Low Intensity Respiratory Muscle Training in COVID-19 Patients after Invasive Mechanical Ventilation: A Retrospective Case-Series Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Ethical Requirements
2.3. Participants
2.4. Descriptive Data
2.5. Outcome Measurements
2.5.1. Respiratory Muscle Strength
2.5.2. Dyspnea Level
2.5.3. Health-Related Quality of Life
2.6. Respiratory Muscle Training Intervention
2.7. Sample Size Calculation
2.8. Statistical Analysis
3. Results
3.1. Baseline Data
3.2. RMT Effect on the Total Sample of COVID-19 Patients
3.3. RMT Effect on the Non-IMV Group
3.4. RMT Effect on the IMV Group
3.5. RMT Effect Comparison between Non-IMV and IMV Groups
3.6. Multivariate Regression Analysis for PImax (%) Prediction
4. Discussion
4.1. Future Studies
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, R.D.; Hall, J.B. Intensive care unit-acquired weakness. Crit. Care Med. 2010, 38, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Elkins, M.; Dentice, R. Inspiratory muscle training facilitates weaning from mechanical ventilation among patients in the intensive care unit: A systematic review. J. Physiother. 2015, 61, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anker, S.D.; Coats, A.J.S.; Morley, J.E.; Rosano, G.; Bernabei, R.; von Haehling, S.; Kalantar-Zadeh, K. Muscle wasting disease: A proposal for a new disease classification. J. Cachexia Sarcopenia Muscle 2014, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Palus, S.; Springer, J.; von Haehling, S. Muscle wasting: An overview of recent developments in basic research. J. Cachexia Sarcopenia Muscle 2014, 5, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schefold, J.C.; Bierbrauer, J.; Weber-Carstens, S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J. Cachexia Sarcopenia Muscle 2010, 1, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supinski, G.S.; Ann Callahan, L. Diaphragm weakness in mechanically ventilated critically ill patients. Crit. Care 2013, 17, R120. [Google Scholar] [CrossRef] [Green Version]
- Dasta, J.F.; McLaughlin, T.P.; Mody, S.H.; Piech, C.T. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit. Care Med. 2005, 33, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Ferguson, N.D.; Meade, M.O.; Frutos-Vivar, F.; Apezteguia, C.; Brochard, L.; Raymondos, K.; Nin, N.; Hurtado, J.; Tomicic, V.; et al. Evolution of mechanical ventilation in response to clinical research. Am. J. Respir. Crit. Care Med. 2008, 177, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Jaber, S.; Petrof, B.J.; Jung, B.; Chanques, G.; Berthet, J.P.; Rabuel, C.; Bouyabrine, H.; Courouble, P.; Koechlin-Ramonatxo, C.; Sebbane, M.; et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am. J. Respir. Crit. Care Med. 2011, 183, 364–371. [Google Scholar] [CrossRef]
- Damuth, E.; Mitchell, J.A.; Bartock, J.L.; Roberts, B.W.; Trzeciak, S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 544–553. [Google Scholar] [CrossRef]
- Powers, S.K.; Andrew Shanely, R.; Coombes, J.S.; Koesterer, T.J.; McKenzie, M.; Van Gammeren, D.; Cicale, M.; Dodd, S.L. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J. Appl. Physiol. 2002, 92, 1851–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.T.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid Disuse Atrophy of Diaphragm Fibers in Mechanically Ventilated Humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Kavazis, A.N.; Levine, S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit. Care Med. 2009, 37, S347–S353. [Google Scholar] [CrossRef] [PubMed]
- Andrew Shanely, R.; Zergeroglu, M.A.; Lennon, S.L.; Sugiura, T.; Yimlamai, T.; Enns, D.; Belcastro, A.; Powers, S.K. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am. J. Respir. Crit. Care Med. 2002, 166, 1369–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, N.; Matecki, S.; Py, G.; Lopez, S.; Mercier, J.; Capdevila, X. Effects of prolonged mechanical ventilation on respiratory muscle ultrastructure and mitochondrial respiration in rabbits. Intensive Care Med. 2003, 29, 111–118. [Google Scholar] [CrossRef]
- Yang, L.; Luo, J.; Bourdon, J.; Lin, M.C.; Gottfried, S.B.; Petrof, B.J. Controlled mechanical ventilation leads to remodeling of the rat diaphragm. Am. J. Respir. Crit. Care Med. 2002, 166, 1135–1140. [Google Scholar] [CrossRef]
