Synthesis of Artemether-Loaded Albumin Nanoparticles and Measurement of Their Anti-Cancer Effects
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of ARM-HSA Nanoparticles
2.3. Investigation of Properties and Structure of Nanoparticles
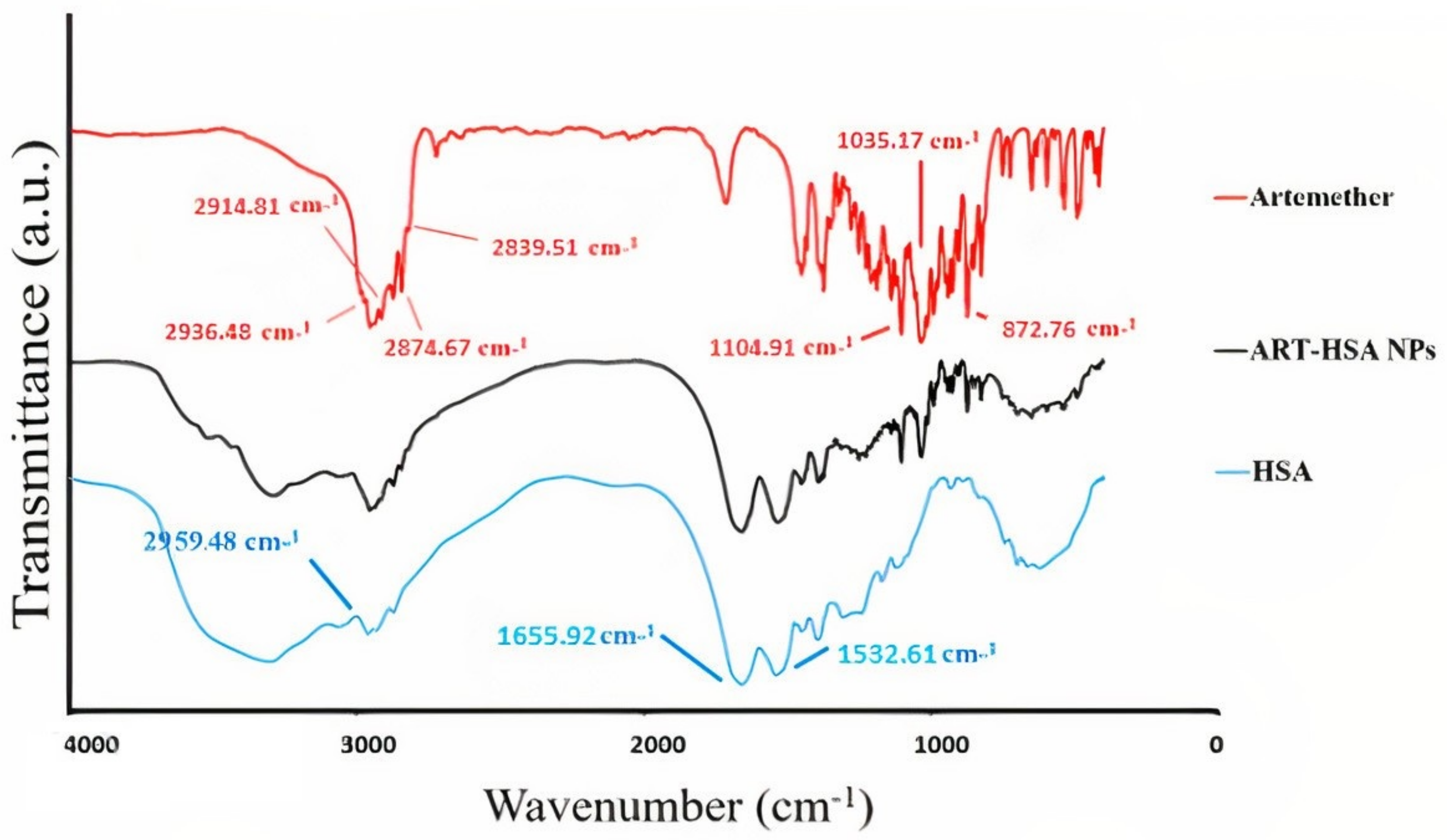
2.3.1. FTIR (Fourier-Transform Infra-Red) Spectroscopy
2.3.2. Investigation of ARM-HSA NPs Morphology by Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.3.3. Investigation of Particle Size, Polydispersity Index and Zeta Potential with DLS
2.3.4. Process Yield and Drug Loading
2.3.5. Solubility Evaluation
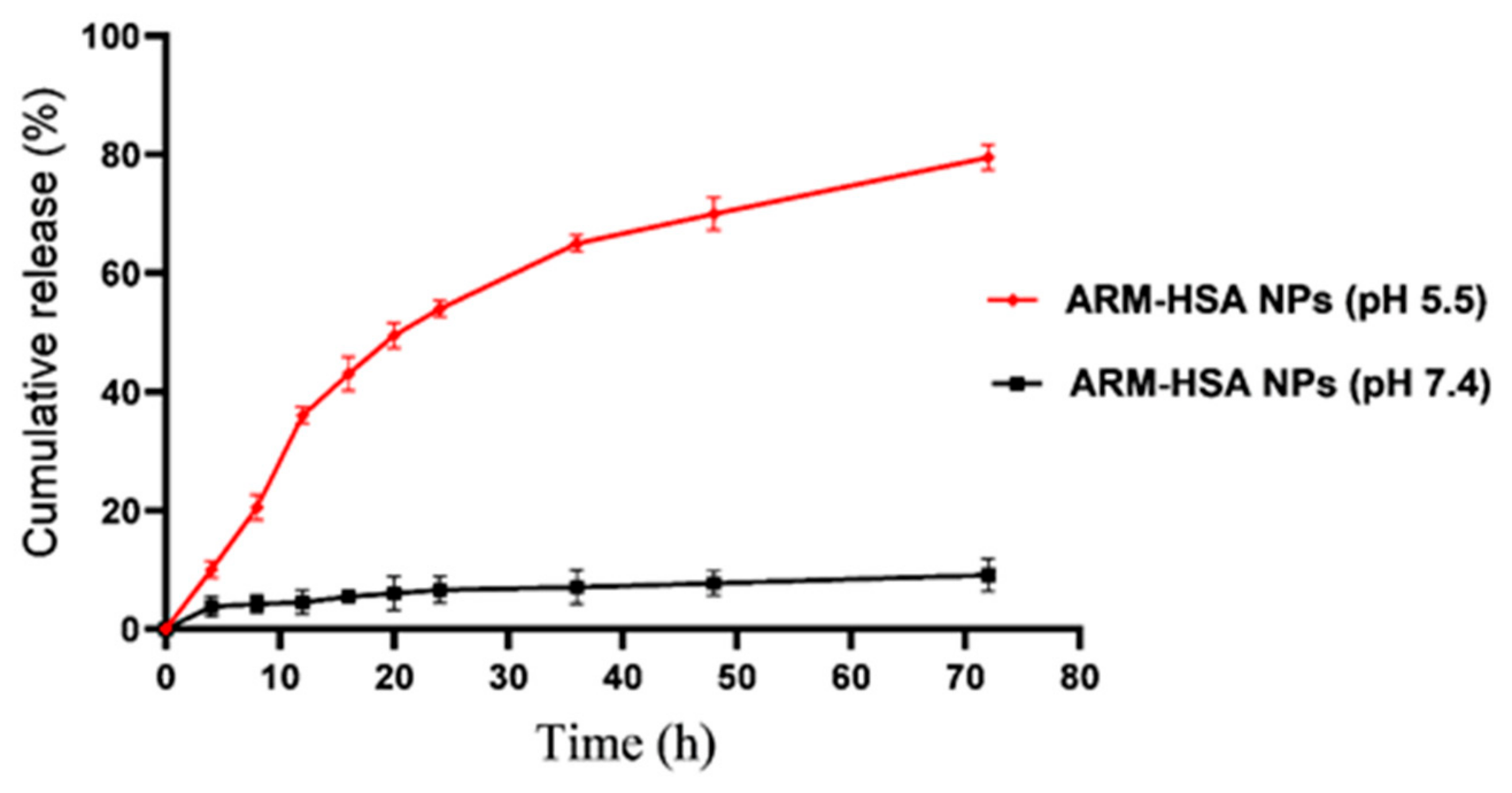
2.3.6. Drug Release Evaluation
2.4. In Vitro Studies
2.4.1. Cell Culture
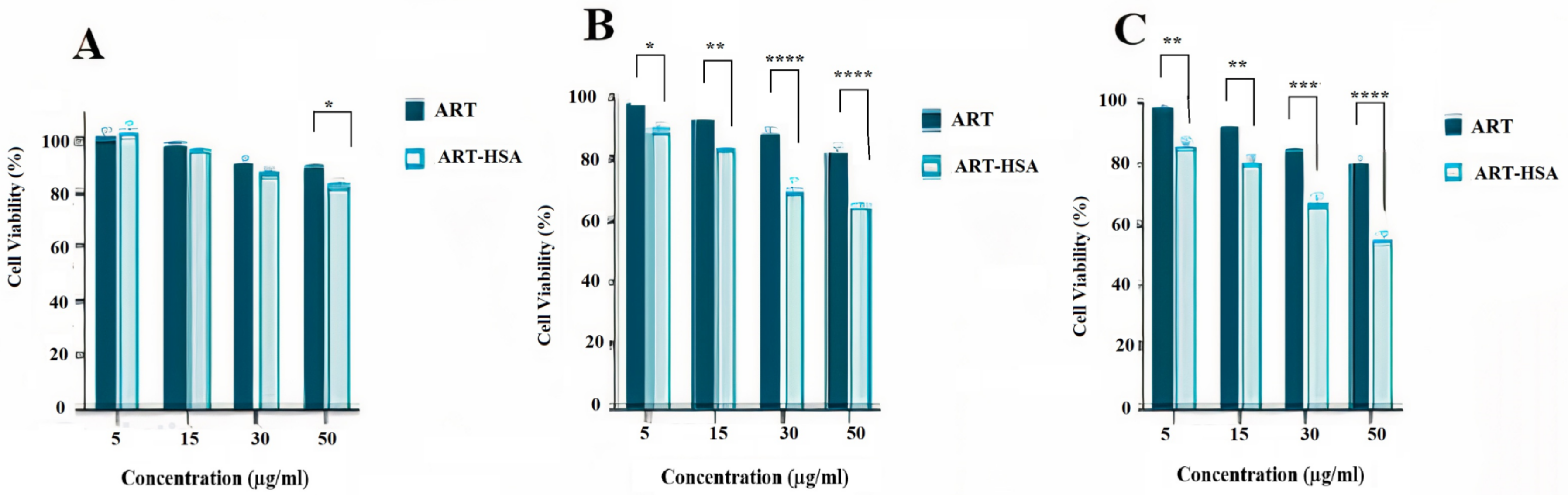
2.4.2. MTT Assay
2.4.3. Apoptosis Assay
2.5. In Vivo Studies
2.5.1. Tumorization of BALB/c Mice
2.5.2. Treatment of Mice with Drugs
2.5.3. Isolation and Culture of MNCs
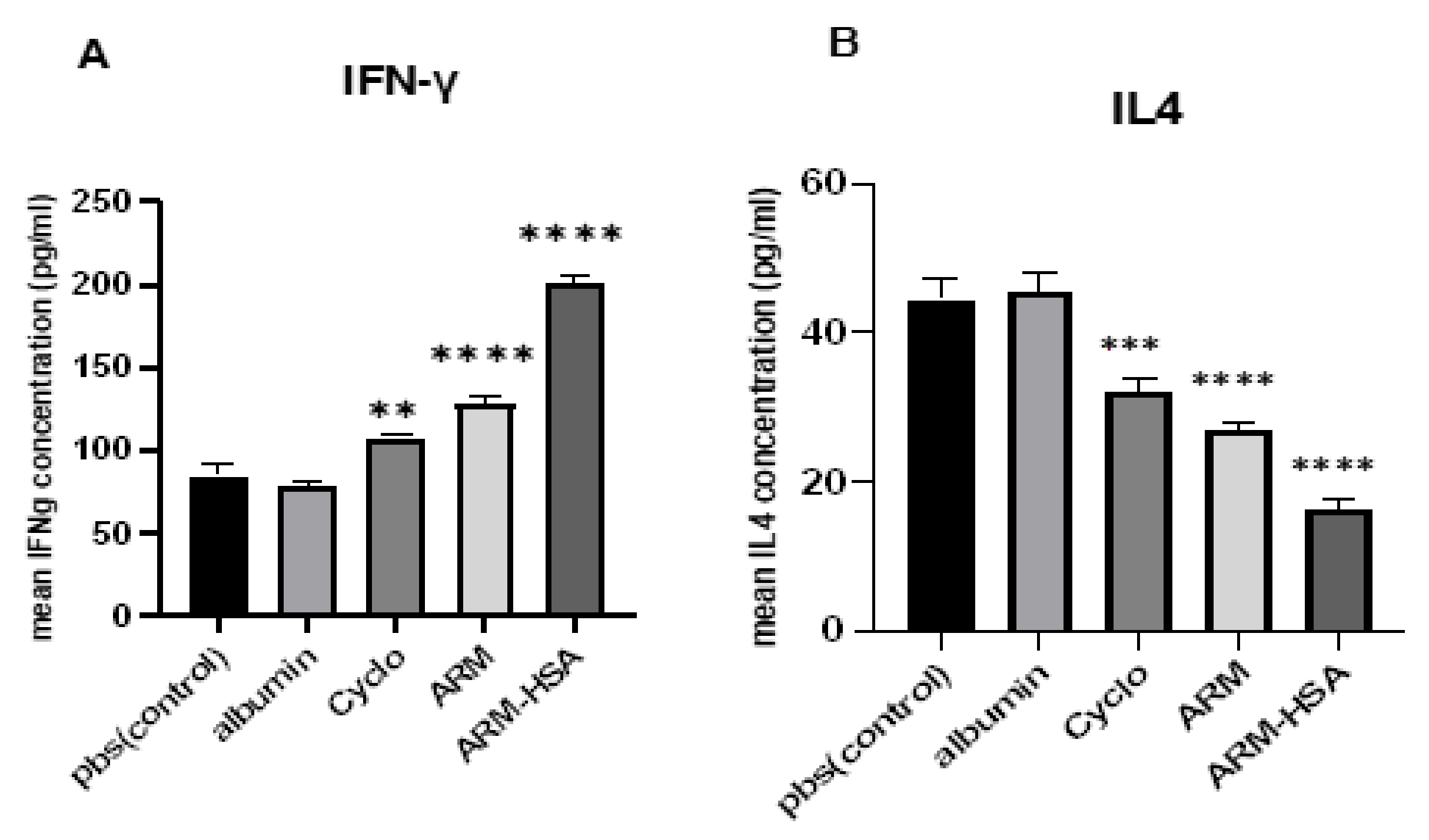
2.5.4. ELISA
2.6. Statistical Analysis
3. Results
3.1. Characterization of Artemether–HSA Nanoparticles
3.1.1. Fourier Transforms Infrared (FTIR) Spectroscopy
3.1.2. Zeta Potential, PDI and Size of Nanoparticles
3.1.3. Analysis of Nanoparticle Shape Using Electron Microscopy (SEM)
3.1.4. The Measure of Encapsulation Efficiency, Drug Loading and Process Yield
3.1.5. The Release and Solubility Profile of Nanoparticles
3.2. MTT Assay
3.3. Apoptosis Analysis
3.4. Results of In Vivo Studies
3.4.1. Effect of Drugs on Tumor Growth
3.4.2. ELISA Assay
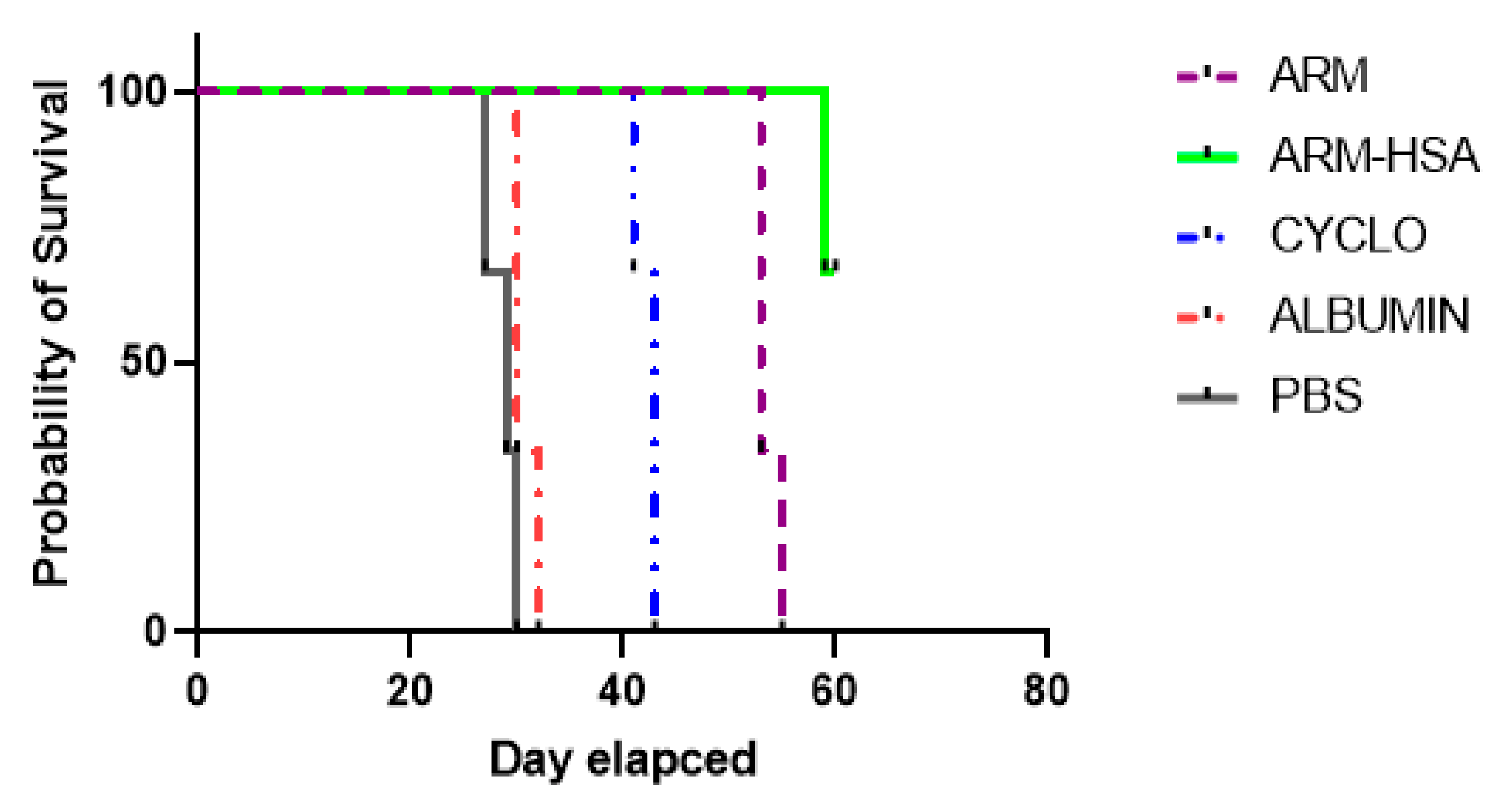
3.4.3. Survival of Tumor Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Amintas, S.; Dupin, C.; Boutin, J.; Beaumont, P.; Moreau-Gaudry, F.; Bedel, A.; Krisa, S.; Vendrely, V.; Dabernat, S. Bioactive food components for colorectal cancer prevention and treatment: A good match. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Katsaounou, K.; Nicolaou, E.; Vogazianos, P.; Brown, C.; Stavrou, M.; Teloni, S.; Hatzis, P.; Agapiou, A.; Fragkou, E.; Tsiaoussis, G. Colon Cancer: From Epidemiology to Prevention. Metabolites 2022, 12, 499. [Google Scholar] [CrossRef]
