Immunomodulation by Hemoadsorption—Changes in Hepatic Biotransformation Capacity in Sepsis and Septic Shock: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design
2.3. Study Protocol
- T1: Preadsorber: (−4 h) before connection to hemoadsorption with CytoSorb®.
- T2: 24 h after the start of CytoSorb® therapy.
- T3: 48 h after the start of CytoSorb® therapy.
- T4: Postadsorber: 24 h after completion of CytoSorb® therapy.
2.4. LiMAx® Test
2.5. CytoSorb®
2.6. Definition of Outcomes
2.7. Statistical Analysis
3. Results
3.1. Demographic Values
3.2. Hepatic Dysfunction
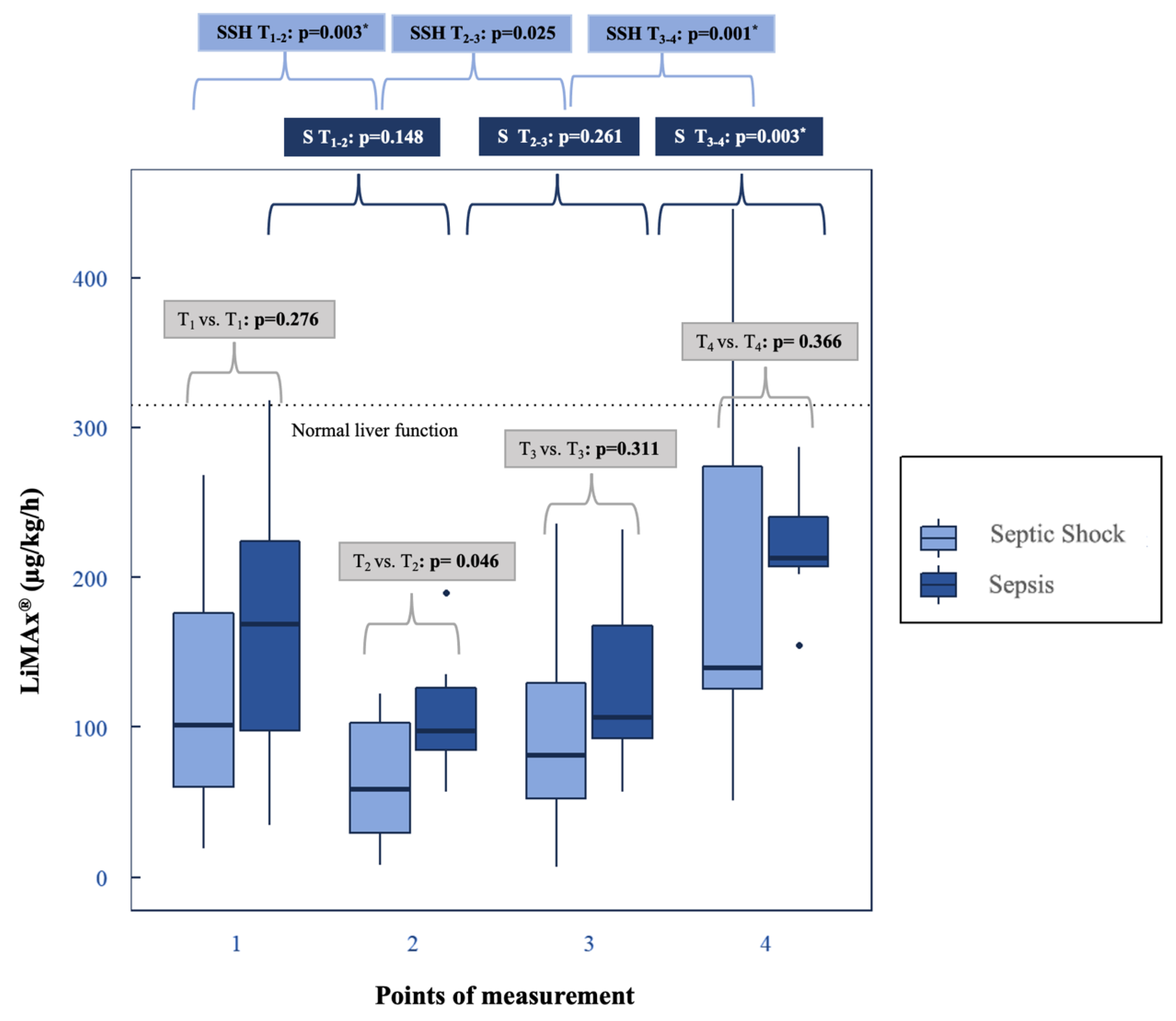
3.2.1. Dynamic Liver Test: LiMAx®
3.2.2. Static Liver Parameters
3.3. Shock Reversal
4. Discussion
4.1. Aspects of Feasibility
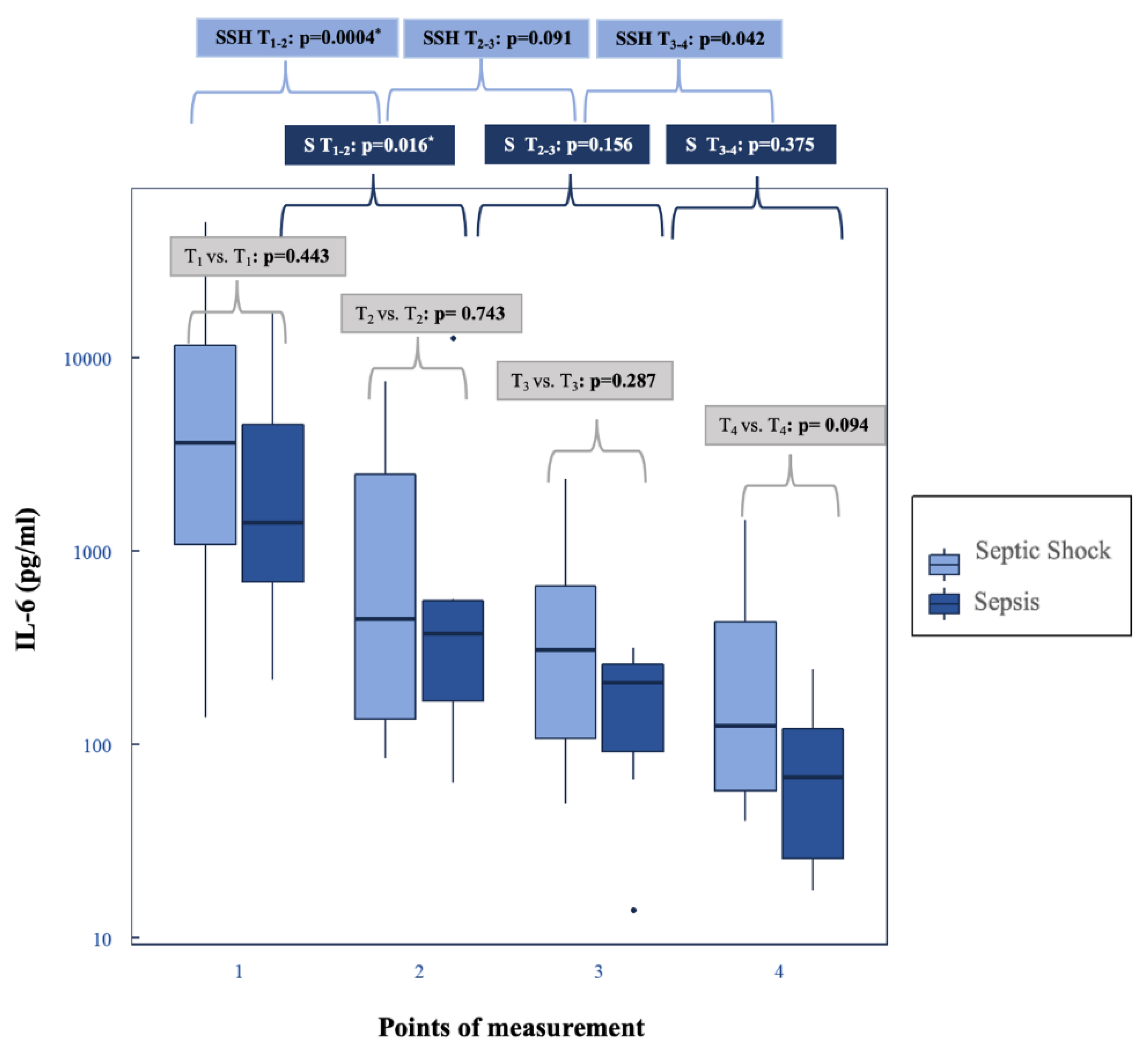
4.2. Decrease in Plasma IL-6 Levels and Shock Reversal
4.3. LiMAx® under Hemoadsorption with CytoSorb®: Progression and Clinical Benefits
4.4. LiMAx® Is Superior to Assess Dysfunction Compared to Static Hepatic Parameters
5. Limitations
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Schwarzkopf, D.; Reinhart, K. Inzidenz der Sepsis in Deutschland und weltweit. Med. Klin.-Intensivmed. Und Notf. 2021, 117, 264–268. [Google Scholar] [CrossRef]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Brienza, N.; Dalfino, L.; Cinnella, G.; Diele, C.; Bruno, F.; Fiore, T. Jaundice in critical illness: Promoting factors of a concealed reality. Intensive Care Med. 2006, 32, 267–274. [Google Scholar] [CrossRef]
- Soultati, A.S.; Dourakis, S.P.; Alexopoulou, A.; Deutsch, M.; Vasilieva, L.; Archimandritis, A.J. Predicting utility of a model for end stage liver disease in alcoholic liver disease. World J. Gastroenterol. 2006, 12, 4020–4025. [Google Scholar] [CrossRef]
- Kramer, L.; Jordan, B.; Druml, W.; Bauer, P.; Metnitz, P.G. Austrian Epidemiologic Study on Intensive Care, A.S.D.I.S.G. Incidence and prognosis of early hepatic dysfunction in critically ill patients—A prospective multicenter study. Crit. Care Med. 2007, 35, 1099–1104. [Google Scholar] [CrossRef]
- Patel, J.J.; Taneja, A.; Niccum, D.; Kumar, G.; Jacobs, E.; Nanchal, R. The association of serum bilirubin levels on the outcomes of severe sepsis. J. Intensive Care Med. 2015, 30, 23–29. [Google Scholar] [CrossRef]
- Zhai, R.; Sheu, C.C.; Su, L.; Gong, M.N.; Tejera, P.; Chen, F.; Wang, Z.; Convery, M.P.; Thompson, B.T.; Christiani, D.C. Serum bilirubin levels on ICU admission are associated with ARDS development and mortality in sepsis. Thorax 2009, 64, 784–790. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Felleiter, P.; Antonelli, M.; Vanhems, P.; Sakr, Y.; Vincent, J.L. Increased mortality in critically ill patients with mild or moderate hyperbilirubinemia. J. Crit. Care 2017, 40, 31–35. [Google Scholar] [CrossRef]
- Nesseler, N.; Launey, Y.; Aninat, C.; Morel, F.; Mallédant, Y.; Seguin, P. Clinical review: The liver in sepsis. Crit. Care 2012, 16, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wlodzimirow, K.A.; Eslami, S.; Abu-Hanna, A.; Nieuwoudt, M.; Chamuleau, R.A. Systematic review: Acute liver failure-one disease, more than 40 definitions. Aliment. Pharmacol. Ther. 2012, 35, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Woźnica, E.A.; Inglot, M.; Woźnica, R.K.; Łysenko, L. Liver dysfunction in sepsis. Adv. Clin. Exp. Med. 2018, 27, 547–551. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Jalan, R.; Gines, P.; Olson, J.C.; Mookerjee, R.P.; Moreau, R.; Garcia-Tsao, G.; Arroyo, V.; Kamath, P.S. Acute-on chronic liver failure. J. Hepatol. 2012, 57, 1336–1348. [Google Scholar] [CrossRef]
- Nesseler, N.; Launey, Y.; Aninat, C.; White, J.; Corlu, A.; Pieper, K.; Malledant, Y.; Seguin, P. Liver Dysfunction Is Associated with Long-Term Mortality in Septic Shock. Am. J. Respir. Crit. Care Med. 2016, 193, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Jäger, B.; Drolz, A.; Michl, B.; Schellongowski, P.; Bojic, A.; Nikfardjam, M.; Zauner, C.; Heinz, G.; Trauner, M.; Fuhrmann, V. Jaundice increases the rate of complications and one-year mortality in patients with hypoxic hepatitis. Hepatology 2012, 56, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, J.; Gomatos, I.P.; Tiniakos, D.G.; Memos, N.; Boutsikou, M.; Garatzioti, A.; Archimandritis, A.; Betrosian, A. Liver histology in ICU patients dying from sepsis: A clinico-pathological study. World J. Gastroenterol. 2008, 14, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Deng, F.; Qi, D.; Hu, Z.; Zhang, L. The Hyperbilirubinemia and Potential Predictors Influence on Long-Term Outcomes in Sepsis: A Population-Based Propensity Score-Matched Study. Front. Med. 2021, 8, 713917. [Google Scholar] [CrossRef]
