Graphene-Based Materials Prove to Be a Promising Candidate for Nerve Regeneration Following Peripheral Nerve Injury
Abstract
:1. Introduction
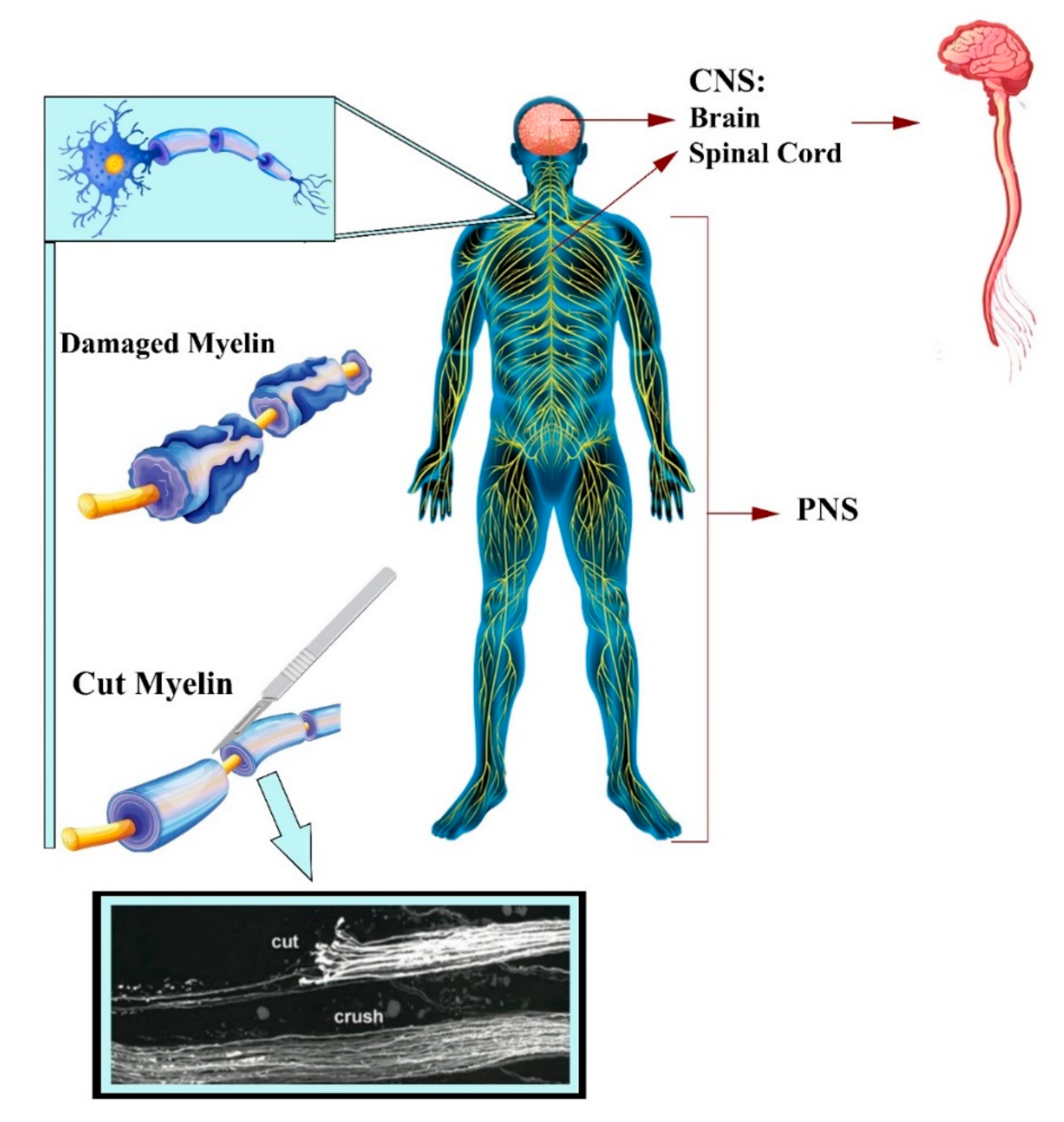


2. Tissue-Engineered Scaffolds with GBM and Neural Tissues
2.1. Electrospun Fibers for Manufacturing 3D Scaffold
2.2. Hydrogels
2.3. 3D Printing and Bioprinting
| Biomaterial(s) | GBMs Concentration | Target and Cell Type | Outcomes | Year | |
|---|---|---|---|---|---|
| Electrospun fiber | Silk/rGO and SF/rGO (Post reduction of Silk/GO) | 5% and 10% | PNS Neuronoma NG108-15 | Conductivity: 4 × 10−5 S/cm (dry), 3 × 10−4 S/cm (hydrated) ↑ metabolic activity and CPM in SF/rGO Neurite extensions up to 100 μm | 2021 [45] |
| Polydopamine/carboxylic GO/PLLA (PDA/CGO/PPy-PLLA) | 0.03% wt | PNS Schwann cells | Surface conductivity: 17.35 S/m Elastic modulus: 260 MPa, ↑ CPM ↑ neural proteins expression 50 mV/cm => ↑∼31% of Schwann cells to align along the direction on mat | 2020 [63] | |
| PCL/G | 1% and 2% | NTE Mouse E12 | ↑ concentration => ↑ fiber diameter, elastic modulus, max stress, and differentiation | 2019 [84] | |
| * GO/ApF/PLCL | 1–2 mg/mL | Sciatic nerve repair, Schwann cells and PC12 | Optimum (mg/mL): 2 ↑ CPM, ↑ PC12 differentiation and FAK expression, ↑ myelination, and repaired 10 mm sciatic nerve defect | 2019 [61] | |
| G/PVA | 1% | PNS PC12 | Orientation Index: aligned scaffold: 28.7° and native nerve: 26.8°, hydrophile, strength: aligned: 29.6 ± 6.7 MPa, + ES => ↑ CPM (aligned > random) | 2018 [58] | |
| G/Sodium alginate/PVA (G/AP) | (0.5–5)% wt | NTE PC12 | Optimum: 1%, ↑ concentration => ↑contact angle, degradation, conductivity (1%): 800 μs, ↑ CPM (1.4 times in 1%) | 2017 [62] | |
| Polypyrrole/G/PLGA (PPy-G/PLGA) | 1 and 6 % PPy-G | Optical NR (Glaucoma) RGCs | Well aligned, +ES => ↑ cell length ↑ cell viability and neurite outgrowth Anti-aging ability of RGCs | 2016 [57] | |
| Hydrogel | * Grafted GO/PEI Core–shell microfiber arrayed hydrogel: chemokine (SDF-1α) and GO-PEI/pDNAs-bFGF microparticles | Mass ratio (GO-PEI): 1:10, 1:40, and 1:70 | Recruit and stimulate the neural-like differentiation BM-MSCs | Optimum ratio: GO-PEI 1:10, ↑ neuronal differentiation, controlled delivering the CXCL12 and GO-PEI/pDNAs-bFGF => endogenous stem cell therapy | 2021 [69] |
| * GO/diacerein-terminated 4-armed polyethylene glycol | 2.5, 5.0, and 7.5 mg/mL | SCI | Optimum concen. (mg/mL): 5.0, Diacerein => ↓↓ inflammatory response and ↓↓, inhibitory microenvironment, conductivity (7.4 S/m) => ↑ neuron growth and axon remyelination | 2020 [70] | |
| Polyacrylamide/GO/gelatin/sodium alginate (PAM/GO/Gel/SA) | 0.5% and 1% | PNS Schwann cells | Optimum %: 0.5, ↑ protein adsorption ↑ NGF, ↑ cytoskeleton related genes expression | 2018 [71] | |
| GO acrylate (GOa) and CNTpega embedded in OPF hydrogel MTAC => rGOaCNTpega-OPF-MTAC | 0.1% w/v | Neuronal proliferation and differentiation PC12 cells | Conductivity: 5.75 × 10−3 S/m ↑ differentiation (+NGF) Robust neurite development Conductive nerve conduits with surficial positive charges | 2017 [72] | |
| GO/polyacrylamide | (0.5–3)% w/v | PNS Schwann cells | Optimum %: 0.4% GO, ↑ biofactors release and larger matrix adsorption | 2016 [73] | |
| Aerogel | Hollow GO/gelatin | 5 mg/mL | NR and CNS P19 mouse cells | Prevent the fibroglial tissue formation Effective differentiation into neural cells | 2020 [85] |
| GO/SA and rGO/SA | 0.5, 1, 3, and 5 mg/mL | CNS NI | ↑ porous and electroconductive Similar MP to CNS (↑ rGO) | 2019 [86] | |
| Foam | G foam/PCL mesoporous coating | 1–7.3 wt% | NTE NI | Conductivity and MP (Young’s modulus): 3.2–108.7 S/m and 0.62–4.50 MPa | 2021 [87] |
| Bioprinting | PU/G or PU/GO (G and GO coated by Pluronic P123) | 10, 25, and 50 ppm | CNS NSCs | Optimum ppm: 25 PU/G > PU/GO: Suitable cell survival rate ↑ CPM ↑ differentiation ↑ oxygen metabolism and ATP production | 2017 [81] |
| gelatin methacrylamide (GelMA)/G | 1 mg/mL | NR PC12 cells (biocompatibility) and NSCs (cell encapsulation) | ↑ CPM ↑ neuron differentiation and neurites elongation | 2016 [82] |
2.4. Conduits for Nerve Guide
| Biomaterial(s) | GBMs Percentage | Target and Cell Type | Animal Model | Outcomes | Year, Ref | |
|---|---|---|---|---|---|---|
| Film (membrane) | PLCL/GO | 1 mg/mL | PNS Schwann cells | SDR | Elastic modulus: 125 MPa ↑ directional migration of single cells along the micropatterns. Macrophage differentiation into the M2. | 2020 [99] |