- Radell, P.; Edström, L.; Stibler, H.; Eriksson, L.I.; Ansved, T. Changes in diaphragm structure following prolonged mechanical ventilation in piglets. Acta Anaesthesiol. Scand. 2004, 48, 430–437. [Google Scholar] [CrossRef]
- Sassoon, C.S.H.; Caiozzo, V.J.; Manka, A.; Sieck, G.C. Altered diaphragm contractile properties with controlled mechanical ventilation. J. Appl. Physiol. 2002, 92, 2585–2595. [Google Scholar] [CrossRef]
- Capdevila, X.; Lopez, S.; Bernard, N.; Rabischong, E.; Ramonatxo, M.; Martinazzo, G.; Prefaut, C. Effects of controlled mechanical ventilation on respiratory muscle contractile properties in rabbits. Intensive Care Med. 2003, 29, 103–110. [Google Scholar] [CrossRef]
- Le Bourdelles, G.; Viires, N.; Boczkowski, J.; Seta, N.; Pavlovic, D.; Aubier, M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am. J. Respir. Crit. Care Med. 1994, 149, 1539–1544. [Google Scholar] [CrossRef]
- Shanely, R.A.; Van Gammeren, D.; DeRuisseau, K.C.; Zergeroglu, A.M.; McKenzie, M.J.; Yarasheski, K.E.; Powers, S.K. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am. J. Respir. Crit. Care Med. 2004, 170, 994–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.T.; Bruells, C.S.; Stabley, J.N.; McCullough, D.J.; Powers, S.K.; Behnke, B.J. Mechanical ventilation reduces rat diaphragm blood flow and impairs oxygen delivery and uptake. Crit. Care Med. 2012, 40, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Zhu, E.; Sassoon, C.S.H. Ventilator-induced diaphragmatic vascular dysfunction. Crit. Care Med. 2012, 40, 2914–2915. [Google Scholar] [CrossRef]
- Watson, A.C.; Hughes, P.D.; Harris, M.L.; Hart, N.; Ware, R.J.; Wendon, J.; Green, M.; Moxham, J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit. Care Med. 2001, 29, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Hermans, G.; Agten, A.; Testelmans, D.; Decramer, M.; Gayan-Ramirez, G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: A prospective observational study. Crit. Care 2010, 14, R127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Padhke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA J. Am. Med. Assoc. 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Morris, P.E.; Berry, M.J.; Files, D.C.; Thompson, J.C.; Hauser, J.; Flores, L.; Dhar, S.; Chmelo, E.; Lovato, J.; Case, L.D.; et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure a randomized clinical trial. JAMA J. Am. Med. Assoc. 2016, 315, 2694–2702. [Google Scholar] [CrossRef]
- Moss, M.; Nordon-Craft, A.; Malone, D.; Van Pelt, D.; Frankel, S.K.; Warner, M.L.; Kriekels, W.; McNulty, M.; Fairclough, D.L.; Schenkman, M. A Randomized Trial of an Intensive Physical Therapy Program for Patients with Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2016, 193, 1101–1110. [Google Scholar] [CrossRef] [Green Version]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Vorona, S.; Sabatini, U.; Al-Maqbali, S.; Bertoni, M.; Dres, M.; Bissett, B.; Van Haren, F.; Martin, A.D.; Urrea, C.; Brace, D.; et al. Inspiratory muscle rehabilitation in critically ill adults a systematic review and meta-analysis. Ann. Am. Thorac. Soc. 2018, 15, 735–744. [Google Scholar] [CrossRef]
- Moodie, L.; Reeve, J.; Elkins, M. Inspiratory muscle training increases inspiratory muscle strength in patients weaning from mechanical ventilation: A systematic review. J. Physiother. 2011, 57, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Bissett, B.M.; Leditschke, I.A.; Neeman, T.; Boots, R.; Paratz, J. Inspiratory muscle training to enhance recovery from mechanical ventilation: A randomised trial. Thorax 2016, 71, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissett, B.; Leditschke, I.A.; Green, M.; Marzano, V.; Collins, S.; Van Haren, F. Inspiratory muscle training for intensive care patients: A multidisciplinary practical guide for clinicians. Aust. Crit. Care 2018, 32, 249–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; Allaire, A.; Aronson, J.; Carpenter, J.; Gagnier, J.; et al. The CARE guidelines: Consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013, 2013, bcr2013201554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18.