- Banoon, S.R.; Ghasemian, A. The characters of graphene oxide nanoparticles and doxorubicin against HCT-116 colorectal cancer cells in vitro. J. Gastrointest. Cancer 2022, 53, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Gurba, A.; Taciak, P.; Sacharczuk, M.; Młynarczuk-Biały, I.; Bujalska-Zadrożny, M.; Fichna, J. Gold (III) Derivatives in Colon Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 724. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Lewis, C.R. Colon Cancer Screening. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer chemotherapy. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Jones, R.; Ocen, J. Cytotoxic chemotherapy: Clinical aspects. Medicine 2020, 48, 97–102. [Google Scholar] [CrossRef]
- Hassanin, I.; Elzoghby, A. Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020, 3, 930. [Google Scholar] [CrossRef]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, K.; Di, L.; Wang, P.; Liu, Z.; Zhang, J.; Yue, P.; Song, W.; Zhang, J.; Chen, T. Traditional herbal medicine and nanomedicine: Converging disciplines to improve therapeutic efficacy and human health. Adv. Drug Deliv. Rev. 2021, 178, 113964. [Google Scholar] [CrossRef]
- Dai, Y.-F.; Zhou, W.-W.; Meng, J.; Du, X.-L.; Sui, Y.-P.; Dai, L.; Wang, P.-Q.; Huo, H.-R.; Sui, F. The pharmacological activities and mechanisms of artemisinin and its derivatives: A systematic review. Med. Chem. Res. 2017, 26, 867–880. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Zhang, R.; Wang, H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J. Cell. Mol. Med. 2009, 13, 1358–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Huang, X.; Tao, C.; Xiao, T.; Li, X.; Zeng, Q.; Ma, M.; Wu, Z. Artemether attenuates the progression of non-small cell lung cancer by inducing apoptosis, cell cycle arrest and promoting cellular senescence. Biol. Pharm. Bull. 2019, 42, 1720–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yang, M.; Song, Z.; Yao, G.; Shi, Q. Artemether inhibits proliferation, invasion and migration of hepatocellular carcinoma cells via targeting of CYP2J2. Oncol. Lett. 2022, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Singh, N.P.; Sasaki, T. Development of artemisinin compounds for cancer treatment. Investig. New Drugs 2013, 31, 230–246. [Google Scholar] [CrossRef]
