Overview of Antiviral Drug Therapy for COVID-19: Where Do We Stand?
Abstract
1. Introduction
2. Methods
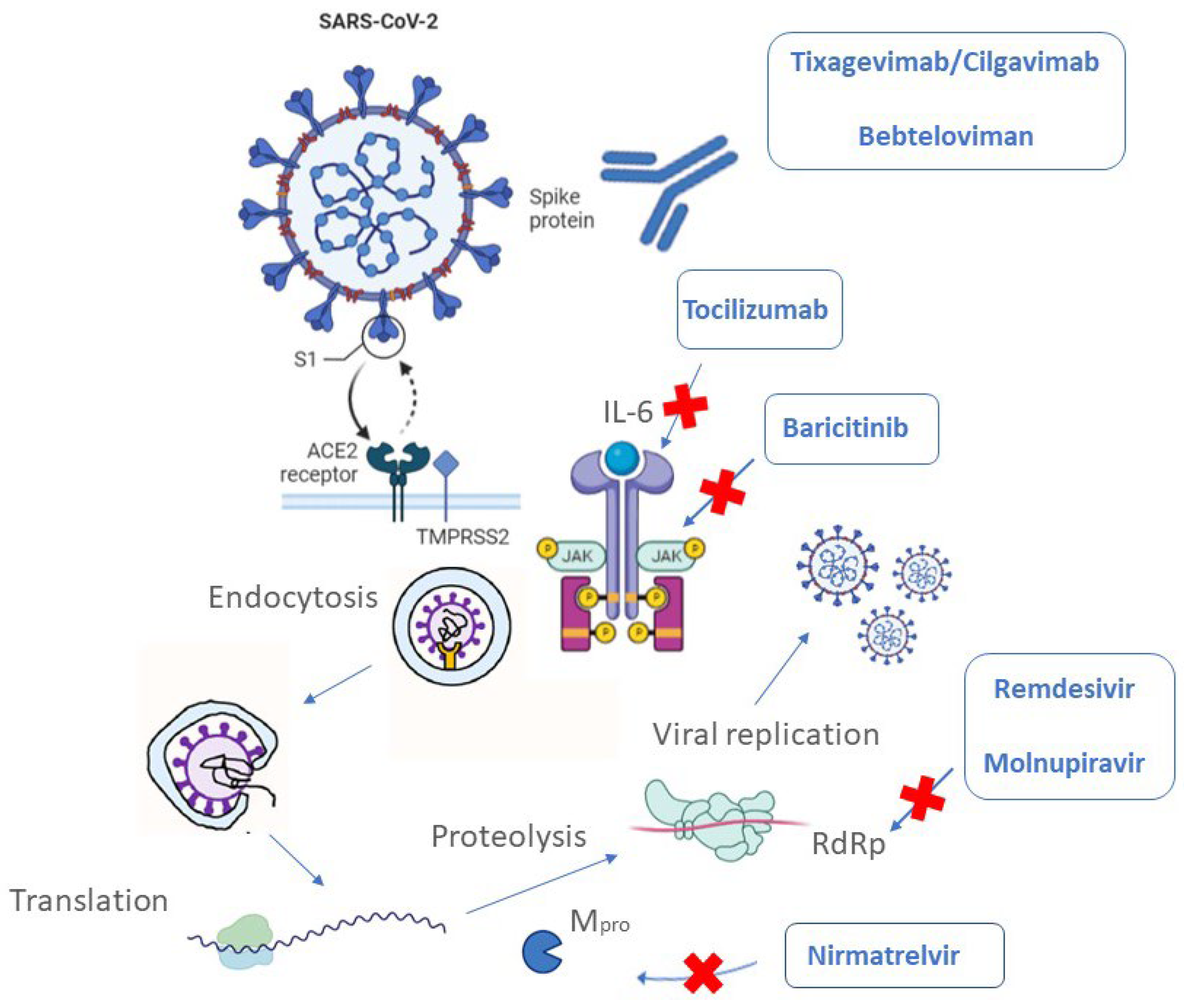
3. Therapeutic Agents Approved in COVID-19 Therapy
3.1. Monoclonal Antibodies (mAbs)
3.2. Antiviral Agents
3.2.1. RNA-Dependent RNA Polymerase (RdRp) Inhibitors
3.2.2. Protease Inhibitors
3.2.3. Drugs for COVID-19 Immune System Regulation
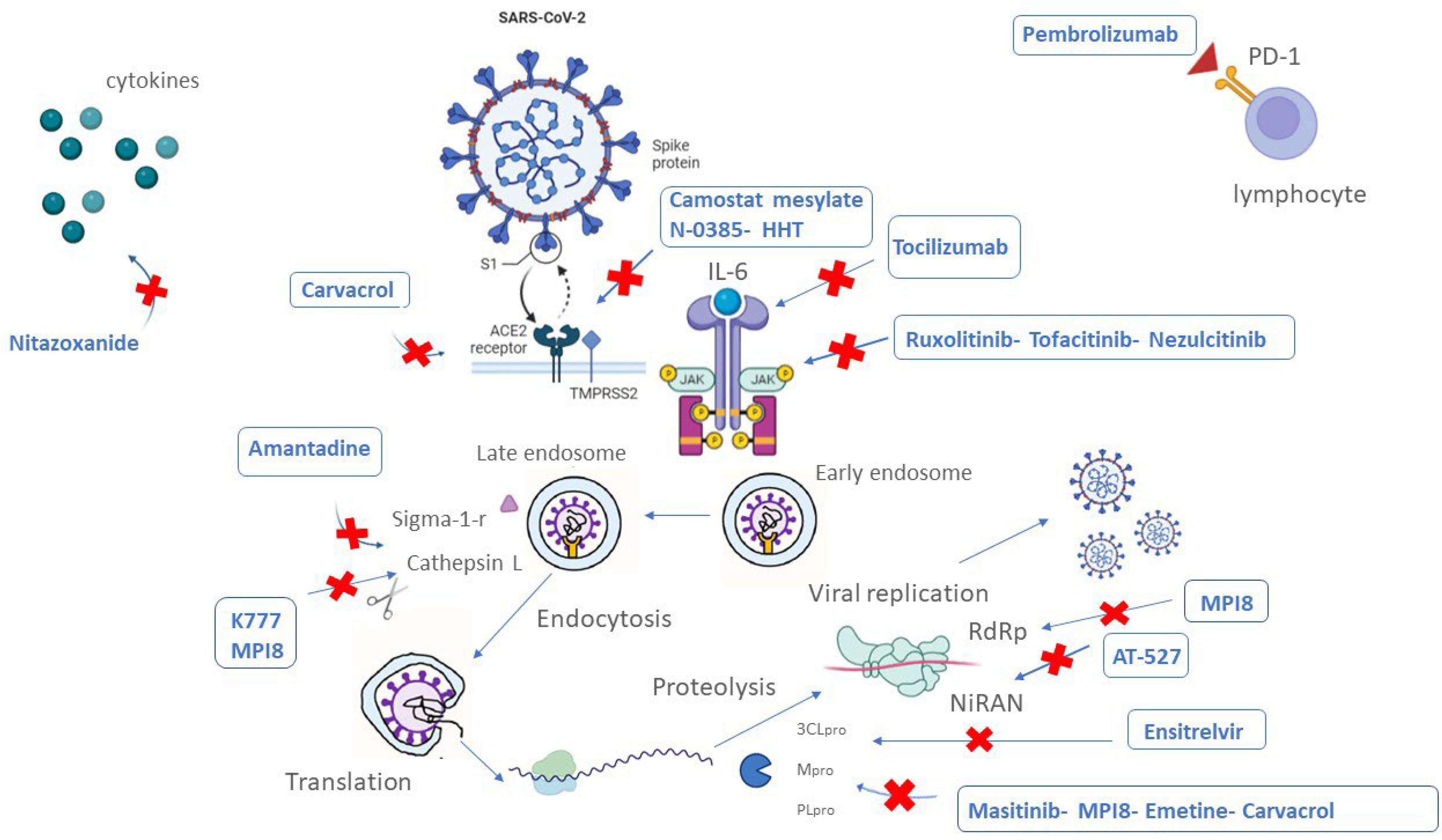
4. Therapeutic Agents in Clinical Trials
4.1. Therapeutic Agents in Phase III Clinical Trials
4.1.1. RNA-Dependent RNA Polymerase (RdRp) Inhibitors
4.1.2. Protease Inhibitors
4.1.3. Drugs for COVID-19 Immune System Regulation
4.1.4. Other Therapeutic Agents
4.2. Therapeutical Agents in Phase II Clinical Trials
4.2.1. Protease Inhibitors
4.2.2. Drugs for COVID-19 Immune System Regulation
5. Preclinical Studies
6. Natural Products and Metal-Based Drugs as Adjuvants Agents in COVID-19 Control
7. Delivery Strategies to Improve COVID-19 Therapy Management
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Center for Systems Science and Engineering. COVID-19 Dashboard Johns Hopkins University. Available online: https://coronavirus.jhu.edu/map.html (accessed on 4 October 2022). WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed on 4 October 2022).