- Spapen, H. Liver perfusion in sepsis, septic shock, and multiorgan failure. Anat. Rec. 2008, 291, 714–720. [Google Scholar] [CrossRef] [PubMed]
- te Boekhorst, T.; Urlus, M.; Doesburg, W.; Yap, S.H.; Goris, R.J. Etiologic factors of jaundice in severely ill patients. A retrospective study in patients admitted to an intensive care unit with severe trauma or with septic intra-abdominal complications following surgery and without evidence of bile duct obstruction. J. Hepatol. 1988, 7, 111–117. [Google Scholar] [CrossRef]
- Trauner, M.; Meier, P.J.; Boyer, J.L. Molecular pathogenesis of cholestasis. N. Engl. J. Med. 1998, 339, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.G. Assessing liver function. Curr. Opin. Crit. Care 2007, 13, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kaffarnik, M.F.; Lock, J.F.; Vetter, H.; Ahmadi, N.; Lojewski, C.; Malinowski, M.; Neuhaus, P.; Stockmann, M. Early diagnosis of sepsis-related hepatic dysfunction and its prognostic impact on survival: A prospective study with the LiMAx test. Crit. Care 2013, 17, R259. [Google Scholar] [CrossRef]
- Stockmann, M.; Lock, J.F.; Riecke, B.; Heyne, K.; Martus, P.; Fricke, M.; Lehmann, S.; Niehues, S.M.; Schwabe, M.; Lemke, A.J.; et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann. Surg. 2009, 250, 119–125. [Google Scholar] [CrossRef]
- Kaffarnik, M.F.; Ahmadi, N.; Lock, J.F.; Wuensch, T.; Pratschke, J.; Stockmann, M.; Malinowski, M. Correlation between plasma endothelin-1 levels and severity of septic liver failure quantified by maximal liver function capacity (LiMAx test). A prospective study. PLoS ONE 2017, 12, e0178237. [Google Scholar] [CrossRef] [PubMed]
- Wicha, S.G.; Frey, O.R.; Roehr, A.C.; Pratschke, J.; Stockmann, M.; Alraish, R.; Wuensch, T.; Kaffarnik, M. Linezolid in liver failure: Exploring the value of the maximal liver function capacity (LiMAx) test in a pharmacokinetic pilot study. Int. J. Antimicrob. Agents 2017, 50, 557–563. [Google Scholar] [CrossRef]
- Kirchner, C.; Sibai, J.; Schwier, E.; Henzler, D.; Eickmeyer, C.; Winde, G.; Köhler, T. Dosing of Antimycotic Treatment in Sepsis-Induced Liver Dysfunction by Functional Liver Testing with LiMAx®. Case Rep. Crit. Care 2019, 2019, 5362514. [Google Scholar] [CrossRef]
- Alraish, R.; Wicha, S.G.; Frey, O.R.; Roehr, A.C.; Pratschke, J.; Stockmann, M.; Wuensch, T.; Kaffarnik, M. Pharmacokinetics of tigecycline in critically ill patients with liver failure defined by maximal liver function capacity test (LiMAx). Ann. Intensive Care 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Buechter, M.; Gerken, G.; Hoyer, D.P.; Bertram, S.; Theysohn, J.M.; Thodou, V.; Kahraman, A. Liver maximum capacity (LiMAx) test as a helpful prognostic tool in acute liver failure with sepsis: A case report. BMC Anesthesiol. 2018, 18, 71. [Google Scholar] [CrossRef]
- Kaffarnik, M.; Stoeger, G.; Liebich, J.; Grieser, C.; Pratschke, J.; Stockmann, M. Liver Function, Quantified by LiMAx Test, After Major Abdominal Surgery. Comparison Between Open and Laparoscopic Approach. World J. Surg. 2018, 42, 557–566. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Krauser, J.A.; Johnson, W.W. Rate-limiting steps in oxidations catalyzed by rabbit cytochrome P450 1A2. Biochemistry 2004, 43, 10775–10788. [Google Scholar] [CrossRef]
- Stockmann, M. Wertigkeit Eines Neu Entwickelten Verfahrens Zur Bestimmung der Leberfunktion in der Leberchirurgie (LiMAx-Test); Charité-Universitätsmedizin Berlin: Berlin, Germany, 2009. [Google Scholar]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Niehues, S.M.; Seehofer, D.; Neuhaus, P. The LiMAx test: A new liver function test for predicting postoperative outcome in liver surgery. HPB 2010, 12, 139–146. [Google Scholar] [CrossRef]
