T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients
Abstract
1. Introduction
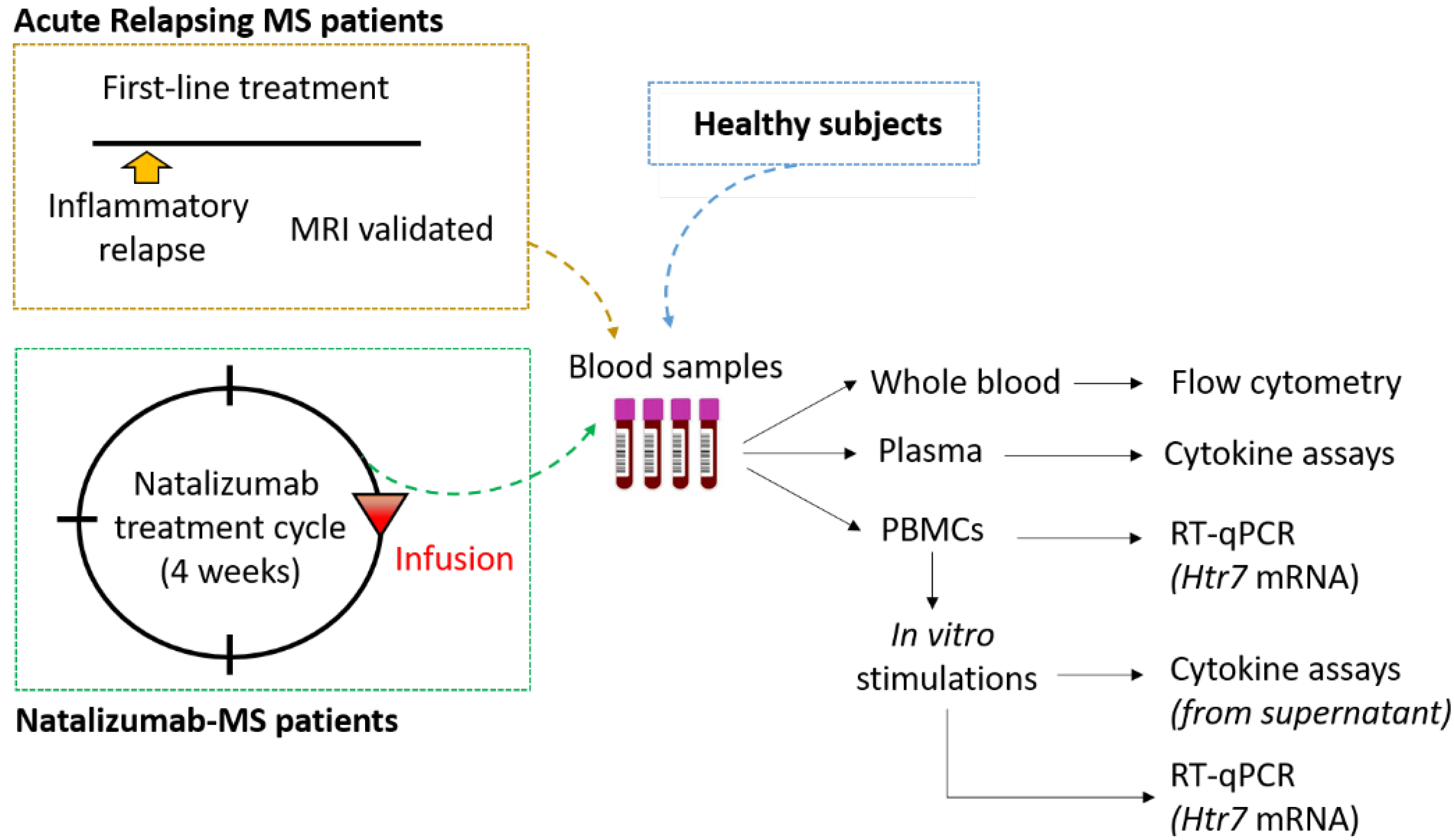
2. Materials and Methods
2.1. Standard Protocol Approvals and Subject Characterization
2.2. Analysis of 5-HT7 Receptor Expression on Lymphocyte Surface
2.3. Isolation and Stimulation of Peripheral Blood Mononuclear Cells
2.4. Cytokine Assays in Plasma Samples and PBMC Supernatants
2.5. Analysis of 5-HT7 Receptor Transcription Level
2.6. Statistical Analysis
3. Results
3.1. High Surface Expression of 5-HT7 Receptor on T Cells in Natalizumab-Treated MS Patients
3.2. Decreased 5-HT7 Receptor Gene Activity in the Absence of a Peripheral Inflammatory Context in Natalizumab-MS Patients
3.3. 5-HT7 Activation Promoted IL-10 Release from PBMCs under Physiological and Pathological Conditions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quintero-Villegas, A.; Valdés-Ferrer, S.I. Role of 5-HT7 receptors in the immune system in health and disease. Mol. Med. 2019, 26. [Google Scholar] [CrossRef]
- Shajib, M.S.; Khan, W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef]
- San Hernandez, A.M.; Singh, C.; Valero, D.J.; Nisar, J.; Ramirez, J.I.T.; Kothari, K.K.; Isola, S.; Gordon, D.K. Multiple Sclerosis and Serotonin: Potential Therapeutic Applications. Cureus 2020, 12, e11293. [Google Scholar] [CrossRef]
- Pegoretti, V.; Swanson, K.A.; Bethea, J.R.; Probert, L.; Eisel, U.L.M.; Fischer, R. Inflammation and Oxidative Stress in Multiple Sclerosis: Consequences for Therapy Development. Oxid. Med. Cell Longev. 2020, 2020, 7191080. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Lenhart, A.; Rosenwald, A.; Monoranu, C.M.; Berberich-Siebelt, F. Lymphoid Aggregates in the CNS of Progressive Multiple Sclerosis Patients Lack Regulatory T Cells. Front. Immunol. 2019, 10, 3090. [Google Scholar] [CrossRef]
- Sacramento, P.M.; Monteiro, C.; Dias, A.S.O.; Kasahara, T.M.; Ferreira, T.B.; Hygino, J.; Wing, A.C.; Andrade, R.M.; Rueda, F.; Sales, M.C.; et al. Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4+ T-cell subsets in multiple sclerosis patients. Eur. J. Immunol. 2018, 48, 1376–1388. [Google Scholar] [CrossRef]
- Hernández-Torres, G.; Enríquez-Palacios, E.; Mecha, M.; Feliú, A.; Rueda-Zubiaurre, A.; Angelina, A.; Martín-Cruz, L.; Martín-Fontecha, M.; Palomares, O.; Guaza, C.; et al. Development of a Fluorescent Bodipy Probe for Visualization of the Serotonin 5-HT1A Receptor in Native Cells of the Immune System. Bioconjug. Chem. 2018, 29, 2021–2027. [Google Scholar] [CrossRef]
- Sviridova, A.; Rogovskii, V.; Kudrin, V.; Pashenkov, M.; Boyko, A.; Melnikov, M. The role of 5-HT2B-receptors in fluoxetine-mediated modulation of Th17- and Th1-cells in multiple sclerosis. J. Neuroimmunol. 2021, 356, 577608. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Front. Immunol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Glass, J.D.; Grossman, G.H.; Farnbauch, L.; Di Nardo, L. Midbrain Raphe Modulation of Nonphotic Circadian Clock Resetting and 5-HT Release in the Mammalian Suprachiasmatic Nucleus. J. Neurosci. 2003, 23, 7451–7460. [Google Scholar] [CrossRef]
- León-Ponte, M.; Ahern, G.P.; O’Connell, P.J. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 2007, 109, 3139–3146. [Google Scholar] [CrossRef]
- Ito, M.; Komai, K.; Mise-Omata, S.; Iizuka-Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Urbina, M.; Arroyo, R.; Lima, L. 5-HT7 receptors and tryptophan hydroxylase in lymphocytes of rats: Mitogen activation, physical restraint or treatment with reserpine. Neuroimmunomodulation 2014, 21, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, A.; Scalia, G.; Ausiello, F.; Moccia, M.; Russo, C.V.; Saccà, F.; De Rosa, A.; Criscuolo, C.; Del Vecchio, L.; Morra, V.B.; et al. CD4/CD8 ratio during natalizumab treatment in multiple sclerosis patients. J. Neuroimmunol. 2017, 309, 47–50. [Google Scholar] [CrossRef]
- Van Kaer, L.; Postoak, J.L.; Wang, C.; Yang, G.; Wu, L. Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell Mol. Immunol. 2019, 16, 531–539. [Google Scholar] [CrossRef]
- González-Oria, M.C.; Márquez-Coello, M.; Girón-Ortega, J.A.; Argente, J.; Moya, M.; Girón-González, J.-A. Monocyte and Lymphocyte Activation and Regulation in Multiple Sclerosis Patients. Therapy Effects. J. Neuroimmune Pharmacol. 2019, 14, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, M.; Sviridova, A.; Rogovskii, V.; Oleskin, A.; Boziki, M.; Bakirtzis, C.; Kesidou, E.; Grigoriadis, N.; Boyko, A. Serotoninergic system targeting in multiple sclerosis: The prospective for pathogenetic therapy. Mult. Scler. Relat. Disord. 2021, 51, 102888. [Google Scholar] [CrossRef]
- Bhat, R.; Mahapatra, S.; Axtell, R.C.; Steinman, L. Amelioration of ongoing experimental autoimmune encephalomyelitis with fluoxetine. J. Neuroimmunol. 2017, 313, 77–81. [Google Scholar] [CrossRef]
- Romme Christensen, J.; Börnsen, L.; Ratzer, R.; Piehl, F.; Khademi, M.; Olsson, T.; Sorensen, P.S.; Sellebjerg, F. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS ONE 2013, 8, e57820. [Google Scholar] [CrossRef] [PubMed]
- Kivisäkk, P.; Healy, B.C.; Viglietta, V.; Quintana, F.J.; Hootstein, M.A.; Weiner, H.L.; Khoury, S.J. Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurology 2009, 72, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Mellergård, J.; Edström, M.; Jenmalm, M.C.; Dahle, C.; Vrethem, M.; Ernerudh, J. Increased B cell and cytotoxic NK cell proportions and increased T cell responsiveness in blood of natalizumab-treated multiple sclerosis patients. PLoS ONE 2013, 8, e81685. [Google Scholar] [CrossRef]
- Börnsen, L.; Christensen, J.R.; Ratzer, R.; Oturai, A.B.; Sørensen, P.S.; Søndergaard, H.B.; Sellebjerg, F. Effect of natalizumab on circulating CD4+ T-cells in multiple sclerosis. PLoS ONE 2012, 7, e47578. [Google Scholar] [CrossRef] [PubMed]
- Frisullo, G.; Iorio, R.; Plantone, D.; Marti, A.; Nociti, V.; Patanella, A.K.; Batocchi, A.P. CD4+T-bet+, CD4+pSTAT3+ and CD8+T-bet+ T cells accumulate in peripheral blood during NZB treatment. Mult. Scler. 2011, 17, 556–566. [Google Scholar] [CrossRef]
- Lepennetier, G.; Hracsko, Z.; Unger, M.; Van Griensven, M.; Grummel, V.; Krumbholz, M.; Berthele, A.; Hemmer, B.; Kowarik, M.C. Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. J. Neuroinflammation 2019, 16, 219. [Google Scholar] [CrossRef]
- Albayrak, A.; Halici, Z.; Cadirci, E.; Polat, B.; Karakus, E.; Bayir, Y.; Unal, D.; Atasoy, M.; Dogrul, A. Inflammation and peripheral 5-HT7 receptors: The role of 5-HT7 receptors in carrageenan induced inflammation in rats. Eur. J. Pharmacol. 2013, 715, 270–279. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Lefkowitz, R.J. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002, 115, 455–465. [Google Scholar] [CrossRef]
- Guthrie, C.R.; Murray, A.T.; Franklin, A.A.; Hamblin, M.W. Differential agonist-mediated internalization of the human 5-hydroxytryptamine 7 receptor isoforms. J. Pharmacol. Exp. Ther. 2005, 313, 1003–1010. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Jia, A.; Cui, Y.; Feng, J. Cerebrospinal fluid cells immune landscape in multiple sclerosis. J. Transl. Med. 2021, 19, 125. [Google Scholar] [CrossRef]
- Quintero-Villegas, A.; Valdés-Ferrer, S.I. Central nervous system effects of 5-HT7 receptors: A potential target for neurodegenerative diseases. Mol. Med. 2022, 28, 70. [Google Scholar] [CrossRef]
