Transcriptome Analysis of Protection by Dendrobium nobile Alkaloids (DNLA) against Chronic Alcoholic Liver Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
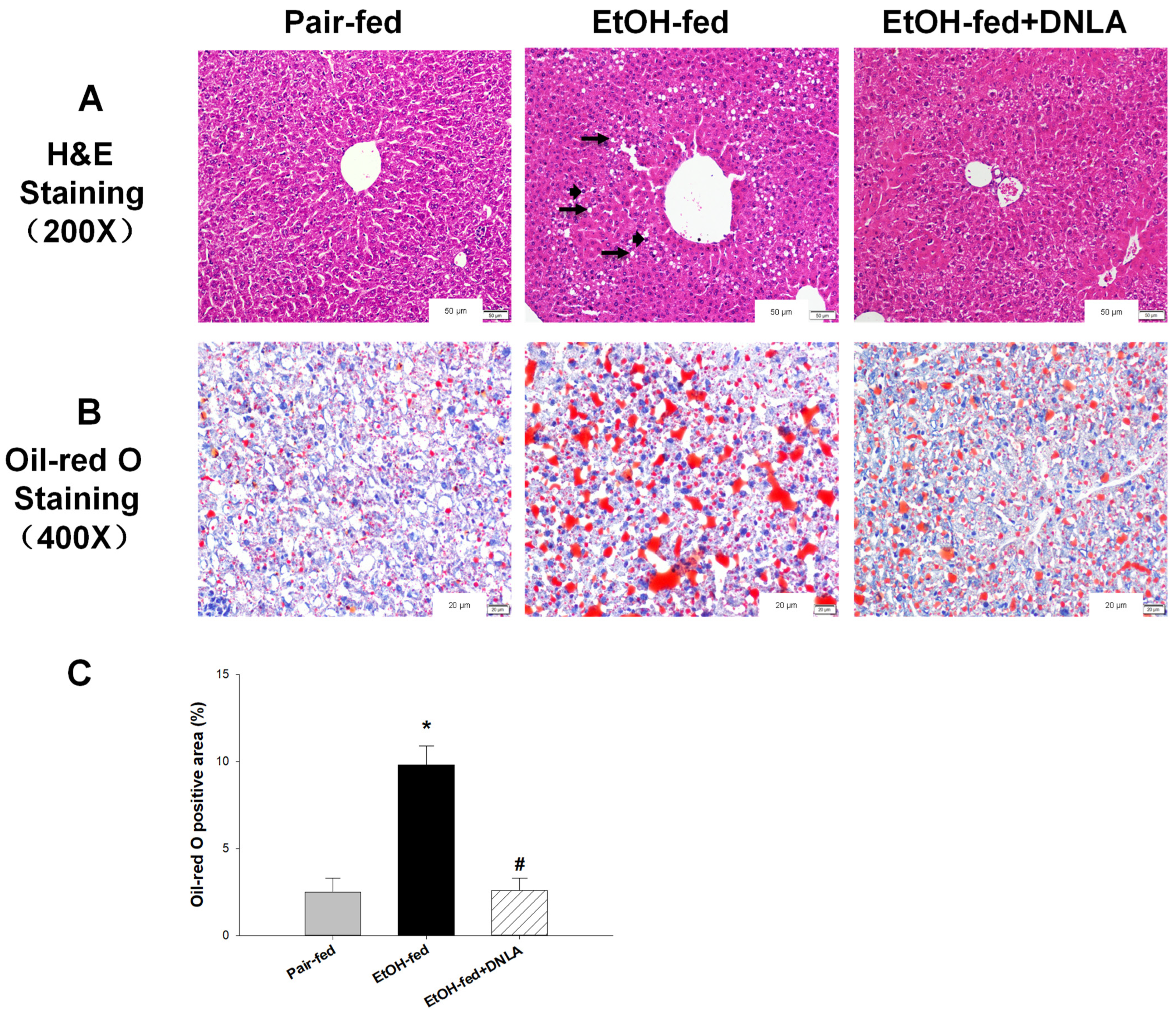
2.3. Histopathology Examination
2.4. Serum Enzyme Activities and Triglyceride Levels
2.5. RNA Isolation and Sequencing
2.6. Bioinformatic Analysis
2.7. Quantitative Real-Time PCR
2.8. Western Blot
2.9. Statistical Analysis
3. Results
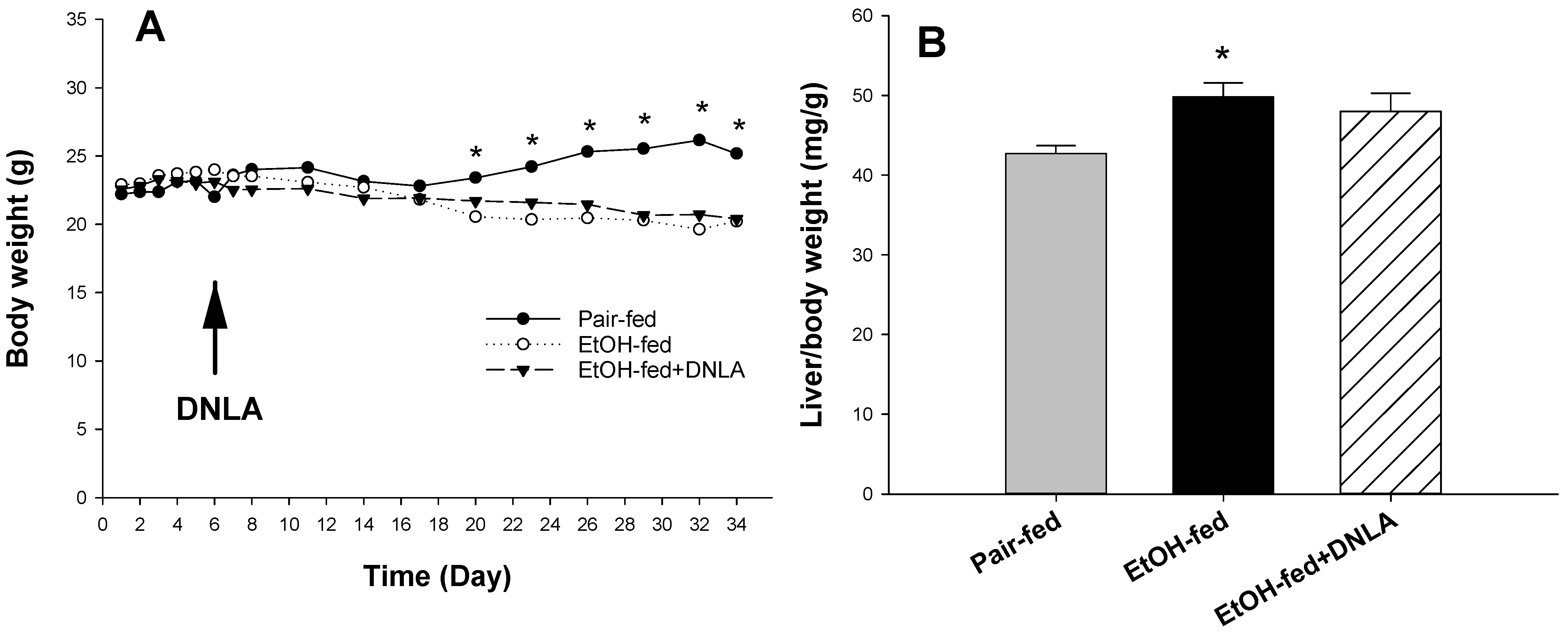
3.1. DNLA Protected against Chronic Alcohol-Induced Fatty Liver Disease
3.2. DNLA Improved Alcohol-Induced Histopathology
3.3. DNLA Alleviated Chronic Alcohol-Induced Aberrant Gene Expression
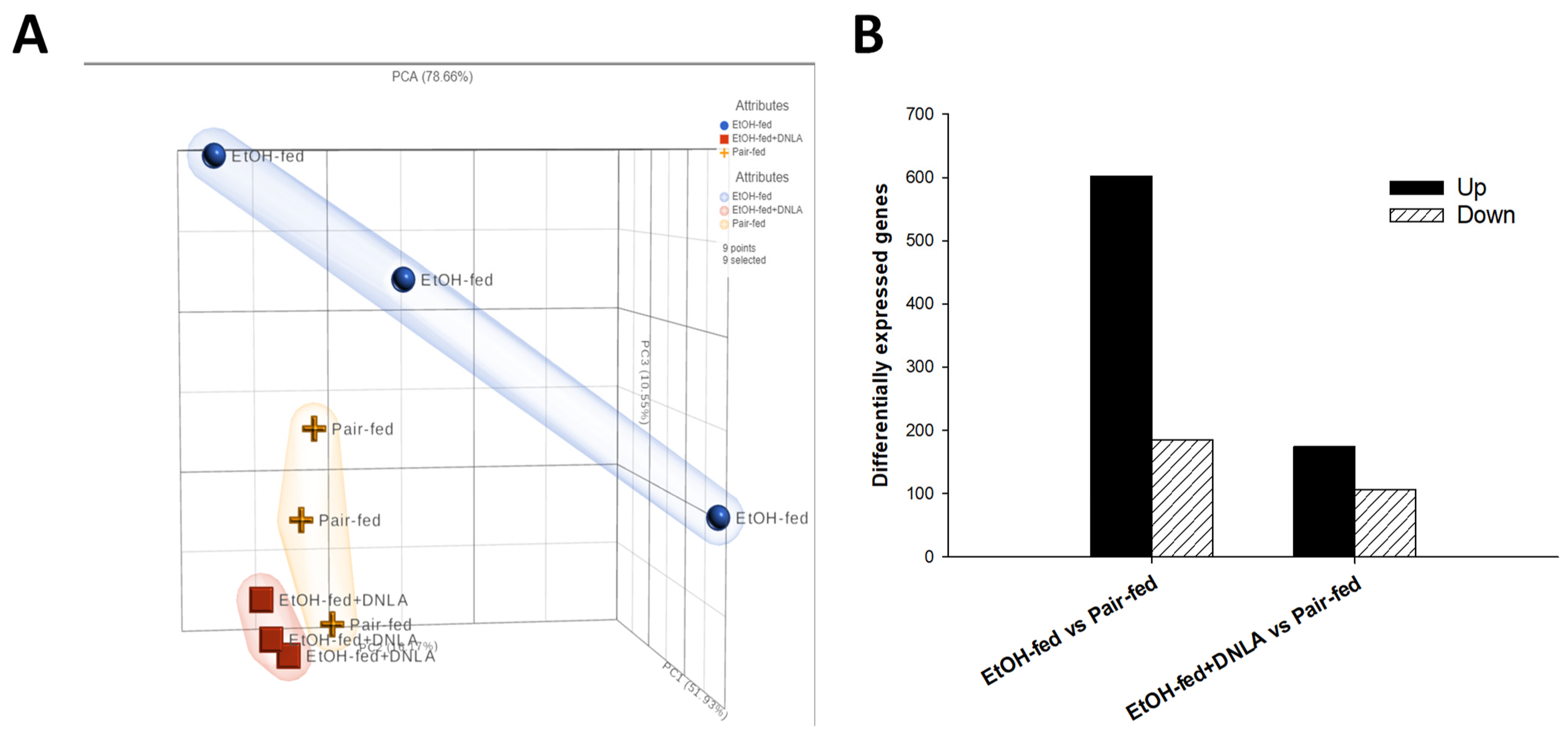
3.3.1. Gene Expression Pattern Analysis
3.3.2. Differentially Expressed Gene Analysis
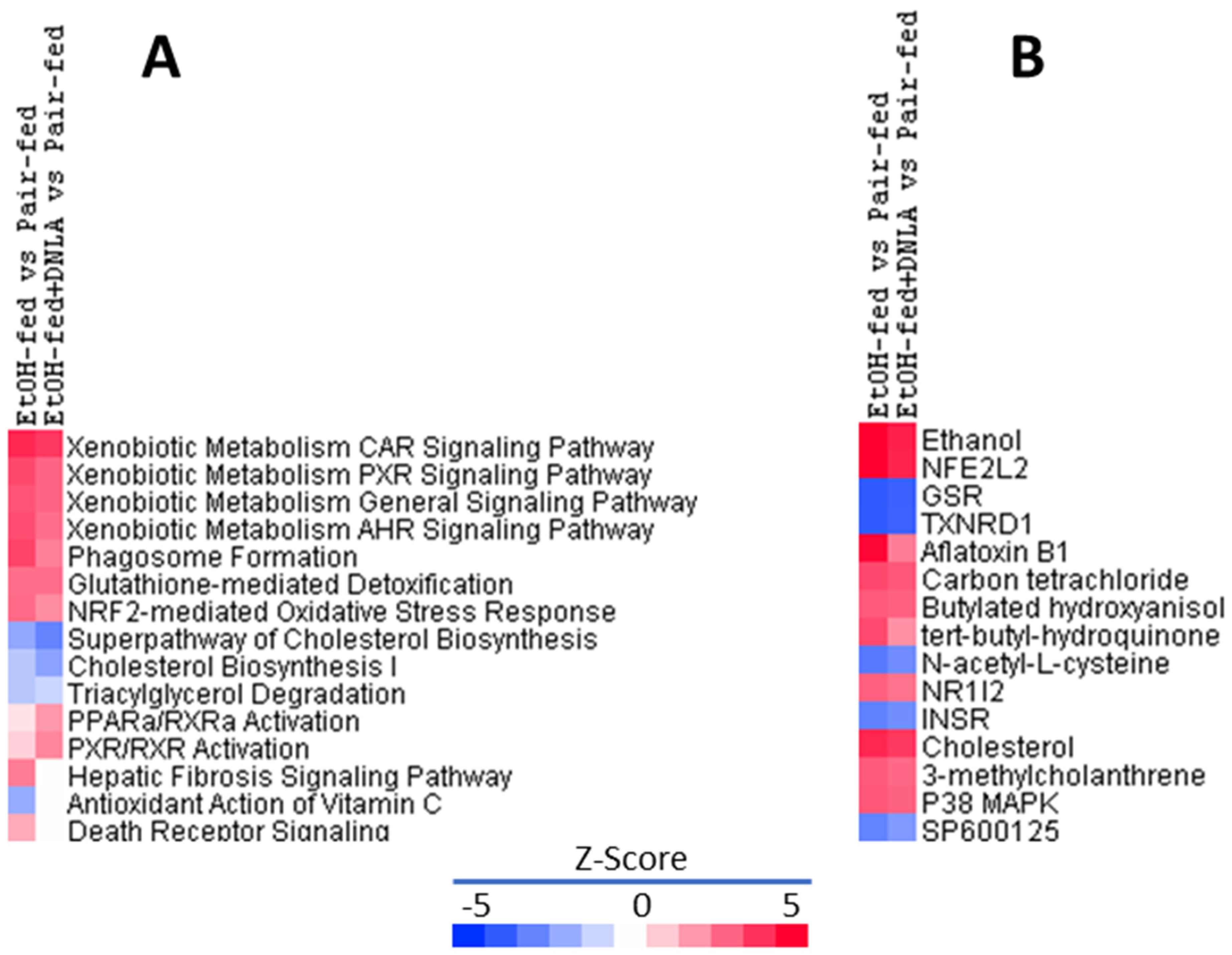
3.3.3. Ingenuity Pathways Analysis (IPA) of DEGs
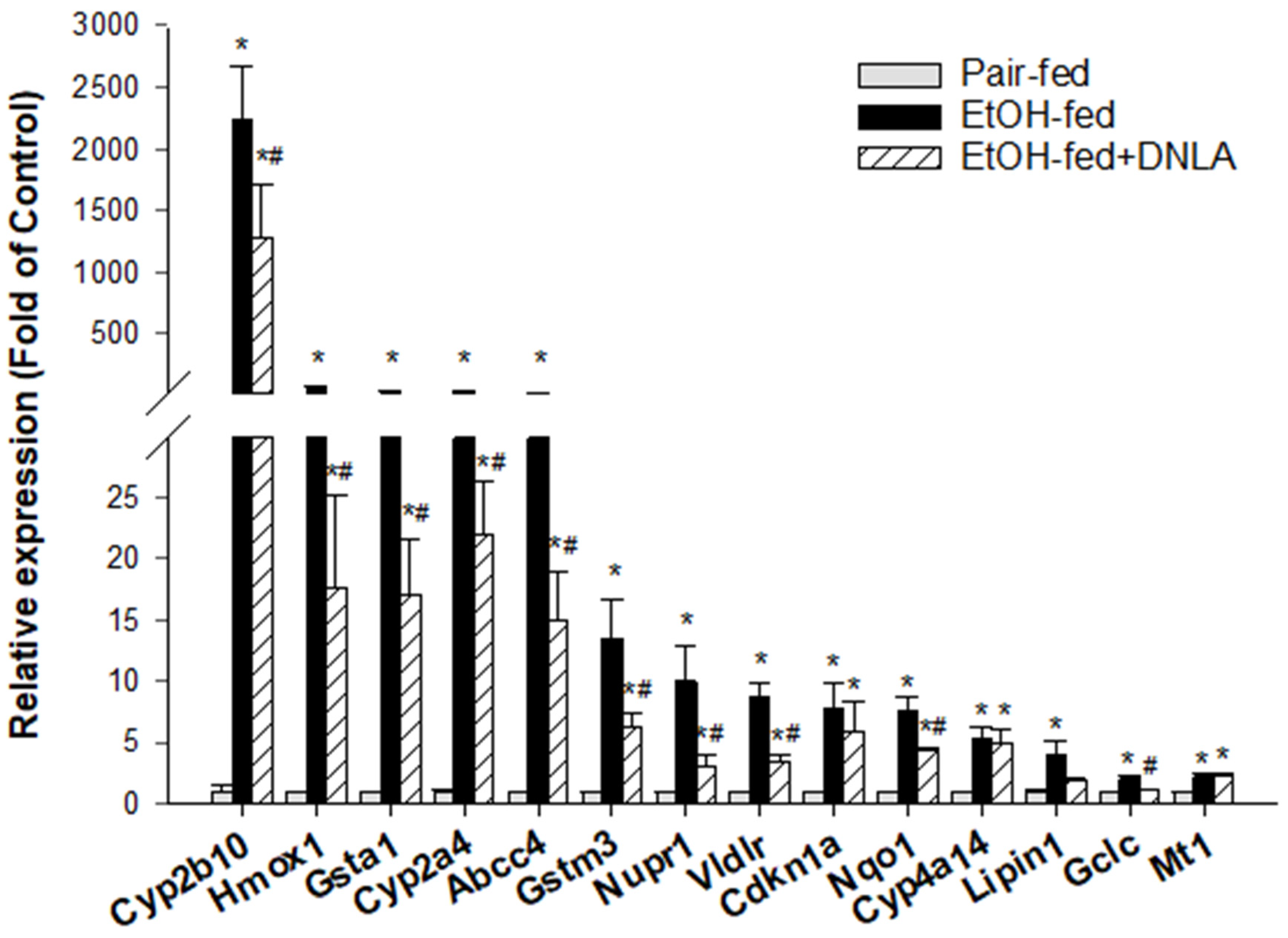
3.3.4. The Upregulated DEGs Verified by qPCR
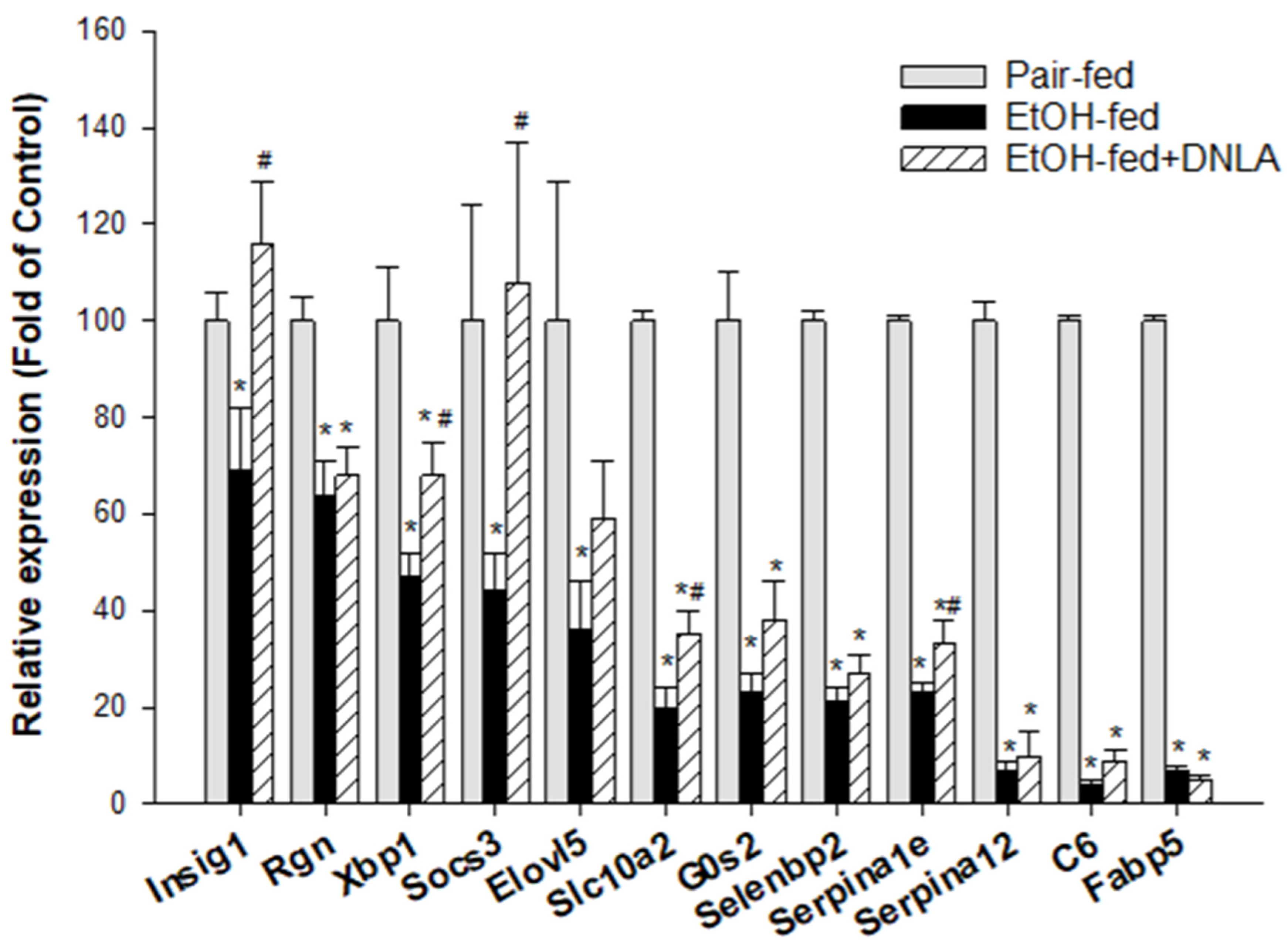
3.3.5. The Downregulated DEGs Verified by qPCR
3.4. Western-Blot Analysis of Selected Proteins
4. Discussion
4.1. DNLA Protected against Chronic Alcohol-Induced Fatty Liver
4.2. DNLA Alleviated Chronic Alcohol-Induced Aberrant Gene Expression
4.3. DNLA Attenuation of Overexpressed Genes
4.4. DNLA Ameliorated Downregulated Genes
4.5. Beneficial Effects of DNLA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuster, D.; Samet, J.H. Alcohol Use in Patients with Chronic Liver Disease. N. Engl. J. Med. 2018, 379, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Axley, P.D.; Richardson, C.T.; Singal, A.K. Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease. Clin. Liver Dis. 2019, 23, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Edenberg, H.J.; McClintick, J.N. Alcohol Dehydrogenases, Aldehyde Dehydrogenases, and Alcohol Use Disorders: A Critical Review. Alcohol Clin. Exp. Res. 2018, 42, 2281–2297. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.M.; Lu, Y. Alcoholic Liver Disease: From CYP2E1 to CYP2A5. Curr. Mol. Pharmacol. 2017, 10, 172–178. [Google Scholar] [CrossRef]
- Wang, Z.D.; Zhang, Y.; Dai, Y.D.; Ren, K.; Han, C.; Wang, H.X.; Yi, S.Q. Tamarix chinensis Lour inhibits chronic ethanol-induced liver injury in mice. World J. Gastroenterol. 2020, 26, 1286–1297. [Google Scholar] [CrossRef]
- Yang, S.; Huang, X.Y.; Zhou, N.; Wu, Q.; Liu, J.; Shi, J.S. RNA-Seq Analysis of Protection against Chronic Alcohol Liver Injury by Rosa roxburghii Fruit Juice (Cili) in Mice. Nutrients 2022, 14, 1974. [Google Scholar] [CrossRef]
- Singh, S.; Osna, N.A.; Kharbanda, K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J. Gastroenterol. 2017, 23, 6549–6570. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Ng, T.B. The medicinal and pharmaceutical importance of Dendrobium species. Appl. Microbiol. Biotechnol. 2017, 101, 2227–2239. [Google Scholar] [CrossRef]
- State Pharmacopoeia Commission of the PRC. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House: Beijing, China, 2020; Volume 1. [Google Scholar]
- Nie, X.; Chen, Y.; Li, W.; Lu, Y. Anti-aging properties of Dendrobium nobile Lindl.: From molecular mechanisms to potential treatments. J. Ethnopharmacol. 2020, 257, 112839. [Google Scholar] [CrossRef]