| PCL/carbon and G nanoparticles | 0.5% | PNS NI | Lewis rats | ↑ CPM ↑ Flexibility => ↑ stump positioning accuracy ↑ myelinated axons Muscle atrophy protection (12 weeks) | 2017 [100] | |
| Electrospun fiber | PCL/collagen/G | 0.5%, 1%, 1.5%, and 2% | Sciatic nerve repair MSCs | SDR | Well aligned Optimum %: 1 ↑ concentration => ↑ conductivity; Conductivity (1%): 5.27 × 10−6 S/m ↑ concentration => ↓ tensile strength and elastic modulus | 2020 [54] |
| Dual-electrospun: PCL, gelatin, and polyaniline/G (PAG) | 0–3% wt | PNS BM-MSCs | NI | Optimum: 2% PAG => ↑ CPM Conductivity: 10.8 × 10−5 S/cm | 2020 [101] | |
| Foam | G/PCL | 2% wt | PNS PC12 cells | NI | Elastic modulus: 2.67 MPa Conductivity: 25 S/m ↑ porosity ↑ cell proliferation and extension No cytotoxicity | 2021 [102] |
| PVDF/GO | 0.5%, 1%, 3%, and 5% wt | PNS PC12 cells | NI | ↑ piezoelectricity and electrical conductivity High flexibility => easy and appropriate NGC formation ↑ CPM (particularly 0.5% and 1%) | 2019 [103] | |
| Hydrogel | GMT/ hydrogel with netrin-1 | 0.05% | PNS Schwann cells | SDR | Elastic modulus: 720 kPa Conductivity: 6.8 S/m GMT => orientation of PNR, O2 and nutrition transport. ↑ levels of S100 and Sox10 (↑PNR). | 2021 [95] |
| GO/GelMA then chemically reduced => r(GO/GelMA) | 0.1% | PC12 cells | SDR | Conductivity: 4.4 × 10−3 S/m (GO/GelMA) and 8.7 × 10−3 S/m (rGO/GelMA) ↑ neuritogenesis: r(GO/GelMA) > GO/GelMA ↑ PNR ↑ regrowth with myelination | 2020 [97] | |
| Chitosan/oxidized hydroxyethyl cellulose (CS/OHEC) hydrogel loaded with asiaticoside liposome and rGO | 0%, 1%, 2%, 4%, 6%, 8%, and 10% | PNS PC12 cells | NI | Optimum: 8% Conductivity (5.27 × 10−4 S/cm) + ES => ↑ differentiation and ↑ CPM. Asiaticoside released => no growth and collagen secretion of fibroblasts => No scars for NR. | 2020 [93] | |
| Cryogel | Amino-functionalized G/collagen | 0.1%, 0.5%, and 1% w/v | SCI BM-MSCs | Organotypic spinal explant culture (spinal cord from SDR) | Optimum %: 0.5% Conductivity: 3.8 × 10−3 S/cm and mechanical cues: 100–347 kPa Young Modulus => SC and NR ↑ ATP secretion ↑ MAP-2 kinase and β-tubulin III expression ↑ CD90 and CD73 gene expression | 2021 [98] |
| Multiple techniques | Electrospinning, molding, and freeze drying: ApF/PLCL/GO | 2% | PNS Schwann cells and PC12 cells (differentiation) | SDR | Effective guiding interface => ↑ CPM and ↑ myelination Tailored degradation and complete degradation at 12 weeks ↑ axonal regrowth and remyelination | 2020 [96] |
| Aligned electrospun and film: carboxylic GO-polypyrrole/poly-L-lactic acid (C-GO/PPy/PLLA) | 0.05% w/v | PNSPC12 and L929 fibroblasts | SDR | ↑ CPMConductivity: ~4.6 S/cm (after 4 weeks of immersion) + ES => re-innervated gastrocnemius muscle, nerve conduction, and neurite alignment (59%) at 12 weeks | 2019 [104] | |
| Molding, phase separation (conduit), and 3D printing (circuit): Gelatin and G/PLA filament | Not reported | PNI MSCs | NI | + ES => ↑ transdifferentiation into Schwann cell-like phenotypes ↑ CPM | 2019 [83] | |
| 3D printing-film: Polydopamine (PDA)/RGD and single-layered G (SG) or multi-layered G (MG)/PCL | 1% | PNS Schwann cells | SDR | Conductivity: 8.92 × 10−3 S/cm (SG) and 6.37 × 10−3 S/cm (MG) Elastic modulus: 68.74 MPa (SG) and 58.63 MPa (MG) ↑ neural expression (SG>MG) ↑ axonal regrowth and remyelination | 2018 [25] | |
| Molding/jet spraying/3D printing: GO/PCL | 0.5%, 1%, 2%, and 4% | PNS Schwann cell | SDR | Optimum %: 1% Conductivity: 4.55 × 10−5 S/cm Elastic modulus: 48.32 MPa ↑ CPM Neural characteristics maintenance Angiogenic capability | 2018 [28] |
2.5. Associated Challenges of GBMs in Clinical Studies
2.6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ADSCs | Adipose-derived stem cells |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| DBS | Dodecyl benzenesulfonate |
| DRG | Dorsal root ganglion |
| ECM | Extracellular matrix |
| ES | Electrical stimulation |
| FGO | Functionalized graphene oxide |
| G | Graphene |
| GBMs | Graphene-based materials |
| GBN | Graphene-based nano scaffolds |
| GelMA | Gelatinmethacryloyl |
| GFAP | Glial fibrillary acidic protein |
| GMT | Graphene mesh tube |
| GO | Graphene oxide |
| iPSCs | Induced pluripotent stem cells |
| M1 | Direct macrophage inflammatory |
| M2 | Anti-inflammatory |