- Holt, G.R. Declaration of Helsinki-the world’s document of conscience and responsibility. South. Med. J. 2014, 107, 407. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef]
- Debouche, S.; Pitance, L.; Robert, A.; Liistro, G.; Reychler, G. Reliability and Reproducibility of Chest Wall Expansion Measurement in Young Healthy Adults. J. Manip. Physiol. Ther. 2016, 39, 443–449. [Google Scholar] [CrossRef]
- Irwig, L.; Groeneveld, H.; Becklake, M. Relationship of lung function loss to level of initial function: Correcting for measurement error using the reliability coefficient. J. Epidemiol. Community Health 1988, 42, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; Macintyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.L.; Steenbruggen, I.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; Miller, M.R.; et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, C.; Barbero-Iglesias, F.J.; Amor-Esteban, V.; Martin-Nogueras, A.M. Comparison between two inspiratory muscle training protocols, low loads versus high loads, in institutionalized elderly women: A double-blind randomized controlled trial. Gerontology 2021, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.M. Standardised questionnaire on respiratory symptoms: A statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score). Br. Med. J. 1960, 2, 1665. [Google Scholar]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Pinto, L.M.; Morogan, A.; Bourbeau, J. The COPD assessment test: A systematic review. Eur. Respir. J. 2014, 44, 873–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco, E.; Ramírez-Sarmiento, A.L.; Coloma, A.; Sartor, M.; Comin-Colet, J.; Vila, J.; Enjuanes, C.; Bruguera, J.; Escalada, F.; Gea, J.; et al. High-intensity vs. sham inspiratory muscle training in patients with chronic heart failure: A prospective randomized trial. Eur. J. Heart Fail. 2013, 15, 892–901. [Google Scholar] [CrossRef]
- Messaggi-Sartor, M.; Guillen-Solà, A.; Depolo, M.; Duarte, E.; Rodríguez, D.A.; Barrera, M.-C.; Barreiro, E.; Escalada, F.; Orozco-Levi, M.; Marco, E. Inspiratory and expiratory muscle training in subacute stroke: A randomized clinical trial. Neurology 2015, 85, 564–572. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Sim, J.; Reid, N. Statistical Inference by Confidence Intervals: Issues of Interpretation and Utilization. Phys. Ther. 1999, 79, 186–195. [Google Scholar] [CrossRef]