- Li, D.D.; Zheng, C.Q.; Zhang, F.; Shi, J.S. Potential neuroprotection by Dendrobium nobile Lindl alkaloid in Alzheimer’s disease models. Neural Regen. Res. 2022, 17, 972–977. [Google Scholar]
- Li, D.D.; Wang, G.Q.; Wu, Q.; Shi, J.S.; Zhang, F. Dendrobium nobile Lindl alkaloid attenuates 6-OHDA-induced dopamine neurotoxicity. Biotechnol. Appl. Biochem. 2021, 68, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Xu, Y.S.; Wang, Y.; Wu, Q.; Lu, Y.F.; Liu, J.; Shi, J.S. Dendrobium nobile Lindl. alkaloids regulate metabolism gene expression in livers of mice. J. Pharm. Pharmacol. 2017, 69, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, Q.; Liu, H.; Ling, H.; He, Y.; Wang, C.; Wang, Z.; Lu, Y.; Lu, Y. Alkaloids of Dendrobium nobile lindl. Altered hepatic lipid homeostasis via regulation of bile acids. J. Ethnopharmacol. 2019, 241, 111976. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, J.; Xu, S.; Li, J.; Liu, J.; Lu, Y.; Shi, J.; Zhou, S.; Wu, Q. Induction of Nrf2 pathway by Dendrobium nobile Lindl. alkaloids protects against carbon tetrachloride induced acute liver injury. Biomed. Pharmacother. 2019, 117, 109073. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Li, S.; Zhou, Q.; Lu, Y.; Shi, J.; Liu, J.; Wu, Q.; Zhou, S. Dendrobium nobile Lindl. alkaloids-mediated protection against CCl4-induced liver mitochondrial oxidative damage is dependent on the activation of Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 129, 110351. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Liu, J.; Li, S.; Zhou, S.; Zhang, C.; Wang, Y.; Shi, J.; Liu, J.; Wu, Q. RNA-Seq analysis of the protection by Dendrobium nobile alkaloids against carbon tetrachloride hepatotoxicity in mice. Biomed. Pharmacother. 2021, 137, 111307. [Google Scholar] [CrossRef]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Wang, M.; Chen, W.S.; Su, H.; Wang, Y.T.; Wan, J.B. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef]
- Xiong, Z.E.; Dong, W.G.; Wang, B.Y.; Tong, Q.Y.; Li, Z.Y. Curcumin attenuates chronic ethanol-induced liver injury by inhibition of oxidative stress via mitogen-activated protein kinase/nuclear factor E2-related factor 2 pathway in mice. Pharmacogn. Mag. 2015, 11, 707–715. [Google Scholar]
- Zhang, Y.P.; Yang, X.Q.; Yu, D.K.; Xiao, H.Y.; Du, J.R. Nrf2 signalling pathway and autophagy impact on the preventive effect of green tea extract against alcohol-induced liver injury. J. Pharm. Pharmacol. 2021, 73, 986–995. [Google Scholar] [CrossRef]
- Guo, F.; Zheng, K.; Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. The Lieber-DeCarli Diet-A Flagship Model for Experimental Alcoholic Liver Disease. Alcohol Clin. Exp. Res. 2018, 42, 1828–1840. [Google Scholar] [CrossRef]
- Nie, J.; Tian, Y.; Zhang, Y.; Lu, Y.L.; Li, L.S.; Shi, J.S. Dendrobium alkaloids prevent Abeta25-35-induced neuronal and synaptic loss via promoting neurotrophic factors expression in mice. PeerJ 2016, 4, e2739. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Lu, Y.L.; Nie, J.; Xu, Y.Y.; Zhang, W.; Yang, W.J.; Gong, Q.H.; Lu, Y.F.; Lu, Y.; Shi, J.S. Dendrobium nobile Lindl alkaloid, a novel autophagy inducer, protects against axonal degeneration