- Huang, W.; Liu, N.; Niu, H. Effect of artesunate on cell apoptosis and cell cycle of human colon cancer cell hct-8. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 225–228. [Google Scholar]
- Wang, X.-M.; Zhang, L.; Ding, G.-F.; Wang, Q.-Z. Inhibitory effect of dihydroartemisinin on the growth of human prostate cancer PC-3M cells and its mechanism. Zhonghua Nan Ke Xue Natl. J. Androl. 2012, 18, 590–594. [Google Scholar]
- Wu, Z.-P.; Gao, C.-W.; Wu, Y.-G.; Zhu, Q.-S.; Chen, Y.; Liu, X.; Liu, C. Inhibitive effect of artemether on tumor growth and angiogenesis in the rat C6 orthotopic brain gliomas model. Integr. Cancer Ther. 2009, 8, 88–92. [Google Scholar] [CrossRef]
- Zhang, L.X.; Liu, Z.N.; Ye, J.; Sha, M.; Qian, H.; Bu, X.H.; Luan, Z.Y.; Xu, X.L.; Huang, A.h.; Yuan, D.l. Artesunate exerts an anti-immunosuppressive effect on cervical cancer by inhibiting PGE2 production and Foxp3 expression. Cell Biol. Int. 2014, 38, 639–646. [Google Scholar] [CrossRef]
- Michaelsen, F.-W.; Saeed, M.E.; Schwarzkopf, J.; Efferth, T. Activity of Artemisia annua and artemisinin derivatives, in prostate carcinoma. Phytomedicine 2015, 22, 1223–1231. [Google Scholar] [CrossRef]
- Tian, L.; Liu, J.; Jia, Q.; Ying, Y.; Yang, Z.; Huang, G. Preparation and evaluation of artemether liposomes for enhanced anti-tumor therapy. AAPS PharmSciTech 2018, 19, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Samandari, B.M.R.; Sarhadi, S.; Esmaeili, M.S. Artemether effect and its interaction with vincristine and doxorubicin on human breast carcinoma MCF-7 cells. Physiol. Pharmacol. 2016, 20, 117–121. [Google Scholar]
- Silamut, K.; Newton, P.N.; Teja-Isavadharm, P.; Suputtamongkol, Y.; Siriyanonda, D.; Rasameesoraj, M.; Pukrittayakamee, S.; White, N.J. Artemether bioavailability after oral or intramuscular administration in uncomplicated falciparum malaria. Antimicrob. Agents Chemother. 2003, 47, 3795–3798. [Google Scholar] [CrossRef]