- Jara, M.; Bednarsch, J.; Valle, E.; Lock, J.F.; Malinowski, M.; Schulz, A.; Seehofer, D.; Jung, T.; Stockmann, M. Reliable assessment of liver function using LiMAx. J. Surg. Res. 2015, 193, 184–189. [Google Scholar] [CrossRef]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Seehofer, D.; Puhl, G.; Pratschke, J.; Neuhaus, P. How to define initial poor graft function after liver transplantation?—A new functional definition by the LiMAx test. Transpl. Int. 2010, 23, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Pletz, M.W.; Altmann, S.; Kirchner, C.; Schwier, E.; Henzler, D.; Winde, G.; Eickmeyer, C. Pericarditis Caused by Enterococcus faecium with acute liver failure treated by a multifaceted approach including antimicrobials and hemoadsorption. Case Rep. Crit. Care 2021, 2021, 8824050. [Google Scholar] [CrossRef]
- Gemelli, C.; Cuoghi, A.; Magnani, S.; Atti, M.; Ricci, D.; Siniscalchi, A.; Mancini, E.; Faenza, S. Removal of Bilirubin with a New Adsorbent System: In Vitro Kinetics. Blood Purif. 2019, 47, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Gruda, M.C.; Ruggeberg, K.G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Venkataraman, R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit. Care Med. 2004, 32, 801–805. [Google Scholar] [CrossRef]
- Köhler, T.; Schwier, E.; Praxenthaler, J.; Kirchner, C.; Henzler, D.; Eickmeyer, C. Therapeutic Modulation of the Host Defense by Hemoadsorption with CytoSorb®-Basics, Indications and Perspectives-A Scoping Review. Int. J. Mol. Sci. 2021, 22, 12786. [Google Scholar] [CrossRef]
- Malard, B.; Lambert, C.; Kellum, J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Poli, E.C.; Rimmelé, T.; Schneider, A.G. Hemoadsorption with CytoSorb®. Intensive Care Med. 2019, 45, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Reiter, K.; Bordoni, V.; Dall’Olio, G.; Ricatti, M.G.; Soli, M.; Ruperti, S.; Soffiati, G.; Galloni, E.; D’Intini, V.; Bellomo, R.; et al. In vitro removal of therapeutic drugs with a novel adsorbent system. Blood Purif. 2002, 20, 380–388. [Google Scholar] [CrossRef]
- Rieckmann, J.C.; Geiger, R.; Hornburg, D.; Wolf, T.; Kveler, K.; Jarrossay, D.; Sallusto, F.; Shen-Orr, S.S.; Lanzavecchia, A.; Mann, M.; et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat. Immunol. 2017, 18, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.M.; Hoste, E.; Molnár, Z.; Jacobs, R.; Joannes-Boyau, O.; Malbrain, M.L.N.G.; Forni, L.G. Cytokine removal in human septic shock: Where are we and where are we going? Ann. Intensive Care 2019, 9, 56. [Google Scholar] [CrossRef]
- Koehler, T.; Schwier, E.; Henzler, D.; Eickmeyer, C. Does adjunctive hemoadsorption with CytoSorb affect survival of COVID-19 patients on ECMO? A critical statement. J. Crit. Care 2021, 66, 187–188. [Google Scholar] [CrossRef]
- König, C.; Röhr, A.C.; Frey, O.R.; Brinkmann, A.; Roberts, J.A.; Wichmann, D.; Braune, S.; Kluge, S.; Nierhaus, A. In vitro removal of anti-infective agents by a novel cytokine adsorbent system. Int. J. Artif. Organs 2019, 42, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kogelmann, K.; Jarczak, D.; Scheller, M.; Drüner, M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit. Care 2017, 21, 74. [Google Scholar] [CrossRef]
- Brouwer, W.P.; Duran, S.; Kuijper, M.; Ince, C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: A propensity-score-weighted retrospective study. Crit. Care 2019, 23, 317. [Google Scholar] [CrossRef]
- Rugg, C.; Klose, R.; Hornung, R.; Innerhofer, N.; Bachler, M.; Schmid, S.; Fries, D.; Ströhle, M. Hemoadsorption with CytoSorb in Septic Shock Reduces Catecholamine Requirements and In-Hospital Mortality: A Single-Center Retrospective ‘Genetic’ Matched Analysis. Biomedicines 2020, 8, 539. [Google Scholar] [CrossRef]
- Schultz, P.; Schwier, E.; Eickmeyer, C.; Henzler, D.; Köhler, T. High-dose CytoSorb hemoadsorption is associated with improved survival in patients with septic shock: A retrospective cohort study. J. Crit. Care 2021, 64, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Dhokia, V.D.; Madhavan, D.; Austin, A.; Morris, C.G. Novel use of Cytosorb™ haemadsorption to provide biochemical control in liver impairment. J. Intensive Care Soc. 2019, 20, 174–181. [Google Scholar] [CrossRef]
