The Organization of Somatostatin-Immunoreactive Cells in the Visual Cortex of the Gerbil
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Tissue Preparation
2.2. Horseradish Peroxidase Staining
2.3. Fluorescence Immunocytochemistry
2.4. Quantitative Analysis
2.5. Synaptic Identification
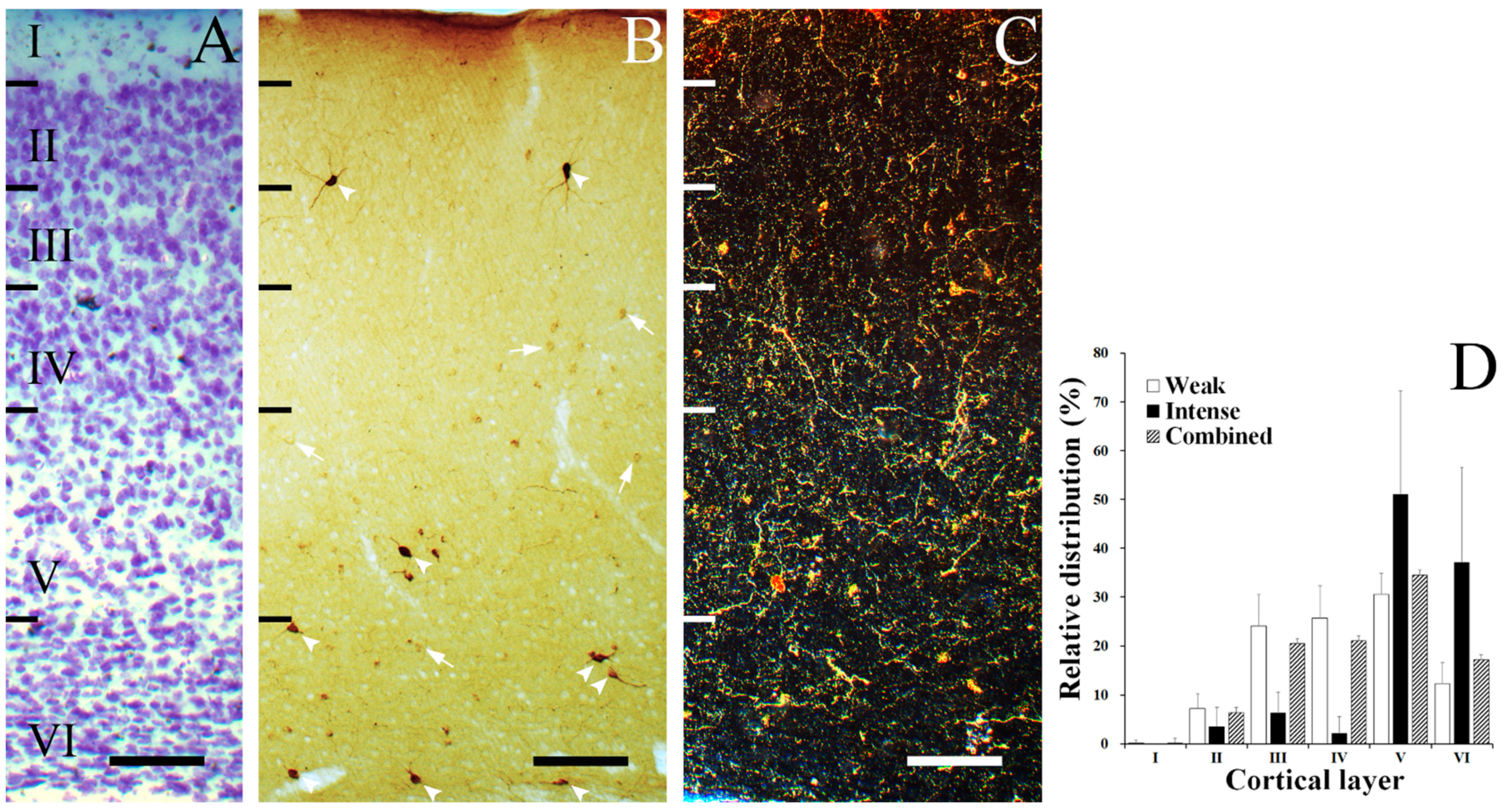
3. Results
3.1. Laminar Distribution of SST Neurons
3.2. Morphology of SST Neurons
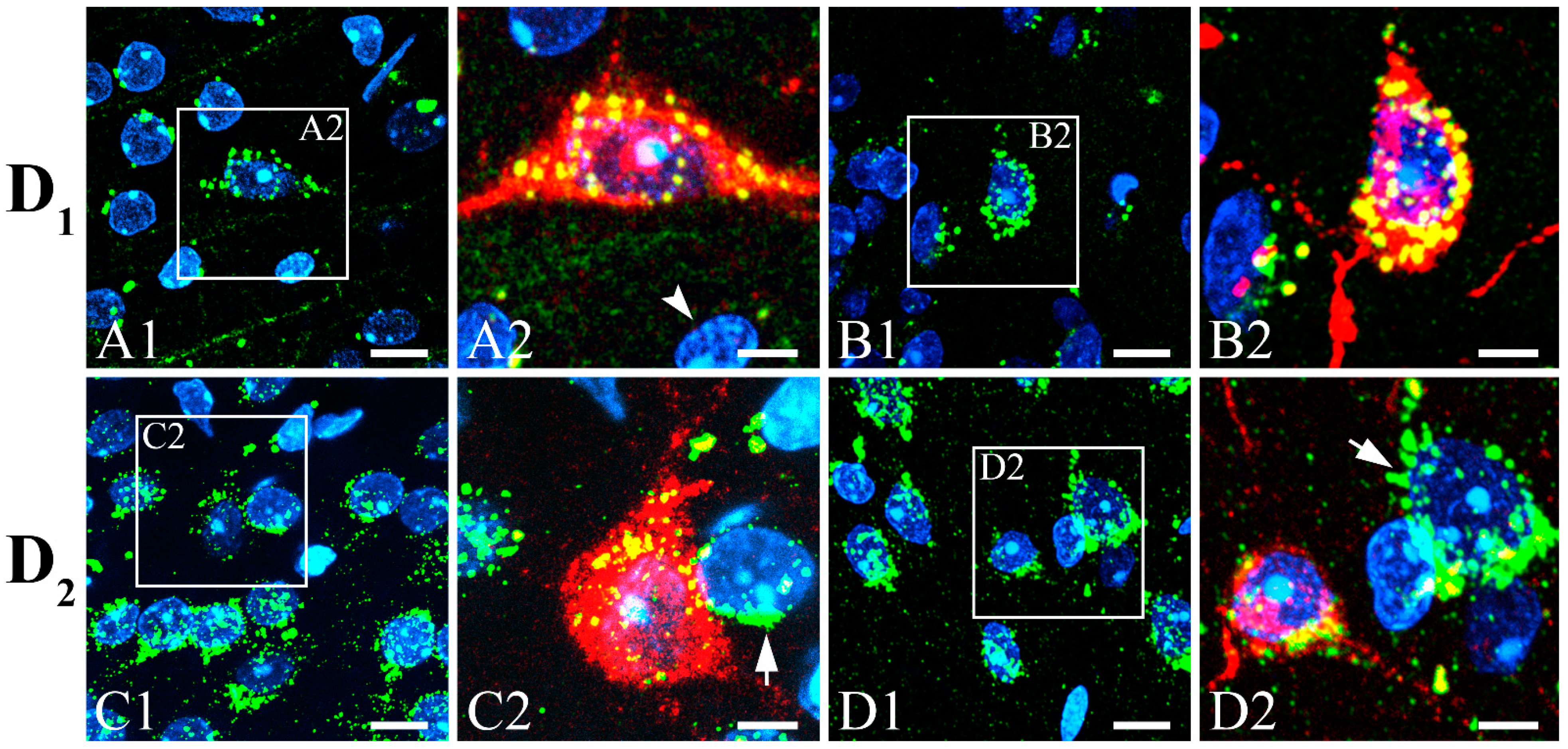
3.3. Colocalization of SST with GABA, CBPs, NOS, NPY, CaMKII, Dopamine Receptors, and ChAT with nAChRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef]
- Strowski, M.Z.; Blake, A.D. Function and expression of somatostatin receptors of the endocrine pancreas. Mol. Cell. Endocrinol. 2008, 286, 169–179. [Google Scholar] [CrossRef]
- Piqueras, L.; Martínez, V. Role of somatostatin receptors on gastric acid secretion in wild-type and somatostatin receptor type 2 knockout mice. Naunyn-Schmiedebergs Arch. Pharmacol. 2004, 370, 510–520. [Google Scholar] [CrossRef]
- Liguz-Lecznar, M.; Urban-Ciecko, J.; Kossut, M. Somatostatin and Somatostatin-Containing Neurons in Shaping Neuronal Activity and Plasticity. Front. Neural Circuits 2016, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.G.; Poort, J.; Chadwick, A.; Blot, A.; Sahani, M.; Mrsic-Flogel, T.D.; Hofer, S.B. Distinct learning-induced changes in stimulus selectivity and interactions of GABAergic interneuron classes in visual cortex. Nat. Neurosci. 2018, 21, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Honoré, E.; Khlaifia, A.; Bosson, A.; Lacaille, J.C. Hippocampal Somatostatin Interneurons, Long-Term Synaptic Plasticity and Memory. Front. Neural Circuits 2021, 15, 687558. [Google Scholar] [CrossRef]
- Adler, A.; Zhao, R.; Shin, M.E.; Yasuda, R.; Gan, W.B. Somatostatin-Expressing Interneurons Enable and Maintain Learning-Dependent Sequential Activation of Pyramidal Neurons. Neuron 2019, 102, 202–216.e207. [Google Scholar] [CrossRef]
- Song, Y.H.; Hwang, Y.S.; Kim, K.; Lee, H.R.; Kim, J.H.; Maclachlan, C.; Dubois, A.; Jung, M.W.; Petersen, C.C.H.; Knott, G.; et al. Somatostatin enhances visual processing and perception by suppressing excitatory inputs to parvalbumin-positive interneurons in V1. Sci. Adv. 2020, 6, eaaz0517. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.J.; Dipoppa, M.; Roth, M.M.; Caudill, M.S.; Ingrosso, A.; Miller, K.D.; Scanziani, M. A Disinhibitory Circuit for Contextual Modulation in Primary Visual Cortex. Neuron 2020, 108, 1181–1193.e1188. [Google Scholar] [CrossRef]
- Huang, P.; Xiang, X.; Chen, X.; Li, H. Somatostatin Neurons Govern Theta Oscillations Induced by Salient Visual Signals. Cell Rep. 2020, 33, 108415. [Google Scholar] [CrossRef]
- Hashimoto, T.; Bazmi, H.H.; Mirnics, K.; Wu, Q.; Sampson, A.R.; Lewis, D.A. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am. J. Psychiat. 2008, 165, 479–489. [Google Scholar] [CrossRef]
- Fee, C.; Banasr, M.; Sibille, E. Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biol. Psychiatry 2017, 82, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Singh, S. Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity. Int. J. Mol. Sci. 2020, 21, 2568. [Google Scholar] [CrossRef]
- Gonchar, Y.; Wang, Q.; Burkhalter, A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front. Neuroanat. 2007, 1, 3. [Google Scholar] [CrossRef]
- Rudy, B.; Fishell, G.; Lee, S.; Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011, 71, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef]
- Wamsley, B.; Fishell, G. Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat. Rev. Neurosci. 2017, 18, 299–309. [Google Scholar] [CrossRef]