- Birgersson, S.; Van Toi, P.; Truong, N.T.; Dung, N.T.; Ashton, M.; Hien, T.T.; Abelö, A.; Tarning, J. Population pharmacokinetic properties of artemisinin in healthy male Vietnamese volunteers. Malar. J. 2016, 15, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira Souza, A.C.; Grabe-Guimarães, A.; Cruz, J.d.S.; Santos-Miranda, A.; Farah, C.; Teixeira Oliveira, L.; Lucas, A.; Aimond, F.; Sicard, P.; Mosqueira, V.C.F. Mechanisms of artemether toxicity on single cardiomyocytes and protective effect of nanoencapsulation. Br. J. Pharmacol. 2020, 177, 4448–4463. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, C.Q.; Post, M.; Simard, B.; Deslandes, Y.; Hsieh, T.H. Design of nanoparticles as drug carriers for cancer therapy. Cancer Genom. Proteom. 2006, 3, 147–157. [Google Scholar]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Rosenoer, V.M.; Oratz, M.; Rothschild, M.A. Albumin: Structure, Function and Uses; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Zwain, T.; Taneja, N.; Zwayen, S.; Shidhaye, A.; Palshetkar, A.; Singh, K.K. Albumin nanoparticles—A versatile and a safe platform for drug delivery applications. In Nanoparticle Therapeutics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 327–358. [Google Scholar]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum albumin in health and disease: Esterase, antioxidant, transporting and signaling properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Ascenzi, P. Serum albumin: A multifaced enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef]
- Yu, L.; Hua, Z.; Luo, X.; Zhao, T.; Liu, Y. Systematic interaction of plasma albumin with the efficacy of chemotherapeutic drugs. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2022, 1877, 188655. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Bagherifam, S.; Bekhradnia, S.; Nyström, B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci. Rep. 2017, 7, 5178. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Miao, J.; Soond, S.M.; Rudzińska, M.; Zamyatnin, A.A., Jr. Albumin nanovectors in cancer therapy and imaging. Biomolecules 2019, 9, 218. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Y.; Wu, A.; Rao, Y.; Huang, Y. Roles of albumin-binding proteins in cancer progression and biomimetic targeted drug delivery. ChemBioChem 2018, 19, 1796–1805. [Google Scholar] [CrossRef]
- An, F.-F.; Zhang, X.-H. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics 2017, 7, 3667. [Google Scholar] [CrossRef]