| MAP-2 | Microtubule-associated protein 2 |
| MMP | Matrix metalloproteinase |
| MSCs | Mesenchymal stem cells |
| MTAC | [2-(methacryloyloxy)ethyl]trimethylammonium chloride |
| NCSCs | Neural crest stem cells |
| NGC | Nerve guidance conduit |
| NPs | Nanoparticles |
| NTE | Nerve tissue engineering |
| OPF | Oligo(poly(ethylene glycol) fumarate |
| PCL | Polycaprolactone |
| PEI | Polyethylenimine |
| PHA | Polyhydroxyl alkanoate |
| PLCL | Poly(D,L-lactide-co-caprolactone) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLLA | Poly-l-lactic acid |
| PNI | Peripheral nerve injury |
| PNS | Peripheral nervous system |
| rGO | Reduced graphene oxide |
| ROS | Reactive oxygen species |
| SDF-1α | Stromal cell-derived factor-1α |
| TE | Tissue engineering |
References
- Ahadian, S.; Obregón, R.; Ramón-Azcón, J.; Salazar, G.; Shiku, H.; Ramalingam, M.; Matsue, T. Carbon nanotubes and graphene-based nanomaterials for stem cell differentiation and tissue regeneration. J. Nanosci. Nanotechnol. 2016, 16, 8862–8880. [Google Scholar] [CrossRef]
- Aydin, T.; Gurcan, C.; Taheri, H.; Yilmazer, A. Graphene based materials in neural tissue regeneration. Adv. Exp. Med. Biol. 2018, 1107, 129–142. [Google Scholar] [PubMed]
- Reddy, S.; Xu, X.; Guo, T.; Zhu, R.; He, L.; Ramakrishana, S. Allotropic carbon (graphene oxide and reduced graphene oxide) based biomaterials for neural regeneration. Curr. Opin. Biomed. Eng. 2018, 6, 120–129. [Google Scholar] [CrossRef]
- Soman, S.S.; Vijayavenkataraman, S. Perspectives on 3d bioprinting of peripheral nerve conduits. Int. J. Mol. Sci. 2020, 21, 5792. [Google Scholar] [CrossRef]
- Ko, C.-H.; Shie, M.-Y.; Lin, J.-H.; Chen, Y.-W.; Yao, C.-H.; Chen, Y.-S. Biodegradable bisvinyl sulfonemethyl-crosslinked gelatin conduit promotes regeneration after peripheral nerve injury in adult rats. Sci. Rep. 2017, 7, 17489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrintaj, P.; Zangene, E.; Manouchehri, S.; Amirabad, L.M.; Baheiraei, N.; Hadjighasem, M.R.; Farokhi, M.; Ganjali, M.R.; Walker, B.W.; Saeb, M.R. Conductive biomaterials as nerve conduits: Recent advances and future challenges. Appl. Mater. Today 2020, 20, 100784. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, S.; Shao, H.; Hu, X.; Zhu, B.; Zhang, Y. Graphene trapped silk scaffolds integrate high conductivity and stability. Carbon N. Y. 2019, 148, 16–27. [Google Scholar] [CrossRef]
- Adiguzel, E.; Yaşar, E.; Tecer, D.; Güzelküçük, Ü.; Taşkaynatan, M.A.; Kesikburun, S.; Özgül, A. Peripheral nerve injuries: Long term follow-up results of rehabilitation. J. Back Musculoskelet. Rehabil. 2016, 29, 367–371. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Jalessi, M.; Jameie, S.B.; Khanmohammadi, M.; Bagher, Z.; Namjoo, Z.; Davachi, S.M. More attention on glial cells to have better recovery after spinal cord injury. Biochem. Biophys. Rep. 2021, 25, 100905. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C. Dedifferentiation: Inspiration for devising engineering strategies for regenerative medicine. NPJ Regen. Med. 2020, 5, 14. [Google Scholar] [CrossRef]
- Philips, C.; Cornelissen, M.; Carriel, V. Evaluation methods as quality control in the generation of decellularized peripheral nerve allografts. J. Neural Eng. 2018, 15, 21003. [Google Scholar] [CrossRef]
- Aleemardani, M.; Bagher, Z.; Farhadi, M.; Chahsetareh, H.; Najafi, R.; Eftekhari, B.; Seifalian, A. Can Tissue Engineering Bring Hope to the Development of Human Tympanic Membrane? Tissue Eng. Part B Rev. 2021, 27, 572–589. [Google Scholar] [CrossRef]
- Najafloo, R.; Majidi, J.; Asghari, A.; Aleemardani, M.; Kamrava, S.K.; Simorgh, S.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Mechanism of Anosmia Caused by Symptoms of COVID-19 and Emerging Treatments. ACS Chem. Neurosci. 2021, 12, 3795–3805. [Google Scholar] [CrossRef]
- Bagher, Z.; Asgari, N.; Bozorgmehr, P.; Kamrava, S.K.; Alizadeh, R.; Seifalian, A. Will Tissue-Engineering Strategies Bring New Hope for the Reconstruction of Nasal Septal Cartilage? Curr. Stem Cell Res. Ther. 2020, 15, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Zare, P.; Aleemardani, M.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene Oxide: Opportunities and Challenges in Biomedicine. Nanomaterials 2021, 11, 1083. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R.; Ramakrishna, S.; Mozafari, M. Biomaterials selection for neuroprosthetics. Curr. Opin. Biomed. Eng. 2018, 6, 99–109. [Google Scholar] [CrossRef]