- Markram, H.; Toledo-Rodriguez, M.; Wang, Y.; Gupta, A.; Silberberg, G.; Wu, C. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004, 5, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Elíes, J.; Yáñez, M.; Pereira, T.M.C.; Gil-Longo, J.; MacDougall, D.A.; Campos-Toimil, M. An Update to Calcium Binding Proteins. Adv. Exp. Med. Biol. 2020, 1131, 183–213. [Google Scholar] [CrossRef]
- Baimbridge, K.G.; Celio, M.R.; Rogers, J.H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992, 15, 303–308. [Google Scholar] [CrossRef]
- Sohn, J.; Hioki, H.; Okamoto, S.; Kaneko, T. Preprodynorphin-expressing neurons constitute a large subgroup of somatostatin-expressing GABAergic interneurons in the mouse neocortex. J. Comp. Neurol. 2014, 522, 1506–1526. [Google Scholar] [CrossRef]
- Yavorska, I.; Wehr, M. Somatostatin-Expressing Inhibitory Interneurons in Cortical Circuits. Front. Neural Circuits 2016, 10, 76. [Google Scholar] [CrossRef]
- Karagiannis, A.; Gallopin, T.; Dávid, C.; Battaglia, D.; Geoffroy, H.; Rossier, J.; Hillman, E.M.; Staiger, J.F.; Cauli, B. Classification of NPY-expressing neocortical interneurons. J. Neurosci. 2009, 29, 3642–3659. [Google Scholar] [CrossRef]
- Allen, J.M.; Bloom, S.R. Neuropeptide Y: A putative neurotransmitter. Neurochem. Int. 1986, 8, 1–8. [Google Scholar] [CrossRef]
- Jaglin, X.H.; Hjerling-Leffler, J.; Fishell, G.; Batista-Brito, R. The origin of neocortical nitric oxide synthase-expressing inhibitory neurons. Front. Neural Circuits 2012, 6, 44. [Google Scholar] [CrossRef]
- Niell, C.M. Cell types, circuits, and receptive fields in the mouse visual cortex. Annu. Rev. Neurosci. 2015, 38, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. Neuronal cell types. Curr. Biol. 2004, 14, R497–R500. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.F. Neuronal diversity: Too many cell types for comfort? Curr. Biol. 1998, 8, R708–R710. [Google Scholar] [CrossRef]
- Sawatari, A.; Callaway, E.M. Diversity and cell type specificity of local excitatory connections to neurons in layer 3B of monkey primary visual cortex. Neuron 2000, 25, 459–471. [Google Scholar] [CrossRef]
- Scala, F.; Kobak, D.; Shan, S.; Bernaerts, Y.; Laturnus, S.; Cadwell, C.R.; Hartmanis, L.; Froudarakis, E.; Castro, J.R.; Tan, Z.H.; et al. Layer 4 of mouse neocortex differs in cell types and circuit organization between sensory areas. Nat. Commun. 2019, 10, 4174. [Google Scholar] [CrossRef]
- Hendry, S.H.; Jones, E.G.; Emson, P.C. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J. Neurosci. 1984, 4, 2497–2517. [Google Scholar] [CrossRef]
- Campbell, M.J.; Lewis, D.A.; Benoit, R.; Morrison, J.H. Regional heterogeneity in the distribution of somatostatin-28- and somatostatin-28(1-12)-immunoreactive profiles in monkey neocortex. J. Neurosci. 1987, 7, 1133–1144. [Google Scholar] [CrossRef]
- Demeulemeester, H.; Arckens, L.; Vandesande, F.; Orban, G.A.; Heizmann, C.W.; Pochet, R. Calcium binding proteins and neuropeptides as molecular markers of GABAergic interneurons in the cat visual cortex. Exp. Brain Res. 1991, 84, 538–544. [Google Scholar] [CrossRef]
- Demeulemeester, H.; Vandesande, F.; Orban, G.A.; Brandon, C.; Vanderhaeghen, J.J. Heterogeneity of GABAergic cells in cat visual cortex. J. Neurosci. 1988, 8, 988–1000. [Google Scholar] [CrossRef]
- Demeulemeester, H.; Vandesande, F.; Orban, G.A. Immunocytochemical localization of somatostatin and cholecystokinin in the cat visual cortex. Brain Res. 1985, 332, 361–364. [Google Scholar] [CrossRef]
- Somogyi, P.; Hodgson, A.J.; Smith, A.D.; Nunzi, M.G.; Gorio, A.; Wu, J.Y. Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin- or cholecystokinin-immunoreactive material. J. Neurosci. 1984, 4, 2590–2603. [Google Scholar] [CrossRef] [PubMed]
- Wahle, P. Differential regulation of substance P and somatostatin in Martinotti cells of the developing cat visual cortex. J. Comp. Neurol. 1993, 329, 519–538. [Google Scholar] [CrossRef] [PubMed]