- Kelley, K.; Preacher, K.J. On Effect Size. Psychol. Methods 2012, 17, 137–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanca, M.J.; Alarcón, R.; Arnau, J.; Bono, R.; Bendayan, R. Non-normal data: Is ANOVA still a valid option? Psicothema 2017, 29, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, N.; Beauducel, A. Violation of the Sphericity Assumption and Its Effect on Type-I Error Rates in Repeated Measures ANOVA and Multi-Level Linear Models (MLM). Front. Psychol. 2017, 8, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Vicente-Campos, D.; Sanchez-Jorge, S.; Terrón-Manrique, P.; Guisard, M.; Collin, M.; Castaño, B.; Rodríguez-Sanz, D.; Becerro-De-bengoa-vallejo, R.; Chicharro, J.L.; Calvo-Lobo, C. The Main Role of Diaphragm Muscle as a Mechanism of Hypopressive Abdominal Gymnastics to Improve Non-Specific Chronic Low Back Pain: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 4983. [Google Scholar] [CrossRef]
- Austin, P.C.; Steyerberg, E.W. The number of subjects per variable required in linear regression analyses. J. Clin. Epidemiol. 2015, 68, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Abodonya, A.M.; Abdelbasset, W.K.; Awad, E.A.; Elalfy, I.E.; Salem, H.A.; Elsayed, S.H. Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine 2021, 100, e25339. [Google Scholar] [CrossRef]
- Perrone, A.; Quacquarelli, M.E.; Barbarossa, A.; Cargoni, A.; Magliocco, C.; Masciangelo, V.; Brunori, M. Possibili effetti della ventilazione meccanica non invasiva su drive e muscolatura respiratoria. Clin. Ter. 2007, 158, 11–16. [Google Scholar]
- Fernandez-Rubio, H.; Becerro-de-Bengoa-Vallejo, R.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Vicente-Campos, D.; Chicharro, J.L. Inspiratory Muscle Training in Patients with Heart Failure. J. Clin. Med. 2020, 9, 1710. [Google Scholar] [CrossRef]
- Fernández-Rubio, H.; Becerro-De-bengoa-vallejo, R.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Vicente-Campos, D.; Chicharro, J.L. Unraveling the role of respiratory muscle metaboloreceptors under inspiratory training in patients with heart failure. Int. J. Environ. Res. Public Health 2021, 18, 1697. [Google Scholar] [CrossRef]
