Potential of Disease-Modifying Anti-Rheumatic Drugs to Limit Abdominal Aortic Aneurysm Growth
Abstract
:1. Introduction
2. Disease-Modifying Anti-Rheumatic Drugs
3. Evidence from Animal Models for a Role of TNF and IL in AAA Pathogenesis
3.1. Animal Studies Investigating the Effect of Blocking TNF-α on AAA Development and Growth
3.2. Animal Studies Investigating the Effect of Blocking IL-1β or IL-1R on AAA Development and Growth
3.3. Animal Studies Investigating the Effect of Blocking IL-6 or IL-6R on AAA Development and Growth
3.4. Animal Studies Investigating the Effect of Blocking IL-12 or IL-23 on AAA Development and Growth
3.5. Animal Studies Investigating the Effect of Blocking IL-17 on AAA Development and Growth
4. Evidence from Human Studies for a Role of TNF and IL in AAA Pathogenesis
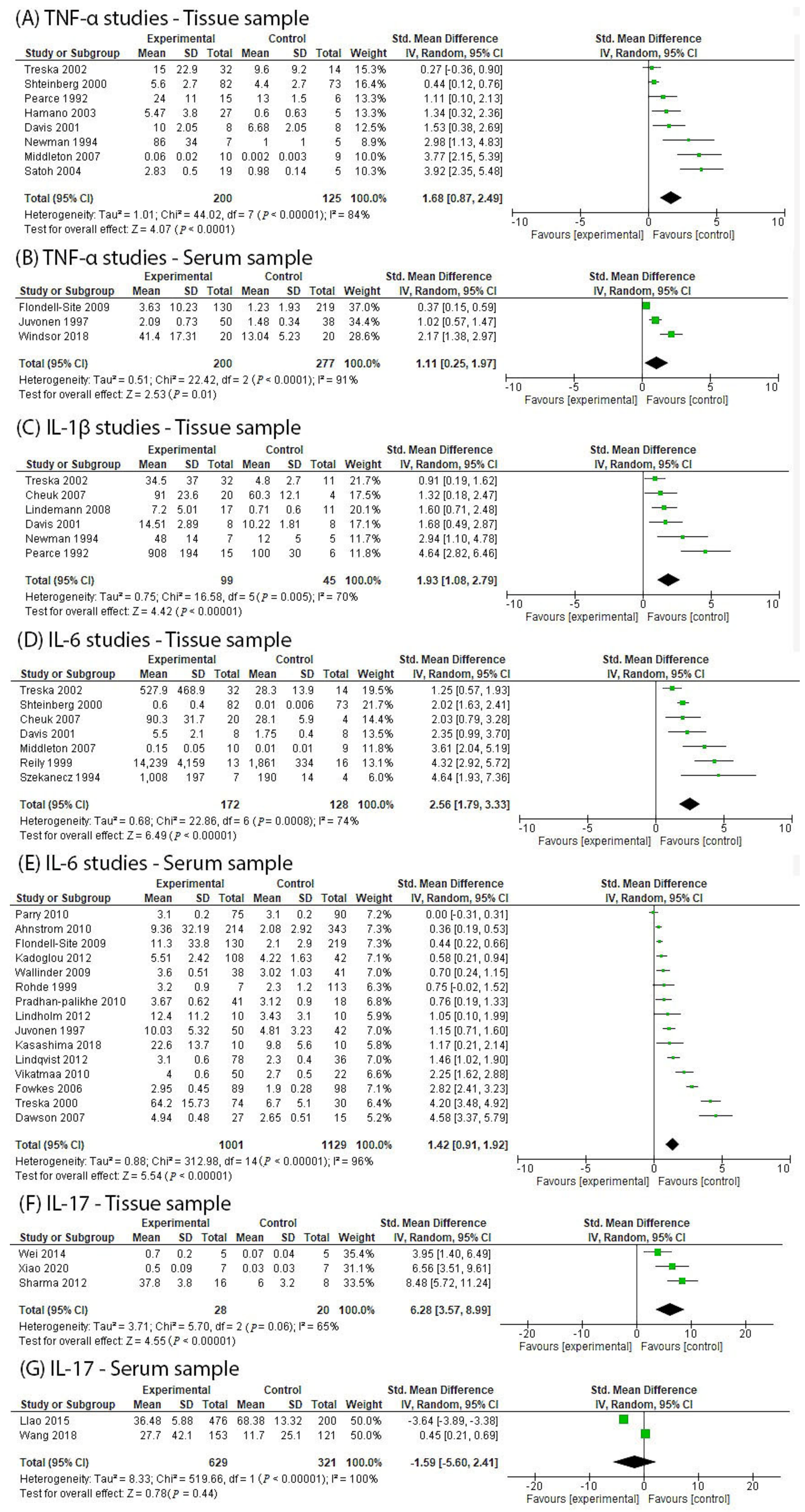
4.1. Human Studies Investigating TNF-α in AAA Participants
4.2. Human Studies Investigating IL-1β in AAA Participants
4.3. Human Studies Investigating IL-6 in AAA Participants
4.4. Human Studies Investigating IL-12/23 in AAA Participants
4.5. Human Studies Investigating IL-17 in AAA Participants
5. Safety Considerations for the Use of bDMARDs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sampson, U.K.; Norman, P.E.; Fowkes, F.G.; Aboyans, V.; Song, Y.; Harrell, F.E., Jr.; Forouzanfar, M.H.; Naghavi, M.; Denenberg, J.O.; McDermott, M.M.; et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob. Heart 2014, 9, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Ullery, B.W.; Hallett, R.L.; Fleischmann, D. Epidemiology and contemporary management of abdominal aortic aneurysms. Abdom. Radiol. 2018, 43, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice: European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom Small Aneurysm Trial Participants; Powell, J.T.; Brady, A.R.; Brown, L.C.; Fowkes, F.G.; Greenhalgh, R.M.; Ruckley, C.V.; Thompson, S.G. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N. Engl. J. Med. 2002, 346, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Reviews. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef]
- Golledge, J.; Muller, J.; Daugherty, A.; Norman, P. Abdominal aortic aneurysm: Pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2605–2613. [Google Scholar] [CrossRef]
- Golledge, J.; Moxon, J.V.; Singh, T.P.; Bown, M.J.; Mani, K.; Wanhainen, A. Lack of an effective drug therapy for abdominal aortic aneurysm. J. Intern. Med. 2020, 288, 6–22. [Google Scholar] [CrossRef]
- Cifani, N.; Proietta, M.; Taurino, M.; Tritapepe, L.; Del Porto, F. Monocyte Subsets, Stanford-A Acute Aortic Dissection, and Carotid Artery Stenosis: New Evidences. J. Immunol. Res. 2019, 2019, 9782594. [Google Scholar] [CrossRef]
- Cifani, N.; Proietta, M.; Tritapepe, L.; Di Gioia, C.; Ferri, L.; Taurino, M.; Del Porto, F. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: A review. Ann. Med. 2015, 47, 441–446. [Google Scholar] [CrossRef]
- Quan, L.-D.; Thiele, G.M.; Tian, J.; Wang, D. The Development of Novel Therapies for Rheumatoid Arthritis. Expert Opin. Pat. 2008, 18, 723–738. [Google Scholar] [CrossRef]
- Benjamin, O.; Goyal, A.; Lappin, S.L. Disease Modifying Anti-Rheumatic Drugs (DMARD); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- FDA. Etanercept. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103795s5503lbl.pdf (accessed on 1 September 2021).
- FDA. Infliximab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf (accessed on 1 September 2021).
- FDA. Adalimumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125057s410lbl.pdf (accessed on 1 September 2021).