- Ayaz, G.; Halici, Z.; Albayrak, A.; Karakus, E.; Cadirci, E. Evaluation of 5-HT7 Receptor Trafficking on In Vivo and In Vitro Model of Lipopolysaccharide (LPS)-Induced Inflammatory Cell Injury in Rats and LPS-Treated A549 Cells. Biochem. Genet. 2017, 55, 34–47. [Google Scholar] [CrossRef]
- Renner, U.; Zeug, A.; Woehler, A.; Niebert, M.; Dityatev, A.; Dityateva, G.; Gorinski, N.; Guseva, D.; Abdel-Galil, D.; Frohlich, M.; et al. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012, 125, 2486–2499. [Google Scholar] [CrossRef]
- Ramos-Cejudo, J.; Oreja-Guevara, C.; Stark Aroeira, L.; Rodriguez de Antonio, L.; Chamorro, B.; Diez-Tejedor, E. Treatment with Natalizumab in Relapsing–Remitting Multiple Sclerosis Patients Induces Changes in Inflammatory Mechanism. J. Clin. Immunol. 2011, 31, 623–631. [Google Scholar] [CrossRef]



| Clinical Characteristics | Natalizumab Treated MS Patients (n = 30) | Acute Relapsing MS Patients (n = 18) | Healthy Subjects (n = 30) |
|---|---|---|---|
| Age, y, mean (SEM) | 37.8 (1.86) | 34.1 (2.13) | 39.2 (1.57) |
| Female, n (%) | 25 (83) | 13 (81) | 25 (83) |
| MS symptom onset, y (SEM) | 8.6 (1.24) | 5.4 (1.03) | / |
| EDSS score, mean (SEM) | 2.3 (0.40) | 2.8 (0.50) | / |
| SSRI treatment, n (%) | 7 (23) | 1 (6) | / |
| Natalizumab infusions, n (SEM) | 49 (7.55) | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reverchon, F.; Guillard, C.; Mollet, L.; Auzou, P.; Gosset, D.; Madouri, F.; Valéry, A.; Menuet, A.; Ozsancak, C.; Pallix-Guyot, M.; et al. T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients. Biomedicines 2022, 10, 2418. https://doi.org/10.3390/biomedicines10102418
Reverchon F, Guillard C, Mollet L, Auzou P, Gosset D, Madouri F, Valéry A, Menuet A, Ozsancak C, Pallix-Guyot M, et al. T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients. Biomedicines. 2022; 10(10):2418. https://doi.org/10.3390/biomedicines10102418
Chicago/Turabian StyleReverchon, Flora, Colleen Guillard, Lucile Mollet, Pascal Auzou, David Gosset, Fahima Madouri, Antoine Valéry, Arnaud Menuet, Canan Ozsancak, Maud Pallix-Guyot, and et al. 2022. "T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients" Biomedicines 10, no. 10: 2418. https://doi.org/10.3390/biomedicines10102418
APA StyleReverchon, F., Guillard, C., Mollet, L., Auzou, P., Gosset, D., Madouri, F., Valéry, A., Menuet, A., Ozsancak, C., Pallix-Guyot, M., & Morisset-Lopez, S. (2022). T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients. Biomedicines, 10(10), 2418. https://doi.org/10.3390/biomedicines10102418