- Ramón, Y.; Cajal-Agüeras, S.; Contamina, P.; Parra, P.; Martinez-Millán, L.; De Carlos, J.A.; Ramo, C. The distribution of somatostatin-immunoreactive neurons in the visual cortex of adult rabbits and during postnatal development. Brain Res. 1985, 359, 379–382. [Google Scholar] [CrossRef]
- Lin, C.S.; Lu, S.M.; Schmechel, D.E. Glutamic acid decarboxylase and somatostatin immunoreactivities in rat visual cortex. J. Comp. Neurol. 1986, 244, 369–383. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.K.; Parnavelas, J.G.; Karamanlidis, A.N.; Brecha, N.; Koenig, J.I. The morphology and distribution of peptide-containing neurons in the adult and developing visual cortex of the rat. I. Somatostatin. J. Neurocytol. 1982, 11, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Gonchar, Y.; Burkhalter, A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb. Cortex 1997, 7, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, G.C.; Parnavelas, J.G.; Cavanagh, M.E. Extensive co-existence of neuropeptides in the rat visual cortex. Brain Res. 1987, 420, 95–99. [Google Scholar] [CrossRef]
- Eadie, L.A.; Parnavelas, J.G.; Franke, E. Development of the ultrastructural features of somatostatin-immunoreactive neurons in the rat visual cortex. J. Neurocytol. 1987, 16, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Karten, H.J. Immunohistochemical analysis of the visual wulst of the pigeon (Columba livia). J. Comp. Neurol. 1990, 300, 346–369. [Google Scholar] [CrossRef]
- Feldman, S.C. Distribution of immunoreactive somatostatin (ISRIF) in the nervous system of the squid, Loligo pealei. J. Comp. Neurol. 1986, 245, 238–257. [Google Scholar] [CrossRef] [PubMed]
- Paspalas, C.D.; Papadopoulos, G.C. Noradrenergic innervation of peptidergic interneurons in the rat visual cortex. Cereb. Cortex 1999, 9, 844–853. [Google Scholar] [CrossRef][Green Version]
- Schmechel, D.E.; Vickrey, B.G.; Fitzpatrick, D.; Elde, R.P. GABAergic neurons of mammalian cerebral cortex: Widespread subclass defined by somatostatin content. Neurosci. Lett. 1984, 47, 227–232. [Google Scholar] [CrossRef]
- Cheal, M.L. The gerbil: A unique model for research on aging. Exp. Aging Res. 1986, 12, 3–21. [Google Scholar] [CrossRef]
- Albers, H.J.; Gordon, S. Comparison of serum cholesterol esters in gerbil and rat. Proc. Soc. Exp. Biol. Med. 1962, 109, 860–863. [Google Scholar] [CrossRef]
- Robinson, P.F. Metabolism of the Gerbil, Meriones unguiculatus. Science 1959, 130, 502–503. [Google Scholar] [CrossRef]
- Gordon, S.; Cekleniak, W.P.; Stolzenberg, S.J.; Benitz, K.F.; Moraski, R.M. Biochemical and morphologic effects of cholesterol and its methyl ether in the gerbil. Toxicol. Appl. Pharmacol. 1961, 3, 315–334. [Google Scholar] [CrossRef]
- Roscoe, H.G.; Fahrenbach, M.J. Cholesterol metabolism in the gerbil. Proc. Soc. Exp. Biol. Med. 1962, 110, 51–55. [Google Scholar] [CrossRef]
- Gordon, S.; Stolzenberg, S.J.; Cekleniak, W.P. Effects of cholesterol and beta-sitosterol in the gerbil. Am. J. Physiol. 1959, 197, 671–673. [Google Scholar] [CrossRef]
- Gordon, S.; Cekleniak, W.P. Serum lipoprotein pattern of the hypercholesteremic gerbil. Am. J. Physiol. 1961, 201, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J.; Brownbill, D.; Lewis, P.D.; Russell, R.W. Cerebral edema following carotid artery ligation in the gerbil. Arch. Neurol. 1973, 28, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Jones-Mumby, C.J.; Axelsson, A. The vascular anatomy of the gerbil cochlea. Am. J. Otolaryngol. 1984, 5, 127–137. [Google Scholar] [CrossRef]