- Ocskay, K.; Tomescu, D.; Faltlhauser, A.; Jacob, D.; Friesecke, S.; Malbrain, M.; Kogelmann, K.; Bogdanski, R.; Bach, F.; Fritz, H.; et al. Hemoadsorption in ‘Liver Indication’-Analysis of 109 Patients’ Data from the CytoSorb International Registry. J. Clin. Med. 2021, 10, 5182. [Google Scholar] [CrossRef]
- Scharf, C.; Liebchen, U.; Paal, M.; Becker-Pennrich, A.; Irlbeck, M.; Zoller, M.; Schroeder, I. Successful elimination of bilirubin in critically ill patients with acute liver dysfunction using a cytokine adsorber and albumin dialysis: A pilot study. Sci. Rep. 2021, 11, 10190. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nakajima, M.; Chiba, K.; Yamamoto, T.; Tani, M.; Ishizaki, T.; Kuroiwa, Y. Inhibitory effects of antiarrhythmic drugs on phenacetin O-deethylation catalysed by human CYP1A2. Br. J. Clin. Pharmacol. 1998, 45, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Batty, K.T.; Davis, T.M.; Ilett, K.F.; Dusci, L.J.; Langton, S.R. The effect of ciprofloxacin on theophylline pharmacokinetics in healthy subjects. Br. J. Clin. Pharmacol. 1995, 39, 305–311. [Google Scholar] [CrossRef]
- Granfors, M.T.; Backman, J.T.; Neuvonen, M.; Neuvonen, P.J. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin. Pharmacol. Ther. 2004, 76, 598–606. [Google Scholar] [CrossRef]
- Danie, W.A.; Syrek, M.; Ryłko, Z.; Wójcikowski, J. Effects of antidepressant drugs on the activity of cytochrome P-450 measured by caffeine oxidation in rat liver microsomes. Pol. J. Pharmacol. 2001, 53, 351–357. [Google Scholar]
- Schwier, E.; Kirchner, C.; Eickmeyer, C.; Winde, G.; Henzler, D.; Kohler, T. Profound decrease of liver maximum function capacity test of isoflurane sedated patients: A report of three cases. Clin. Case Rep. 2021, 9, e04862. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Riecke, B.; Neuhaus, P.; Stockmann, M. Major influence of oxygen supply on 13CO2:12CO2 ratio measurement by nondispersive isotope-selective infrared spectroscopy. Helicobacter 2005, 10, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Palevsky, P.M. Renal replacement therapy in acute kidney injury. Adv. Chronic Kidney Dis. 2013, 20, 76–84. [Google Scholar] [CrossRef]
- Dimski, T.; Brandenburger, T.; Slowinski, T.; Kindgen-Milles, D. Feasibility and safety of combined cytokine adsorption and continuous veno-venous hemodialysis with regional citrate anticoagulation in patients with septic shock. Int. J. Artif. Organs 2020, 43, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Sims, M.; Coats, T.; Thompson, J.P. The microcirculation and its measurement in sepsis. J. Intensive Care Soc. 2017, 18, 221–227. [Google Scholar] [CrossRef]
- Ince, C. The microcirculation is the motor of sepsis. Crit. Care 2005, 9 (Suppl. 4), S13–S19. [Google Scholar] [CrossRef]
- Remick, D.G. Pathophysiology of sepsis. Am. J. Pathol. 2007, 170, 1435–1444. [Google Scholar] [CrossRef]
- Henzler, D.; Scheffler, M.; Westheider, A.; Köhler, T. Microcirculation measurements: Barriers for use in clinical routine. Clin. Hemorheol. Microcirc. 2017, 67, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Friesecke, S.; Stecher, S.S.; Gross, S.; Felix, S.B.; Nierhaus, A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: A prospective single-center study. J. Artif. Organs 2017, 20, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Hawchar, F.; Rao, C.; Akil, A.; Mehta, Y.; Rugg, C.; Scheier, J.; Adamson, H.; Deliargyris, E.; Molnar, Z. The Potential Role of Extracorporeal Cytokine Removal in Hemodynamic Stabilization in Hyperinflammatory Shock. Biomedicines 2021, 9, 768. [Google Scholar] [CrossRef]
- Träger, K.; Fritzler, D.; Fischer, G.; Schröder, J.; Skrabal, C.; Liebold, A.; Reinelt, H. Treatment of post-cardiopulmonary bypass SIRS by hemoadsorption: A case series. Int. J. Artif. Organs 2016, 39, 141–146. [Google Scholar] [CrossRef]
- Träger, K.; Schütz, C.; Fischer, G.; Schröder, J.; Skrabal, C.; Liebold, A.; Reinelt, H. Cytokine Reduction in the Setting of an ARDS-Associated Inflammatory Response with Multiple Organ Failure. Case Rep. Crit. Care 2016, 2016, 9852073. [Google Scholar] [CrossRef]
- Träger, K.; Skrabal, C.; Fischer, G.; Datzmann, T.; Schroeder, J.; Fritzler, D.; Hartmann, J.; Liebold, A.; Reinelt, H. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass-a case series. Int. J. Artif. Organs 2017, 40, 240–249. [Google Scholar] [CrossRef]