- Boateng-Marfo, Y.; Dong, Y.; Loh, Z.H.; Lin, H.; Ng, W.K. Intravenous human serum albumin (HSA)-bound artemether nanoparticles for treatment of severe malaria. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 20–29. [Google Scholar] [CrossRef]
- Sidhaye, A.A.; Bhuran, K.C.; Zambare, S.; Abubaker, M.; Nirmalan, N.; Singh, K.K. Bio-inspired artemether-loaded human serum albumin nanoparticles for effective control of malaria-infected erythrocytes. Nanomedicine 2016, 11, 2809–2828. [Google Scholar] [CrossRef] [Green Version]
- Domingos, R.F.; Baalousha, M.A.; Ju-Nam, Y.; Reid, M.M.; Tufenkji, N.; Lead, J.R.; Leppard, G.G.; Wilkinson, K.J. Characterizing manufactured nanoparticles in the environment: Multimethod determination of particle sizes. Environ. Sci. Technol. 2009, 43, 7277–7284. [Google Scholar] [CrossRef]
- Karajgi, S.; Tanveer, A.; Kalyane, N. Simultaneous determination of artemether and lumefantrine by area under curve UV spectrophotometric method. J. Pharm. Sci. Res. 2016, 8, 506. [Google Scholar]
- Akbarian, A.; Ebtekar, M.; Pakravan, N.; Hassan, Z.M. Folate receptor alpha targeted delivery of artemether to breast cancer cells with folate-decorated human serum albumin nanoparticles. Int. J. Biol. Macromol. 2020, 152, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kayani, Z.; Bordbar, A.-K.; Firuzi, O. Novel folic acid-conjugated doxorubicin loaded β-lactoglobulin nanoparticles induce apoptosis in breast cancer cells. Biomed. Pharmacother. 2018, 107, 945–956. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nanoparticles for targeted delivery to HER-2 positive breast cancer cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Beyond annexin V: Fluorescence response of cellular membranes to apoptosis. Cytotechnology 2013, 65, 157–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farsam, V.; Hassan, Z.M.; Zavaran-Hosseini, A.; Noori, S.; Mahdavi, M.; Ranjbar, M. Antitumor and immunomodulatory properties of artemether and its ability to reduce CD4+ CD25+ FoxP3+ T reg cells in vivo. Int. Immunopharmacol. 2011, 11, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Tomar, D.S.; Bhalala, K.; Wahajuddin, M. Development and investigation of Artemether loaded binary solid lipid nanoparticles: Physicochemical characterization and In-Situ single-pass intestinal permeability. J. Drug Deliv. Sci. Technol. 2020, 60, 102072. [Google Scholar] [CrossRef]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; Von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Wlodkowic, D.; Skommer, J.; Darzynkiewicz, Z. Flow cytometry-based apoptosis detection. In Apoptosis; Springer: Berlin/Heidelberg, Germany, 2009; pp. 19–32. [Google Scholar]
- Darzynkiewicz, Z.; Bedner, E.; Smolewski, P. Flow cytometry in analysis of cell cycle and apoptosis. Semin. Hematol. 2001, 38, 179–193. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [Green Version]
- Usama, S.M.; Park, G.K.; Nomura, S.; Baek, Y.; Choi, H.S.; Burgess, K. Role of albumin in accumulation and persistence of tumor-seeking cyanine dyes. Bioconjug. Chem. 2020, 31, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- Girardot, J.; Girardot, M. Amide cross-linking: An alternative to glutaraldehyde fixation. J. Heart Valve Dis. 1996, 5, 518–525. [Google Scholar] [PubMed]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahobia, S.; Bajpai, J.; Bajpai, A.K. An in-vitro investigation of swelling controlled delivery of insulin from egg albumin nanocarriers. Iran. J. Pharm. Res. IJPR 2016, 15, 695. [Google Scholar]
- Ghosh, P.; Roy, A.S.; Chaudhury, S.; Jana, S.K.; Chaudhury, K.; Dasgupta, S. Preparation of albumin based nanoparticles for delivery of fisetin and evaluation of its cytotoxic activity. Int. J. Biol. Macromol. 2016, 86, 408–417. [Google Scholar] [CrossRef]
- Bansal, A.; Kapoor, D.N.; Kapil, R.; Chhabra, N.; Dhawan, S. Design and development of paclitaxel-loaded bovine serum albumin nanoparticles for brain targeting. Acta Pharm. 2011, 61, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Grobmyer, S.R.; Iwakuma, N.; Sharma, P.; Moudgil, B.M. What is cancer nanotechnology? Cancer Nanotechnol. 2010, 624, 1–9. [Google Scholar]
- Bolaños, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, 14, 6387. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.R.; Agrawal, Y. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Zhao, X.; Guo, X.; Yue, W.; Wang, J.; Yang, J.; Chen, J. Artemether suppresses cell proliferation and induces apoptosis in diffuse large B cell lymphoma cells. Exp. Ther. Med. 2017, 14, 4083–4090. [Google Scholar] [CrossRef] [PubMed]
- Meerloo, J.V.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture; Springer: Berlin/Heidelberg, Germany, 2011; pp. 237–245. [Google Scholar]
- Dose, W.A.R.N.W.A.; Group, I.S. The effect of dose on the antimalarial efficacy of artemether–lumefantrine: A systematic review and pooled analysis of individual patient data. Lancet Infect. Dis. 2015, 15, 692–702. [Google Scholar]
- Lohy Das, J.; Rulisa, S.; de Vries, P.J.; Mens, P.F.; Kaligirwa, N.; Agaba, S.; Tarning, J.; Karlsson, M.O.; Dorlo, T.P. Population pharmacokinetics of artemether, dihydroartemisinin, and lumefantrine in Rwandese pregnant women treated for uncomplicated Plasmodium falciparum malaria. Antimicrob. Agents Chemother. 2018, 62, e00518-18. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-B.; Hu, Y.; Li, Z.; Wang, P.; Xue, Y.-X.; Yao, Y.-L.; Yu, B.; Liu, Y.-H. Artemether combined with shRNA interference of vascular cell adhesion molecule-1 significantly inhibited the malignant biological behavior of human glioma cells. PLoS ONE 2013, 8, e60834. [Google Scholar] [CrossRef]
- Nirachonkul, W.; Ogonoki, S.; Thumvijit, T.; Chiampanichayakul, S.; Panyajai, P.; Anuchapreeda, S.; Tima, S.; Chiampanichayakul, S. CD123-targeted nano-curcumin molecule enhances cytotoxic efficacy in leukemic stem cells. Nanomaterials 2021, 11, 2974. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Hao, M. Effect of artemisinin on proliferation and apoptosis-related protein expression in vivo and in vitro. Saudi J. Biol. Sci. 2018, 25, 1488–1493. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Singh, N.P.; Lai, H.C. Artemisinin induces apoptosis in human cancer cells. Anticancer Res. 2004, 24, 2277–2280. [Google Scholar]
- Mirzaei-Parsa, M.J.; Najafabadi, M.R.H.; Haeri, A.; Zahmatkeshan, M.; Ebrahimi, S.A.; Pazoki-Toroudi, H.; Adel, M. Preparation, characterization, and evaluation of the anticancer activity of artemether-loaded nano-niosomes against breast cancer. Breast Cancer 2020, 27, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altshuler, A.E.; Penn, A.H.; Yang, J.A.; Kim, G.-R.; Schmid-Schönbein, G.W. Protease activity increases in plasma, peritoneal fluid, and vital organs after hemorrhagic shock in rats. PLoS ONE 2012, 7, e32672. [Google Scholar] [CrossRef] [Green Version]
