699 |
| Gestational age at sampling b | 16.0 (4.0) | 16.0 (4.0) | 16.0 (3.0) | 16.0 (2.0) | 0.953 |
| Educational level (%) | |||||
| Low | 17.8% | 16.5% | 16.8% | 20.5% | 0.038 |
| Medium | 47.8% | 40.7% | 45.5% | 59.0% | |
| High | 34.4% | 42.8% | 37.7% | 20.5% | |
| Working (%) | |||||
| Employment | 57.4% | 54.9% | 61.4% | 55.1% | 0.593 |
| Unemployment | 42.6% | 45.1% | 38.6% | 44.9% | |
| Smokers (%) | 20.5% | 15.4% | 20.0% | 27.3% | 0.216 |
| Having children (% yes) | 67.7% | 64.3% | 76.8% | 59.7% | 0.041 |
| Total energy intake b | 1750 (620) | 1667 (674) | 1752 (545) | 1858 (596) | 0.045 |
| MDS b | 4.0 (2.0) | 4.0 (2.0) | 4.0 (2.0) | 4.0 (2.0) | 0.102 |
| Pre-pregnancy weight b | 61.0 (15.2) | 62.0 (16.0) | 59.0 (13.0) | 64.5 (18.3) | 0.012 |
| Pre-pregnancy BMI b | 22.8 (5.1) | 22.8 (4.8) | 22.0 (3.8) | 25.0 (5.7) | 0.002 |
| Pre-pregnancy BMI categories | |||||
| Underweight | 6.7% | 6.6% | 6.9% | 6.4% | <0.001 |
| Normal weight | 64.1% | 68.1% | 77.2% | 42.3% | |
| Overweight | 17.4% | 9.9% | 8.9% | 37.2% | |
| Obese | 11.9% | 15.4% | 7.0% | 14.1% | |
| Weight at delivery b | 74.0 (15.0) | 68.5 (11.5) | 73.0 (12.7) | 82.0 (15.2) | <0.001 |
| Gestational age at delivery b | 39.0 (2.0) | 38.0 (2) | 39.0 (2) | 39.0 (2.0) | 0.383 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; Giunta, G.; Panella, M.; Cianci, A.; Caruso, M.A.T.; Agodi, A.; Barchitta, M. The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort. Biomedicines 2022, 10, 67. https://doi.org/10.3390/biomedicines10010067
Maugeri A, Magnano San Lio R, La Rosa MC, Giunta G, Panella M, Cianci A, Caruso MAT, Agodi A, Barchitta M. The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort. Biomedicines. 2022; 10(1):67. https://doi.org/10.3390/biomedicines10010067
Chicago/Turabian StyleMaugeri, Andrea, Roberta Magnano San Lio, Maria Clara La Rosa, Giuliana Giunta, Marco Panella, Antonio Cianci, Maria Anna Teresa Caruso, Antonella Agodi, and Martina Barchitta. 2022. "The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort" Biomedicines 10, no. 1: 67. https://doi.org/10.3390/biomedicines10010067
APA StyleMaugeri, A., Magnano San Lio, R., La Rosa, M. C., Giunta, G., Panella, M., Cianci, A., Caruso, M. A. T., Agodi, A., & Barchitta, M. (2022). The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort. Biomedicines, 10(1), 67. https://doi.org/10.3390/biomedicines10010067