- Amani, H.; Mostafavi, E.; Arzaghi, H.; Davaran, S.; Akbarzadeh, A.; Akhavan, O.; Pazoki-Toroudi, H.; Webster, T.J. Three-dimensional graphene foams: Synthesis, properties, biocompatibility, biodegradability, and applications in tissue engineering. ACS Biomater. Sci. Eng. 2018, 5, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Steel, E.M.; Sundararaghavan, H.G. Electrically conductive materials for nerve regeneration. In Neural Engineering; Springer: Berlin/Heidelberg, Germany, 2016; pp. 145–179. [Google Scholar]
- Xiaoli, F.; Qiyue, C.; Weihong, G.; Yaqing, Z.; Chen, H.; Junrong, W.; Longquan, S. Toxicology data of graphene-family nanomaterials: An update. Arch. Toxicol. 2020, 94, 1915–1939. [Google Scholar] [CrossRef]
- Devasena, T.; Francis, A.P.; Ramaprabhu, S. Toxicity of Graphene: An Update. Rev. Environ. Contam. Toxicol. 2021, 259, 51–76. [Google Scholar]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verre, A.F.; Faroni, A.; Iliut, M.; Silva, C.; Muryn, C.; Reid, A.J.; Vijayaraghavan, A. Improving the glial differentiation of human Schwann-like adipose-derived stem cells with graphene oxide substrates. Interface Focus 2018, 8, 20180002. [Google Scholar] [CrossRef] [PubMed]
- Henna, T.K.; Nivitha, K.P.; Raphey, V.R.; Sabu, C.; Pramod, K. Functionalized Graphene for Drug Delivery Applications. In Graphene Functionalization Strategies; Springer: Berlin/Heidelberg, Germany, 2019; pp. 247–278. [Google Scholar]
- Portolés, M.T.; Serrano, M.C. Potentiality of Graphene-Based Materials for Neural Repair. In Graphene-Based Materials in Health and Environment; Springer: Berlin/Heidelberg, Germany, 2016; pp. 159–190. [Google Scholar]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Klausen, L.H.; Chen, M.; Dong, M. Electroactive Scaffolds for Neurogenesis and Myogenesis: Graphene-Based Nanomaterials. Small 2018, 14, 801983. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Song, J.; Zhao, X.; Chen, W.; Ouyang, Y.; Yuan, W.; Fan, C. 3D fabrication with integration molding of a graphene oxide/polycaprolactone nanoscaffold for neurite regeneration and angiogenesis. Adv. Sci. 2018, 5, 1700499. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, N.P.; Lottner, M.; Giugliano, M.; Matruglio, A.; D’Amico, F.; Prato, M.; Garrido, J.A.; Ballerini, L.; Scaini, D. Single-layer graphene modulates neuronal communication and augments membrane ion currents. Nat. Nanotechnol. 2018, 13, 755–764. [Google Scholar] [CrossRef]
- Dixon, A.R.; Jariwala, S.H.; Bilis, Z.; Loverde, J.R.; Pasquina, P.F.; Alvarez, L.M. Bridging the gap in peripheral nerve repair with 3D printed and bioprinted conduits. Biomaterials 2018, 186, 44–63. [Google Scholar] [CrossRef]
- Convertino, D.; Fabbri, F.; Mishra, N.; Mainardi, M.; Cappello, V.; Testa, G.; Capsoni, S.; Albertazzi, L.; Luin, S.; Marchetti, L. Graphene promotes axon elongation through local stall of Nerve Growth Factor signaling endosomes. Nano Lett. 2020, 20, 3633–3641. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, Z.; Sun, M.; Fang, S.; Li, H. A study on graphene composites for peripheral nerve injury repair under electrical stimulation. RSC Adv. 2019, 9, 28627–28635. [Google Scholar] [CrossRef] [Green Version]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [Green Version]
- Mahar, M.; Cavalli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, J. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- He, Z.; Zang, H.; Zhu, L.; Huang, K.; Yi, T.; Zhang, S.; Cheng, S. An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int. J. Nanomed. 2019, 14, 721. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Holloway, J.L. Harnessing the immune response to improve functional healing. Sci. Transl. Med. 2018, 10, eaav3889. [Google Scholar] [CrossRef]
- Shende, P.; Pathan, N. Potential of carbohydrate-conjugated graphene assemblies in biomedical applications. Carbohydr. Polym. 2021, 255, 117385. [Google Scholar] [CrossRef]