- Palermo, A.E.; Cahalin, L.P.; Nash, M.S. A case for inspiratory muscle training in SCI: Potential role as a preventative tool in infectious respiratory diseases like COVID-19. Spinal Cord. Ser. Cases 2020, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Vehtari, A.; Gelman, A.; Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 2017, 27, 1413–1432. [Google Scholar] [CrossRef]
| Quantitative Data at Baseline | Total Sample (n = 40) Mean ± SD (95% CI) | Non-IMV (n = 20) Mean ± SD (95% CI) | IMV (n = 20) Mean ± SD (95% CI) | Mean Difference (95% CI) | Statistics | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 56.0 ± 11.3 (52.3–59.6) | 56.4 ± 12.0 (50.7–62.0) | 55.6 ± 10.8 (50.5–60.7) | 0.7 (−6.6–8.1) | t = 0.206 | 0.838 * |
| Hospitalization (days) | 30.6 ± 28.8 (21.4–39.8) | 13.3 ± 9.1 (9.0–17.5) | 47.9 ± 31.4 (33.2–62.6) | −34.6 (−49.8–−19.4) | U = 371.000 | <0.001 † |
| ICU (days) | 15.7 ± 23.5 (8.2–23.2) | N/A | 31.5 ± 24.7 (19.9–43.1) | N/A | N/A | N/A |
| FVC (%) | 78.8 ± 15.3 (73.9–83.8) | 80.9 ± 14.0 (74.3–87.4) | 76.8 ± 16.6 (69.0–84.6) | 4.0 (−5.8–13.9) | U = 169.500 | 0.414 † |
| FEV1(%) | 88.5 ± 16.5 (83.2–93.8) | 88.4 ± 14.4 (81.6–95.1) | 88.7 ± 18.9 (79.9–97.6) | −0.3 (−11.1–10.3) | U = 205.500 | 0.883 † |
| IT (%) | 86.5 ± 6.3 (84.5–88.6) | 84.9 ± 7.0 (81.5–88.2) | 88.2 ± 5.1 (85.8–90.6) | −3.3 (−7.3–0.3) | t = −1.735 | 0.091 * |
| DLCO (%) | 75.1 ± 19.3 (68.9–81.2) | 85.0 ± 19.5 (75.9–94.1) | 65.1 ± 13.3 (58.9–71.4) | 19.8 (9.1–30.5) | t = 3.759 | 0.001 * |
| DL/VA (%) | 89.1 ± 15.4 (84.1–94.0) | 91.7 ± 18.7 (82.8–100.3) | 86.6 ± 11.1 (81.4–91.8) | 4.9 (−4.9–14.9) | t = 1.018 | 0.317 * |
| PEmax (cm H2O) | 99.5 ± 38.0 (87.3–111.7) | 101.1 ± 37.1 (83.7–118.5) | 97.9 ± 39.8 (79.2–116.6) | 3.2 (−21.4–27.8) | t = 0.263 | 0.794 * |
| PEmax (%) | 50.1 ± 16.3 (44.9–55.4) | 52.9 ± 16.1 (45.3–60.4) | 47.4 ± 16.6 (39.6–55.2) | 5.4 (−5.0–15.9) | t = 1.048 | 0.301 * |
| PImax (cm H2O) | 71.3 ± 26.7 (62.8–79.8) | 82.6 ± 29.3 (68.9–96.3) | 60.0 ± 18.3 (51.4–68.6) | 22.5 (6.8–38.3) | t = 2.921 | 0.006 * |
