Pulegone and Eugenol Oral Supplementation in Laboratory Animals: Results from Acute and Chronic Studies
Abstract
:1. Introduction
2. Materials and Methods
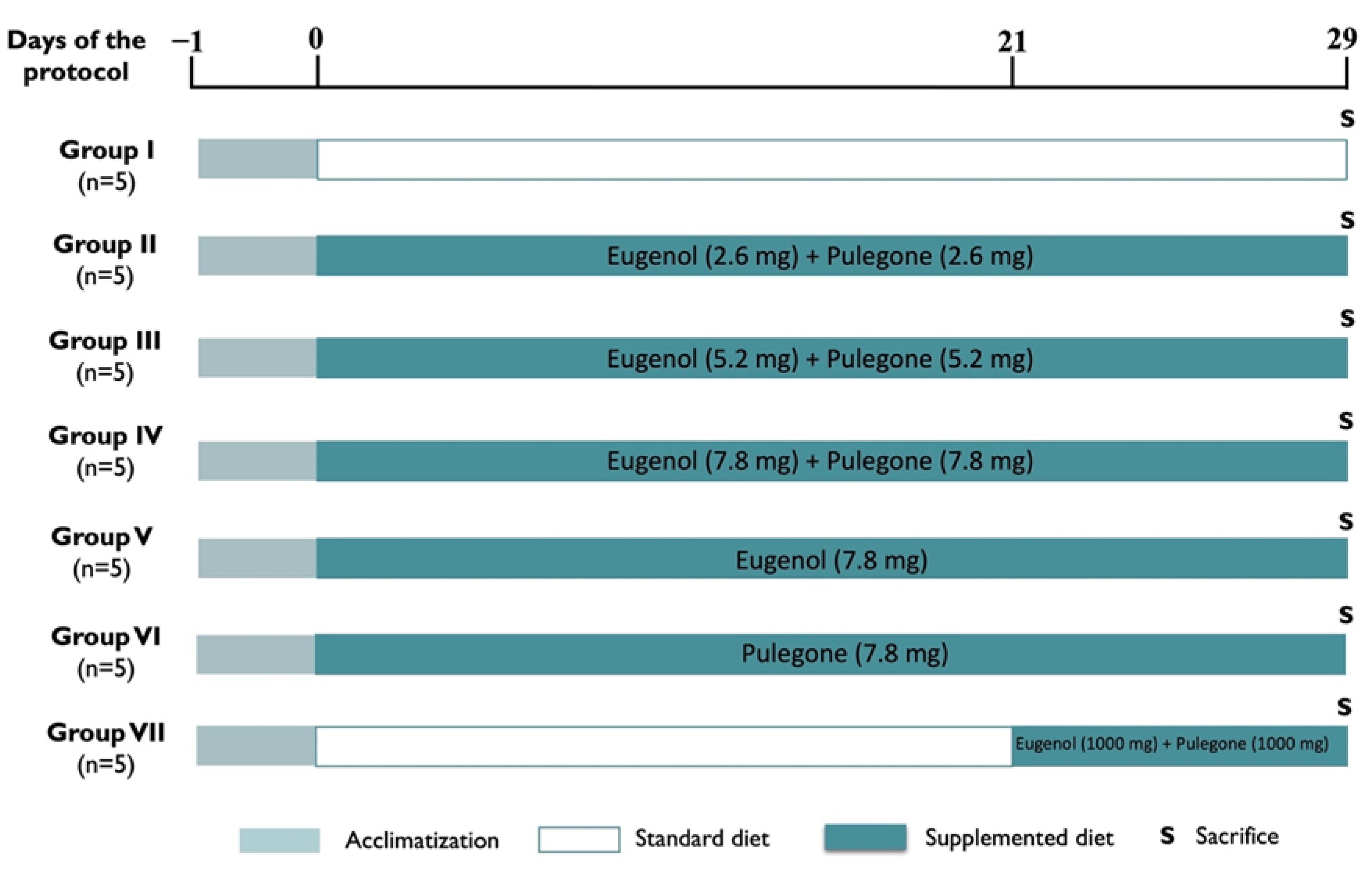
2.1. Experimental Design and Conditions
2.2. Diet Preparation
2.3. Animals’ Monitoring
2.4. Animals’ Sacrifice
2.5. Microhematocrit and Serum Biochemical Analysis
2.6. Comet Assay
2.7. Oxidative Stress Parameters
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results
3.1. General Findings and Humane Endpoints
3.2. Food and Water Consumption
3.3. Body Weight and Ponderal Weight Gain
3.4. Organs’ Weight and Relative Weight
3.5. Microhematocrit and Biochemical Parameters
3.6. Comet Assay
3.7. Oxidative Stress Parameters
3.8. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent Activity of Essential Oils: A Review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Isman, M.B. Plant Essential Oils for Pest and Disease Management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Ootani, M.A.; Aguiar, R.W.; Carlos, A.; Ramos, C.; Brito, R.; Batista, J.; Cajazeira, P. Use of Essential Oils in Agriculture Use of Essential Oils in Agriculture. J. Biotechnol. Biodivers. 2013, 4, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.S.; Kim, T.H.; Lim, J.M.; Song, J.H. Effects of Eugenol on Na+ Currents in Rat Dorsal Root Ganglion Neurons. Brain Res. 2008, 1243, 53–62. [Google Scholar] [CrossRef]
- Barkin, M.E.; Boyd, J.P.; Cohen, S. Acute Allergic Reaction to Eugenol. Oral Surg. Oral Med. Oral Pathol. 1984, 57, 441–442. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mazumdar, A.; Mondhe, D.; Mandal, M. Apoptotic Effect of Eugenol in Human Colon Cancer Cell Lines. Cell Biol. Int. 2011, 35, 607–615. [Google Scholar] [CrossRef]
- Fangjun, L.; Zhijia, Y. Tumor Suppressive Roles of Eugenol in Human Lung Cancer Cells. Thorac. Cancer 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.A.; Rycroft, R.J.G.; White, I.R.; McFadden, J.P. Fragrance as an Occupational Allergen. Occup. Med. (Chic. Ill). 2002, 52, 13–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol--from the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurian, R.; Arulmozhi, D.K.; Veeranjaneyulu, A.; Bodhankar, S.L. Effect of Eugenol on Animal Models of Nociception. Indian J. Pharmacol. 2006, 38, 341. [Google Scholar] [CrossRef]
- Boonchird, C.; Flegel, T.W. In Vitro Antifungal Activity of Eugenol and Vanillin against Candida Albicans and Cryptococcus Neoformans. Can. J. Microbiol. 1982, 28, 1235–1241. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Yousuf, S.; Khan, L.A.; Manzoor, N. Proton Translocating ATPase Mediated Fungicidal Activity of Eugenol and Thymol. Fitoterapia 2010, 81, 1157–1162. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Magalhães, C.B.; Riva, D.R.; Depaula, L.J.; Brando-Lima, A.; Koatz, V.L.G.; Leal-Cardoso, J.H.; Zin, W.A.; Faffe, D.S. In Vivo Anti-Inflammatory Action of Eugenol on Lipopolysaccharide-Induced Lung Injury. J. Appl. Physiol. 2010, 108, 845–851. [Google Scholar] [CrossRef]
- Sarrami, N.; Pemberton, M.N.; Thornhill, M.H.; Theaker, E.D. Adverse Reactions Associated with the Use of Eugenol in Dentistry. Br. Dent. J. 2002, 193, 257–259. [Google Scholar] [CrossRef]