- Papi, M. Graphene-Based Materials: Biological and Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 672. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Barajaa, M.; Tahmasbi Rad, A.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Healthc. Mater. 2021, 10, 2001414. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Aleemardani, M.; Solouk, A.; Akbari, S.; Dehghan, M.M.; Moeini, M. Silk-derived oxygen-generating electrospun patches for enhancing tissue regeneration: Investigation of calcium peroxide role and its effects in controlled oxygen delivery. Materialia 2020, 14, 100877. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, M.; Li, Y.; Guo, Z.; Li, H. Reduced graphene oxide-coated electrospun fibre: Effect of orientation, coverage and electrical stimulation on Schwann cells behavior. J. Mater. Chem. B 2021, 9, 2656–2665. [Google Scholar] [CrossRef]
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C 2021, 119, 111632. [Google Scholar] [CrossRef]
- Aval, N.A.; Emadi, R.; Valiani, A.; Kharaziha, M.; Karimipour, M.; Rahbarghazi, R. Nano-featured poly (lactide-co-glycolide)-graphene microribbons as a promising substrate for nerve tissue engineering. Compos. Part B Eng. 2019, 173, 106863. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Niu, C.; Wei, Z.; Shi, J.; Li, G.; Yang, Y.; Wang, H. A new electrospun graphene-silk fibroin composite scaffolds for guiding Schwann cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 2171–2185. [Google Scholar] [CrossRef]
- Jaswal, R.; Shrestha, S.; Shrestha, B.K.; Kumar, D.; Park, C.H.; Kim, C.S. Nanographene enfolded AuNPs sophisticatedly synchronized polycaprolactone based electrospun nanofibre scaffold for peripheral nerve regeneration. Mater. Sci. Eng. C 2020, 116, 111213. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, G.; Liu, X.; Yang, M.; Xing, S.; Du, Y.; Xiong, X. Synthesis of an electrospun PHA/RGO/Au scaffold for peripheral nerve regeneration: An in vitro study. Appl. Nanosci. 2020, 10, 687–694. [Google Scholar] [CrossRef]
- Du, J.; Zhen, G.; Chen, H.; Zhang, S.; Qing, L.; Yang, X.; Lee, G.; Mao, H.-Q.; Jia, X. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials 2018, 181, 347–359. [Google Scholar] [CrossRef]
- Zhao, S.; Mehta, A.S.; Zhao, M. Biomedical applications of electrical stimulation. Cell. Mol. Life Sci. 2020, 77, 2681–2699. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019, 84, 98–113. [Google Scholar] [CrossRef]
- Shang, L.; Huang, Z.; Pu, X.; Yin, G.; Chen, X. Preparation of graphene oxide-doped polypyrrole composite films with stable conductivity and their effect on the elongation and alignment of neurite. ACS Biomater. Sci. Eng. 2019, 5, 1268–1278. [Google Scholar] [CrossRef]
- Dong, C.; Qiao, F.; Hou, W.; Yang, L.; Lv, Y. Graphene-based conductive fibrous scaffold boosts sciatic nerve regeneration and functional recovery upon electrical stimulation. Appl. Mater. Today 2020, 21, 100870. [Google Scholar] [CrossRef]
- Mohseni, M.; Ahmad Ramazani, S.A.; Shirazi, F.H.; Nemati, N.H. Preparation and characterization of self-electrical stimuli conductive gellan based nano scaffold for nerve regeneration containing chopped short spun nanofibers of PVDF/MCM41 and polyaniline/graphene nanoparticles: Physical, mechanical and morphological. Int. J. Biol. Macromol. 2021, 167, 881–893. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Z.; Pu, X.; Shang, L.; Yin, G.; Chen, X.; Cheng, S. Fabrication of carboxylic graphene oxide-composited polypyrrole film for neurite growth under electrical stimulation. Front. Mater. Sci. 2019, 13, 258–267. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, B.; Liu, X.; Li, X.; Zeng, C.; Shi, H.; Xu, X.; Lin, T.; Dai, L.; Liu, Y. Aligned nanofibers from polypyrrole/graphene as electrodes for regeneration of optic nerve via electrical stimulation. ACS Appl. Mater. Interfaces 2016, 8, 6834–6840. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Fathi, M.; Larson, B.L.; Giatsidis, G.; Masoumi, N. Anisotropic architecture and electrical stimulation enhance neuron cell behaviour on a tough graphene embedded PVA: Alginate fibrous scaffold. RSC Adv. 2018, 8, 6381–6389. [Google Scholar] [CrossRef] [Green Version]
- Girao, A.F.; Sousa, J.; Dominguez-Bajo, A.; Gonzalez-Mayorga, A.; Bdikin, I.; Pujades-Otero, E.; Casan-Pastor, N.; Hortigüela, M.J.; Otero-Irurueta, G.; Completo, A. 3D Reduced Graphene Oxide Scaffolds with a Combinatorial Fibrous-Porous Architecture for Neural Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 38962–38975. [Google Scholar] [CrossRef]