induced by Aβ(25-35) in hippocampus neurons in vitro. CNS Neurosci. Ther. 2017, 23, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A. The application of principal component analysis to drug discovery and biomedical data. Drug Discov. Today 2017, 22, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, Z.; Wang, X.; Zeng, S.; Huang, W. The nuclear receptor CAR modulates alcohol-induced liver injury. Lab. Investig. 2011, 91, 1136–1145. [Google Scholar] [CrossRef]
- Choi, S.; Neequaye, P.; French, S.W.; Gonzalez, F.J.; Gyamfi, M.A. Pregnane X receptor promotes ethanol-induced hepatosteatosis in mice. J. Biol. Chem. 2018, 293, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, G.; Nie, Y.; Han, S.; Li, J.; Zhong, X.B.; Zhang, L. Epigenetic Memory Is Involved in the Persistent Alterations of Drug-Processing Genes in Adult Mice due to PCN-Activated PXR during Early Life. Toxicol. Sci. 2019, 72, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhang, J.; Dowhan, D.H.; Han, Y.; Moore, D.D. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenom. J. 2002, 2, 117–126. [Google Scholar] [CrossRef]
- Yao, R.; Yasuoka, A.; Kamei, A.; Ushiama, S.; Kitagawa, Y.; Rogi, T.; Shibata, H.; Abe, K.; Misaka, T. Nuclear receptor-mediated alleviation of alcoholic fatty liver by polyphenols contained in alcoholic beverages. PLoS ONE 2014, 9, e87142. [Google Scholar] [CrossRef]
- Mohs, A.; Otto, T.; Schneider, K.M.; Peltzer, M.; Boekschoten, M.; Holland, C.H.; Hudert, C.A.; Kalveram, L.; Wiegand, S.; Saez-Rodriguez, J.; et al. Hepatocyte-specific NRF2 activation controls fibrogenesis and carcinogenesis in steatohepatitis. J. Hepatol. 2021, 74, 638–648. [Google Scholar] [CrossRef]
- Wang, Z.; Dou, X.; Li, S.; Zhang, X.; Sun, X.; Zhou, Z.; Song, Z. Nuclear factor (erythroid-derived 2)-like 2 activation-induced hepatic very-low-density lipoprotein receptor overexpression in response to oxidative stress contributes to alcoholic liver disease in mice. Hepatology 2014, 59, 1381–1392. [Google Scholar] [CrossRef]
- Yan, X.; Liu, X.; Wang, Y.; Ren, X.; Ma, J.; Song, R.; Wang, X.; Dong, Y.; Fan, Q.; Wei, J.; et al. Multi-omics integration reveals the hepatoprotective mechanisms of ursolic acid intake against chronic alcohol consumption. Eur. J. Nutr. 2021, 61, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Brind, A.M.; Hurlstone, A.; Edrisinghe, D.; Gilmore, I.; Fisher, N.; Pirmohamed, M.; Fryer, A.A. The role of polymorphisms of glutathione S-transferases GSTM1, M3, P1, T1 and A1 in susceptibility to alcoholic liver disease. Alcohol Alcohol. 2004, 39, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, H.; Thompson, D.C.; Shertzer, H.G.; Nebert, D.W.; Vasiliou, V. Glutathione defense mechanism in liver injury: Insights from animal models. Food Chem. Toxicol. 2013, 60, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Horváth, B.; Rajesh, M.; Varga, Z.V.; Gariani, K.; Ryu, D.; Cao, Z.; Holovac, E.; Park, O.; Zhou, Z.; et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J. Hepatol. 2017, 66, 589–600. [Google Scholar] [CrossRef]