- Quirce, S.; Fernández-Nieto, M.; Del Pozo, V.; Sastre, B.; Sastre, J. Occupational Asthma and Rhinitis Caused by Eugenol in a Hairdresser. Allergy 2008, 63, 137–138. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Nóbrega, F.F.F.; de Lima, M.R.V.; de Almeida, R.N. Pharmacological Activity of (R)-(+)-Pulegone, a Chemical Constituent of Essential Oils. Z. Naturforsch. C. 2011, 66, 353–359. [Google Scholar] [CrossRef]
- Miraj, S.; Kiani, S. Study of Pharmacological Effect of Mentha Pulegium: A Review. Der Pharm. Lett. 2016, 8, 242–245. [Google Scholar]
- Chen, L.J.; Lebetkin, E.H.; Burka, L.T. Comparative Disposition of (R)-(+)-Pulegone in B6C3F1 Mice and F344 Rats. Drug Metab. Dispos. 2003, 31, 892–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, T.A.; Antunes, R.M.P.; Catão, R.M.R.; Lima, E.O.; Sousa, D.P.; Nunes, X.P.; Pereira, M.S.V.; Barbosa-Filho, J.M.; da Cunha, E.V.L. Preliminary Study of the Antimicrobial Activity of Mentha x Villosa Hudson Essential Oil, Rotundifolone and Its Analogues. Rev. Bras. Farmacogn. 2006, 16, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Franzios, G.; Mirotsou, M.; Hatziapostolou, E.; Kral, J.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and Genotoxic Activities of Mint Essential Oils. J. Agric. Food Chem. 1997, 45, 2690–2694. [Google Scholar] [CrossRef]
- Harwood, S.H.; Moldenke, A.F.; Berry, R.E. Toxicity of Peppermint Monoterpenes to the Variegated Cutworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 1990, 83, 1761–1767. [Google Scholar] [CrossRef]
- Roy, A.; Park, H.-J.; Abdul, Q.A.; Jung, H.A.; Choi, J.S. Pulegone Exhibits Anti-Inflammatory Activities through the Regulation of NF-ΚB and Nrf-2 Signaling Pathways in LPS-Stimulated RAW 264.7 Cells. Nat. Prod. Sci. 2018, 24, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Luo, J.; Lv, H.; Wen, T.; Shi, B.; Liu, X.; Zeng, N. Pulegone Inhibits Inflammation via Suppression of NLRP3 Inflammasome and Reducing Cytokine Production in Mice. Immunopharmacol. Immunotoxicol. 2019, 41, 420–427. [Google Scholar] [CrossRef]
- de Urbina, A.V.O.; Martin, M.L.; Montero, M.J.; Carron, R.; Sevilla, M.A.; San Roman, L. Antihistaminic Activity of Pulegone on the Guinea-Pig Ileum. J. Pharm. Pharmacol. 1990, 42, 295–296. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and Antifungal Properties of Essential Oil Components. J. Essent. Oil Res. 2011, 1, 119–128. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Puleio, R.; Morelli, I.; Panizzi, L. Antimicrobial Activity of the Essential Oil of Calamintha Nepeta and Its Constituent Pulegone against Bacteria and Fungi. Phyther. Res. 1999, 13, 349–351. [Google Scholar] [CrossRef]
- de Urbina, A.V.O.; Martín, M.L.; Montero, M.J.; Morán, A.; Román, L.S. Sedating and Antipyretic Activity of the Essential Oil of Calamintha Sylvatica Subsp. Ascendens. J. Ethnopharmacol. 1989, 25, 165–171. [Google Scholar] [CrossRef]
- Aviles-Moreno, J.R.; UreÑa Horno, E.; Partal UreÑa, F.; LÓpez GonzÁlez, J.J. IR-Raman-VCD Study of R-(+)-Pulegone: Influence of the Solvent. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 767–776. [Google Scholar] [CrossRef]