- Guo, Z.; Liang, J.; Ankone, M.J.K.; Poot, A.A.; Grijpma, D.W.; Chen, H. Fabrication of poly (trimethylene carbonate)/reduced graphene oxide-graft-poly (trimethylene carbonate) composite scaffolds for nerve regeneration. Biomed. Mater. 2019, 14, 24104. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, W.; Chen, L.; Zhu, T.; Shen, W.; Fan, C.; Wang, H.; Mo, X. Enhancement of Schwann cells function using graphene-oxide-modified nanofiber scaffolds for peripheral nerve regeneration. ACS Biomater. Sci. Eng. 2019, 5, 2444–2456. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon N. Y. 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Pu, X.; Chen, X.; Yin, G.; Wang, Y.; Miao, D.; Fan, J.; Mu, J. Polydopamine/carboxylic graphene oxide-composited polypyrrole films for promoting adhesion and alignment of Schwann cells. Colloids Surf. B Biointerfaces 2020, 191, 110972. [Google Scholar] [CrossRef]
- Diban, N.; Sánchez-González, S.; Lázaro-Díez, M.; Ramos-Vivas, J.; Urtiaga, A. Facile fabrication of poly (ε-caprolactone)/graphene oxide membranes for bioreactors in tissue engineering. J. Memb. Sci. 2017, 540, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Soltani, S.; Ebrahimian-Hosseinabadi, M.; Kharazi, A.Z. Chitosan/graphene and poly (D, L-lactic-co-glycolic acid)/graphene nano-composites for nerve tissue engineering. Tissue Eng. Regen. Med. 2016, 13, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Ghafaralahi, S.; Ebrahimian-Hosseinabadi, M.; Zargar Kharazi, A. Poly (glycerol-sebacate)/poly (caprolactone)/graphene nanocomposites for nerve tissue engineering. J. Bioact. Compat. Polym. 2018, 33, 529–542. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, X.; Song, J.; Chen, W.; Chen, S.; Jin, Y.; Ouyang, Y.; Yuan, W.-E.; Fan, C. Preclinical assessment on neuronal regeneration in the injury-related microenvironment of graphene-based scaffolds. NPJ Regen. Med. 2021, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Aleemardani, M.; Solouk, A.; Mirzadeh, H.; Teuschl, A.H.; Redl, H. Crosslinking strategies for silk fibroin hydrogels: Promising biomedical materials. Biomed. Mater. 2021, 16, 22004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, T.-J.; Tan, M.-H.; Xu, X.-H.; Huang, Y.-F.; Peng, L.-H. Smart graphene-based hydrogel promotes recruitment and neural-like differentiation of bone marrow derived mesenchymal stem cells in rat skin. Biomater. Sci. 2021, 9, 2146–2161. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Jin, J.; Dong, J.; Li, L.; Xue, B.; Wang, W.; Jiang, Q.; Cao, Y. Injectable, anti-inflammatory and conductive hydrogels based on graphene oxide and diacerein-terminated four-armed polyethylene glycol for spinal cord injury repair. Mater. Des. 2020, 196, 109092. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Niu, C.; Zhang, L.; Li, G.; Yang, Y. Construction of polyacrylamide/graphene oxide/gelatin/sodium alginate composite hydrogel with bioactivity for promoting Schwann cells growth. J. Biomed. Mater. Res. Part A 2018, 106, 1951–1964. [Google Scholar] [CrossRef]
- Liu, X.; Miller, A.L.; Park, S.; Waletzki, B.E.; Zhou, Z.; Terzic, A.; Lu, L. Functionalized carbon nanotube and graphene oxide embedded electrically conductive hydrogel synergistically stimulates nerve cell differentiation. ACS Appl. Mater. Interfaces 2017, 9, 14677–14690. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.; Zhang, L.; Gao, M.; Kong, Y.; Yang, Y. Preparation of graphene oxide/polyacrylamide composite hydrogel and its effect on Schwann cells attachment and proliferation. Colloids Surf. B Biointerfaces 2016, 143, 547–556. [Google Scholar] [CrossRef]
- Hsieh, T.; Huang, W.; Kang, Y.; Chu, C.; Liao, W.; Chen, Y.; Chen, S. Neurotensin-conjugated reduced graphene oxide with multi-stage near-infrared-triggered synergic targeted neuron gene transfection in vitro and in vivo for neurodegenerative disease therapy. Adv. Healthc. Mater. 2016, 5, 3016–3026. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, M.; Knight, A.M.; Lu, L.; Windebank, A.J.; Yaszemski, M.J. Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials 2009, 30, 3874–3881. [Google Scholar] [CrossRef] [Green Version]