- Träger, K.; Skrabal, C.; Fischer, G.; Schroeder, J.; Marenski, L.; Liebold, A.; Reinelt, H.; Datzmann, T. Hemoadsorption treatment with CytoSorb in patients with extracorporeal life support therapy: A case series. Int. J. Artif. Organs 2019, 43, 422–429. [Google Scholar] [CrossRef]
- Henrion, J.; Schapira, M.; Luwaert, R.; Colin, L.; Delannoy, A.; Heller, F.R. Hypoxic hepatitis: Clinical and hemodynamic study in 142 consecutive cases. Medicine 2003, 82, 392–406. [Google Scholar] [CrossRef]
- Lescot, T.; Karvellas, C.; Beaussier, M.; Magder, S. Acquired liver injury in the intensive care unit. Anesthesiology 2012, 117, 898–904. [Google Scholar] [CrossRef]
- Tapper, E.B.; Sengupta, N.; Bonder, A. The Incidence and Outcomes of Ischemic Hepatitis: A Systematic Review with Meta-analysis. Am. J. Med. 2015, 128, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Elferink, M.G.; Olinga, P.; Draaisma, A.L.; Merema, M.T.; Faber, K.N.; Slooff, M.J.; Meijer, D.K.; Groothuis, G.M. LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1008–G1016. [Google Scholar] [CrossRef] [PubMed]
- Geier, A.; Fickert, P.; Trauner, M. Mechanisms of disease: Mechanisms and clinical implications of cholestasis in sepsis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Moseley, R.H. Sepsis and cholestasis. Clin. Liver Dis. 2004, 8, 83–94. [Google Scholar] [CrossRef]
- Trauner, M.; Fickert, P.; Stauber, R.E. Inflammation-induced cholestasis. J. Gastroenterol. Hepatol. 1999, 14, 946–959. [Google Scholar] [CrossRef]
- Horn, P. Leberwertveränderungen und Leberdysfunktion bei Sepsis. Intensiv-Und Notf. 2020, 45, 159. [Google Scholar] [CrossRef]
- Kasper, P.; Tacke, F.; Steffen, H.-M.; Michels, G. Leberfunktionsstörungen bei Sepsis. Med. Klin.-Intensivmed. Und Notf. 2020, 115, 609–619. [Google Scholar] [CrossRef]
- Sponholz, C.; Gonnert, F.A.; Kortgen, A.; Bauer, M. Monitoring of liver function in the critically ill. Anaesthesist 2014, 63, 603–612. [Google Scholar] [CrossRef]
- Lock, J.F.; Kotobi, A.N.; Malinowski, M.; Schulz, A.; Jara, M.; Neuhaus, P.; Stockmann, M. Predicting the prognosis in acute liver failure: Results from a retrospective pilot study using the LiMAx test. Ann. Hepatol. 2013, 12, 556–562. [Google Scholar] [CrossRef]
- Stockmann, M.; Vondran, F.W.R.; Fahrner, R.; Tautenhahn, H.M.; Mittler, J.; Bektas, H.; Malinowski, M.; Jara, M.; Klein, I.; Lock, J.F.; et al. Randomized clinical trial comparing liver resection with and without perioperative assessment of liver function. BJS Open 2018, 2, 301–309. [Google Scholar] [CrossRef]
- Alraish, R.; Wicha, S.G.; Frey, O.R.; Roehr, A.C.; Pratschke, J.; Stockmann, M.; Wuensch, T.; Kaffarnik, M. Liver function, quantified by the LiMAx test, as a predictor for the clinical outcome of critically ill patients treated with linezolid. Technol. Health Care 2022, 30, 309–321. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Thamm, K.; Schmidt, B.M.W.; Falk, C.S.; Kielstein, J.T. Effect of extracorporeal cytokine removal on vascular barrier function in a septic shock patient. J. Intensive Care 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Ziegeler, S.; Kindgen-Milles, D. Rationale of Hemoadsorption during Extracorporeal Membrane Oxygenation Support. Blood Purif. 2019, 48, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Rimmelé, T.; Kellum, J.A. Clinical review: Blood purification for sepsis. Crit. Care 2011, 15, 205. [Google Scholar] [CrossRef]
- Friesecke, S.; Träger, K.; Schittek, G.A.; Molnar, Z.; Bach, F.; Kogelmann, K.; Bogdanski, R.; Weyland, A.; Nierhaus, A.; Nestler, F.; et al. International registry on the use of the CytoSorb® adsorber in ICU patients: Study protocol and preliminary results. Med. Klin. Intensivmed. Notfmed. 2019, 114, 699–707. [Google Scholar] [CrossRef]
- Nemeth, E.; Kovacs, E.; Racz, K.; Soltesz, A.; Szigeti, S.; Kiss, N.; Csikos, G.; Koritsanszky, K.B.; Berzsenyi, V.; Trembickij, G.; et al. Impact of intraoperative cytokine adsorption on outcome of patients undergoing orthotopic heart transplantation-an observational study. Clin. Transplant. 2018, 32, e13211. [Google Scholar] [CrossRef]