- Gordon, W.P.; Forte, A.J.; McMurtry, R.J.; Gal, J.; Nelson, S.D. Hepatotoxicity and Pulmonary Toxicity of Pennyroyal Oil and Its Constituent Terpenes in the Mouse. Toxicol. Appl. Pharmacol. 1982, 65, 413–424. [Google Scholar] [CrossRef]
- Thorup, I.; Würtzen, G.; Carstensen, J.; Olsen, P. Short Term Toxicity Study in Rats Dosed with Pulegone and Menthol. Toxicol. Lett. 1983, 19, 207–210. [Google Scholar] [CrossRef]
- Imaizumi, K.; Hanada, K.; Mawatari, K.; Sugano, M. Effect of Essential Oils on the Concentration of Serum Lipids and Apolipoproteins in Rats. Agric. Biol. Chem. 2014, 49, 2795–2796. [Google Scholar] [CrossRef] [Green Version]
- Umezu, T. Evidence for Dopamine Involvement in Ambulation Promoted by Pulegone in Mice. Pharmacol. Biochem. Behav. 2010, 94, 497–502. [Google Scholar] [CrossRef]
- Anderson, I.B.; Mullen, W.H.; Meeker, J.E.; Khojasteh-Bakht, S.C.; Oishi, S.; Nelson, S.D.; Blanc, P.D. Pennyroyal Toxicity: Measurement of Toxic Metabolite Levels in Two Cases and Review of the Literature. Ann. Intern. Med. 1996, 124, 726–734. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Olfert, E.; Bhasin, J.; Latt, R.; Macallum, E.; McCutcheon, K.; Rainnie, D.; Schunk, M. Guidelines on: Choosing an Appropriate Endpoint in Experiments Using Animals for Research, Teaching and Testing; Canadian Council on Animal Care: Ottawa, ON, Canada, 1998. [Google Scholar]
- Faustino-Rocha, A.I.; Ginja, M.; Ferreira, R.; Oliveira, P.A. Studying Humane Endpoints in a Rat Model of Mammary Carcinogenesis. Iran. J. Basic Med. Sci. 2019, 22, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Silva-Reis, R.; Faustino-Rocha, A.I.; Gonçalves, M.; Ribeiro, C.C.; Ferreira, T.; Ribeiro-Silva, C.; Gonçalves, L.; Antunes, L.; Venâncio, C.; Ferreira, R.; et al. Refinement of Animal Model of Colorectal Carcinogenesis through the Definition of Novel Humane Endpoints. Animal 2021, 11, 985. [Google Scholar] [CrossRef] [PubMed]
- Forbes, D.; Blom, H.; Kostomitsopulos, N.; Moore, G.; Perretta, G. Euroguide: On the Accommodation and Care of Animals Used for Experimental and Other Scientific Purposes; Federation of European Laboratory Animal Science Associations: London, UK, 2007. [Google Scholar]
- Collins, A.R.; Azqueta, A. Single-Cell Gel Electrophoresis Combined with Lesion-Specific Enzymes to Measure Oxidative Damage to DNA. Methods Cell Biol. 2012, 112, 69–92. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of Serum Proteins by Means of the Biuret Reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Paya, M.; Halliwell, B.; Hoult, J.R.S. Interactions of A Series of Coumarins with Reactive Oxygen Species—Scavenging of Superoxide, Hypochlorous Acid and Hydroxyl Radicals. Biochem. Pharmacol. 1992, 44, 205–214. [Google Scholar] [CrossRef]
- del Río, L.A.; Ortega, M.G.; López, A.L.; Gorgé, J.L. A More Sensitive Modification of the Catalase Assay with the Clark Oxygen Electrode: Application to the Kinetic Study of the Pea Leaf Enzyme. Anal. Biochem. 1977, 80, 409–415. [Google Scholar] [CrossRef]
- Hatton, P.J.; Cole, D.J.; Edwards, R. Influence of Plant Age on Glutathione Levels and Glutathione Transferases Involved in Herbicide Detoxification in Corn (Zea Mays L.) and Giant Foxtail (Setaria Faberi Herrm). Pestic. Biochem. Physiol. 1996, 54, 199–209. [Google Scholar] [CrossRef]