- Elliott, P.; Bodinier, B.; Eales, O.; Wang, H.; Haw, D.; Elliott, J.; Whitaker, M.; Jonnerby, J.; Tang, D.; Walters, C.E.; et al. Rapid increase in Omicron infections in England during December 2021: REACT-1 study. Science 2022, 375, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; De Marco, A.; Ferreira, I.A.T.M.; Meng, B.; Datir, R.P.; Walls, A.C.; Kemp, S.A.; Bassi, J.; Pinto, D.; Silacci-Fregni, C.; et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 2021, 593, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Muik, A.; Lui, B.G.; Bacher, M.; Wallisch, A.-K.; Toker, A.; Finlayson, A.; Krüger, K.; Ozhelvaci, O.; Grikscheit, K.; Hoehl, S.; et al. Omicron BA.2 breakthrough infection enhances cross-neutralization of BA.2.12.1 and BA.4/BA.5. Sci. Immunol. 2022, eade2283. [Google Scholar] [CrossRef]
- Punekar, M.; Kshirsagar, M.; Tellapragada, C.; Patil, K. Repurposing of antiviral drugs for COVID-19 and impact of repurposed drugs on the nervous system. Microb. Pathog. 2022, 168, 105608. [Google Scholar] [CrossRef]
- Kato, Y.; Nishiyama, K.; Nishimura, A.; Noda, T.; Okabe, K.; Kusakabe, T.; Kanda, Y.; Nishida, M. Drug repurposing for the treatment of COVID-19. J. Pharmacol. Sci. 2022, 149, 108–114. [Google Scholar] [CrossRef]
- Marcianò, G.; Roberti, R.; Palleria, C.; Mirra, D.; Rania, V.; Casarella, A.; De Sarro, G.; Gallelli, L. SARS-CoV-2 Treatment: Current Therapeutic Options and the Pursuit of Tailored Therapy. Appl. Sci. 2021, 11, 7457. [Google Scholar] [CrossRef]
- Rahmah, L.; Abarikwu, S.O.; Arero, A.G.; Jibril, A.T.; Fal, A.; Flisiak, R.; Makuku, R.; Marquez, L.; Mohamed, K.; Ndow, L.; et al. Oral antiviral treatments for COVID-19: Opportunities and challenges. Pharmacol. Rep. 2022, 25, 1–24. [Google Scholar] [CrossRef]
- Jaworski, J.P. Neutralizing monoclonal antibodies for COVID-19 treatment and prevention. Biomed. J. 2021, 44, 7–17. [Google Scholar] [CrossRef]
- Madan, M.; Mohan, A.; Madan, K.; Hadda, V.; Tiwari, P.; Guleria, R.; Mittal, S. Timing of Anti-Viral Therapy in COVID-19: Key to Success. Adv. Respir. Med. 2021, 89, 237–239. [Google Scholar] [CrossRef]
- Moeinafshar, A.; Yazdanpanah, N.; Rezaei, N. Immune-based therapeutic approaches in COVID-19. Biomed. