Effects of the Phenethylamine 2-Cl-4,5-MDMA and the Synthetic Cathinone 3,4-MDPHP in Adolescent Rats: Focus on Sex Differences
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
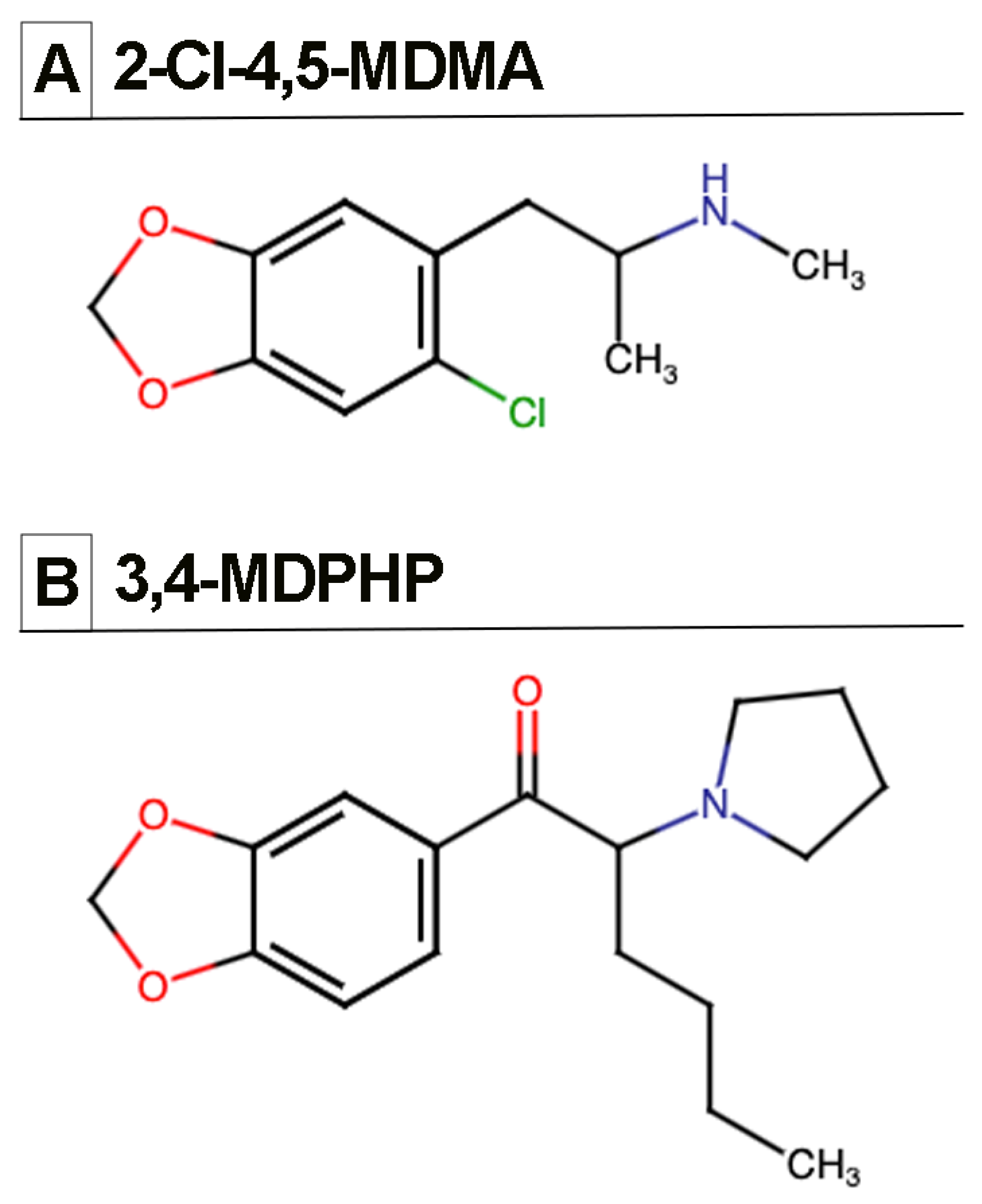
2.2. Drugs
2.3. Surgery
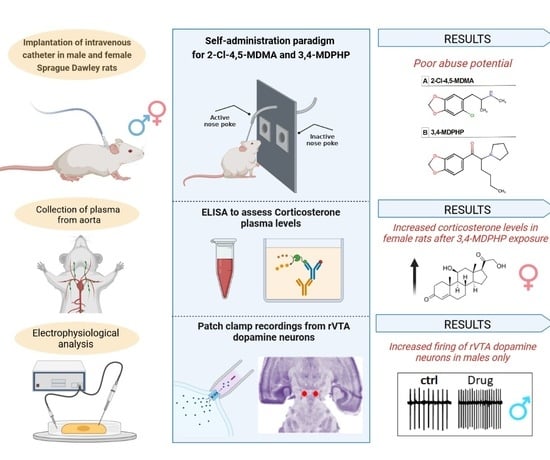
2.4. Self-Administration Apparatus and Procedure
2.5. Plasma Corticosterone Levels
2.6. Preparation of Brain Slices
2.7. Electrophysiology
2.8. Statistical Analyses
3. Results
3.1. Intravenous Drug Self-Administration
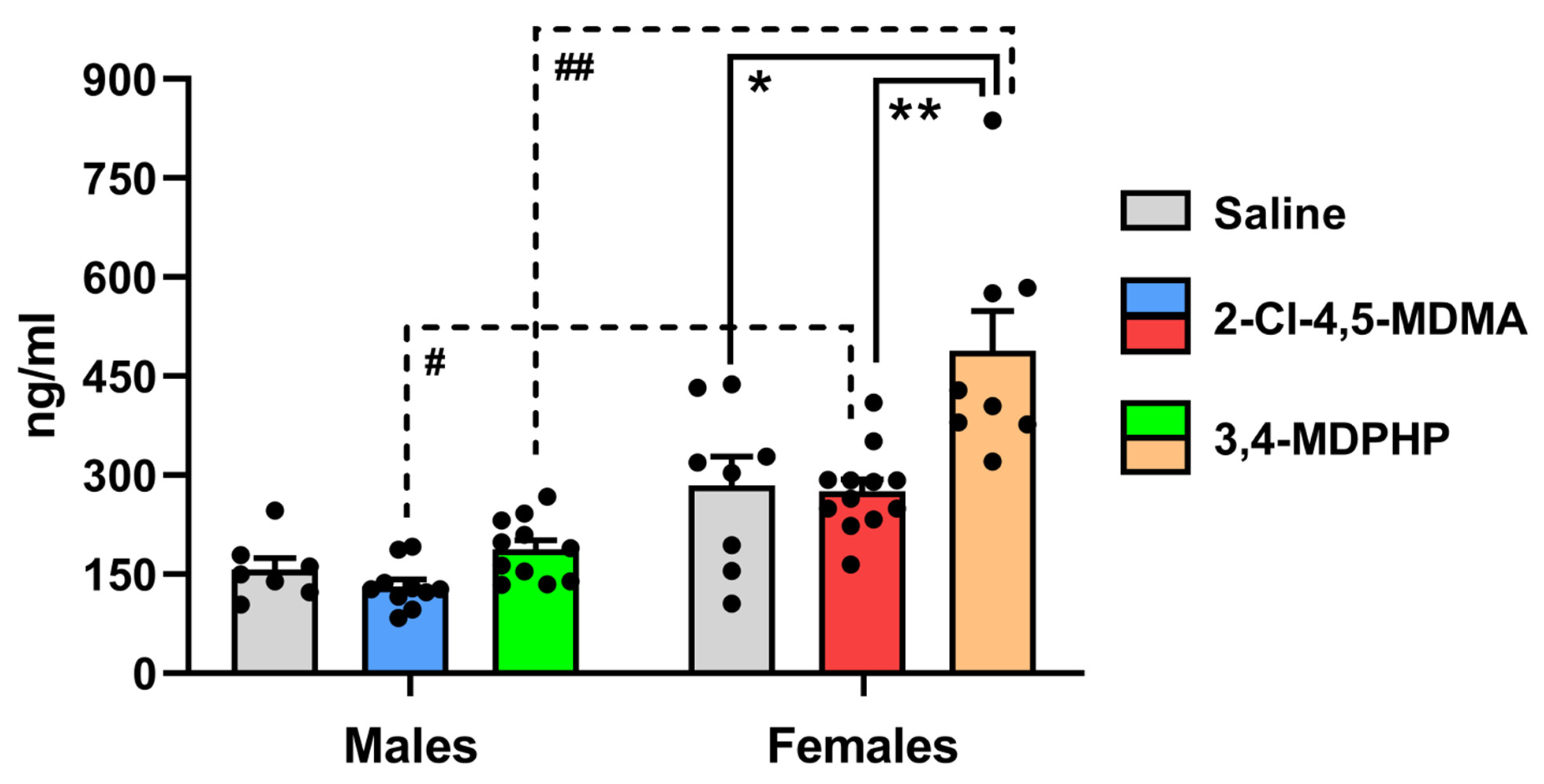
3.2. Plasma Corticosterone Levels
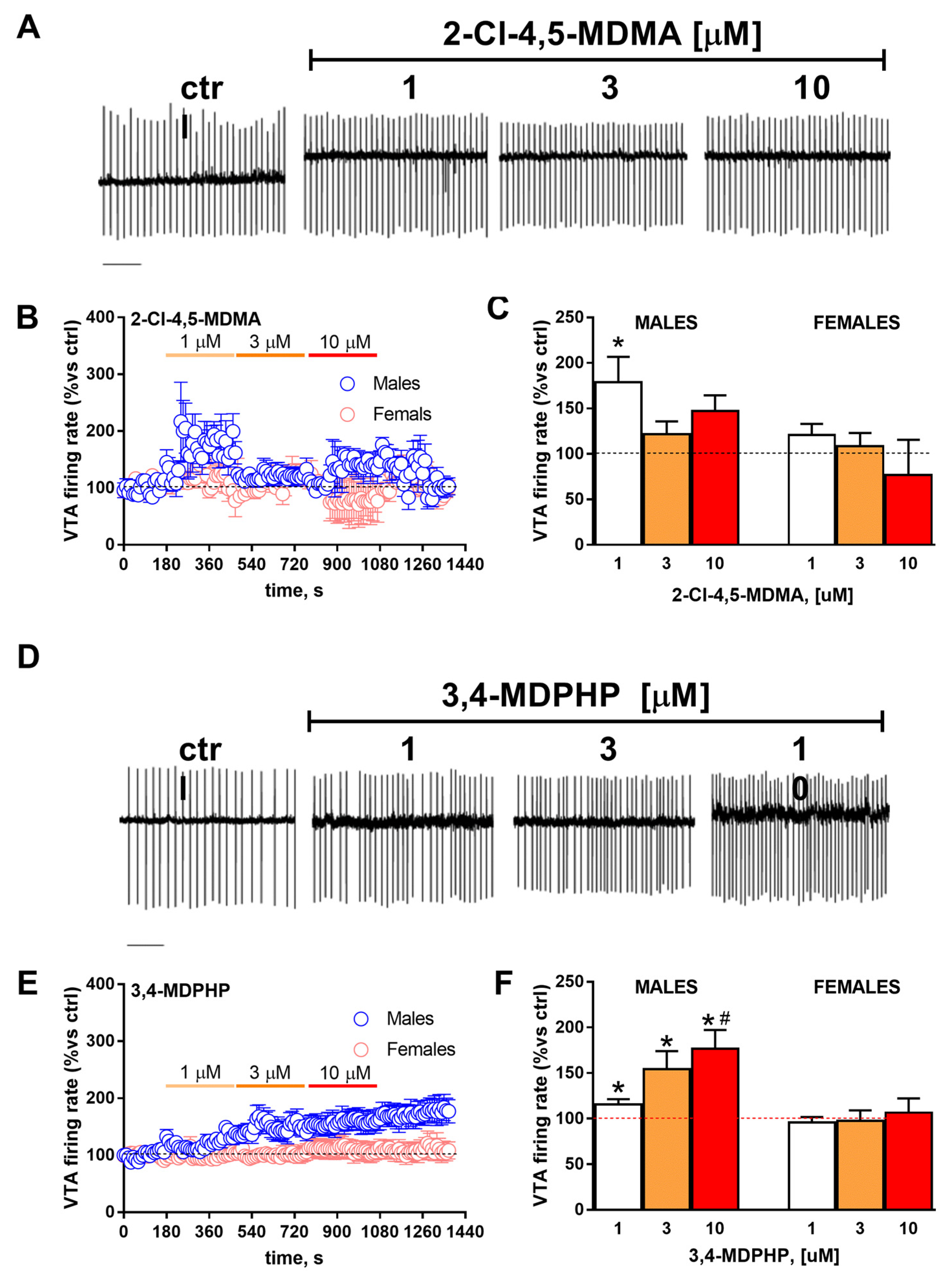
3.3. rVTA Dopamine Neurons Recordings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Drug Report 2021; United Nations Publication, Sales No. E. 21; United Nations: New York, NY, USA, 2021. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2021: Trends and Developments; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- UNODC. Regional Diversity and the Impact of Scheduling on NPS Trends—Global SMART Update; UNODC: Vienna, Austria, 2021; Volume 25. [Google Scholar]
- Bijlsma, L.; Celma, A.; Castiglioni, S.; Salgueiro-González, N.; Bou-Iserte, L.; Baz-Lomba, J.A.; Reid, M.J.; Dias, M.J.; Lopes, A.; Matias, J.; et al. Monitoring psychoactive substance use at six European festivals through wastewater and pooled urine analysis. Sci. Total Environ. 2020, 725, 138376. [Google Scholar] [CrossRef]
- Castiglioni, S.; Salgueiro-González, N.; Bijlsma, L.; Celma, A.; Gracia-Lor, E.; Beldean-Galea, M.S.; Mackuľak, T.; Emke, E.; Heath, E.; Kasprzyk-Hordern, B.; et al. New psychoactive substances in several European populations assessed by wastewater-based epidemiology. Water Res. 2021, 195, 116983. [Google Scholar] [CrossRef]
- Costa, G.; De Luca, M.A.; Piras, G.; Marongiu, J.; Fattore, L.; Simola, N. Neuronal and peripheral damages induced by synthetic psychoactive substances: An update of recent findings from human and animal studies. Neural Regen. Res. 2020, 15, 802–816. [Google Scholar] [PubMed]
- Seely, K.A.; Lapoint, J.; Moran, J.H.; Fattore, L. Spice drugs are more than harmless herbal blends: A review of the pharmacology and toxicology of synthetic cannabinoids. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Miliano, C.; Margiani, G.; Fattore, L.; De Luca, M.A. Sales and Advertising Channels of New Psychoactive Substances (NPS): Internet, Social Networks, and Smartphone Apps. Brain Sci. 2018, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.M.; Rosca, P.; Fattore, L.; London, E.D. Synthetic Cathinone and Cannabinoid Designer Drugs Pose a Major Risk for Public Health. Front. Psychiatry 2017, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Zanda, M.T.; Fadda, P.; Chiamulera, C.; Fratta, W.; Fattore, L. Methoxetamine, a novel psychoactive substance with serious adverse pharmacological effects: A review of case reports and preclinical findings. Behav. Pharmacol. 2016, 27, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.I.; Chen, L.Y.; Chen, H.Y.; Yu, J.H.; Su, Y.J.; Liu, S.W.; Tracy, D.K.; Chen, Y.C.; Lin, C.C.; Fang, C.C. Gender differences in clinical characteristics of emergency department patients involving illicit drugs use with analytical confirmation. J. Formos. Med. Assoc. 2022, 121, 1832–1840. [Google Scholar] [CrossRef]
- Wronikowska, O.; Zykubek, M.; Kurach, Ł.; Michalak, A.; Boguszewska-Czubara, A.; Budzyńska, B. Vulnerability factors for mephedrone-induced conditioned place preference in rats-the impact of sex differences, social-conditioning and stress. Psychopharmacology 2021, 238, 2947–2961. [Google Scholar] [CrossRef]
- Fattore, L.; Marti, M.; Mostallino, R.; Castelli, M.P. Sex and Gender Differences in the Effects of Novel Psychoactive Substances. Brain Sci. 2020, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.; Shi, X.; Goodair, C.; Cheeta, S. Trends in Mortality from Novel Psychoactive Substances as “Legal Highs”: Gender Differences in Manner of Death and Implications for Risk Differences for Women. Front. Psychiatry 2022, 13, 890840. [Google Scholar] [CrossRef]
- Manke, H.N.; Nelson, K.H.; Vlachos, A.; Bailey, J.M.; Maradiaga, K.J.; Weiss, T.D.; Rice, K.C.; Riley, A.L. Assessment of aversive effects of methylone in male and female Sprague-Dawley rats: Conditioned taste avoidance, body temperature and activity/stereotypies. Neurotoxicol. Teratol. 2021, 86, 106977. [Google Scholar] [CrossRef] [PubMed]
- Miliano, C.; Marti, M.; Pintori, N.; Castelli, M.P.; Tirri, M.; Arfè, R.; De Luca, M.A. Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe. Front. Pharmacol. 2019, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Chartoff, E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 2019, 44, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Fattore, L.; Melis, M.; Fadda, P.; Fratta, W. Sex differences in addictive disorders. Front. Neuroendocrinol. 2014, 35, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Reed, D.; Service, A.G.; Langford, A.M. The identification of 2-chloro-4,5-methylenedioxymethylamphetamine in an illicit drug seizure. J. Forensic Sci. 2000, 45, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Maresova, V.; Hampl, J.; Chundela, Z.; Zrcek, F.; Polasek, M.; Chadt, J. The identification of a chlorinated MDMA. J. Anal. Toxicol. 2005, 29, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Plummer, C.M.; Breadon, T.W.; Pearson, J.R.; Jones, O.A.H. The synthesis and characterisation of MDMA derived from a catalytic oxidation of material isolated from black pepper reveals potential route specific impurities. Sci. Justice 2016, 56, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Fowble, K.L.; Shepard, J.R.E.; Musah, R.A. Identification and classification of cathinone unknowns by statistical analysis processing of direct analysis in real time-high resolution mass spectrometry-derived “neutral loss” spectra. Talanta 2018, 179, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Grapp, M.; Kaufmann, C.; Schwelm, H.M.; Neukamm, M.A.; Blaschke, S.; Eidizadeh, A. Intoxication cases associated with the novel designer drug 3′,4′-methylenedioxy-α-pyrrolidinohexanophenone and studies on its human metabolism using high-resolution mass spectrometry. Drug Test. Anal. 2020, 12, 1320–1335. [Google Scholar] [CrossRef] [PubMed]
- Beck, O.; Bäckberg, M.; Signell, P.; Helander, A. Intoxications in the STRIDA project involving a panorama of psychostimulant pyrovalerone derivatives, MDPV copycats. Clin. Toxicol. 2018, 56, 256–263. [Google Scholar] [CrossRef]
- Adamowicz, P.; Hydzik, P. Fetal death associated with the use of 3,4-MDPHP and α-PHP. Clin. Toxicol. 2019, 57, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Di Candia, D.; Boracchi, M.; Ciprandi, B.; Giordano, G.; Zoja, R. A unique case of death by MDPHP with no other co-ingestion: A forensic toxicology case. Int. J. Legal Med. 2022, 136, 1291–1296. [Google Scholar] [CrossRef]
- Sogos, V.; Caria, P.; Porcedda, C.; Mostallino, R.; Piras, F.; Miliano, C.; de Luca, M.A.; Castelli, M.P. Human Neuronal Cell Lines as An In Vitro Toxicological Tool for the Evaluation of Novel Psychoactive Substances. Int. J. Mol. Sci. 2021, 22, 6785. [Google Scholar] [CrossRef] [PubMed]
- Aarde, S.M.; Taffe, M.A. Predicting the Abuse Liability of Entactogen-Class, New and Emerging Psychoactive Substances via Preclinical Models of Drug Self-administration. Curr. Top. Behav. Neurosci. 2017, 32, 145–164. [Google Scholar] [PubMed]
- Gannon, B.M.; Baumann, M.H.; Walther, D.; Jimenez-Morigosa, C.; Sulima, A.; Rice, K.C.; Collins, G.T. The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 2018, 43, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: Para-halogenated amphetamines and pyrovalerone cathinones. Eur. Neuropsychopharmacol. 2015, 25, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.L.; Nelson, K.H.; To, P.; López-Arnau, R.; Xu, P.; Wang, D.; Wang, Y.; Shen, H.W.; Kuhn, D.M.; Angoa-Perez, M.; et al. Abuse potential and toxicity of the synthetic cathinones (i.e., “Bath salts”). Neurosci. Biobehav. Rev. 2020, 110, 150–173. [Google Scholar] [CrossRef]
- Simmler, L.D.; Liechti, M.E. Pharmacology of MDMA- and Amphetamine-Like New Psychoactive Substances. Handb. Exp. Pharmacol. 2018, 252, 143–164. [Google Scholar] [PubMed]
- Solis, E., Jr.; Partilla, J.S.; Sakloth, F.; Ruchala, I.; Schwienteck, K.L.; de Felice, L.J.; Eltit, J.M.; Glennon, R.A.; Negus, S.S.; Baumann, M.H. N-Alkylated Analogs of 4-Methylamphetamine (4-MA) Differentially Affect Monoamine Transporters and Abuse Liability. Neuropsychopharmacology 2017, 42, 1950–1961. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Velasco, M.; Reguilón, M.D.; Bellot, M.; Nadal-Gratacós, N.; Berzosa, X.; Gómez-Canela, C.; Rodríguez-Arias, M.; Camarasa, J.; Escubedo, E.; Pubill, D.; et al. Repeated administration of N-ethyl-pentedrone induces increased aggression and impairs social exploration after withdrawal in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2022, 117, 110562. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Piras, G.; De Luca, M.A.; Perra, V.; Bordi, F.; Borsini, F.; Frau, R.; Di Chiara, G. Evidence for a role of a dopamine/5-HT6 receptor interaction in cocaine reinforcement. Neuropharmacology 2013, 65, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, A.; Lecca, D.; Valentini, V.; Bahi, A.; Dreyer, J.L.; Cacciapaglia, F.; Scifo, A.; Piras, G.; Cadoni, C.; Di Chiara, G. Impairment of acquisition of intravenous cocaine self-administration by RNA-interference of dopamine D1-receptors in the nucleus accumbens shell. Neuropharmacology 2015, 89, 398–411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boero, G.; Pisu, M.G.; Biggio, F.; Muredda, L.; Carta, G.; Banni, S.; Paci, E.; Follesa, P.; Concas, A.; Porcu, P.; et al. Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology 2018, 133, 242–253. [Google Scholar] [CrossRef]
- Talani, G.; Biggio, G.; Sanna, E. Enhanced sensitivity to ethanol induced inhibition of LTP in CA1 pyramidal neurons of socially isolated C57BL/6J mice: Role of neurosteroids. Front. Endocrinol. 2011, 2, 56. [Google Scholar] [CrossRef]
- Talani, G.; Licheri, V.; Biggio, F.; Locci, V.; Mostallino, M.C.; Secci, P.P.; Melis, V.; Dazzi, L.; Carta, G.; Banni, S.; et al. Enhanced glutamatergic synaptic plasticity in the hippocampal ca1 field of food-restricted rats: Involvement of CB1 receptors. Neuropsychopharmacology 2016, 41, 1308–1318. [Google Scholar] [CrossRef]
- Bassareo, V.; Talani, G.; Frau, R.; Porru, S.; Rosas, M.; Kasture, S.B.; Peana, A.T.; Loi, E.; Sanna, E.; Acquas, E. Inhibition of Morphine- and Ethanol-Mediated Stimulation of Mesolimbic Dopamine Neurons by Withania somnifera. Front. Neurosci. 2019, 13, 545. [Google Scholar] [CrossRef]
- Porcu, P.; Locci, A.; Santoru, F.; Berretti, R.; Morrow, A.L.; Concas, A. Failure of acute ethanol administration to alter cerebrocortical and hippocampal allopregnanolone levels in C57BL/6J and DBA/2J mice. Alcohol Clin. Exp. Res. 2014, 38, 948–958. [Google Scholar] [CrossRef]
- O’Connor, E.C.; Chapman, K.; Butler, P.; Mead, A.N. The predictive validity of the rat self-administration model for abuse liability. Neurosci. Biobehav. Rev. 2011, 35, 912–938. [Google Scholar] [CrossRef]
- Zanda, M.T.; Fattore, L. Old and new synthetic cannabinoids: Lessons from animal models. Drug Metab. Rev. 2018, 50, 54–64. [Google Scholar] [CrossRef]
- Schenk, S.; Gittings, D.; Johnstone, M.; Daniela, E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology 2003, 169, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ratzenboeck, E.; Saria, A.; Kriechbaum, N.; Zernig, G. Reinforcing effects of MDMA (“ecstasy”) in drug-naive and cocaine-trained rats. Pharmacology 2001, 62, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Creehan, K.M.; Vandewater, S.A.; Taffe, M.A. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology 2015, 92, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Vandewater, S.A.; Creehan, K.M.; Taffe, M.A. Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology 2015, 99, 538–545. [Google Scholar] [CrossRef]
- Aarde, S.M.; Huang, P.K.; Creehan, K.M.; Dickerson, T.J.; Taffe, M.A. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: Self-administration and locomotor activity in rats. Neuropharmacology 2013, 71, 130–140. [Google Scholar] [CrossRef]
- Watterson, L.R.; Kufahl, P.R.; Nemirovsky, N.E.; Sewalia, K.; Grabenauer, M.; Thomas, B.F.; Marusich, J.A.; Wegner, S.; Olive, M.F. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict. Biol. 2014, 19, 165–174. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ma, S.X.; Hur, K.H.; Lee, Y.; Ko, Y.H.; Lee, B.R.; Kim, S.K.; Sung, S.J.; Kim, K.M.; Kim, H.C.; et al. New designer phenethylamines 2C-C and 2C-P have abuse potential and induce neurotoxicity in rodents. Arch. Toxicol. 2021, 95, 1413–1429. [Google Scholar] [CrossRef]
- Lai, M.; Fu, D.; Xu, Z.; Du, H.; Liu, H.; Wang, Y.; Xu, P.; Zhou, W. Relative reinforcing effects of dibutylone, ethylone, and N-ethylpentylone: Self-administration and behavioral economics analysis in rats. Psychopharmacology 2022, 239, 2875–2884. [Google Scholar] [CrossRef]
- Schindler, C.W.; Thorndike, E.B.; Goldberg, S.R.; Lehner, K.R.; Cozzi, N.V.; Brandt, S.D.; Baumann, M.H. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology 2016, 233, 1981–1990. [Google Scholar] [CrossRef]
- Custodio, R.J.P.; Sayson, L.V.; Botanas, C.J.; Abiero, A.; Kim, M.; Lee, H.J.; Ryu, H.W.; Lee, Y.S.; Kim, H.J.; Cheong, J.H. Two newly-emerging substituted phenethylamines MAL and BOD induce differential psychopharmacological effects in rodents. J. Psychopharmacol. 2020, 34, 1056–1067. [Google Scholar] [CrossRef]
- Gerra, G.; Bassignana, S.; Zaimovic, A.; Moi, G.; Bussandri, M.; Caccavari, R.; Brambilla, F.; Molina, E. Hypothalamic-pituitary-adrenal axis responses to stress in subjects with 3,4-methylenedioxy-methamphetamine (‘ecstasy’) use history: Correlation with dopamine receptor sensitivity. Psychiatry Res. 2003, 120, 115–124. [Google Scholar] [CrossRef]
- Dumont, G.J.; Verkes, R.J. A review of acute effects of 3,4-methylenedioxymethamphetamine in healthy volunteers. J. Psychopharmacol. 2006, 20, 176–187. [Google Scholar] [CrossRef]
- Mas, M.; Farré, M.; de la Torre, R.; Roset, P.N.; Ortuño, J.; Segura, J.; Camí, J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J. Pharmacol. Exp. Ther. 1999, 290, 136–145. [Google Scholar] [PubMed]
- Graham, D.L.; Herring, N.R.; Schaefer, T.L.; Vorhees, C.V.; Williams, M.T. Glucose and corticosterone changes in developing and adult rats following exposure to (+/−)-3,4-methylendioxymethamphetamine or 5-methoxydiisopropyltryptamine. Neurotoxicol. Teratol. 2010, 32, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Walker, Q.D.; Williams, C.N.; Jotwani, R.P.; Waller, S.T.; Francis, R.; Kuhn, C.M. Sex differences in the neurochemical and functional effects of MDMA in Sprague-Dawley rats. Psychopharmacology 2007, 189, 435–445. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, J.P.; Yeh, C.M.; Hsu, K.S. Sex difference in stress-induced enhancement of hippocampal CA1 long-term depression during puberty. Hippocampus 2012, 22, 1622–1634. [Google Scholar] [CrossRef]
- Sze, Y.; Gill, A.C.; Brunton, P.J. Sex-dependent changes in neuroactive steroid concentrations in the rat brain following acute swim stress. J. Neuroendocrinol. 2018, 30, e12644. [Google Scholar] [CrossRef]
- Critchlow, V.; Liebelt, R.A.; Bar-Sela, M.; Mountcastle, W.; Lipscomb, H.S. Sex difference in resting pituitary-adrenal function in the rat. Am. J. Physiol. 1963, 205, 807–815. [Google Scholar] [CrossRef]
- Atkinson, H.C.; Waddell, B.J. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: Sexual dimorphism and changes across the estrous cycle. Endocrinology 1997, 138, 3842–3848. [Google Scholar] [CrossRef]
- Quirós Cognuck, S.; Reis, W.L.; Silva, M.; Debarba, L.K.; Mecawi, A.S.; de Paula, F.J.A.; Rodrigues Franci, C.; Elias, L.L.K.; Antunes-Rodrigues, J. Sex differences in body composition, metabolism-related hormones, and energy homeostasis during aging in Wistar rats. Physiol. Rep. 2020, 8, e14597. [Google Scholar] [CrossRef]