- Peckova, R.; Sak, B.; Kvetonova, D.; Kvac, M.; Koritakova, E.; Foitova, I. The course of experimental giardiasis in Mongolian gerbil. Parasitol. Res. 2018, 117, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Belosevic, M.; Faubert, G.M.; MacLean, J.D.; Law, C.; Croll, N.A. Giardia lamblia infections in Mongolian gerbils: An animal model. J. Infect. Dis. 1983, 147, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Keplinger, S.; Beiderbeck, B.; Michalakis, S.; Biel, M.; Grothe, B.; Kunz, L. Optogenetic Control of Neural Circuits in the Mongolian Gerbil. Front. Cell. Neurosci. 2018, 12, 111. [Google Scholar] [CrossRef]
- Lee, T.K.; Chen, B.H.; Lee, J.C.; Shin, M.C.; Cho, J.H.; Lee, H.A.; Choi, J.H.; Hwang, I.K.; Kang, I.J.; Ahn, J.H.; et al. Agedependent decreases in insulinlike growth factorI and its receptor expressions in the gerbil olfactory bulb. Mol. Med. Rep. 2018, 17, 8161–8166. [Google Scholar] [CrossRef]
- Depner, M.; Tziridis, K.; Hess, A.; Schulze, H. Sensory cortex lesion triggers compensatory neuronal plasticity. BMC. Neurosci. 2014, 15, 57. [Google Scholar] [CrossRef][Green Version]
- Thomas, H.; Tillein, J.; Heil, P.; Scheich, H. Functional organization of auditory cortex in the mongolian gerbil (Meriones unguiculatus). I. Electrophysiological mapping of frequency representation and distinction of fields. Eur. J. Neurosci. 1993, 5, 882–897. [Google Scholar] [CrossRef]
- Henschke, J.U.; Oelschlegel, A.M.; Angenstein, F.; Ohl, F.W.; Goldschmidt, J.; Kanold, P.O.; Budinger, E. Early sensory experience influences the development of multisensory thalamocortical and intracortical connections of primary sensory cortices. Brain Struct. Funct. 2018, 223, 1165–1190. [Google Scholar] [CrossRef]
- Bertorelli, R.; Adami, M.; Ongini, E. The Mongolian gerbil in experimental epilepsy. Ital. J. Neurol. Sci. 1995, 16, 101–106. [Google Scholar] [CrossRef]
- Levine, S.; Payan, H. Effects of ischemia and other procedures on the brain and retina of the gerbil (Meriones unguiculatus). Exp. Neurol. 1966, 16, 255–262. [Google Scholar] [CrossRef]
- Kitabatake, T.T.; Marini, L.C.; Goncalves, R.B.; Bertolino, G.; de Souza, H.C.D.; de Araujo, J.E. Behavioral effects and neural changes induced by continuous and not continuous treadmill training, post bilateral cerebral ischemia in gerbils. Behav. Brain Res. 2015, 291, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, D.D.; Goar, S. Stereotaxic atlas of the hypothalamus of the Mongolian gerbil (Meriones unguiculatus). J. Comp. Neurol. 1970, 140, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Radtke-Schuller, S.; Schuller, G.; Angenstein, F.; Grosser, O.S.; Goldschmidt, J.; Budinger, E. Brain atlas of the Mongolian gerbil (Meriones unguiculatus) in CT/MRI-aided stereotaxic coordinates. Brain Struct. Funct. 2016, 221 (Suppl. 1), 1–272. [Google Scholar] [CrossRef]
- Mankin, E.A.; Thurley, K.; Chenani, A.; Haas, O.V.; Debs, L.; Henke, J.; Galinato, M.; Leutgeb, J.K.; Leutgeb, S.; Leibold, C. The hippocampal code for space in Mongolian gerbils. Hippocampus 2019, 29, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Reme, C.E.; Wirz-Justice, A.; Terman, M. The visual input stage of the mammalian circadian pacemaking system: I. Is there a clock in the mammalian eye? J. Biol. Rhythms 1991, 6, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Govardovskii, V.I.; Rohlich, P.; Szel, A.; Khokhlova, T.V. Cones in the retina of the Mongolian gerbil, Meriones unguiculatus: An immunocytochemical and electrophysiological study. Vision Res. 1992, 32, 19–27. [Google Scholar] [CrossRef]
- Bytyqi, A.H.; Layer, P.G. Lamina formation in the Mongolian gerbil retina (Meriones unguiculatus). Anat. Embryol. 2005, 209, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.G.; Emerson, V.F. Grating acuity of the Mongolian gerbil (Meriones unguiculatus). Behav. Brain Res. 1983, 8, 195–209. [Google Scholar] [CrossRef]