- Woolf, A. Essential Oil Poisoning. J. Toxicol. Clin. Toxicol. 2004, 37, 721–727. [Google Scholar] [CrossRef]
- Rates, S. Plants as Source of Drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Hanif, M.; Nisar, S.; Khan, G.; Mushtaq, Z. Essential Oils. In Essential Oil Research; Springer: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989. [Google Scholar] [CrossRef] [Green Version]
- PubChem Eugenol|C10H12O2—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Eugenol (accessed on 15 August 2022).
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-Inflammatory Potential and Antioxidant Profile of Eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Serag, E.S.; Sneed, K.B.; Zhou, S.F. Herbal Bioactivation, Molecular Targets and the Toxicity Relevance. Chem. Biol. Interact. 2011, 192, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Mahler, J.F.; Stokes, W.; Mann, P.C.; Takaoka, M.; Maronpot, R.R. Spontaneous Lesions in Aging FVB/N Mice*. Toxicol Pathol. 1996, 24, 710–716. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Carcinogenesis Studies of Eugenol (CAS No. 97-53-0) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 1983, 223, 1–159. [Google Scholar]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Pulegone (CAS No. 89-82-7) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 2011, 1–201.
- Greaves, P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, 4th ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 1–886. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Zarei, A.; Changizi-Ashtiyani, S.; Masmouei, B.; Rasekh, F.; Sokhandani, M.; Jahangir, F. The Physiological and Pharmacological Effects of Ziziphoratenuior, L.: A Review Study. Avicenna J. Phytomedicine 2022, 12, 16. [Google Scholar] [CrossRef]
- Harb, A.A.; Bustanji, Y.K.; Almasri, I.M.; Abdalla, S.S. Eugenol Reduces LDL Cholesterol and Hepatic Steatosis in Hypercholesterolemic Rats by Modulating TRPV1 Receptor. Sci. Rep. 2019, 9, 14003. [Google Scholar] [CrossRef] [Green Version]
- Godarzi, M.; Abbasi, M.S.; Mousavi, Z.M.K. Morphine Withdrawal Symptoms Improvement by Ziziphora Tenuior Ethanoic Extract in Male Mice. Koomesh J. 2016, 17, 990–995. [Google Scholar]
- Dehkordi, H.S.; Mobarakeh, H.I.; Dehkordi, M.J.; Khamesipour, F. Studying the Effect of the Ziziphora Tenuior L. Plant on Some Biochemical Factors of Serum in Rats. Int. J. Biol. 2014, 6, p131. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, Y.; Yang, Y.; Liu, X.; Zhang, J.; Li, B.; Zhou, X.; Niu, J.; Wei, X.; Liu, Z. A 15-Day Oral Dose Toxicity Study of Aspirin Eugenol Ester in Wistar Rats. Food Chem. Toxicol. 2012, 50, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Escobar, F.M.; Sabini, M.C.; Cariddi, L.N.; Sabini, L.I.; Mañas, F.; Cristofolini, A.; Bagnis, G.; Gallucci, M.N.; Cavaglieri, L.R. Safety Assessment of Essential Oil from Minthostachys Verticillata (Griseb.) Epling (Peperina): 90-Days Oral Subchronic Toxicity Study in Rats. Regul. Toxicol. Pharmacol. 2015, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yogalakshmi, B.; Viswanathan, P.; Anuradha, C.V. Investigation of Antioxidant, Anti-Inflammatory and DNA-Protective Properties of Eugenol in Thioacetamide-Induced Liver Injury in Rats. Toxicology 2010, 268, 204–212. [Google Scholar] [CrossRef]