- Azzu, V.; Vacca, M.; Kamzolas, I.; Hall, Z.; Leslie, J.; Carobbio, S.; Virtue, S.; Davies, S.E.; Lukasik, A.; Dale, M.; et al. Suppression of insulin-induced gene 1 (INSIG1) function promotes hepatic lipid remodelling and restrains NASH progression. Mol. Metab. 2021, 48, 101210. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Murata, T. Involvement of regucalcin in lipid metabolism and diabetes. Metabolism 2013, 62, 1045–1051. [Google Scholar] [CrossRef]
- Sugaya, Y.; Satoh, H. Liver-specific G(0)/G(1) switch gene 2 (G0s2) expression promotes hepatic insulin resistance by exacerbating hepatic steatosis in male Wistar rats. J. Diabetes 2017, 9, 754–763. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.S.; Liu, H.M.; Lee, T.Y. Ursodeoxycholic Acid Regulates Hepatic Energy Homeostasis and White Adipose Tissue Macrophages Polarization in Leptin-Deficiency Obese Mice. Cells 2019, 8, 253. [Google Scholar] [CrossRef]
- Glimcher, L.H.; Lee, A.H. From sugar to fat: How the transcription factor XBP1 regulates hepatic lipogenesis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. 1), E2–E9. [Google Scholar] [CrossRef]
- Tripathy, S.; Lytle, K.A.; Stevens, R.D.; Bain, J.R.; Newgard, C.B.; Greenberg, A.S.; Huang, L.S.; Jump, D.B. Fatty acid elongase-5 (Elovl5) regulates hepatic triglyceride catabolism in obese C57BL/6J mice. J. Lipid Res. 2014, 55, 1448–1464. [Google Scholar] [CrossRef]
- Arab, J.P.; Cabrera, D.; Sehrawat, T.S.; Jalan-Sakrikar, N.; Verma, V.K.; Simonetto, D.; Cao, S.; Yaqoob, U.; Leon, J.; Freire, M.; et al. Hepatic stellate cell activation promotes alcohol-induced steatohepatitis through Igfbp3 and SerpinA12. J. Hepatol. 