- Huber, G.; Heynen, S.; Imsand, C.; vom Hagen, F.; Muehlfriedel, R.; Tanimoto, N.; Feng, Y.; Hammes, H.P.; Grimm, C.; Peichl, L.; et al. Novel rodent models for macular research. PLoS ONE 2010, 5, e13403. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.J.; Jeon, C.J. Localization of melanopsin-immunoreactive cells in the Mongolian gerbil retina. Neurosci. Res. 2015, 100, 6–16. [Google Scholar] [CrossRef]
- Yang, S.; Luo, X.; Xiong, G.; So, K.F.; Yang, H.; Xu, Y. The electroretinogram of Mongolian gerbil (Meriones unguiculatus): Comparison to mouse. Neurosci. Lett. 2015, 589, 7–12. [Google Scholar] [CrossRef]
- Macharadze, T.; Budinger, E.; Brosch, M.; Scheich, H.; Ohl, F.W.; Henschke, J.U. Early Sensory Loss Alters the Dendritic Branching and Spine Density of Supragranular Pyramidal Neurons in Rodent Primary Sensory Cortices. Front. Neural Circuits 2019, 13, 61. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef]
- Lin, L.C.; Sibille, E. Reduced brain somatostatin in mood disorders: A common pathophysiological substrate and drug target? Front. Pharmacol. 2013, 4, 110. [Google Scholar] [CrossRef]
- Koukouli, F.; Changeux, J.P. Do Nicotinic Receptors Modulate High-Order Cognitive Processing? Trends Neurosci. 2020, 43, 550–564. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, J.Y.; Jeon, C.J. Immunocytochemical localization of calretinin in the superficial layers of the cat superior colliculus. Neurosci. Res. 2002, 44, 325–335. [Google Scholar] [CrossRef]
- Lee, J.E.; Ahn, C.H.; Lee, J.Y.; Chung, E.S.; Jeon, C.J. Nitric oxide synthase and calcium-binding protein-containing neurons in the hamster visual cortex. Mol. Cells. 2004, 18, 30–39. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, W.T.; Jeon, C.J. Organization of Neuropeptide Y-Immunoreactive Cells in the Mongolian gerbil (Meriones unguiculatus) Visual Cortex. Cells 2021, 10, 311. [Google Scholar] [CrossRef]
- López-Mascaraque, L.; Ramo, C.; Contamina-Gonzalvo, P.; de Carlos, J. Layers I and VI of the visual cortex in the rabbit. A correlated Golgi and immunocytochemical study of somatostatin and vasoactive intestinal peptide containing neurons. J. Brain Res.-J. Hirnforsch. 1989, 30, 163–173. [Google Scholar]
- Kosaka, T.; Wu, J.Y.; Benoit, R. GABAergic neurons containing somatostatin-like immunoreactivity in the rat hippocampus and dentate gyrus. Exp. Brain Res. 1988, 71, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Wouterlood, F.G.; Pothuizen, H. Sparse colocalization of somatostatin- and GABA-immunoreactivity in the entorhinal cortex of the rat. Hippocampus 2000, 10, 77–86. [Google Scholar] [CrossRef]
- Tomioka, R.; Okamoto, K.; Furuta, T.; Fujiyama, F.; Iwasato, T.; Yanagawa, Y.; Obata, K.; Kaneko, T.; Tamamaki, N. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur. J. Neurosci. 2005, 21, 1587–1600. [Google Scholar] [CrossRef]
- Dodd, J.; Kelly, J.S. Is somatostatin an excitatory transmitter in the hippocampus? Nature 1978, 273, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Delfs, J.R.; Dichter, M.A. Effects of somatostatin on mammalian cortical neurons in culture: Physiological actions and unusual dose response characteristics. J. Neurosci. 1983, 3, 1176–1188. [Google Scholar] [CrossRef]
- Stornetta, R.L.; Rosin, D.L.; Wang, H.; Sevigny, C.P.; Weston, M.C.; Guyenet, P.G. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. J. Comp. Neurol. 2003, 455, 499–512. [Google Scholar] [CrossRef]
- Thek, K.R.; Ong, S.J.M.; Carter, D.C.; Bassi, J.K.; Allen, A.M.; McDougall, S.J. Extensive Inhibitory Gating of Viscerosensory Signals by a Sparse Network of Somatostatin Neurons. J. Neurosci. 2019, 39, 8038–8050. [Google Scholar] [CrossRef]
- Cattaneo, S.; Zaghi, M.; Maddalena, R.; Bedogni, F.; Sessa, A.; Taverna, S. Somatostatin-Expressing Interneurons Co-Release GABA and Glutamate onto Different Postsynaptic Targets in the Striatum. bioRxiv 2019, 566984. [Google Scholar] [CrossRef]