- Tiku, A.B.; Abraham, S.K.; Kale, R.K. Eugenol as An In Vivo Radioprotective Agent. J. Radiat. Res. 2004, 45, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffray, M.; Cohen, G.M. Apoptosis and Necrosis in Toxicology: A Continuum or Distinct Modes of Cell Death? Pharmacol. Ther. 1997, 75, 153–177. [Google Scholar] [CrossRef]
- Costa, S.; Paulo Teixeira, J. Comet Assay. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Oxford, UK, 2014; pp. 1020–1023. [Google Scholar] [CrossRef]
- Beedanagari, S. Genetic Toxicology. In Comprehensive Medicinal Chemistry III; Rotella, D., Ward, S.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 195–203. ISBN 978-0-12-803201-5. [Google Scholar]
- Mohammadi Nejad, S.; Özgüneş, H.; Başaran, N. Pharmacological and Toxicological Properties of Eugenol. Turkish J. Pharm. Sci. 2017, 14, 201. [Google Scholar] [CrossRef]
- Pulla Reddy, A.C.; Lokesh, B.R. Alterations in Lipid Peroxides in Rat Liver by Dietary N-3 Fatty Acids: Modulation of Antioxidant Enzymes by Curcumin, Eugenol, and Vitamin E. J. Nutr. Biochem. 1994, 5, 181–188. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Garabadu, D.; Shah, A.; Singh, S.; Krishnamurthy, S. Protective Effect of Eugenol against Restraint Stress-Induced Gastrointestinal Dysfunction: Potential Use in Irritable Bowel Syndrome. Pharm. Biol. 2015, 53, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Liu, Y.; Lu, Y.; Ma, C. Anti-Inflammatory Effects of Eugenol on Lipopolysaccharide-Induced Inflammatory Reaction in Acute Lung Injury via Regulating Inflammation and Redox Status. Int. Immunopharmacol. 2015, 26, 265–271. [Google Scholar] [CrossRef]
- Kaur, G.; Athar, M.; Sarwar Alam, M. Eugenol Precludes Cutaneous Chemical Carcinogenesis in Mouse by Preventing Oxidative Stress and Inflammation and by Inducing Apoptosis. Mol. Carcinog. 2010, 49, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Abd El Motteleb, D.M.; Selim, S.A.; Mohamed, A.M. Differential Effects of Eugenol against Hepatic Inflammation and Overall Damage Induced by Ischemia/Re-Perfusion Injury. J. Immunotoxicol. 2014, 11, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Mnafgui, K.; Hajji, R.; Derbali, F.; Gammoudi, A.; Khabbabi, G.; Ellefi, H.; Allouche, N.; Kadri, A.; Gharsallah, N. Anti-Inflammatory, Antithrombotic and Cardiac Remodeling Preventive Effects of Eugenol in Isoproterenol-Induced Myocardial Infarction in Wistar Rat. Cardiovasc. Toxicol. 2016, 16, 336–344. [Google Scholar] [CrossRef] [PubMed]
- McMillian, M.; Nie, A.Y.; Parker, J.B.; Leone, A.; Bryant, S.; Kemmerer, M.; Herlich, J.; Liu, Y.; Yieh, L.; Bittner, A.; et al. A Gene Expression Signature for Oxidant Stress/Reactive Metabolites in Rat Liver. Biochem. Pharmacol. 2004, 68, 2249–2261. [Google Scholar] [CrossRef]
- Rabinovich, A.; Romanoff, N.; Mordvinov, D.; Ivanov, M.; Russian, P.; Andrew, M. The Effects of Oral Administration of Pulegone in Carbon Tetrachloride-Induced Oxidative Stress in Wistar Rats. GMJ Med. 2019, 3, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Adefegha, S.A.; Oyeleye, S.I.; Okeke, B.M.; Oboh, G. Influence of Eugenol on Oxidative Stress Biomarkers in the Liver of Carrageenan-Induced Arthritis Rats. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 185–193. [Google Scholar] [CrossRef]