2020, 73, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Huenchullan, S.F.; Shipsey, I.; Hatchwell, L.; Min, D.; Twigg, S.M.; Larance, M. Blockade of High-Fat Diet Proteomic Phenotypes Using Exercise as Prevention or Treatment. Mol. Cell Proteom. 2021, 20, 100027. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Günther, I.; Chin, D.; Rimbach, G. In Contrast to Dietary Restriction, Application of Resveratrol in Mice Does not Alter Mouse Major Urinary Protein Expression. Nutrients 2020, 12, 815. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Yang, S.; Sun, J.; Li, X.; Zhou, S.-Y.; Shi, J.-S.; Liu, J.; Wu, Q. Transcriptome Analysis of Protection by Dendrobium nobile Alkaloids (DNLA) against Chronic Alcoholic Liver Injury in Mice. Biomedicines 2022, 10, 2800. https://doi.org/10.3390/biomedicines10112800
Huang X, Yang S, Sun J, Li X, Zhou S-Y, Shi J-S, Liu J, Wu Q. Transcriptome Analysis of Protection by Dendrobium nobile Alkaloids (DNLA) against Chronic Alcoholic Liver Injury in Mice. Biomedicines. 2022; 10(11):2800. https://doi.org/10.3390/biomedicines10112800
Chicago/Turabian StyleHuang, Xianyu, Shan Yang, Jian Sun, Xia Li, Shao-Yu Zhou, Jing-Shan Shi, Jie Liu, and Qin Wu. 2022. "Transcriptome Analysis of Protection by Dendrobium nobile Alkaloids (DNLA) against Chronic Alcoholic Liver Injury in Mice" Biomedicines 10, no. 11: 2800. https://doi.org/10.3390/biomedicines10112800
APA StyleHuang, X., Yang, S., Sun, J., Li, X., Zhou, S.-Y., Shi, J.-S., Liu, J., & Wu, Q. (2022). Transcriptome Analysis of Protection by Dendrobium nobile Alkaloids (DNLA) against Chronic Alcoholic Liver Injury in Mice. Biomedicines, 10(11), 2800. https://doi.org/10.3390/biomedicines10112800