- Kubota, Y.; Hattori, R.; Yui, Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994, 649, 159–173. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kubota, Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 1997, 7, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Roby, K.D.; Callaway, E.M. Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 2010, 518, 389–404. [Google Scholar] [CrossRef]
- Xu, X.; Roby, K.D.; Callaway, E.M. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J. Comp. Neurol. 2006, 499, 144–160. [Google Scholar] [CrossRef]
- Wang, Y.; Gupta, A.; Toledo-Rodriguez, M.; Wu, C.Z.; Markram, H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb. Cortex 2002, 12, 395–410. [Google Scholar] [CrossRef]
- Riedemann, T.; Schmitz, C.; Sutor, B. Immunocytochemical heterogeneity of somatostatin-expressing GABAergic interneurons in layers II and III of the mouse cingulate cortex: A combined immunofluorescence/design-based stereologic study. J. Comp. Neurol. 2016, 524, 2281–2299. [Google Scholar] [CrossRef]
- Wasilewska, B.; Robak, A.; Równiak, M.; Bogus-Nowakowska, K.; Najdzion, J.; Zakowski, W.; Majewski, M. Distribution and chemical coding pattern of somatostatin immunoreactivity in the dorsal striatum of the guinea pig. Folia Histochem. Cytobiol. 2011, 49, 690–699. [Google Scholar] [CrossRef][Green Version]
- Figueredo-Cardenas, G.; Morello, M.; Sancesario, G.; Bernardi, G.; Reiner, A. Colocalization of somatostatin, neuropeptide Y, neuronal nitric oxide synthase and NADPH-diaphorase in striatal interneurons in rats. Brain Res. 1996, 735, 317–324. [Google Scholar] [CrossRef]
- Jinno, S.; Kosaka, T. Patterns of colocalization of neuronal nitric oxide synthase and somatostatin-like immunoreactivity in the mouse hippocampus: Quantitative analysis with optical disector. Neuroscience 2004, 124, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Post, R.J.; Warden, M.R. Depression: The search for separable behaviors and circuits. Curr. Opin. Neurobiol. 2018, 49, 192–200. [Google Scholar] [CrossRef]
- Lin, L.C.; Sibille, E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol. Psychiatr. 2015, 20, 377–387. [Google Scholar] [CrossRef]
- Noudoost, B.; Moore, T. The role of neuromodulators in selective attention. Trends Cogn. Sci. 2011, 15, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Khlghatyan, J.; Quintana, C.; Parent, M.; Beaulieu, J.M. High Sensitivity Mapping of Cortical Dopamine D2 Receptor Expressing Neurons. Cereb. Cortex 2019, 29, 3813–3827. [Google Scholar] [CrossRef]
- Le Moine, C.; Normand, E.; Bloch, B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc. Natl. Acad. Sci. USA 1991, 88, 4205–4209. [Google Scholar] [CrossRef]
- Engber, T.M.; Boldry, R.C.; Kuo, S.; Chase, T.N. Dopaminergic modulation of striatal neuropeptides: Differential effects of D1 and D2 receptor stimulation on somatostatin, neuropeptide Y, neurotensin, dynorphin and enkephalin. Brain Res. 1992, 581, 261–268. [Google Scholar] [CrossRef]
- Mueller, A.; Krock, R.M.; Shepard, S.; Moore, T. Dopamine Receptor Expression Among Local and Visual Cortex-Projecting Frontal Eye Field Neurons. Cereb. Cortex 2020, 30, 148–164. [Google Scholar] [CrossRef]
- Anastasiades, P.G.; Boada, C.; Carter, A.G. Cell-Type-Specific D1 Dopamine Receptor Modulation of Projection Neurons and Interneurons in the Prefrontal Cortex. Cereb. Cortex 2019, 29, 3224–3242. [Google Scholar] [CrossRef]
- Adesnik, H.; Bruns, W.; Taniguchi, H.; Huang, Z.J.; Scanziani, M. A neural circuit for spatial summation in visual cortex. Nature 2012, 490, 226–231. [Google Scholar] [CrossRef]