- Vamvakopoulos, N.C.; Chrousos, G.P. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimorphism of the stress response and immune/inflammatory reaction. J. Clin. Investig. 1993, 92, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Porceddu, P.F.; Serra, M.; Casu, M.A.; Schiano, V.; Napolitano, F.; Pinna, A.; Usiello, A.; Morelli, M. Lack of Rhes Increases MDMA-Induced Neuroinflammation and Dopamine Neuron Degeneration: Role of Gender and Age. Int. J. Mol. Sci. 2019, 20, 1556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, S.; Yang, C.; Jin, G.; Zhen, X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology 2008, 199, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cunningham, K.A.; Thomas, M.L. Estrogen regulation of gene expression in the brain: A possible mechanism altering the response to psychostimulants in female rats. Brain Res. Mol. Brain Res. 2002, 100, 75–83. [Google Scholar] [CrossRef]
- Han, X.; Jing, M.Y.; Zhao, T.Y.; Wu, N.; Song, R.; Li, J. Role of dopamine projections from ventral tegmental area to nucleus accumbens and medial prefrontal cortex in reinforcement behaviors assessed using optogenetic manipulation. Metab. Brain Dis. 2017, 32, 1491–1502. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisanu, A.; Lo Russo, G.; Talani, G.; Bratzu, J.; Siddi, C.; Sanna, F.; Diana, M.; Porcu, P.; De Luca, M.A.; Fattore, L. Effects of the Phenethylamine 2-Cl-4,5-MDMA and the Synthetic Cathinone 3,4-MDPHP in Adolescent Rats: Focus on Sex Differences. Biomedicines 2022, 10, 2336. https://doi.org/10.3390/biomedicines10102336
Pisanu A, Lo Russo G, Talani G, Bratzu J, Siddi C, Sanna F, Diana M, Porcu P, De Luca MA, Fattore L. Effects of the Phenethylamine 2-Cl-4,5-MDMA and the Synthetic Cathinone 3,4-MDPHP in Adolescent Rats: Focus on Sex Differences. Biomedicines. 2022; 10(10):2336. https://doi.org/10.3390/biomedicines10102336
Chicago/Turabian StylePisanu, Augusta, Giacomo Lo Russo, Giuseppe Talani, Jessica Bratzu, Carlotta Siddi, Fabrizio Sanna, Marco Diana, Patrizia Porcu, Maria Antonietta De Luca, and Liana Fattore. 2022. "Effects of the Phenethylamine 2-Cl-4,5-MDMA and the Synthetic Cathinone 3,4-MDPHP in Adolescent Rats: Focus on Sex Differences" Biomedicines 10, no. 10: 2336. https://doi.org/10.3390/biomedicines10102336
APA StylePisanu, A., Lo Russo, G., Talani, G., Bratzu, J., Siddi, C., Sanna, F., Diana, M., Porcu, P., De Luca, M. A., & Fattore, L. (2022). Effects of the Phenethylamine 2-Cl-4,5-MDMA and the Synthetic Cathinone 3,4-MDPHP in Adolescent Rats: Focus on Sex Differences. Biomedicines, 10(10), 2336. https://doi.org/10.3390/biomedicines10102336