- Vidhya, N.; Niranjali Devaraj, S. Antioxidant Effect of Eugenol in Rat Intestine. Indian J. Exp. Biol. 1999, 37, 1192–1195. [Google Scholar]
- Magalhães, C.B.; Casquilho, N.V.; Machado, M.N.; Riva, D.R.; Travassos, L.H.; Leal-Cardoso, J.H.; Fortunato, R.S.; Faffe, D.S.; Zin, W.A. The Anti-Inflammatory and Anti-Oxidative Actions of Eugenol Improve Lipopolysaccharide-Induced Lung Injury. Respir. Physiol. Neurobiol. 2019, 259, 30–36. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Z.; Zeng, J.; Chen, L.; Wu, Q.; Mo, J.; Zhang, G.; Song, L.; Xu, W.; Zhang, S.; et al. Eugenol Inhibits Non-Small Cell Lung Cancer by Repressing Expression of NF-ΚB-Regulated TRIM. Phytother. Res. 2019, 33, 1562–1569. [Google Scholar] [CrossRef]





| Parameter | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Body condition | Normal | Changed body condition | Emaciated | --- |
| Body weight | Weight loss of <10% | Weight loss of 10–20% | Weight loss of >20% | |
| Posture | Normal | Curved | --- | --- |
| Hair appearance and grooming | Normal | Lack of grooming | Bad hair condition, chromodachryorrhea | Hair in very bad condition, severe chromodachryorrhea |
| Mucosa | Normal | Slightly anemic | Moderately anemic | Severely anemic (Euthanasia) |
| Eyes, ears, and whiskers | Normal | Eyes partially closed, dropped ears, whiskers flattened and elongated to the tip of the nose | Eyes completely closed, dropped ears, whiskers flattened and elongated to the tip of the nose | --- |
| Mental status | Normal | Inactive, stereotyped behavior | Stupor | Coma (Euthanasia) |
| Response to external stimuli | Normal | Moderate | Moderate with vocalization | Violent |
| Hydration status (skin pinch) | Normal | Abnormal skin pinch test (>2 s) | --- | --- |
| Respiratory rate | Normal | Increased | Decreased | Abdominal breathing |
| Heart rate | Normal | Increased | Decreased | ---- |
| Stool appearance | Normal | Diarrhea, changed color | Black (with digested blood) | With blood in nature (Euthanasia) |
| Convulsions | Absence | Presence | --- | --- |
| Group (n = 5) | Mean Score of Humane Endpoints | |||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | |
| I (control) | 0 | 0 | 0 | 0 |
| II (2.6 mg eugenol + 2.6 mg pulegone) | 0 | 0.4 | 0.8 | 0.8 |
| III (5.2 mg eugenol + 5.2 mg pulegone) | 0 | 0 | 0 | 0 |
| IV (7.8 mg eugenol + 7.8 mg pulegone) | 1 | 0.6 | 0.2 | 1 |
| V (7.8 mg eugenol) | 0 | 0 | 0 | 0 |
| VI (7.8. mg pulegone) | 0 | 0.2 | 0.2 | 0.6 |
| VII (1000 mg eugenol + 1000 mg pulegone) | 0 | 0 | 0 | 6.8 |
| Changed Parameter | Score | Week of the Protocol | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Body condition | 1 | --- | --- | --- | Group VII (n = 4; 80%) |
| Body weight | 2 | Group IV (n = 1; 20%) | Group IV (n = 1; 20%) | Group II (n = 1; 20%) | --- |
| 3 | Group IV (n = 1; 20%) | --- | --- | Group VII (n = 5; 100%) | |
| Posture | 1 | --- | Group IV (n = 1; 20%) Group VI (n = 1; 20%) | Group IV (n = 1; 20%) Group VI (n = 1; 20%) | Group VII (n = 4; 80%) |