- FDA. Certolizumab Pegol. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125160s270lbl.pdf (accessed on 1 September 2021).
- FDA. Golimumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf (accessed on 1 September 2021).
- FDA. Canakinumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/BLA125319_858687lbl.pdf (accessed on 1 September 2021).
- FDA. Anakinra. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/anakamg111401LB.pdf (accessed on 1 September 2021).
- FDA. Tocilizumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125276s114lbl.pdf (accessed on 1 September 2021).
- FDA. Sarilumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf (accessed on 1 September 2021).
- FDA. Ustekizumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf (accessed on 1 September 2021).
- FDA. Guselkumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf (accessed on 1 September 2021).
- FDA. Tildrakizumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761067s000lbl.pdf (accessed on 1 September 2021).
- FDA. Secukinumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125504s013lbl.pdf (accessed on 1 September 2021).
- FDA. Brodalumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf (accessed on 1 September 2021).
- FDA. Ixekizumab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125521s004lbl.pdf (accessed on 1 September 2021).
- Xiong, W.; MacTaggart, J.; Knispel, R.; Worth, J.; Persidsky, Y.; Baxter, B.T. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J. Immunol. 2009, 183, 2741–2746. [Google Scholar] [CrossRef]
- Kaneko, H.; Anzai, T.; Horiuchi, K.; Kohno, T.; Nagai, T.; Anzai, A.; Takahashi, T.; Sasaki, A.; Shimoda, M.; Maekawa, Y.; et al. Tumor necrosis factor-α converting enzyme is a key mediator of abdominal aortic aneurysm development. Atherosclerosis 2011, 218, 470–478. [Google Scholar] [CrossRef]
- Hingorani, A.; Ascher, E.; Scheinman, M.; Yorkovich, W.; DePippo, P.; Ladoulis, C.T.; Salles-Cunha, S. The effect of tumor necrosis factor binding protein and interleukin-1 receptor antagonist on the development of abdominal aortic aneurysms in a rat model. J. Vasc. Surg. 1998, 28, 522–526. [Google Scholar] [CrossRef]
- Kaneko, H.; Anzai, T.; Horiuchi, K.; Kohno, T.; Shimoda, M.; Maekawa, Y.; Shimizu, H.; Yoshikawa, T.; Yozu, R.; Okada, Y.; et al. Temporal Systemic Deletion of Tumor Necrosis Factor-alpha Converting Enzyme Inhibits Development of Abdominal Aortic Aneurysm. Circulation 2010, 122, A13646. [Google Scholar]
- Batra, R.; Suh, M.K.; Carson, J.S.; Dale, M.A.; Meisinger, T.M.; Fitzgerald, M.; Opperman, P.J.; Luo, J.; Pipinos, I.I.; Xiong, W.; et al. IL-1β (Interleukin-1β) and TNF-α (Tumor Necrosis Factor-α) Impact Abdominal Aortic Aneurysm Formation by Differential Effects on Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 457–463. [Google Scholar] [CrossRef]
- Da Ros, F.; Carnevale, R.; Cifelli, G.; Bizzotto, D.; Casaburo, M.; Perrotta, M.; Carnevale, L.; Vinciguerra, I.; Fardella, S.; Iacobucci, R.; et al. Targeting Interleukin-1β Protects from Aortic Aneurysms Induced by Disrupted Transforming Growth Factor β Signaling. Immunity 2017, 47, 959–973.e959. [Google Scholar] [CrossRef]
- Isoda, K.; Akita, K.; Kitamura, K.; Sato-Okabayashi, Y.; Kadoguchi, T.; Isobe, S.; Ohtomo, F.; Sano, M.; Shimada, K.; Iwakura, Y.; et al. Inhibition of interleukin-1 suppresses angiotensin II-induced aortic inflammation and aneurysm formation. Int. J. Cardiol. 2018, 270, 221–227. [Google Scholar] [CrossRef]
- Johnston, W.F.; Salmon, M.; Su, G.; Lu, G.; Stone, M.L.; Zhao, Y.; Owens, G.K.; Upchurch, G.R.; Ailawadi, G. Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 294–304. [Google Scholar] [CrossRef]
- Kokje, V.B.C.; Gäbel, G.; Koole, D.; Northoff, B.H.; Holdt, L.M.; Hamming, J.F.; Lindeman, J.H.N. IL-6: A Janus-like factor in abdominal aortic aneurysm disease. Atherosclerosis 2016, 251, 139–146. [Google Scholar] [CrossRef]
- Nishihara, M.; Aoki, H.; Ohno, S.; Furusho, A.; Hirakata, S.; Nishida, N.; Ito, S.; Hayashi, M.; Imaizumi, T.; Fukumoto, Y. The role of IL-6 in pathogenesis of abdominal aortic aneurysm in mice. PLoS ONE 2017, 12, e0185923. [Google Scholar] [CrossRef] [Green Version]
- Paige, E.; Clément, M.; Lareyre, F.; Sweeting, M.; Raffort, J.; Grenier, C.; Finigan, A.; Harrison, J.; Peters, J.E.; Sun, B.B.; et al. Interleukin-6 Receptor Signaling and Abdominal Aortic Aneurysm Growth Rates. Circ. Genom. Precis. Med. 2019, 12, e002413. [Google Scholar] [CrossRef]
- Pope, N.H.; Salmon, M.; Johnston, W.F.; Lu, G.; Lau, C.L.; Upchurch, G.R., Jr.; Ailawadi, G. Interleukin-6 receptor inhibition prevents descending thoracic aortic aneurysm formation. Ann. Thorac. Surg. 2015, 100, 1620–1626. [Google Scholar] [CrossRef]
- Sharma, N.; Dev, R.; Belenchia, A.M.; Aroor, A.R.; Whaley-Connell, A.; Pulakat, L.; Hans, C.P. Deficiency of IL12p40 (Interleukin 12 p40) Promotes Ang II (Angiotensin II)-Induced Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 212–223. [Google Scholar] [CrossRef]
- Yan, H.; Hu, Y.; Akk, A.; Ye, K.; Bacon, J.; Pham, C.T.N. Interleukin-12 and -23 blockade mitigates elastase-induced abdominal aortic aneurysm. Sci. Rep. 2019, 9, 10447. [Google Scholar] [CrossRef]
- Sharma, A.K.; Lu, G.; Jester, A.; Johnston, W.F.; Zhao, Y.; Hajzus, V.A.; Reza Saadatzadeh, M.; Su, G.; Bhamidipati, C.M.; Mehta, G.S.; et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation 2012, 126, S38–S45. [Google Scholar] [CrossRef]
- Xiao, J.; Wei, Z.; Chen, X.; Chen, W.; Zhang, H.; Yang, C.; Shang, Y.; Liu, J. Experimental abdominal aortic aneurysm growth is inhibited by blocking the JAK2/STAT3 pathway. Int. J. Cardiol. 2020, 312, 100–106. [Google Scholar] [CrossRef]
- Meher, A.K.; Spinosa, M.; Davis, J.P.; Pope, N.; Laubach, V.E.; Su, G.; Serbulea, V.; Leitinger, N.; Ailawadi, G.; Upchurch, G.R., Jr. Novel Role of IL (Interleukin)-1β in Neutrophil Extracellular Trap Formation and Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 843–853. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlanda, C.; Dinarello Charles, A.; Mantovani, A. The Interleukin-1 Family: Back to the Future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Davis, V.A.; Persidskaia, R.N.; Baca-Regen, L.M.; Fiotti, N.; Halloran, B.G.; Baxter, B.T. Cytokine pattern in aneurysmal and occlusive disease of the aorta. J. Surg. Res. 2001, 101, 152–156. [Google Scholar] [CrossRef]
- Hamano, K.; Li, T.S.; Takahashi, M.; Kobayashi, T.; Shirasawa, B.; Ito, H.; Zempo, N. Enhanced tumor necrosis factor- alpha expression in small sized abdominal aortic aneurysms. World J. Surg. 2003, 27, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Middleton, R.K.; Lloyd, G.M.; Bown, M.J.; Cooper, N.J.; London, N.J.; Sayers, R.D. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: A protein array study. J. Vasc. Surg. 2007, 45, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.M.; Jean-Claude, J.; Li, H.; Ramey, W.G.; Tilson, M.D. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation 1994, 90, Ii224–Ii227. [Google Scholar] [PubMed]
- Pearce, W.H.; Sweis, I.; Yao, J.S.T.; McCarthy, W.J.; Koch, A.E. Interleukin-1β and tumor necrosis factor-α release in normal and diseased human infrarenal aortas. J. Vasc. Surg. 1992, 16, 784–789. [Google Scholar] [CrossRef]
- Satoh, H.; Nakamura, M.; Satoh, M.; Nakajima, T.; Izumoto, H.; Maesawa, C.; Kawazoe, K.; Masuda, T.; Hiramori, K. Expression and localization of tumour necrosis factor-alpha and its converting enzyme in human abdominal aortic aneurysm. Clin. Sci. 2004, 106, 301–306. [Google Scholar] [CrossRef]
- Shteinberg, D.; Halak, M.; Shapiro, S.; Kinarty, A.; Sobol, E.; Lahat, N.; Karmeli, R. Abdominal Aortic Aneurysm and Aortic Occlusive Disease: A Comparison of Risk Factors and Inflammatory Response. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 462–465. [Google Scholar] [CrossRef]
- Treska, V.; Kocova, J.; Boudova, L.; Neprasova, P.; Topolcan, O.; Pecen, L.; Tonar, Z. Inflammation in the wall of abdominal aortic aneurysm and its role in the symptomatology of aneurysm. Cytokines Cell. Mol. Ther. 2002, 7, 91–97. [Google Scholar] [CrossRef]
- Windsor, M.T.; Bailey, T.G.; Perissiou, M.; Greaves, K.; Jha, P.; Leicht, A.S.; Russell, F.D.; Golledge, J.; Askew, C.D. Acute Inflammatory Responses to Exercise in Patients with Abdominal Aortic Aneurysm. Med. Sci. Sports Exerc. 2018, 50, 649–658. [Google Scholar] [CrossRef]
- Flondell-Sité, D.; Lindblad, B.; Kölbel, T.; Gottsäter, A. Cytokines and systemic biomarkers are related to the size of abdominal aortic aneurysms. Cytokine 2009, 46, 211–215. [Google Scholar] [CrossRef]
- Juvonen, J.; Surcel, H.M.; Satta, J.; Teppo, A.M.; Bloigu, A.; Syrjala, H.; Airaksinen, J.; Leinonen, M.; Saikku, P.; Juvonen, T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2843–2847. [Google Scholar] [CrossRef]
- Cheuk, B.L.; Cheng, S.W. Differential secretion of prostaglandin E(2), thromboxane A(2) and interleukin-6 in intact and ruptured abdominal aortic aneurysms. Int. J. Mol. Med. 2007, 20, 391–395. [Google Scholar] [CrossRef]
- Lindeman, J.H.N.; Abdul-Hussien, H.; Schaapherder, A.F.M.; Van Bockel, J.H.; Von Der Thüsen, J.H.; Roelen, D.L.; Kleemann, R. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin. Sci. 2008, 114, 687–697. [Google Scholar] [CrossRef]
- Reilly, J.M.; Miralles, M.; Wester, W.N.; Sicard, G.A. Differential expression of prostaglandin E2 and interleukin-6 in occlusive and aneurysmal aortic disease. Surgery 1999, 126, 624–628. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Shah, M.R.; Pearce, W.H.; Koch, A.E. Intercellular adhesion molecule-1 (ICAM-1) expression and soluble ICAM-1 (sICAM-1) production by cytokine-activated human aortic endothelial cells: A possible role for ICAM-1 and sICAM-1 in atherosclerotic aortic aneurysms. Clin. Exp. Immunol. 1994, 98, 337–343. [Google Scholar] [CrossRef]
- Ahnström, J.; Gottsäter, A.; Lindblad, B.; Dahlbäck, B. Plasma concentrations of apolipoproteins A-I, B and M in patients with abdominal aortic aneurysms. Clin. Biochem. 2010, 43, 407–410. [Google Scholar] [CrossRef]
- Dawson, J.; Cockerill, G.W.; Choke, E.; Belli, A.-M.; Loftus, I.; Thompson, M.M. Aortic aneurysms secrete interleukin-6 into the circulation. J. Vasc. Surg. 2007, 45, 350–356. [Google Scholar] [CrossRef]
- Fowkes, F.G.R.; Anandan, C.L.C.; Lee, A.J.; Smith, F.B.; Tzoulaki, I.; Rumley, A.; Powell, J.T.; Lowe, G.D.O. Reduced lung function in patients with abdominal aortic aneurysm is associated with activation of inflammation and hemostasis, not smoking or cardiovascular disease. J. Vasc. Surg. 2006, 43, 474–480. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Papadakis, I.; Moulakakis, K.G.; Ikonomidis, I.; Alepaki, M.; Moustardas, P.; Lampropoulos, S.; Karakitsos, P.; Lekakis, J.; Liapis, C.D. Arterial stiffness and novel biomarkers in patients with abdominal aortic aneurysms. Regul. Pept. 2012, 179, 50–54. [Google Scholar] [CrossRef]
- Kasashima, S.; Kawashima, A.; Zen, Y.; Ozaki, S.; Kasashima, F.; Endo, M.; Matsumoto, Y.; Kawakami, K. Upregulated interleukins (IL-6, IL-10, and IL-13) in immunoglobulin G4-related aortic aneurysm patients. J. Vasc. Surg. 2018, 67, 1248–1262. [Google Scholar] [CrossRef]
- Lindholm, E.; Seljeflot, I.; Aune, E.; Kirkebøen, K.A. Proinflammatory cytokines and complement activation in salvaged blood from abdominal aortic aneurism surgery and total hip replacement surgery. Transfusion 2012, 52, 1761–1769. [Google Scholar] [CrossRef]
- Lindqvist, M.; Wallinder, J.; Henriksson, A.E. Soluble urokinase plasminogen activator receptor in patients with abdominal aortic aneurysm. Thromb. Res. 2012, 130, 511–513. [Google Scholar] [CrossRef]
- Parry, D.J.; Al-Barjas, H.S.; Chappell, L.; Rashid, S.T.; Ariëns, R.A.S.; Scott, D.J.A. Markers of inflammation in men with small abdominal aortic aneurysm. J. Vasc. Surg. 2010, 52, 145–151. [Google Scholar] [CrossRef]
- Pradhan-Palikhe, P.; Vikatmaa, P.; Lajunen, T.; Palikhe, A.; Lepäntalo, M.; Tervahartiala, T.; Salo, T.; Saikku, P.; Leinonen, M.; Pussinen, P.J.; et al. Elevated MMP-8 and Decreased Myeloperoxidase Concentrations Associate Significantly with the Risk for Peripheral Atherosclerosis Disease and Abdominal Aortic Aneurysm1. Scand. J. Immunol. 2010, 72, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Třeška, V.; Topolčan, O.; Pecen, L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin. Chem. Lab. Med. 2000, 38, 1161–1164. [Google Scholar] [CrossRef]
- Vikatmaa, P.; Lajunen, T.; Ikonen, T.S.; Pussinen, P.J.; Lepäntalo, M.; Leinonen, M.; Saikku, P. Chlamydial lipopolysaccharide (cLPS) is present in atherosclerotic and aneurysmal arterial wall—cLPS levels depend on disease manifestation. Cardiovasc. Pathol. 2010, 19, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wallinder, J.; Bergqvist, D.; Henriksson, A.E. Proinflammatory and anti-inflammatory cytokine balance in patients with abdominal aortic aneurysm and the impact of aneurysm size. Vasc. Endovasc. Surg. 2009, 43, 258–261. [Google Scholar] [CrossRef]
- Rohde, L.E.P.; Arroyo, L.H.; Rifai, N.; Creager, M.A.; Libby, P.; Ridker, P.M.; Lee, R.T. Plasma Concentrations of Interleukin-6 and Abdominal Aortic Diameter Among Subjects Without Aortic Dilatation. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1695–1699. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Zhang, K.; Liao, Y.; Ye, P.; Wu, J.; Wang, Y.; Li, F.; Yao, Y.; Zhou, Y.; et al. Inhibiting the Th17/IL-17A-Related inflammatory responses with digoxin confers protection against experimental abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2429–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, M.; Liu, C.-L.; Lv, B.-J.; Zhang, J.-Y.; Cheng, L.; Cheng, X.; Lindholt, J.S.; Rasmussen, L.M.; Shi, G.-P. Plasma cytokine levels and risks of abdominal aortic aneurysms: A population-based prospective cohort study. Ann. Med. 2015, 47, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Green, L.A.; Gutwein, A.R.; Drucker, N.A.; Motaganahalli, R.L.; Gupta, A.K.; Fajardo, A.; Murphy, M.P. Description of human AAA by cytokine and immune cell aberrations compared to risk-factor matched controls. Surgery 2018, 164, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012, 122, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.M.; Peritt, D.; Scallon, B.J.; Heavner, G.A.; Shealy, D.J.; Giles-Komar, J.M.; Mascelli, M.A. Discovery and mechanism of ustekinumab: A human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. mAbs 2011, 3, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Gaffen, S.L. Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications. Cold Spring Harb. Perspect Biol. 2018, 10, a028522. [Google Scholar] [CrossRef] [PubMed]
- Lenk, G.M.; Tromp, G.; Weinsheimer, S.; Gatalica, Z.; Berguer, R.; Kuivaniemi, H. Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMC Genom. 2007, 8, 237. [Google Scholar] [CrossRef]
- Choke, E.; Cockerill, G.W.; Laing, K.; Dawson, J.; Wilson, W.R.W.; Loftus, I.M.; Thompson, M.M. Whole Genome-expression Profiling Reveals a Role for Immune and Inflammatory Response in Abdominal Aortic Aneurysm Rupture. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 305–310. [Google Scholar] [CrossRef]
- Holmberg, A.; Bergqvist, D.; Westman, B.; Siegbahn, A. Cytokine and Fibrinogen Response in Patients Undergoing Open Abdominal Aortic Aneurysm Surgery. Eur. J. Vasc. Endovasc. Surg. 1999, 17, 294–300. [Google Scholar] [CrossRef]
- Lindeman, J.H.; Matsumura, J.S. Pharmacologic Management of Aneurysms. Circ. Res. 2019, 124, 631–646. [Google Scholar] [CrossRef]
- Garbers, C.; Heink, S.; Korn, T.; Rose-John, S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Reviews. Drug Discov. 2018, 17, 395–412. [Google Scholar] [CrossRef]
- Takagi, H.; Watanabe, T.; Mizuno, Y.; Kawai, N.; Umemoto, T. Circulating interleukin-6 levels are associated with abdominal aortic aneurysm presence: A meta-analysis and meta-regression of case-control studies. Ann. Vasc. Surg. 2014, 28, 1913–1922. [Google Scholar] [CrossRef]
- Harrison, S.C.; Smith, A.J.; Jones, G.T.; Swerdlow, D.I.; Rampuri, R.; Bown, M.J.; Folkersen, L.; Baas, A.F.; de Borst, G.J.; Blankensteijn, J.D.; et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur. Heart J. 2013, 34, 3707–3716. [Google Scholar] [CrossRef]
- Lis, K.; Kuzawińska, O.; Bałkowiec-Iskra, E. Tumor necrosis factor inhibitors—State of knowledge. Arch. Med. Sci. 2014, 10, 1175–1185. [Google Scholar] [CrossRef]
- Saag, K.G.; Teng, G.G.; Patkar, N.M.; Anuntiyo, J.; Finney, C.; Curtis, J.R.; Paulus, H.E.; Mudano, A.; Pisu, M.; Elkins-Melton, M.; et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008, 59, 762–784. [Google Scholar] [CrossRef]
- Perry, L.M.; Winthrop, K.L.; Curtis, J.R. Vaccinations for rheumatoid arthritis. Curr. Rheumatol. Rep. 2014, 16, 431. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. ACZ885 for the Treatment of Abdominal Aortic Aneurysm (AAA); Novartis Pharmaceuticals: Basel, Switzerland, 2013. [Google Scholar]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Ramiro, S.; Sepriano, A.; Chatzidionysiou, K.; Nam, J.L.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; Bijlsma, J.W.; Burmester, G.R.; et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1101–1136. [Google Scholar] [CrossRef]
- Burmester, G.R.; Panaccione, R.; Gordon, K.B.; McIlraith, M.J.; Lacerda, A.P. Adalimumab: Long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann. Rheum. Dis. 2013, 72, 517–524. [Google Scholar] [CrossRef]
- Saougou, I.; Markatseli, T.E.; Papagoras, C.; Voulgari, P.V.; Alamanos, Y.; Drosos, A.A. Sustained clinical response in psoriatic arthritis patients treated with anti-TNF agents: A 5-year open-label observational cohort study. Semin. Arthritis Rheum. 2011, 40, 398–406. [Google Scholar] [CrossRef]
- Leonardi, C.; Reich, K.; Foley, P.; Torii, H.; Gerdes, S.; Guenther, L.; Gooderham, M.; Ferris, L.K.; Griffiths, C.E.M.; ElMaraghy, H.; et al. Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials. Dermatol. Ther. 2020, 10, 431–447. [Google Scholar] [CrossRef] [Green Version]
- Deodhar, A.; Mease, P.J.; McInnes, I.B.; Baraliakos, X.; Reich, K.; Blauvelt, A.; Leonardi, C.; Porter, B.; Das Gupta, A.; Widmer, A.; et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: Integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res. Ther. 2019, 21, 111. [Google Scholar] [CrossRef]
- Rowbotham, S.E.; Krishna, S.M.; Moran, C.S.; Golledge, J. Fenofibrate and Telmisartan in the Management of Abdominal Aortic Aneurysm. Curr. Drug Targets 2018, 19, 1241–1246. [Google Scholar] [CrossRef]
- McHugh, J. Long-term safety of canakinumab in systemic JIA. Nat. Rev. Rheum. 2018, 14, 622. [Google Scholar] [CrossRef]
- Genovese, M.C.; van der Heijde, D.; Lin, Y.; St John, G.; Wang, S.; van Hoogstraten, H.; Gómez-Reino, J.J.; Kivitz, A.; Maldonado-Cocco, J.A.; Seriolo, B.; et al. Long-term safety and efficacy of sarilumab plus methotrexate on disease activity, physical function and radiographic progression: 5 years of sarilumab plus methotrexate treatment. RMD Open 2019, 5, e000887. [Google Scholar] [CrossRef]
- Reich, K.; Warren, R.B.; Iversen, L.; Puig, L.; Pau-Charles, I.; Igarashi, A.; Ohtsuki, M.; Falqués, M.; Harmut, M.; Rozzo, S.; et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: Pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br. J. Dermatol. 2020, 182, 605–617. [Google Scholar] [CrossRef]
- Lan, J.L.; Tseng, C.H.; Chen, J.H.; Cheng, C.F.; Liang, W.M.; Tsay, G.J. Reduced risk of all-cancer and solid cancer in Taiwanese patients with rheumatoid arthritis treated with etanercept, a TNF-α inhibitor. Medicine 2017, 96, e6055. [Google Scholar] [CrossRef]
- Buchbinder, R.; Van Doornum, S.; Staples, M.; Lassere, M.; March, L. Malignancy risk in Australian rheumatoid arthritis patients treated with anti-tumour necrosis factor therapy: Analysis of the Australian Rheumatology Association Database (ARAD) prospective cohort study. BMC Musculoskelet Disord. 2015, 16, 309. [Google Scholar] [CrossRef] [PubMed]
- Mercer, L.K.; Lunt, M.; Low, A.L.S.; Dixon, W.G.; Watson, K.D.; Symmons, D.P.M.; Hyrich, K.L.; Consortium, B.C.C. Risk of solid cancer in patients exposed to anti-tumour necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 2015, 74, 1087–1093. [Google Scholar] [CrossRef]
- Solomon, D.H.; Kremer, J.M.; Fisher, M.; Curtis, J.R.; Furer, V.; Harrold, L.R.; Hochberg, M.C.; Reed, G.; Tsao, P.; Greenberg, J.D. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin. Arthritis Rheum. 2014, 43, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.L.; Emery, P.; Porter, D.; Reynolds, A.; Young, A.; Boyd, H.; Poole, C.D.; Currie, C.J. Treatment of rheumatoid arthritis with etanercept with reference to disease-modifying anti-rheumatic drugs: Long-term safety and survival using prospective, observational data. Rheumatology 2014, 53, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Drug Name | Trade Name | Specificity | Route of Administration | Indications for Use |
|---|---|---|---|---|
| Tumor Necrosis Factor inhibitors | ||||
| Etanercept [13] | ENBREL | sTNF, tmTNF, lymphotoxin A | SC injection | RA, JIA, PsA, AS, PPs, paediatric PPs |
| Infliximab [14] | REMICADE | sTNF, tmTNF | IV injection | RA, PJIA, AS, PsA, psoriasis, CD, paediatric CD, UC |
| Adalimumab [15] | HUMIRA | sTNF, tmTNF | SC injection | RA, adult and paediatric CD, UC, paediatric UC, AS, PsA, psoriasis |
| Certolizumab pegol [16] | CIMZIA | sTNF, tmTNF | SC injection | RA |
| Golimumab [17] | SYMPONI | sTNF, tmTNF | SC injection | RA, AS, PsA |
| Interleukin inhibitors | ||||
| Canakinumab [18] | ILARIS, previously ACZ885 | IL-1β | SC injection | CAPS, FCAS, MWS, TRAPS, HIDS/MKD, Familial Mediterranean Fever |
| Anakinra [19] | KINERET | IL-1 Receptor A | SC injection | Moderate-to-severe active RA in patients 18 years of age or older who have failed one or more DMARDs |
| Tocilizumab [20] | ACTEMRA | IL-6 | IV or SC injection | RA, pJIA and sJIA, Tocilizumab may be used alone or in combination with methotrexate; and in RA, other DMARDs may be used |
| Sarilumab [21] | KEVZARA | IL-6 Receptor | SC injection | For patients with moderate-to-severe active RA who have had an inadequate response or intolerance to one or more DMARDs |
| Ustekinumab [22] | STELARA | IL-12/23 | IV or SC injection | Moderate-to-severe plaque psoriasis, active PsA, moderately to severely active CD |
| Guselkumab [23] | TREMFYA | IL-23 | SC injection | Moderate-to-severe plaque psoriasis candidates for systemic therapy or phototherapy |
| Tildrakizumab [24] | ILUMYA | IL-23 | SC injection | Moderate-to-severe plaque psoriasis patients who are candidates for systemic therapy or phototherapy |
| Secukinumab [25] | COSENTYX | IL-17A | SC injection | Moderate-to-severe plaque psoriasis patients who are candidates for systemic therapy or phototherapy, PsA, AS |
| Brodalumab [26] | SILIQ | IL-17 Receptor A | SC injection | Moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and who have failed to respond or have lost response to other systemic therapies |
| Ixekizumab [27] | TALTZ | IL-17A | SC injection | Moderate-to-severe plaque psoriasis patients who are candidates for systemic therapy or phototherapy, active PsA |
| Ref | Animal | AAA Model | Aortic Diameter (Intervention vs. AAA Control) | Intervention | Intervention Started after AAA Induction | Dose/Frequency of Intervention | Assessment Period | Post-Intervention Cytokine Change | p Value (TNF or IL Inhibition vs. AAA Controls) | Mechanisms Implicated in Protection from AAA Development or Growth |
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Necrosis Factor-α | ||||||||||
| [28] | B6129SF2 mice | Periaortic application of CaCl2 | 9.8 ± 0.3 vs. 5.8 ± 0.1 mm | TNF alpha gene knockout Infliximab | NA Yes | NA 10 μg/g body weight, once weekly | 6 weeks 6 weeks | ↓ ↓ | <0.01 0.03 | Reduced elastic fiber disruption, macrophage infiltration, and MMP-2 and MMP-9 expression in aortic tissue |
| [29] | Mx-1 Cre transgenic mice | Periaortic application of CaCl2 | 1.3 ± 0.1 vs. 0.8 ± 0.1 mm ^ | TACE gene knockout | No | 250 μg on alternate days, starting 2 weeks prior to the operation | 6 weeks | ↓ | 0.05 | Attenuated inflammation, oxidative stress, neoangiogenesis and extracellular matrix disruption |
| [30] | WKY | Elastase perfusion | 2.7 ± 0.1 vs. 1.4 ± 0.1 mm | TNF-BP | No | 1 mg/kg diluted in vehicle prior, 48 & 96 h | 6 days | ↓ | <0.01 | Elastin fragmentation and smooth muscle cell loss in the media of the aortic wall was prevented |
| Interleukin-1b/1R | ||||||||||
| [30] | WKY | Elastase perfusion | 2.3 ± 0.2 vs. 2.2 ± 0.3 mm | IL-1R-a | No | Dose: 100 mg/kg diluted in vehicle Frequency: 20 min prior to surgery, and every 8 h | 6 days | ↑ | >0.05 | NA |
| [32] | C57BL/6J mice | Periaortic application of CaCl2 | 58.2 ± 5.2 vs. 35.5 ± 3.5% ^ | Genetic deletion of IL1β | No | NA | 6 weeks | ↓ | 0.01 | NA |
| [34] | C57BL/6J mice | Ang-II infusion + IL-1Ra-deficient mice | 0.9 ± 0.1 vs. 0.5 ± 0.0 mm | IL-1β mAb | Yes | 7.5 mg/kg, twice a week | 14 days | ↓ | <0.01 | Prevented destruction of the elastic lamina and degeneration of SMCs in the abdominal aorta |
| [44] | C57BL/6J mice | Elastase perfusion | 110% increase in AAA cases vs. self-controls | IL-1β knockout | No | NA | 3, 7 and 14 days | ↓ | 0.05 | Attenuated ceramide synthesis in aortic infiltrated neutrophils prevents NETosis |
| [33] | C57BL/6J mice | Ang-II infusion + SMC selective Smad4 deletion in IL1-R1−/− | 1.2 ± 0.0 vs. 1.5 ± 0.1 mm | IL-1β antibody | No | 10 mg/kg body/weight, once weekly | 16 weeks | ↓ | <0.01 | Monocyte infiltration was blocked and aneurysm progression ameliorated |
| [35] | C57BL/6J mice | Elastase perfusion + IL-1β gene knockout | 38 ± 20.4 vs. 89.5 ± 13.1% 52.9 ± 3.2 vs. 82.4 ± 15.3% | IL-1R gene knockout IL-1R antagonist (anakinra) | No Yes | Anakinra administered at day 3 post-AAA induction at 100 mg/kg per day | 14 days | NA | NA | Decreased macrophage and elastin fragmentation |
| Interleukin-6 | ||||||||||
| [37] | C57BL/6J mice | Periaortic application of CaCl2 | 0.9 ± 0.0 vs. 1.1 ± 0.0 mm | murine anti-IL-6R | Prior and post induction | 0.25 mg MR16-1 every week | 6 weeks | ↓ | <0.01 | Suppressed STAT3 activation and AAA expansion |
| [36] | C57BL/6J mice | Elastase perfusion | 50 ± 20.9 vs. 82.7 ± 25.1 mm ^ | Anti-IL-6 antibody | Yes | 4 mg/kg, initiated at day 3 | 14 days | ↓ | <0.03 | Reduced AAA progression |
| [39] | C57BL/6J mice | Elastase perfusion | 101.2 ± 20.1 vs. 101.2 ± 18.4% ^ | IL-6 knockout | No | NA | 14 days | ↔ (Unchanged) | 0.73 | NA |
| [38] | C57BL/6J mice | elastase + anti-TGF-β model | 1.6 ± 0.3 vs. 1.9 ± 0.5 | sgp130Fc | Yes | 10µg thrice a week initiated on the day of experiment | 7 days | ↓ | <0.01 | Increased collagen content of the arterial wall |
| Interleukin-12/23 | ||||||||||
| [40] | C57BL/6J mice | Ang-II infusion | 1.4 ± 0.1 vs. 1.1 ± 0.1 mm ^ | IL-12p40 knockout | No | 150 μL 2 timesat 3-day interval | 14 days | ↓ | <0.01 | Augmented TGFβ2-mediated MMP2 expression |
| [41] | C57BL/6J mice | Elastase perfusion | 0.5 ± 0.1 vs. 0.7 ± 0.1 mm | IL-12p40/IL-23p19 mAb | Yes | 250 μg on days 3 and 8 | 14 days | ↓ | <0.001 | Reduced M1 and M2 macrophages |
| Interleukin-17 | ||||||||||
| [43] | ApoE−/− mice | Ang-II infusion | 1.4 ± 0.1 vs. 1.7 ± 0.1 mm | IL-17A siRNA | No | 3μg/kg | 28 days | ↓ | 0.05 | Reduced VEGFA, MMP-2, MMP-9 and JAK2 protein levels. |
| [42] | C57BL/6J mice | Elastase perfusion | 89.4 ± 7.4 vs. 141.1 ± 16.1% | IL-17−/− | No | NA | 14 days | ↓ | <0.05 | Reduced MCP-1, RANTES, KC, TNF-α, MIP-1α and IFN-γ |
| Ref | Ethics Approval | Animal Strain and Number | Animal Age/Weight | AAA Model | Controls Used | Aortic Diameter | AAA Measurement Methods | Reproducibility of Measurements | Randomisation | Blinding of Assessors |
|---|---|---|---|---|---|---|---|---|---|---|
| [28] | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No |
| [29] | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No |
| [30] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| [32] | Yes | Yes | No | Yes | Yes | No | Yes | No | No | No |
| [34] | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | No |
| [44] | Yes | Yes | No | Yes | Yes | No | Yes | No | No | No |
| [33] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| [35] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| [37] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| [36] | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes |
| [39] | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No |
| [38] | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No |
| [40] | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No |
| [41] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| [43] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| [42] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Ref | Number of AAA vs. Control Cases | Age of AAA vs. Control Cases (p Value), Years | Male Gender % (AAA vs. Control Cases) | Aortic Diameter in AAA (mm) | Method of Assessment | Cytokine Concentration in AAA Cases | Cytokine Concentration in Control Cases | p Value |
|---|---|---|---|---|---|---|---|---|
| Tumor Necrosis Factor-α | ||||||||
| [56] | 32 vs. 11 | 70.5 ± 7.5 vs. 59.5 ± 4.5 | 74.4 vs. 75 ⁿ | ≥50 mm | IHC | 15.0 ± 22.9 pg/mg | 9.6 ± 9.2 pg/mg | <0.002 |
| [50] | 27 vs. 5 | NR | NR | NR | IHC | 5.5 ± 3.5 pg/mg | 0.6 ± 0.63 pg/mg | <0.05 |
| [51] | 10 vs. 9 | 73 (67–81) vs. 55(44–74) x | 100 vs. 66.7 | 75 (56 to 93) mm x | Antibody based protein array | 60 ± 20 × 10−3 (SI) | 2 ± 3 × 10−3 (SI) | <0.01 |
| [61] | 17 vs. 11 | 72.4 ± 6.2 vs. 55.6 ± 10.2 | 82.3 vs. 63.6 | 6.7 ± 1.1 cm | RT-PCR | 0.2 (0.0–0.8) | ND | <0.01 |
| [53] | 15 vs. 6 | 70 ± 6 vs. 41 ± 14 | NR | NR | ELISA | 24 ± 11 pg/mL | 13 ± 1.5 pg/mL | NS |
| [52] | 7 vs. 5 | NR | NR | NR | ELISA | 86 ± 34 pg/mg | 1 ± 1 pg/mg | <0.01 |
| [49] | 8 vs. 8 | 64.8 ± 2.9 vs. 60.8 ± 3.6 | 87.5 vs. 100 | NR | ELISA | 10 ± 1.6 ng/mL ^ | 6.68 ± 2.05 ng/mL ^ | <0.05 |
| [55] | 82 vs. 73 | 73 (50–88) vs. 62 (43–82) (p < 0.01) x | 90 vs. 85 | >5 cm | RT-PCR | 5.6 ± 2.7 × 10−4 am/μL | 4.4 ± 2.7 × 10−5 am/μL | <0.01 |
| [83] | 6 vs. 7 | 66.8 ± 5.9 vs. 62 ± 14.5 ᵇ | 60 vs. 70 | NR | Affymetrix and illumina microarray | Relative expression to controls—0.65 | <0.05 | |
| [54] | 19 vs. 5 | 72 ± 6 vs. 46 ± 4 | 89.4 vs. 80 | NR | RT-PCR | 2.8 ± 0.5 (GAPDH ratio) | 1.0 ± 0.1 (GAPDH ratio) | <0.05 |
| Interleukin-1β/1R | ||||||||
| [56] | 32 vs. 11 | 70.5 ± 7.5 vs. 59.5 ± 4.5 | 74.4 vs. 75 ⁿ | ≥50 mm | IHC | 34.5 ± 37.5 pg/mg | 4.8 ± 2.7 pg/mg | <0.01 |
| [51] | 10 vs. 9 | 73 (67–81) vs. 55(44–74) x | 100 vs. 66.7 | 75 (56 to 93) mm | Antibody based protein array | upregulated | ND | <0.01 |
| [61] | 17 vs. 11 | 72.4 ± 6.2 vs. 55.6 ± 10.2 | 82.3 vs. 63.6 | 6.7 ± 1.1 cm | RT-PCR | 7.2 ± 5.01 pg/mg x | 0.71 ± 0.60 pg/mg x | <0.01 |
| [53] | 15 vs. 6 | 70 ± 6 vs. 41 ± 14 | NR | NR | ELISA | 908 ± 194 pg/mL | 100 ± 30 pg/mL | 0.05 |
| [52] | 7 vs. 5 | NR | NR | NR | ELISA | 48 ± 14 pg/mg | 12 ± 5 pg/mg | <0.05 |
| [49] | 8 vs. 8 | 64.8 ± 2.9 vs. 60.8 ± 3.6 | 87.5 vs. 100 | NR | ELISA | 14.5 ± 2.9 ng/mL ^ | 10.2 ± 1.8 ng/mL ^ | <0.05 |
| [83] | 6 vs. 7 | 66.8 ± 5.9 vs. 62 ± 14.5 ᵇ | 60 vs. 70 | NR | Affymetrix and illumine microarray | Relative expression to controls—1.6 | <0.01 | |
| [60] | 20 vs. 4 | 77.3 vs. 60.5 ^ | 80 vs. 50 | 7.5 (5–10) cm | ELISA | 91 ± 23.6 pg/mg | 60.3 ± 12.1 pg/mg | NS |
| [84] | 10 vs. 10 | 75 (61–82) x | NR | >5 cm | Affymetrix Human Genome microarray | Fold change—3.94 | 0.05 | |
| Interleukin-6 | ||||||||
| [56] | 32 vs. 11 | 70.5 ± 7.5 vs. 59.5 ± 4.5 | 74.4 vs. 75 ⁿ | ≥50 mm | IHC | 527.9 ± 468.9 ng/mL | 28.3 ± 13.9 ng/mL | <0.01 |
| [51] | 10 vs. 9 | 73 (67–81) vs. 55(44–74) x | 100 vs. 66.7 | 75 (56–93) mm | Antibody based protein array | 150 ± 500 × 10−3 (SI) | 10 ± 10 × 10−3 (SI) | <0.01 |
| [49] | 8 vs. 8 | 64.8 ± 2.9 vs. 60.8 ± 3.6 | 87.5 vs. 100 | NR | ELISA | 5.5 ± 2.15 ng/mL ^ | 1.7 ± 0.4 ng/mL ^ | <0.05 |
| [55] | 82 vs. 73 | 73 (50–88) vs. 62 (43–82) (<0.01) x | 90 vs. 85 | >5 cm | RT-PCR | 0.6 ± 0.4 am/μL | 0.01 ± 0.01 am/μL | 0.02 |
| [60] | 20 vs. 4 | 77.3 vs. 60.5 ^ | 80 vs. 50 | 7.5 (5–10) cm | ELISA | 90.3 ± 31.7 ng/mL | 28.1 ± 5.9 ng/mL | NS |
| [63] | 7 vs. 4 | NR | NR | NR | ELISA | 1008 ± 197 ng/mL | 190 ± 14 ng/mL | <0.05 |
| [62] | 13 vs. 16 | NR | NR | NR | ELISA | 14,329 ± 4159 U/mL | 1861 ± 334 U/mL | 0.02 |
| [84] | 10 vs. 10 | 75 (61–82) x | NR | >5 cm | Affymetrix Human Genome microarray | Fold change—6.9 | <0.05 | |
| Interleukin-12/23 | ||||||||
| [51] | 10 vs. 9 | 73 (67–81) vs. 55(44–74) x | 100 vs. 66.7 | 75 (56 to 93) mm | Antibody based protein array | 0.04 [0.01 to 0.07] x | 0.00 [0.00 to 0.04] x | 0.02 |
| [61] | 17 vs. 11 | 72.4 ± 6.2 vs. 55.6 ± 10.2 | 82.3 vs. 63.6 | 6.7 ± 1.1 cm | RT-PCR | 0.05 (0–0.3) x | ND | NS |
| [83] | 6 vs. 7 | 66.8 ± 5.9 vs. 62 ± 14.5 ᵇ | 60 vs. 70 | NR | Affymetrix and illumina microarray | Relative expression to controls—1.3 | <0.01 | |
| Interleukin-17 | ||||||||
| [43] | 7 vs. 7 | NR | NR | NR | Western blotting | 0.5 ± 0.08 | 0.03 ± 0.03 | <0.01 |
| [42] | 16 vs. 8 | NR | 100 vs. 100 | NR | Multiplex cytokine panel assay | 37.8 ± 3.8 pg/mL | 6.0 ± 3.2 pg/mL | < 0.05 |
| [77] | 5 vs. 5 | NR | NR | NR | Western blotting | 0.7 ± 0.2 | 0.07 ± 0.04 | <0.01 |
| Ref | Sample Size Estimate Reported | Age-Matched Controls | Sex-Matched Controls | Comorbidities Were Adjusted for in Analyses | Analysis by Blinded Observer | Controls and AAA Cases Imaged | Method and Mode of Aortic Diameter Imaging |
|---|---|---|---|---|---|---|---|
| [43] | No | No | No | No | No | No | NA |
| [42] | No | No | No | No | No | No | NA |
| [56] | No | Yes | Yes | No | No | No | NA |
| [50] | No | NR | NR | No | No | No | NA |
| [51] | No | Yes | Yes | No | No | No | NA |
| [61] | No | Yes | Yes | No | No | No | NA |
| [53] | No | Yes | NR | No | No | No | NA |
| [52] | No | NR | NR | No | No | No | NA |
| [49] | No | Yes | Yes | No | No | No | NA |
| [55] | No | Yes | Yes | No | No | No | NA |
| [83] | No | Yes | Yes | No | No | No | NA |
| [54] | No | Yes | Yes | No | No | No | NA |
| [59] | No | Yes | Yes | No | No | Yes | Ultrasonography * |
| [57] | Yes | Yes | Yes | No | No | Yes | Ultrasound * |
| [60] | No | Yes | Yes | No | No | No | NA |
| [84] | No | Yes | Yes | No | No | No | NA |
| [63] | No | NR | NR | No | No | No | NA |
| [62] | No | NR | NR | No | No | No | NA |
| [75] | No | Yes | Yes | No | No | Yes | Ultrasonography * |
| [65] | No | Yes | Yes | No | Yes | Yes | Computed tomography ** |
| [66] | No | Yes | Yes | No | No | Yes | Ultrasound * |
| [73] | No | Yes | Yes | No | No | Yes | Ultrasonography * |
| [64] | No | Yes | Yes | No | No | Yes | Ultrasound * |
| [67] | No | Yes | Yes | No | No | Yes | Ultrasound or computed tomography # |
| [72] | No | No | No | No | No | No | NA |
| [58] | Yes | Yes | Yes | No | No | Yes | Ultrasonography * |
| [85] | No | Yes | Yes | No | No | Yes | Ultrasonography * |
| [69] | No | Yes | No | No | No | No | NA |
| [70] | No | Yes | Yes | No | No | Yes | Ultrasonography * |
| [71] | Yes | Yes | Yes | Yes | No | Yes | Ultrasonography * |
| [76] | No | Yes | Yes | Yes | No | Yes | Ultrasonography * |
| [74] | No | No | No | No | No | No | NA |
| [68] | No | Yes | Yes | No | No | Yes | Contrast-enhanced computed tomography |
| [77] | No | No | No | No | No | No | NA |
| [79] | No | Yes | Yes | No | No | Yes | Ultrasound * |
| [78] | No | Yes | Yes | Yes | Yes | Yes | Computed tomography * |
| Ref | Number of AAA vs. Control Cases | Age of AAA vs. Control Cases (p Value), Years | Male Gender % (AAA vs. Control Cases) | Aortic Diameter in AAA (mm) | Method of Assessment | Cytokine Concentration in AAA Cases | Cytokine Concentration in Control Cases | p Value |
|---|---|---|---|---|---|---|---|---|
| Tumor Necrosis Factor-α | ||||||||
| [59] | 50 vs. 42 | 58.6 ± 6.6 vs. 58.1 ± 6.3 * | 80 vs. 44.7 | 48 (33–66) mm | Solid phase radioimmunoassay | 2.1 ± 0.7 pmol/L ^ | 1.5 ± 0.3 pmol/L ^ | <0.05 |
| [57] | 20 vs. 20 | 74 ± 6 vs. 72 ± 5 | 100 vs. 100 | <45 mm | ELISA | 41.4 ± 17.3 pg/mL | 13.1 ± 5.2 pg/mL | <0.05 |
| [58] | 130 vs. 219 z | 75 ± 8 vs. 68 (53–80) | 82.6 vs. 90 | >55 mm | ELISA | 3.6 ± 10.2 pg/mL | 1.23 ± 1.93 pg/mL | <0.01 |
| Interleukin-1β | ||||||||
| [59] | 50 vs. 42 | 58.6 ± 6.6 vs. 58.05 ± 6.3 * | 80 vs. 44.7 | 48 (33–66) mm | Solid phase radioimmunoassay | 19.3 pmol/L | 2.1 pmol/L | <0.01 |
| Interleukin-6 | ||||||||
| [59] | 50 vs. 42 | 58.6 ± 6.6 vs. 58.05 ± 6.3 * | 80 vs. 44.7 | 48 (33–66) mm | Solid phase radioimmunoassay | 10.0 ± 5.3 pmol/L ^ | 4.8 ± 3.2 pmol/L ^ | <0.05 |
| [75] | 38 vs. 41 | 70(66–76) vs. 72(67–79) x | 71 vs. 80.5 | 4.0 (3.5–4.3) cm | ELISA | 3.6 ± 0.51 pg/mL x | 3.0 ± 1.03 pg/mL x | NS |
| [65] | 27 vs. 15 | 73 (58–91) vs. 50 (32–74) (p < 0.01) x | 100 vs. 20 | 64 (51–100) mm | ELISA | 4.9 ± 0.4 pg/mL | 2.6 ± 0.5 pg/mL | <0.05 |
| [66] | 89 vs. 98 | 73.5 ± 0.5 vs. 73.5 ± 0.5 | 71.9 vs. 71.4 | 4.5 (3.9 to 5.1) cm | ELISA | 2.9 ± 0.4 pg/mL x | 1.9 ± 0.2 pg/mL x | <0.05 |
| [73] | 74 vs. 30 | 70.7 (56–82) vs. NR | 80 vs. NR | 5 (5–8), vs. NR cm | ELISA | 64.2 ± 15.7 pg/mL | 6.7 ± 5.1 pg/mL | <0.05 |
| [64] | 214 vs. 343 | 74 ± 8 vs. 68 ± 2 (p < 0.01) | 79 vs. 46.3 (p < 0.01) | 62.8 ± 14.6 mm | ELISA | 9.4 ± 32.2 pg/mL | 2.1 ± 2.9 pg/mL | <0.01 |
| [67] | 108 vs. 42 | 72 ± 4 vs. 69 ± 8 | 100 vs. 100 | 6.3 ± 0.8 cm | Immunoassay | 5.5 ± 2.4 pg/mL | 4.2 ± 1.6 pg/mL | 0.04 |
| [72] | 41 vs. 18 | 72.0 (63.4–77.8) vs. 59.6 (51.4–69.4) | 92.7 vs. 55.6 | 61.6 (40–112) mm | ELISA | 3.7 ± 0.6 pg/mL x | 3.1 ± 0.9 pg/mL x | NS |
| [58] | 130 vs. 219 z | 75 ± 8 vs. 68 (53–80) x | 82.6 vs. 90 | >55 mm | ELISA | 11.3 ± 33.8 pg/mL | 2.1 ± 2.9 pg/mL | <0.01 |
| [85] | 23 vs. 20 | 72 (54–83) vs. 72 (66–79) | 100 vs. 80 | 60 (43–75) x mm | ELISA | 940 x ng/mL | 793 x ng/mL | <0.01 |
| [69] | 10 vs. 10 | 72 (62–75) vs. 72 (62–75) | 80 vs. 20 | NR | ELISA | 12.4 ± 11.2 pg/mL | 3.4 ± 3.1 pg/mL | 0.02 |
| [70] | 78 vs. 36 | 71 (66–78) vs. 72 (67–78) | 79.5 vs. 83.3 | 49 (40–61) x mm | ELISA | 3.1 ± 0.6 ng/mL | 2.3 ± 0.4 ng/mL | <0.01 |
| [71] | 75 vs. 90 | 72 ± 7 vs. 72 ± 6 | 100 vs. 100 | 41 (35–46) mm | ELISA | 3.1 ±0.2 x ng/mL | 3.1 ± 0.2 x ng/mL | 0.98 |
| [76] | 7 vs. 113 | 65 ± 9 (both groups combined) | 52.5 vs. 67.5 | 2.1 ± 0.6 cm/m2 | ELISA | 3.2 ± 0.9 pg/mL | 2.3 ± 1.2 pg/mL | 0.04 |
| [74] | 50 vs. 22 | 72.0 (54–85) vs. 59.6 (44–78) | 90 vs. 54.5 | 61.6 (40–112) mm | ELISA | 4 ± 0.6 x pg/mL | 2.7 ± 0.5 x pg/mL | <0.01 |
| [68] | 10 vs. 10 | 76.5 (65–85) vs. 70.5 (59–81) | 80 vs. 80 | 56.1 (48–83) mm | ELISA | 22.6 ± 13.7 x pg/mL | 9.8 ± 5.6 x pg/mL | <0.05 |
| Interleukin-17 | ||||||||
| [79] | 153 vs. 121 | 68.9 ± 4.9 vs. 69.4 ± 6.4 | 96.7 vs. 99.2 | 49.4 mm ^ | ELISA | 27.7 ± 42.1 pg/mL | 11.7 ± 25.1 pg/mL | <0.01 |
| [78] | 476 vs. 200 | 69.9± 2.8 vs. 69.6 ± 2.8 | 100 vs. 100 | 50 mm | ELISA | 36.5 ± 5.9 pg/mL | 68.4 ± 13.3 pg/mL | 0.02 |
| Drug Class | Safety Warnings from FDA | Contraindications |
|---|---|---|
| Infliximab [14] |
|
|
| Etanercept [13] |
|
|
| Adalimumab [15] |
|
|
| Certolizumab pegol [16] |
|
|
| Golimumab [17] |
|
|
| Canakinumab [18] |
|
|
| Anakinra [19] |
|
|
| Tocilizumab [20] |
|
|
| Sarilumab [21] |
|
|
| Ustekizumab [22] |
|
|
| Guselkumab [23] |
|
|
| Tildrakizumab [24] |
|
|
| Secukinumab [25] |
|
|
| Brodalumab [26] |
|
|
| Ixekizumab [27] |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanigaimani, S.; Ibrahim, M.; Golledge, J. Potential of Disease-Modifying Anti-Rheumatic Drugs to Limit Abdominal Aortic Aneurysm Growth. Biomedicines 2022, 10, 2409. https://doi.org/10.3390/biomedicines10102409
Thanigaimani S, Ibrahim M, Golledge J. Potential of Disease-Modifying Anti-Rheumatic Drugs to Limit Abdominal Aortic Aneurysm Growth. Biomedicines. 2022; 10(10):2409. https://doi.org/10.3390/biomedicines10102409
Chicago/Turabian StyleThanigaimani, Shivshankar, Muhammad Ibrahim, and Jonathan Golledge. 2022. "Potential of Disease-Modifying Anti-Rheumatic Drugs to Limit Abdominal Aortic Aneurysm Growth" Biomedicines 10, no. 10: 2409. https://doi.org/10.3390/biomedicines10102409