| PImax (%) | 70.4 ± 24.1 (62.7–78.2) | 85.1 ± 22.0 (74.8–95.4) | 55.7 ± 16.0 (48.2–63.3) | 29.3 (16.9–41.7) | t = 4.805 | <0.001 * |
| CAT (scores) | 12.1 ± 8.2 (9.4–14.7) | 10.3 ± 7.0 (7.0–13.6) | 13.8 ± 9.0 (9.6–18.0) | −3.5 (−8.7–1.7) | U = 254.000 | 0.149 † |
| MRC (scores) | 1.4 ± 1.0 (1.0–1.7) | 0.9 ± 0.7 (0.6–1.2) | 1.9 ± 1.1 (1.3–1.9) | −0.9 (−1.5–−0.3) | U = 314.000 | 0.002 † |
| Categorical Data at Baseline | Total Sample (n = 40) n (%) | Non-IMV (n = 20) n (%) | IMV (n = 20) n (%) | Statistics | p-Value † | |
|---|---|---|---|---|---|---|
| Sex | Female | 26 (65%) | 12 (60%) | 14 (70%) | χ2 = 0.440 | 0.741 |
| Male | 14 (35%) | 8 (40%) | 6 (30%) | |||
| Hypertension | No | 24 (60%) | 13 (65%) | 11 (55%) | χ2 = 0.417 | 0.748 |
| Yes | 16 (40%) | 7 (35%) | 9 (45%) | |||
| Diabetes | No | 33 (82.5%) | 18 (90%) | 15 (75%) | χ2 = 1.558 | 0.407 |
| Yes | 7 (17.5%) | 2 (10%) | 5 (25%) | |||
| Dyslipidemia | No | 34 (85%) | 19 (95%) | 15 (75%) | χ2 = 3.137 | 0.182 |
| Yes | 6 (15%) | 1 (5%) | 5 (25%) | |||
| CAD | No | 37 (92.5%) | 17 (85%) | 20 (100%) | χ2 = 3.243 | 0.231 |
| Yes | 3 (7.5%) | 3 (15%) | 0 (0%) | |||
| COPD | No | 36 (90%) | 17 (85%) | 19 (95%) | χ2 = 1.111 | 0.605 |
| Yes | 4 (10%) | 3 (15%) | 1 (5%) | |||
| Smoker | No | 31 (77.5%) | 16 (80%) | 15 (75%) | χ2 = 0.143 | 1.000 |
| Yes | 9 (22.5%) | 4 (20%) | 5 (25%) | |||
| Obesity | No | 36 (90%) | 19 (95%) | 17 (85%) | χ2 = 1.111 | 0.605 |
| Yes | 4 (10%) | 1 (5%) | 3 (15%) | |||
| CKD | No | 30 (75%) | 16 (80%) | 14 (70%) | χ2 = 0.533 | 0.716 |
| Yes | 10 (25%) | 4 (20%) | 6 (30%) | |||
| Hypothyroidism | No | 39 (97.5%) | 19 (95%) | 20 (100%) | χ2 = 1.026 | 1.000 |
| Yes | 1 (2.5%) | 1 (5%) | 0 (0%) | |||
| Outcome Measurements | Baseline (n = 40) Mean ± SD (95% CI) | After RMT (n = 40) Mean ± SD (95% CI) | Mean Difference (95% CI) | Statistics | p-Value | Effect Size (Cohen d) |
|---|---|---|---|---|---|---|
| PEmax (cm H2O) | 99.5 ± 38.0 (87.3–111.7) | 108.9 ± 34.0 (98.0–119.8) | 9.3 (−2.7–21.5) | W = 288.000 | 0.101 † | d = 0.24 |
| PEmax (%) | 50.1 ± 16.3 (44.9–55.4) | 57.2 ± 16.9 (51.8–62.6) | 7.0 (1.1–13.0) | t = 2.408 | 0.021 * | d = 0.38 |
| PImax (cm H2O) | 71.3 ± 26.7 (62.8–79.8) | 83.8 ± 31.8 (73.6–94.0) | 12.4 (2.7–22.2) | t = 2.583 | 0.014 * | d = 0.40 |
| PImax (%) | 70.4 ± 24.1 (62.7–78.2) | 85.9 ± 33.1 (75.3–96.5) | 15.5 (7.4–23.5) | W = 143.000 | <0.001 † | d = 0.61 |
| CAT (scores) | 12.1 ± 8.2 (9.4–14.7) | 6.7 ± 6.7 (4.6–8.9) | −5.3 (−7.5–−3.1) | W = 618.500 | <0.001 † | d = 0.78 |
| MRC (scores) | 1.4 ± 1.0 (1.0–1.7) | 1.0 ± 4.2 (1.0–1.7) | −0.8 (−1.1–−0.5) | W = 558.000 | <0.001 † | d = 0.97 |
| Outcome Measurements (Scores) | Baseline (n = 20) Mean ± SD (95% CI) | After RMT (n = 20) Mean ± SD (95% CI) | Mean Difference (95% CI) | Statistics | p-Value | Effect Size (Cohen d) |
|---|---|---|---|---|---|---|
| PEmax (cm H2O) | 101.1 ± 37.1 (83.7–118.5) | 104.5 ± 29.5 (90.7–118.3) | 3.3 (−12.7–19.4) | W = 99.000 | 0.823 † | d = 0.09 |
| PEmax (%) | 52.9 ± 16.1 (45.3–60.4) | 58.8 ± 17.3 (50.7–66.9) | 5.9 (−1.1–13.0) | W = 69.000 | 0.179 † | d = 0.39 |
| PImax (cm H2O) | 82.6 ± 29.3 (68.9–96.3) | 88.6 ± 32.5 (73.3–103.8) | 5.9 (−9.7–21.6) | t = 0.797 | 0.435 * | d = 0.17 |
| PImax (%) | 85.1 ± 22.0 (74.8–95.4) | 98.6 ± 35.1 (82.2–115.1) | 13.5 (−0.3–27.4) | W = 54.000 | 0.057 † | d = 0.45 |
| CAT (scores) | 10.3 ± 7.0 (7.0–13.6) | 3.4 ± 3.6 (1.6–5.1) | −6.9 (−9.9–−3.9) | W = 165.000 | 0.001 † | d = 1.09 |
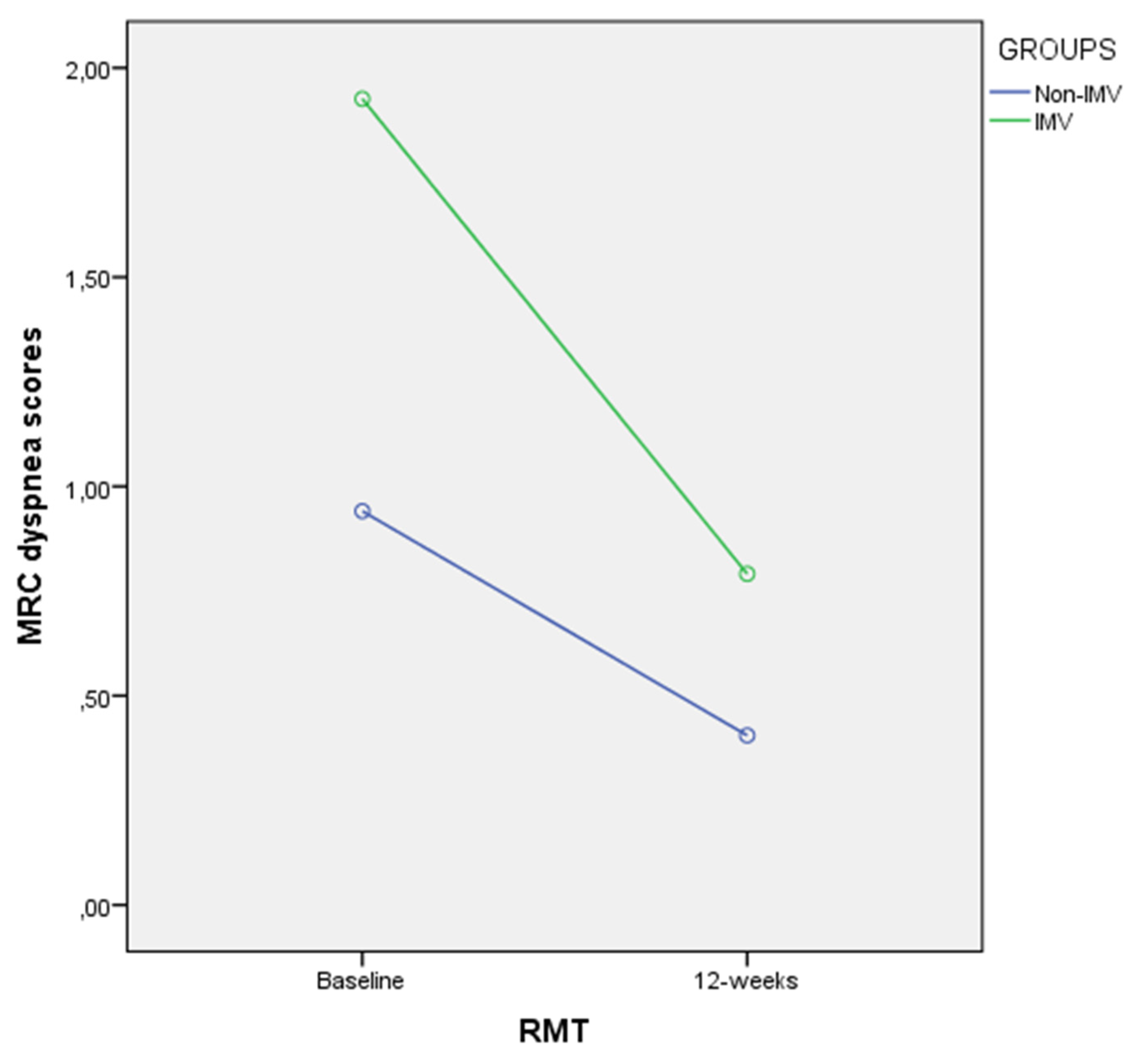
| MRC (scores) | 0.9 ± 0.7 (0.6–1.2) | 0.4 ± 0.3 (0.2–0.5) | −0.5 (−0.8–−0.2) | W = 118.000 | 0.001 † | d = 0.94 |
| Outcome Measurements | Baseline (n = 20) Mean ± SD (95% CI) | After RMT (n = 20) Mean ± SD (95% CI) | Mean Difference (95% CI) | Statistics | p-Value | Effect Size (Cohen d) |
|---|---|---|---|---|---|---|
| PEmax (cm H2O) | 97.9 ± 39.8 (79.2–116.6) | 113.3 ± 38.3 (95.4–131.2) | 15.4 (−3.9–34.7) | t = 1.663 | 0.113 * | d = 0.37 |
| PEmax (%) | 47.4 ± 16.6 (39.6–55.2) | 55.7 ± 16.8 (47.8–63.6) | 8.2 (−1.9–18.4) | t = 1.685 | 0.108 * | d = 0.37 |
| PImax (cm H2O) | 60.0 ± 18.3 (51.4–68.6) | 79.0 ± 31.2 (64.4–93.7) | 19.0 (6.5–31.4) | t = 3.192 | 0.005 * | d = 0.71 |
| PImax (%) | 55.7 ± 16.0 (48.2–63.3) | 73.2 ± 26.0 (61.0–85.4) | 17.4 (7.8–27.1) | t = 3.811 | 0.001 * | d = 0.85 |
| CAT (scores) | 13.8 ± 9.0 (9.6–18.0) | 4.4 ± 3.6 (1.6–5.1) | −3.7 (−7.0–0.4) | W = 149.000 | 0.029 † | d = 0.54 |
| MRC (scores) | 1.9 ± 1.1 (1.3–1.9) | 0.7 ± 4.2 (0.5–1.0) | −1.1 (−1.6–−0.6) | W = 0.000 | <0.001 † | d = 1.13 |
| Outcome Differences after RMT | Non-IMV (n = 20) Mean ± SD (95% CI) | IMV (n = 20) Mean ± SD (95% CI) | Mean Difference (95% CI) | Statistics | p-Value | Effect Size (Cohen d) |
|---|---|---|---|---|---|---|
| PEmax (cm H2O) | 3.3 ± 34.3 (−12.7–19.4) | 15.4 ± 41.4 (−3.9–34.7) | −12.0 (−36.3–12.3) | t = −0.999 | 0.324 * | d = 0.31 |
| PEmax (%) | 5.9 ± 15.2 (−1.1–13.0) | 8.2 ± 21.8 (−1.9–18.4) | −2.2 (−14.3–9.7) | t = −0.385 | 0.702 * | d = 0.12 |
| PImax (cm H2O) | 5.9 ± 33.4 (−9.7–21.6) | 19.0 ± 26.6 (6.5–31.4) | −13.0 (−32.4–6.3) | t = −1.365 | 0.180 * | d = 0.43 |
| PImax (%) | 13.5 ± 29.6 (−0.3–27.4) | 17.4 ± 20.5 (7.8–27.1) | −3.9 (−20.2–12.3) | U = 235.500 | 0.341 † | d = 0.15 |
| CAT (scores) | −6.9 ± 6.3 (−9.9–−3.9) | −3.7 ± 6.9 (−7.0–−0.4) | 3.2 (−7.4–1.07) | t = −1.517 | 0.138 * | d = 0.48 |
| MRC (scores) | −0.5 ± 0.5 (−0.8–−0.2) | −1.1 ± 1.0 (−1.6–−0.6) | 0.5 (0.7–1.1) | U = 114.000 | 0.020 † | d = 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villelabeitia-Jaureguizar, K.; Calvo-Lobo, C.; Rodríguez-Sanz, D.; Vicente-Campos, D.; Castro-Portal, J.A.; López-Cañadas, M.; Becerro-de-Bengoa-Vallejo, R.; Chicharro, J.L. Low Intensity Respiratory Muscle Training in COVID-19 Patients after Invasive Mechanical Ventilation: A Retrospective Case-Series Study. Biomedicines 2022, 10, 2807. https://doi.org/10.3390/biomedicines10112807
Villelabeitia-Jaureguizar K, Calvo-Lobo C, Rodríguez-Sanz D, Vicente-Campos D, Castro-Portal JA, López-Cañadas M, Becerro-de-Bengoa-Vallejo R, Chicharro JL. Low Intensity Respiratory Muscle Training in COVID-19 Patients after Invasive Mechanical Ventilation: A Retrospective Case-Series Study. Biomedicines. 2022; 10(11):2807. https://doi.org/10.3390/biomedicines10112807
Chicago/Turabian StyleVillelabeitia-Jaureguizar, Koldo, César Calvo-Lobo, David Rodríguez-Sanz, Davinia Vicente-Campos, José Adrián Castro-Portal, Marta López-Cañadas, Ricardo Becerro-de-Bengoa-Vallejo, and José López Chicharro. 2022. "Low Intensity Respiratory Muscle Training in COVID-19 Patients after Invasive Mechanical Ventilation: A Retrospective Case-Series Study" Biomedicines 10, no. 11: 2807. https://doi.org/10.3390/biomedicines10112807