| Hair appearance and grooming | 1 | --- | --- | --- | Group IV (n = 5; 100%) Group VI (n = 3; 60%) Group VII (n = 4; 80%) |
| Eyes, ears, and whiskers | 1 | --- | --- | --- | Group VII (n = 1; 20%) |
| Mental status | 1 | --- | Group II (n = 2; 40%) | Group II (n = 2; 40%) | Group II (n = 4; 80%) Group VII (n = 3; 60%) |
| 3 | --- | --- | --- | Group VII (n = 1; 20%) | |
| Group (n = 5) | Food Consumption (g) | Water Consumption (g) | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| I (control) | 5.70 | 4.60 | 5.36 | 4.33 |
| II (2.6 mg eugenol + 2.6 mg pulegone) | 5.77 | 5.17 | 5.90 | 4.97 |
| III (5.2 mg eugenol + 5.2 mg pulegone) | 4.40 | 4.39 | 6.24 | 4.95 |
| IV (7.8 mg eugenol + 7.8 mg pulegone) | 3.09 | 3.31 | 9.89 | 7.11 |
| V (7.8 mg eugenol) | 5.62 | 4.76 | 5.72 | 5.54 |
| VI (7.8. mg pulegone) | 3.93 | 3.75 | 6.67 | 5.43 |
| VII (1000 mg eugenol + 1000 mg pulegone) | 4.44 | 0.40 | 5.61 | 1.39 |
| Group (n = 5) | Body Weight (g) | PWG (%) | |
|---|---|---|---|
| Initial | Final | ||
| I (control) | 25.83 ± 1.09 | 29.51 ± 1.95 a | 15.05 b |
| II (2.6 mg eugenol + 2.6 mg pulegone) | 24.99 ± 2.21 | 26.89 ± 2.80 | 11.19 |
| III (5.2 mg eugenol + 5.2 mg pulegone) | 26.33 ± 2.32 | 28.42 ± 1.92 | 10.73 |
| IV (7.8 mg eugenol + 7.8 mg pulegone) | 26.89 ± 1.59 | 25.66 ± 1.42 | −0.88 c |
| V (7.8 mg eugenol) | 26.27 ± 1.54 | 27.73 ± 1.50 | 9.04 |
| VI (7.8. mg pulegone) | 24.99 ± 1.73 | 26.59 ± 1.32 | 5.56 |
| VII (1000 mg eugenol + 1000 mg pulegone) | 28.00 ± 1.02 | 18.18 ± 1.45 | −48.77 |
| Group (n = 5) | Organs’ Weight (g) | |||||
|---|---|---|---|---|---|---|
| Heart | Lung | Right Kidney | Left Kidney | Spleen | Liver | |
| I (control) | 0.144 ± 0.029 | 0.206 ± 0.026 | 0.192 ± 0.018 | 0.198 ± 0.011 | 0.128 ± 0.019 | 1.432 ± 0.089 |
| II (2.6 mg eugenol + 2.6 mg pulegone) | 0.134 ± 0.015 | 0.182 ± 0.026 | 0.178 ± 0.028 | 0.178 ± 0.025 | 0.140 ± 0.02 | 1.384 ± 0.199 |
| III (5.2 mg eugenol + 5.2 mg pulegone) | 0.128 ± 0.029 | 0.184 ± 0.022 | 0.170 ± 0.014 | 0.174 ± 0.015 | 0.174 ± 0.015 | 1.546 ± 0.083 a |
| IV (7.8 mg eugenol + 7.8 mg pulegone) | 0.112 ± 0.004 | 0.188 ± 0.011 | 0.144 ± 0.027 | 0.158 ± 0.016 | 0.082 ± 0.016 | 1.418 ± 0.082 |
| V (7.8 mg eugenol) | 0.148 ± 0.022 | 0.214 ± 0.030 | 0.178 ± 0.008 | 0.176 ± 0.037 | 0.134 ± 0.021 | 1.432 ± 0.094 |
| VI (7.8. mg pulegone) | 0.120 ± 0.016 | 0.190 ± 0.012 | 0.154 ± 0.009 | 0.152 ± 0.008 | 0.110 ± 0.020 | 1.430 ± 0.080 |
| VII (1000 mg eugenol + 1000 mg pulegone) | 0.100 ± 0.016 | 0.188 ± 0.024 | 0.133 ± 0.013 | 0.133 ± 0.010 | 0.060 ± 0.018 | 0.848 ± 0.097 b |
| Group (n = 5) | Score | Number of Animals (%) | |||
|---|---|---|---|---|---|
| Inflammatory Infiltrate | Necrosis/Apoptosis | ||||
| Liver | Kidneys | Lung | Liver | ||
| I (control) | 0 | 4 (80%) | 4 (80%) | 3 (60%) | 5 (100%) |
| 1 | 1 (20%) | 1 (20%) | 2 (40%) | --- | |
| 2 | --- | --- | --- | --- | |
| Mean ± SE | 0.20 ± 0.45 | 0.20 ± 0.45 | 0.40 ± 0.55 a | 0.00 ± 0.00 | |
| II (2.6 mg eugenol + 2.6 mg pulegone) | 0 | 2 (40%) | 4 (80%) | 4 (80%) | 5 (100%) |
| 1 | 3 (60%) | 1 (20%) | 1 (20%) | --- | |
| 2 | --- | --- | --- | --- | |
| Mean ± SE | 0.60 ± 0.55 | 0.20 ± 0.45 | 0.20 ± 0.45 a | 0.00 ± 0.00 | |
| III (5.2 mg eugenol + 5.2 mg pulegone) | 0 | 2 (40%) | 2 (40%) | 3 (60%) | 5 (100%) |