- Chen, S.X.; Kim, A.N.; Peters, A.J.; Komiyama, T. Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat. Neurosci. 2015, 18, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Zoli, M.; Clementi, F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol. Sci. 2006, 27, 482–491. [Google Scholar] [CrossRef]
- Sun, Q.; Li, X.; Ren, M.; Zhao, M.; Zhong, Q.; Ren, Y.; Luo, P.; Ni, H.; Zhang, X.; Zhang, C.; et al. A whole-brain map of long-range inputs to GABAergic interneurons in the mouse medial prefrontal cortex. Nat. Neurosci. 2019, 22, 1357–1370. [Google Scholar] [CrossRef]
- Obermayer, J.; Heistek, T.S.; Kerkhofs, A.; Goriounova, N.A.; Kroon, T.; Baayen, J.C.; Idema, S.; Testa-Silva, G.; Couey, J.J.; Mansvelder, H.D. Lateral inhibition by Martinotti interneurons is facilitated by cholinergic inputs in human and mouse neocortex. Nat. Commun. 2018, 9, 4101. [Google Scholar] [CrossRef] [PubMed]
- Demars, M.P.; Morishita, H. Cortical parvalbumin and somatostatin GABA neurons express distinct endogenous modulators of nicotinic acetylcholine receptors. Mol. Brain 2014, 7, 75. [Google Scholar] [CrossRef]
- Krubitzer, L.; Campi, K.L.; Cooke, D.F. All rodents are not the same: A modern synthesis of cortical organization. Brain Behav. Evol. 2011, 78, 51–93. [Google Scholar] [CrossRef]
- Campi, K.L.; Krubitzer, L. Comparative studies of diurnal and nocturnal rodents: Differences in lifestyle result in alterations in cortical field size and number. J. Comp. Neurol. 2010, 518, 4491–4512. [Google Scholar] [CrossRef] [PubMed]
- Kaas, J.H.; Hall, W.C.; Diamond, I.T. Visual cortex of the grey squirrel (Sciurus carolinensis): Architectonic subdivisions and connections from the visual thalamus. J. Comp. Neurol. 1972, 145, 273–305. [Google Scholar] [CrossRef]
- Heimel, J.A.; Van Hooser, S.D.; Nelson, S.B. Laminar organization of response properties in primary visual cortex of the gray squirrel (Sciurus carolinensis). J. Neurophysiol. 2005, 94, 3538–3554. [Google Scholar] [CrossRef] [PubMed]
- Van Hooser, S.D.; Heimel, J.A.; Chung, S.; Nelson, S.B.; Toth, L.J. Orientation selectivity without orientation maps in visual cortex of a highly visual mammal. J. Neurosci. 2005, 25, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Van Hooser, S.D.; Heimel, J.A.; Nelson, S.B. Functional cell classes and functional architecture in the early visual system of a highly visual rodent. Prog. Brain Res. 2005, 149, 127–145. [Google Scholar] [CrossRef]
- Ferreiro, D.N.; Conde-Ocazionez, S.A.; Patriota, J.H.N.; Souza, L.C.; Oliveira, M.F.; Wolf, F.; Schmidt, K.E. Spatial clustering of orientation preference in primary visual cortex of the large rodent agouti. iScience 2021, 24, 101882. [Google Scholar] [CrossRef] [PubMed]
- Bennett-Clarke, C.A.; Joseph, S.A. Immunocytochemical localization of somatostatin in human brain. Peptides 1986, 7, 877–884. [Google Scholar] [CrossRef]
- Reubi, J.C.; Probst, A.; Cortes, R.; Palacios, J.M. Distinct topographical localisation of two somatostatin receptor subpopulations in the human cortex. Brain Res. 1987, 406, 391–396. [Google Scholar] [CrossRef]
- Selmer, I.-S.; Schindler, M.; Humphrey, P.P.A.; Waldvogel, H.J.; Faull, R.L.M.; Emson, P.C. First localisation of somatostatin sst4 receptor protein in selected human brain areas: An immunohistochemical study. Mol. Brain Res. 2000, 82, 114–125. [Google Scholar] [CrossRef]
- Mengod, G.; Rigo, M.; Savasta, M.; Probst, A.; Palacios, J.M. Regional distribution of neuropeptide somatostatin gene expression in the human brain. Synapse 1992, 12, 62–74. [Google Scholar] [CrossRef]
- Thoss, V.S.; Pérez, J.; Probst, A.; Hoyer, D. Expression of five somatostatin receptor mRNAs in the human brain and pituitary. Naunyn-Schmiedebergs Arch. Pharmacol. 1996, 354, 411–419. [Google Scholar] [CrossRef]