- Scharf, C.; Schroeder, I.; Paal, M.; Winkels, M.; Irlbeck, M.; Zoller, M.; Liebchen, U. Can the cytokine adsorber CytoSorb((R)) help to mitigate cytokine storm and reduce mortality in critically ill patients? A propensity score matching analysis. Ann. Intensive Care 2021, 11, 115. [Google Scholar] [CrossRef]
- Shiga, H.; Hirasawa, H.; Nishida, O.; Oda, S.; Nakamura, M.; Mashiko, K.; Matsuda, K.; Kitamura, N.; Kikuchi, Y.; Fuke, N. Continuous hemodiafiltration with a cytokine-adsorbing hemofilter in patients with septic shock: A preliminary report. Blood Purif. 2014, 38, 211–218. [Google Scholar] [CrossRef]
- Bauer, M.; Press, A.T.; Trauner, M. The liver in sepsis: Patterns of response and injury. Curr. Opin. Crit. Care 2013, 19, 123–127. [Google Scholar] [CrossRef]
- Bolder, U.; Ton-Nu, H.T.; Schteingart, C.D.; Frick, E.; Hofmann, A.F. Hepatocyte transport of bile acids and organic anions in endotoxemic rats: Impaired uptake and secretion. Gastroenterology 1997, 112, 214–225. [Google Scholar] [CrossRef]
- Crawford, J.M.; Boyer, J.L. Clinicopathology conferences: Inflammation-induced cholestasis. Hepatology 1998, 28, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Drolz, A.; Trauner, M.; Fuhrmann, V. Liver Injury and Failure in Critical Illness. Hepatology 2019, 70, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver-guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.Y.; Li, L.; Zhao, X.; Ding, F.; Hou, X.; Peng, Z. Hemoperfusion with CytoSorb(R) in Critically Ill COVID-19 Patients. Blood Purif. 2021, 51, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, H.; Thelen, P.; Stroben, F.; Pigorsch, M.; Keller, T.; Krannich, A.; Spies, C.; Treskatsch, S.; Ocken, M.; Kunz, J.V.; et al. CytoSorb Rescue for COVID-19 Patients With Vasoplegic Shock and Multiple Organ Failure: A Prospective, Open-Label, Randomized Controlled Pilot Study. Crit. Care Med. 2022, 50, 964–976. [Google Scholar] [CrossRef]
- Supady, A.; Zahn, T.; Kuhl, M.; Maier, S.; Benk, C.; Kaier, K.; Böttiger, B.W.; Bode, C.; Lother, A.; Staudacher, D.L.; et al. Cytokine adsorption in patients with post-cardiac arrest syndrome after extracorporeal cardiopulmonary resuscitation (CYTER)-A single-centre, open-label, randomised, controlled trial. Resuscitation 2022, 173, 169–178. [Google Scholar] [CrossRef]
- Kogelmann, K.; Hübner, T.; Schwameis, F.; Drüner, M.; Scheller, M.; Jarczak, D. First Evaluation of a New Dynamic Scoring System Intended to Support Prescription of Adjuvant CytoSorb Hemoadsorption Therapy in Patients with Septic Shock. J. Clin. Med. 2021, 10, 2939. [Google Scholar] [CrossRef] [PubMed]
- Rubin, T.M.; Heyne, K.; Luchterhand, A.; Jan, B.; Vondran, F.W.R.; Polychronidis, G.; Malinowski, M.; Nikolic, A.; Tautenhahn, H.M.; Jara, M.; et al. Kinetic validation of the LiMAx test during 10 000 intravenous (13)C-methacetin breath tests. J. Breath Res. 2017, 12, 016005. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Kneidinger, N.; Herkner, H.; Heinz, G.; Nikfardjam, M.; Bojic, A.; Schellongowski, P.; Angermayr, B.; Schöniger-Hekele, M.; Madl, C.; et al. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011, 37, 1302–1310. [Google Scholar] [CrossRef]
- Recknagel, P.; Gonnert, F.A.; Westermann, M.; Lambeck, S.; Lupp, A.; Rudiger, A.; Dyson, A.; Carré, J.E.; Kortgen, A.; Krafft, C.; et al. Liver dysfunction and phosphatidylinositol-3-kinase signalling in early sepsis: Experimental studies in rodent models of peritonitis. PLoS Med. 2012, 9, e1001338. [Google Scholar] [CrossRef]
- Carcillo, J.A.; Doughty, L.; Kofos, D.; Frye, R.F.; Kaplan, S.S.; Sasser, H.; Burckart, G.J. Cytochrome P450 mediated-drug metabolism is reduced in children with sepsis-induced multiple organ failure. Intensive Care Med. 2003, 29, 980–984. [Google Scholar] [CrossRef]
- Frye, R.F.; Schneider, V.M.; Frye, C.S.; Feldman, A.M. Plasma levels of TNF-alpha and IL-6 are inversely related to cytochrome P450-dependent drug metabolism in patients with congestive heart failure. J. Card. Fail. 2002, 8, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Buechter, M.; Thimm, J.; Baba, H.A.; Bertram, S.; Willuweit, K.; Gerken, G.; Kahraman, A. Liver Maximum Capacity: A Novel Test to Accurately Diagnose Different Stages of Liver Fibrosis. Digestion 2019, 100, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Buechter, M.; Kersting, S.; Gerken, G.; Kahraman, A. Enzymatic liver function measured by LiMAx-a reliable diagnostic and prognostic tool in chronic liver disease. Sci. Rep. 2019, 9, 13577. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; van der Poll, T. Coagulation and sepsis. Thromb. Res. 2017, 149, 38–44. [Google Scholar] [CrossRef]