- Cassado, A.D.A.; D’Império Lima, M.R.; Bortoluci, K.R. Revisiting mouse peritoneal macrophages: Heterogeneity, development, and function. Front. Immunol. 2015, 6, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Chong, M.M.; Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Shurin, M.R.; Lu, L.; Kalinski, P.; Stewart-Akers, A.M.; Lotze, M.T. Th1/Th2 balance in cancer, transplantation and pregnancy. In Springer Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 1999; pp. 339–359. [Google Scholar]
- Woods, A.; Patel, A.; Spina, D.; Riffo-Vasquez, Y.; Babin-Morgan, A.; De Rosales, R.; Sunassee, K.; Clark, S.; Collins, H.; Bruce, K. In vivo biocompatibility, clearance, and biodistribution of albumin vehicles for pulmonary drug delivery. J. Control. Release 2015, 210, 1–9. [Google Scholar] [CrossRef]





| Nanoparticles | Zeta Potential ± SD (mV) | PDI ± SD | Size ± SD (nm) |
|---|---|---|---|
| ARM-HSA NPs | −19.1 ± 0.82 | 0.132 ± 0.006 | 171.3 ± 5.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirali-Hamedani, Z.; Abbasi, A.; Hassan, Z.M. Synthesis of Artemether-Loaded Albumin Nanoparticles and Measurement of Their Anti-Cancer Effects. Biomedicines 2022, 10, 2713. https://doi.org/10.3390/biomedicines10112713
Pirali-Hamedani Z, Abbasi A, Hassan ZM. Synthesis of Artemether-Loaded Albumin Nanoparticles and Measurement of Their Anti-Cancer Effects. Biomedicines. 2022; 10(11):2713. https://doi.org/10.3390/biomedicines10112713
Chicago/Turabian StylePirali-Hamedani, Zeynab, Ardeshir Abbasi, and Zuhair Mohammad Hassan. 2022. "Synthesis of Artemether-Loaded Albumin Nanoparticles and Measurement of Their Anti-Cancer Effects" Biomedicines 10, no. 11: 2713. https://doi.org/10.3390/biomedicines10112713
APA StylePirali-Hamedani, Z., Abbasi, A., & Hassan, Z. M. (2022). Synthesis of Artemether-Loaded Albumin Nanoparticles and Measurement of Their Anti-Cancer Effects. Biomedicines, 10(11), 2713. https://doi.org/10.3390/biomedicines10112713