- Lewis, D.A.; Sweet, R.A. Schizophrenia from a neural circuitry perspective: Advancing toward rational pharmacological therapies. J. Clin. Investig. 2009, 119, 706–716. [Google Scholar] [CrossRef]



| Primary | Type | Dilution | Manufacturer | |
|---|---|---|---|---|
| SST | RtM | 1:200 | Millipore, Burlington, MA, USA | |
| GABA | MM | 1:500 | Millipore | |
| CB | MM | 1:500 | Sigma-Aldrich, Saint Louis, MO, USA | |
| CR | MM | 1:500 | Sigma-Aldrich | |
| CR | RbP | 1:100 | Sigma-Aldrich | |
| PV | MM | 1:500–1000 | Millipore | |
| NPY | RbP | 1:500 | Immunostar, Hudson, WI, USA | |
| NOS | MM | 1:200 | BD Biosciences, San Jose, CA, USA | |
| CaMKII | RbP | 1:500 | Proteintech, Rosemont, IL, USA | |
| D1 | MM | 1:200 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | |
| D2 | MM | 1:200 | Santa Cruz Biotechnology, Inc. | |
| ChAT | MM | 1:250 | Millipore | |
| α7 | RbP | 1:200 | Santa Cruz Biotechnology, Inc. | |
| β2 | RbP | 1:200 | Santa Cruz Biotechnology, Inc. | |
| Secondary | Conjugation | Dilution | Target | Manufacturer |
| HRP | ||||
| Goat anti-rat IgG | Biotinylated | 1:200 | SST | Vector laboratories, Inc., Burlingame, CA, USA |
| Fluorescence | ||||
| Goat anti-rat IgG | Cy3 | 1:200 | SST | Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA |
| Horse anti-mouse IgG | FITC | 1:200 | CB, CR(MM), PV, GABA, ChAT, D1, D2, NOS | Vector laboratories, Inc. |
| Goat anti-rabbit IgG | FITC | 1:200 | CR(RbP), NPY, CaMKII | Jackson ImmunoResearch Laboratories, Inc. |
| Goat anti-rabbit IgG | Cy5 | 1:200 | α7, β2 | Jackson ImmunoResearch Laboratories, Inc. |
| Antibodies | Animal | No. Sections | No. SST Cells | No. Double | % Double (Mean ± S.D.) |
|---|---|---|---|---|---|
| GABA | #1 | 3 | 70 | 23 | 32.85 ± 5.59 |
| #2 | 3 | 73 | 23 | 31.50 ± 3.38 | |
| #3 | 3 | 54 | 20 | 37.03 ± 6.75 | |
| GABA total | 9 | 197 | 66 | 33.50 ± 6.04 | |
| CB | #1 | 3 | 78 | 19 | 24.35 ± 0.56 |
| #2 | 3 | 63 | 16 | 25.39 ± 1.55 | |
| #3 | 3 | 72 | 16 | 22.22 ± 5.69 | |
| CB total | 9 | 212 | 51 | 24.05 ± 3.73 | |
| CR | #1 | 3 | 58 | 9 | 15.51 ± 4.29 |
| #2 | 3 | 84 | 14 | 16.66 ± 0.56 | |
| #3 | 3 | 97 | 17 | 17.52 ± 0.65 | |
| CR total | 9 | 239 | 40 | 16.73 ± 2.77 | |
| PV | #1 | 3 | 67 | 0 | 0 |
| #2 | 3 | 65 | 0 | 0 | |
| #3 | 3 | 61 | 0 | 0 | |
| PV total | 9 | 193 | 0 | 0 | |
| NPY | #1 | 3 | 84 | 71 | 84.52 ± 3.73 |
| #2 | 3 | 103 | 73 | 70.87 ± 2.49 | |
| #3 | 3 | 84 | 73 | 86.90 ± 3.47 | |
| NPY total | 9 | 271 | 217 | 80.07 ± 7.80 | |
| NOS | #1 | 3 | 68 | 55 | 80.88 ± 2.40 |
| #2 | 3 | 103 | 73 | 67.20 ± 5.71 | |
| #3 | 3 | 84 | 73 | 84.72 ± 5.42 | |
| NOS total | 9 | 265 | 200 | 75.41 ± 8.64 | |
| CaMKII | #1 | 3 | 86 | 54 | 62.79 ± 8.53 |
| #2 | 3 | 85 | 50 | 58.82 ± 5.11 | |
| #3 | 3 | 83 | 60 | 72.29 ± 6.03 | |
| CaMKII total | 9 | 254 | 164 | 64.57 ± 8.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, K.-M.; Lee, M.-J.; Chung, H.-S.; Pak, J.-H.; Jeon, C.-J. The Organization of Somatostatin-Immunoreactive Cells in the Visual Cortex of the Gerbil. Biomedicines 2022, 10, 92. https://doi.org/10.3390/biomedicines10010092
Kwon K-M, Lee M-J, Chung H-S, Pak J-H, Jeon C-J. The Organization of Somatostatin-Immunoreactive Cells in the Visual Cortex of the Gerbil. Biomedicines. 2022; 10(1):92. https://doi.org/10.3390/biomedicines10010092
Chicago/Turabian StyleKwon, Kyung-Min, Myung-Jun Lee, Han-Saem Chung, Jae-Hong Pak, and Chang-Jin Jeon. 2022. "The Organization of Somatostatin-Immunoreactive Cells in the Visual Cortex of the Gerbil" Biomedicines 10, no. 1: 92. https://doi.org/10.3390/biomedicines10010092
APA StyleKwon, K.-M., Lee, M.-J., Chung, H.-S., Pak, J.-H., & Jeon, C.-J. (2022). The Organization of Somatostatin-Immunoreactive Cells in the Visual Cortex of the Gerbil. Biomedicines, 10(1), 92. https://doi.org/10.3390/biomedicines10010092