- Wheeler, A.P.; Bernard, G.R. Treating patients with severe sepsis. N. Engl. J. Med. 1999, 340, 207–214. [Google Scholar] [CrossRef]
- Levi, M.; de Jonge, E.; van der Poll, T. Sepsis and disseminated intravascular coagulation. J. Thromb. Thrombolysis 2003, 16, 43–47. [Google Scholar] [CrossRef]
- Khan, R.; Koppe, S. Modern management of acute liver failure. Gastroenterol. Clin. 2018, 47, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Schiodt, F.V.; Atillasoy, E.; Shakil, A.O.; Schiff, E.R.; Caldwell, C.; Kowdley, K.V.; Stribling, R.; Crippin, J.S.; Flamm, S.; Somberg, K.A.; et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transplant. Surg. 1999, 5, 29–34. [Google Scholar] [CrossRef]
- Ostapowicz, G.; Fontana, R.J.; Schiødt, F.V.; Larson, A.; Davern, T.J.; Han, S.H.; McCashland, T.M.; Shakil, A.O.; Hay, J.E.; Hynan, L.; et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002, 137, 947–954. [Google Scholar] [CrossRef]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin. Chem. 2000, 46, 2027–2049. [Google Scholar] [CrossRef]
- Jenniskens, M.; Langouche, L.; Vanwijngaerden, Y.M.; Mesotten, D.; Van den Berghe, G. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med. 2016, 42, 16–27. [Google Scholar] [CrossRef] [Green Version]
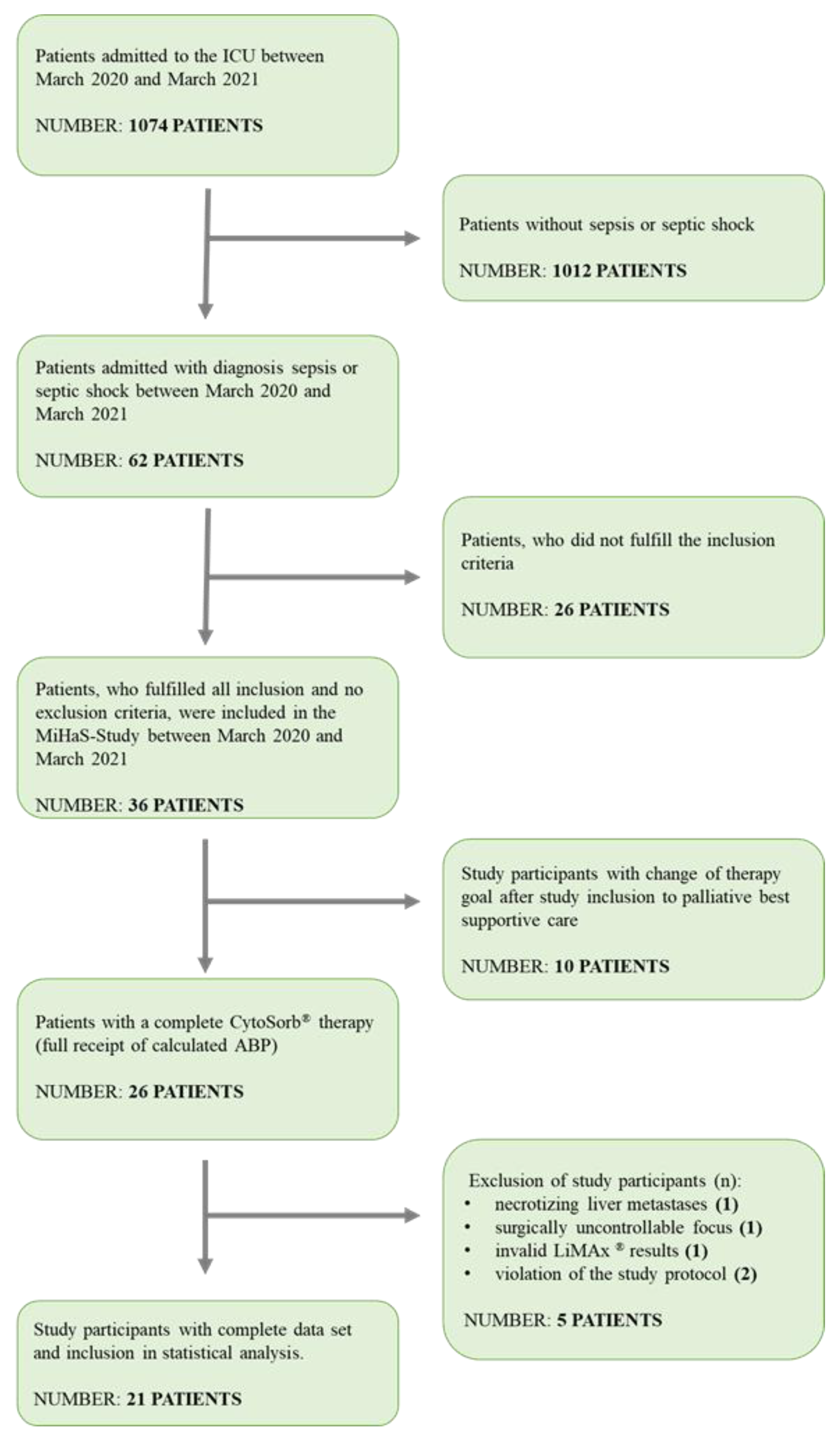
| Parameter | Total Cohort | Sepsis | Septic Shock | p-Value * |
|---|---|---|---|---|
| Age (years) | 74 (58–80) | 60 (54–79.5) | 76.5 (60–80.75) | 0.28 |
| Gender n (%) female male | 12 (57%) 9 (43%) | 6 (86%) 1 (14%) | 6 (42%) 8 (58%) | 0.16 |
| Weight (kg) | 72.52 ± 19.40 | 71.14 ± 25.29 | 73.21 ± 16.80 | 0.85 |
| Height (cm) | 166.57 ± 9.61 | 163.57 ± 10.00 | 168.07 ± 9.41 | 0.34 |
| BMI (kg/m2) | 26.05 ± 6.46 | 26.45 ± 9.07 | 25.85 ± 5.10 | 0.87 |
| APACHE II score | 31.19 ± 4.19 | 30.43 ± 2.7 | 31.57 ± 4.82 | 0.5 |
| Predicted mortality APACHE II (%) | 83.73 ± 7.94 | 83.17 ± 5.72 | 84.02 ± 9.03 | 0.8 |
| SOFA score | 14 (12–15) | 13 (12–14.5) | 15 (13.25–15) | 0.28 |
| LOS in ICU (days) 28 d observed mortality n (%) ICU observed mortality n (%) 90 d observed mortality n (%) | 24.38 ± 15.67 7 (33%) 8 (38%) 8 (38%) | 22.29 ± 17.73 0 (0%) 0 (0%) 0 (0%) | 25.43 ± 15.13 7 (50%) 8 (57%) 8 (57%) | 0.70 0.032 0.018 0.018 |
| Septic focus n (%) Abdominal Others: pulmonary pelvinal lower extremity | 15 (71%) 6 (29%) 3 (14%) 2 (10%) 1 (5%) | 6 (86%) 1 (14%) 1 (14%) 0 0 | 9 (65%) 5 (35%) 2 (14%) 2 (14%) 1 (7%) | 0.34 |
| Duration of CytoSorb® application/patient (h) | 82.51 ± 18.02 | 80.74 ± 24.56 | 83.39 ± 14.79 | 0.8 |
| Number of CytoSorb® adsorbers/patient (n) | 5.10 ± 2.17 | 4.14 ± 2.12 | 5.57 ± 2.10 | 0.17 |
| ABP (L/kg) | 13.23 ± 1.71 | 13.52 ± 1.05 | 13.08 ± 1.98 | 0.52 |
| Level of sepsis (Sepsis-3) Sepsis Septic shock | 7 (33%) 14 (67%) | 0 14 | 7 0 | |
| Lactate mmol/L | 2.27 (1.79–3.99) | 1.39 (1.29–1.71) | 3.75 (2.4–4.28) | 0.00002 # |
| Parameter | Point of Measurement | Sepsis | Septic Shock | Sepsis vs. Septic Shock p-Value | Sepsis p-Value | Septic Shock p-Value | |
|---|---|---|---|---|---|---|---|
| LiMAx® (µg/kg/h) | Preadsorber | T1 | 166.43 ± 99.53 * (97.5–224) | 117.36 ± 73.76 * (60–176) | 0.276 | ||
| Hemoadsorption (CytoSorb®) | T2 | 109.43 ± 43.95 * (84.5–126) | 64.86 ± 40.62 * (29.75–102.75) | 0.046 | 0.148 | 0.003 # | |
| T3 | 130.86 ± 66.12 * (92.5–167.5) | 98.14 ± 68.66 * (52.25–129.75) | 0.311 | 0.261 | 0.025 | ||
| Postadsorber | T4 | 221.43 ± 40.95 * (207–240.5) | 188.36 ± 120.09 * (125.25–274.25) | 0.366 | 0.003 # | 0.001 # | |
| ALT (U/L) (Reference range < 50) | Preadsorber | T1 | 34 * (16.5–53) | 33.5 (15.25–161) | 0.502 | ||
| Hemoadsorption (CytoSorb®) | T2 | 25 * (19–33) | 48 (14.25–403.25) | 0.412 | 0.208 | 0.195 | |
| T3 | 21 * (16–28) | 58.5 (16.75–246.75) | 0.117 | 0.025 | 0.03 | ||
| Postadsorber | T4 | 21 * (16.5–30.5) | 34 (13.25–181.25) | 0.654 | 0.593 | 0.055 | |
| Alkaline Phosphatase (U/L) (Reference range: 40–129) | Preadsorber | T1 | 64 (50–66) | 101 * (65.5–145) | 0.073 | ||