| 1 | 3 (60%) | 2 (40%) | 2 (40%) | --- | |
| 2 | --- | 1 (20%) | --- | --- | |
| Mean ± SE | 0.60 ± 0.55 | 0.80 ± 0.84 | 0.40 ± 0.55 a | 0.00 ± 0.00 | |
| IV (7.8 mg eugenol + 7.8 mg pulegone) | 0 | 2 (40%) | 1 (20%) | --- | 5 (100%) |
| 1 | 2 (40%) | 3 (60%) | 1 (20%) | --- | |
| 2 | 1 (20%) | 1 (20%) | 4 (80%) | --- | |
| Mean ± SE | 0.80 ± 0.84 | 1.00 ± 0.71 | 1.80 ± 0.45 | 0.00 ± 0.00 | |
| V (7.8 mg eugenol) | 0 | --- | 1 (20%) | --- | 5 (100%) |
| 1 | 5 (100%) | 3 (60%) | 3 (60%) | --- | |
| 2 | --- | 1 (20%) | 2 (40%) | --- | |
| Mean ± SE | 1.00 ± 0.00 | 1.00 ± 0.71 | 1.40 ± 0.55 | 0.00 ± 0.00 | |
| VI (7.8. mg pulegone) | 0 | 1 (20%) | 1 (20%) | 1 (20%) | 5 (100%) |
| 1 | 4 (80%) | 4 (80%) | 4 (80%) | --- | |
| 2 | --- | --- | --- | --- | |
| Mean ± SE | 0.80 ± 0.45 | 0.80 ± 0.45 | 0.80 ± 0.45 | 0.00 ± 0.00 | |
| VII (1000 mg eugenol + 1000 mg pulegone) | 0 | 1 (20%) | 4 (80%) | 1 (20%) | 3 (60%) |
| 1 | 4 (80%) | 1 (20%) | 4 (80%) | 2 (40%) | |
| 2 | --- | --- | --- | --- | |
| Mean ± SE | 0.80 ± 0.45 | 0.20 ± 0.45 | 0.80 ± 0.45 | 0.40 ± 0.55 | |
| Group (n = 5) | Number of Animals (%) | ||
|---|---|---|---|
| Centrilobular Hepatocelular Hypertrophy | Marked Centrilobular Hepatocelular Hypertrophy | Hepatocyte Karyomegaly | |
| I (control) | 3 (60%) | --- | 3 (60%) |
| II (2.6 mg eugenol + 2.6 mg pulegone) | 3 (60%) | --- | 3 (60%) |
| III (5.2 mg eugenol + 5.2 mg pulegone) | 4 (80%) | --- | 4 (80%) |
| IV (7.8 mg eugenol + 7.8 mg pulegone) | 4 (80%) | --- | 4 (80%) |
| V (7.8 mg eugenol) | 4 (80%) | --- | 4 (80%) |
| VI (7.8. mg pulegone) | 3 (60%) | --- | 3 (60%) |
| VII (1000 mg eugenol + 1000 mg pulegone) | --- | 5 (100%) | 5 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro-Silva, C.M.; Faustino-Rocha, A.I.; Gil da Costa, R.M.; Medeiros, R.; Pires, M.J.; Gaivão, I.; Gama, A.; Neuparth, M.J.; Barbosa, J.V.; Peixoto, F.; et al. Pulegone and Eugenol Oral Supplementation in Laboratory Animals: Results from Acute and Chronic Studies. Biomedicines 2022, 10, 2595. https://doi.org/10.3390/biomedicines10102595
Ribeiro-Silva CM, Faustino-Rocha AI, Gil da Costa RM, Medeiros R, Pires MJ, Gaivão I, Gama A, Neuparth MJ, Barbosa JV, Peixoto F, et al. Pulegone and Eugenol Oral Supplementation in Laboratory Animals: Results from Acute and Chronic Studies. Biomedicines. 2022; 10(10):2595. https://doi.org/10.3390/biomedicines10102595
Chicago/Turabian StyleRibeiro-Silva, Carla M., Ana I. Faustino-Rocha, Rui M. Gil da Costa, Rui Medeiros, Maria J. Pires, Isabel Gaivão, Adelina Gama, Maria J. Neuparth, Joana V. Barbosa, Francisco Peixoto, and et al. 2022. "Pulegone and Eugenol Oral Supplementation in Laboratory Animals: Results from Acute and Chronic Studies" Biomedicines 10, no. 10: 2595. https://doi.org/10.3390/biomedicines10102595
APA StyleRibeiro-Silva, C. M., Faustino-Rocha, A. I., Gil da Costa, R. M., Medeiros, R., Pires, M. J., Gaivão, I., Gama, A., Neuparth, M. J., Barbosa, J. V., Peixoto, F., Magalhães, F. D., Bastos, M. M. S. M., & Oliveira, P. A. (2022). Pulegone and Eugenol Oral Supplementation in Laboratory Animals: Results from Acute and Chronic Studies. Biomedicines, 10(10), 2595. https://doi.org/10.3390/biomedicines10102595