- Madigan, N.N.; Chen, B.K.; Knight, A.M.; Rooney, G.E.; Sweeney, E.; Kinnavane, L.; Yaszemski, M.J.; Dockery, P.; O’Brien, T.; McMahon, S.S. Comparison of cellular architecture, axonal growth, and blood vessel formation through cell-loaded polymer scaffolds in the transected rat spinal cord. Tissue Eng. Part A 2014, 20, 2985–2997. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.K.; Knight, A.M.; Madigan, N.N.; Gross, L.; Dadsetan, M.; Nesbitt, J.J.; Rooney, G.E.; Currier, B.L.; Yaszemski, M.J.; Spinner, R.J. Comparison of polymer scaffolds in rat spinal cord: A step toward quantitative assessment of combinatorial approaches to spinal cord repair. Biomaterials 2011, 32, 8077–8086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binan, L.; Ajji, A.; De Crescenzo, G.; Jolicoeur, M. Approaches for neural tissue regeneration. Stem Cell Rev. Rep. 2014, 10, 44–59. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P. Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Huang, Q.; Cai, Y.; Yang, X.; Li, W.; Pu, H.; Liu, Z.; Liu, H.; Tamtaji, M.; Xu, F.; Sheng, L. Graphene foam/hydrogel scaffolds for regeneration of peripheral nerve using ADSCs in a diabetic mouse model. Nano Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Huang, C.T.; Kumar Shrestha, L.; Ariga, K.; Hsu, S.H. A graphene-polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells. J. Mater. Chem. B 2017, 5, 8854–8864. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Harris, B.T.; Zhang, L.G. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4185–4188. [Google Scholar]
- Uz, M.; Donta, M.; Mededovic, M.; Sakaguchi, D.S.; Mallapragada, S.K. Development of gelatin and graphene-based nerve regeneration conduits using three-dimensional (3D) printing strategies for electrical transdifferentiation of mesenchymal stem cells. Ind. Eng. Chem. Res. 2019, 58, 7421–7427. [Google Scholar] [CrossRef] [Green Version]
- Ginestra, P. Manufacturing of polycaprolactone-Graphene fibers for nerve tissue engineering. J. Mech. Behav. Biomed. Mater. 2019, 100, 103387. [Google Scholar] [CrossRef] [Green Version]
- Zeinali, K.; Khorasani, M.T.; Rashidi, A.; Daliri Joupari, M. Preparation and characterization of graphene oxide aerogel/gelatin as a hybrid scaffold for application in nerve tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 674–683. [Google Scholar] [CrossRef]
- Mansouri, N.; Al-Sarawi, S.F.; Mazumdar, J.; Losic, D. Advancing fabrication and properties of three-dimensional graphene–alginate scaffolds for application in neural tissue engineering. RSC Adv. 2019, 9, 36838–36848. [Google Scholar] [CrossRef] [Green Version]
- Tolou, N.B.; Salimijazi, H.; Dikonimos, T.; Faggio, G.; Messina, G.; Tamburrano, A.; Aurora, A.; Lisi, N. Fabrication of 3D monolithic graphene foam/polycaprolactone porous nanocomposites for bioapplications. J. Mater. Sci. 2021, 56, 5581–5594. [Google Scholar] [CrossRef]
- Allbright, K.O.; Bliley, J.M.; Havis, E.; Kim, D.; Dibernardo, G.A.; Grybowski, D.; Waldner, M.; James, I.B.; Sivak, W.N.; Rubin, J.P. Delivery of adipose-derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle Nerve 2018, 58, 251–260. [Google Scholar] [CrossRef]
- Carriel, V.; Garrido-Gómez, J.; Hernández-Cortés, P.; Garzón, I.; García-García, S.; Sáez-Moreno, J.A.; del Carmen Sanchez-Quevedo, M.; Campos, A.; Alaminos, M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J. Neural Eng. 2013, 10, 26022. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Wieringa, P.; Moroni, L.; Navarro, X.; Valle, J. Del PEOT/PBT guides enhance nerve regeneration in long gap defects. Adv. Healthc. Mater. 2017, 6, 1600298. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, C.; Hai, B.; Ma, T.; Zhang, W.; Tan, J.; Fu, X.; Wang, H.; Xu, Y.; Song, C. Chitosan conduits filled with simvastatin/Pluronic F-127 hydrogel promote peripheral nerve regeneration in rats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 787–799. [Google Scholar] [CrossRef]
- Zheng, F.; Li, R.; He, Q.; Koral, K.; Tao, J.; Fan, L.; Xiang, R.; Ma, J.; Wang, N.; Yin, Y. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro. Mater. Sci. Eng. C 2020, 109, 110560. [Google Scholar] [CrossRef]
- Qing, L.; Chen, H.; Tang, J.; Jia, X. Exosomes and their microRNA cargo: New players in peripheral nerve regeneration. Neurorehabil. Neural Repair 2018, 32, 765–776. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Cai, Y.; Zhang, X.; Liu, J.; Liu, Z.; Li, B.; Wong, H.; Xu, F.; Sheng, L.; Sun, D. Aligned graphene mesh-supported double network natural hydrogel conduit loaded with netrin-1 for peripheral nerve regeneration. ACS Appl. Mater. Interfaces 2021, 13, 112–122. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Wang, H.; Wang, Y.; Zhang, K.; Fan, C.; Wang, H.; Mo, X. Biomimetic and hierarchical nerve conduits from multifunctional nanofibers for guided peripheral nerve regeneration. Acta Biomater. 2020, 117, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeon, J.; Kim, B.; Lee, M.S.; Park, S.; Lim, J.; Yi, J.; Lee, H.; Yang, H.S.; Lee, J.Y. Electrically Conductive Hydrogel Nerve Guidance Conduits for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2003759. [Google Scholar] [CrossRef]
- Agarwal, G.; Kumar, N.; Srivastava, A. Highly elastic, electroconductive, immunomodulatory graphene crosslinked collagen cryogel for spinal cord regeneration. Mater. Sci. Eng. C 2021, 118, 111518. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, Y.; Duan, Y.; Yu, X.; Shi, H.; Nakkala, J.R.; Zuo, X.; Hong, L.; Mao, Z.; Gao, C. Surface-Anchored Graphene Oxide Nanosheets on Cell-Scale Micropatterned Poly (D, L-lactide-co-caprolactone) Conduits Promote Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 7915–7930. [Google Scholar] [CrossRef]
- Assaf, K.; Leal, C.V.; Derami, M.S.; de Rezende Duek, E.A.; Ceragioli, H.J.; de Oliveira, A.L.R. Sciatic nerve repair using poly (ε-caprolactone) tubular prosthesis associated with nanoparticles of carbon and graphene. Brain Behav. 2017, 7, e00755. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Ramazani SaadatAbadi, A.; Mashayekhan, S.; Sanaei, R. Conductive multichannel PCL/gelatin conduit with tunable mechanical and structural properties for peripheral nerve regeneration. J. Appl. Polym. Sci. 2020, 137, 49219. [Google Scholar] [CrossRef]
- Tolou, N.B.; Salimijazi, H.; Kharaziha, M.; Faggio, G.; Chierchia, R.; Lisi, N. A three-dimensional nerve guide conduit based on graphene foam/polycaprolactone. Mater. Sci. Eng. C 2021, 126, 112110. [Google Scholar] [CrossRef]
- Abzan, N.; Kharaziha, M.; Labbaf, S. Development of three-dimensional piezoelectric polyvinylidene fluoride-graphene oxide scaffold by non-solvent induced phase separation method for nerve tissue engineering. Mater. Des. 2019, 167, 107636. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Huang, Z.; Pu, X.; Shang, L.; Yin, G.; Xue, C. Preparation of carboxylic graphene oxide-composited polypyrrole conduits and their effect on sciatic nerve repair under electrical stimulation. J. Biomed. Mater. Res. Part A 2019, 107, 2784–2795. [Google Scholar] [CrossRef]
- Bei, H.P.; Yang, Y.; Zhang, Q.; Tian, Y.; Luo, X.; Yang, M.; Zhao, X. Graphene-based nanocomposites for neural tissue engineering. Molecules 2019, 24, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magaz, A.; Faroni, A.; Gough, J.E.; Reid, A.J.; Li, X.; Blaker, J.J. Bioactive Silk-Based Nerve Guidance Conduits for Augmenting Peripheral Nerve Repair. Adv. Healthc. Mater. 2018, 7, 1800308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A. Graphene: Safe or toxic? The two faces of the medal. Angew. Chemie Int. Ed. 2013, 52, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Seifalian, A.M.; Hancock, S. Composite Material and Its Method of Production. GB Patent Application No. 1811090; WO Patent Application No. 2019008381A1; EP Patent Application No. 3648808A1. U.S. Patent Application No. 20200123381A1, 5 July 2018. [Google Scholar]
- Nezakati, T. Development of a Graphene-POSS-Nanocomposite Conductive Material for Biomedical Application. Ph.D. Thesis, London University College, London, UK, 2016. Supervisor Professor Alexander Seifalian. [Google Scholar]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleemardani, M.; Zare, P.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene-Based Materials Prove to Be a Promising Candidate for Nerve Regeneration Following Peripheral Nerve Injury. Biomedicines 2022, 10, 73. https://doi.org/10.3390/biomedicines10010073
Aleemardani M, Zare P, Seifalian A, Bagher Z, Seifalian AM. Graphene-Based Materials Prove to Be a Promising Candidate for Nerve Regeneration Following Peripheral Nerve Injury. Biomedicines. 2022; 10(1):73. https://doi.org/10.3390/biomedicines10010073
Chicago/Turabian StyleAleemardani, Mina, Pariya Zare, Amelia Seifalian, Zohreh Bagher, and Alexander M. Seifalian. 2022. "Graphene-Based Materials Prove to Be a Promising Candidate for Nerve Regeneration Following Peripheral Nerve Injury" Biomedicines 10, no. 1: 73. https://doi.org/10.3390/biomedicines10010073