| Hemoadsorption (CytoSorb®) | T2 | 59 (54.5–64.5) | 88 (70.25–110.5) | 0.062 | 1 | 0.124 | |
| T3 | 76 * (59–97) | 109 * (73.75–141.5) | 0.156 | 0.059 | 0.028 | ||
| Postadsorber | T4 | 121 * (81.5–166.5) | 147.5 (80.25–202) | 0.765 | 0.034 | 0.019 | |
| Bilirubin (mg/dL) (Reference range: <1.2) | Preadsorber | T1 | 0.43 * (0.35–0.87) | 0.51 (0.47–0.63) | 0.737 | ||
| Hemoadsorption (CytoSorb®) | T2 | 0.48 * (0.25–0.64) | 0.45 (0.37–0.94) | 0.391 | 0.02 | 0.326 | |
| T3 | 0.36 * (0.24–0.42) | 0.56 * (0.42–0.69) | 0.015 | 0.248 | 0.51 | ||
| Postadsorber | T4 | 0.37 * (0.29–0.42) | 0.62 * (0.46–1.04) | 0.037 | 0.537 | 0.091 | |
| INR | Preadsorber | T1 | 1.24 * (1.21–1.32) | 1.28 (1.19–1.45) | 0.55 | ||
| Hemoadsorption (CytoSorb®) | T2 | 1.38 * (1.33–1.48) | 1.61 * (1.45–1.72) | 0.062 | 0.0002 # | 0.006# | |
| T3 | 1.40 * (1.25–1.44) | 1.37 * (1.24–1.57) | 0.654 | 0.203 | 0.03 | ||
| Postadsorber | T4 | 1.04 * (1.00–1.08) | 1.22 * (1.07–1.29) | 0.04 | 0.002 | 0.018 | |
| Parameter | Point of Measurement | Sepsis | Septic Shock | |
|---|---|---|---|---|
| LiMAx® | Preadsorber | T1 | 3 (43%) | 5 (36%) |
| Impaired liver function | Hemoadsorption (CytoSorb®) | T2 | 1 (14%) | 0 (0%) |
| (314–140 µg/kg/h) | T3 | 2 (28%) | 3 (21%) | |
| Postadsorber | T4 | 7 (100%) | 5 (36%) | |
| LiMAx® | Preadsorber | T1 | 3 (43%) | 9 (64%) |
| Severe liver damage | Hemoadsorption (CytoSorb®) | T2 | 6 (86%) | 14 (100%) |
| (0–139 µg/kg/h) | T3 | 5 (71%) | 11 (79%) | |
| Postadsorber | T4 | 0 (0%) | 7 (50%) | |
| Elevated ALT | Preadsorber | T1 | 2 (29%) | 6 (43%) |
| (≥50 U/L) | Hemoadsorption (CytoSorb®) | T2 | 1 (14%) | 7 (50%) |
| T3 | 0 (0%) | 7 (50%) | ||
| Postadsorber | T4 | 1 (14%) | 5 (36%) | |
| Elevated alkaline phosphatase | Preadsorber | T1 | 1 (14%) | 5 (36%) |
| (≥129 U/L) | Hemoadsorption (CytoSorb®) | T2 | 1 (14%) | 3 (21%) |
| T3 | 1 (14%) | 4 (29%) | ||
| Postadsorber | T4 | 3 (43%) | 8 (57%) | |
| Hyperbilirubinemia | Preadsorber | T1 | 0 (0%) | 3 (21%) |
| (≥1.2 mg/dL) | Hemoadsorption (CytoSorb®) | T2 | 0 (0%) | 3 (21%) |
| T3 | 0 (0%) | 0 (0%) | ||
| Postadsorber | T4 | 0 (0%) | 3 (21%) | |
| INR (≥1.5) | Preadsorber | T1 | 0 (0%) | 3 (21%) |
| Hemoadsorption (CytoSorb®) | T2 | 2 (29%) | 10 (71%) | |
| T3 | 1 (14%) | 6 (43%) | ||
| Postadsorber | T4 | 0 (0%) | 2 (14%) | |
| Parameter | Point of Measurement | Sepsis | Septic Shock | Sepsis vs. Septic Shock p-Value | Sepsis p-Value | Septic Shock p-Value | |
|---|---|---|---|---|---|---|---|
| Interleukin-6 (pg/mL) (Reference range: <7) | Preadsorber | T1 | 1402 (727.75–5317) | 4713 (1089.5–11717.5) | 0.443 | ||
| Hemoadsorption (CytoSorb®) | T2 | 374.20 (175.15–553.2) | 448.35 (135.75–2593.25) | 0.743 | 0.016 # | 0.0004 # | |
| T3 | 210.4 * (97.15–263.90)) | 329.4 (107.68–668.33) | 0.287 | 0.156 | 0.091 | ||
| Postadsorber | T4 | 68.30 (27.35–139.80) | 128.75 (57.58–446.38) | 0.094 | 0.375 | 0.042 | |
| Norepinephrine dose (µg/kg/min) | Preadsorber | T1 | 0.09 * (0.07–0.19) | 0.22 * (0.11–0.65) | 0.19 | ||
| Hemoadsorption (CytoSorb®) | T2 | 0.24 * (0.14–0.37) | 0.56 * (0.43–0.73) | 0.007 # | 0.09 | 0.005 # | |
| T3 | 0.09 * (0.05–0.10) | 0.16 (0.11–0.19) | 0.038 | 0.016 # | 0.0001 # | ||
| Postadsorber | T4 | 0.05 * (0.02–0.09) | 0.05 (0.02–0.08) | 0.97 | 0.721 | 0.0006 # | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praxenthaler, J.; Schwier, E.; Altmann, S.; Kirchner, C.; Bialas, J.; Henzler, D.; Köhler, T. Immunomodulation by Hemoadsorption—Changes in Hepatic Biotransformation Capacity in Sepsis and Septic Shock: A Prospective Study. Biomedicines 2022, 10, 2340. https://doi.org/10.3390/biomedicines10102340
Praxenthaler J, Schwier E, Altmann S, Kirchner C, Bialas J, Henzler D, Köhler T. Immunomodulation by Hemoadsorption—Changes in Hepatic Biotransformation Capacity in Sepsis and Septic Shock: A Prospective Study. Biomedicines. 2022; 10(10):2340. https://doi.org/10.3390/biomedicines10102340
Chicago/Turabian StylePraxenthaler, Janina, Elke Schwier, Simon Altmann, Carmen Kirchner, Julian Bialas, Dietrich Henzler, and Thomas Köhler. 2022. "Immunomodulation by Hemoadsorption—Changes in Hepatic Biotransformation Capacity in Sepsis and Septic Shock: A Prospective Study" Biomedicines 10, no. 10: 2340. https://doi.org/10.3390/biomedicines10102340





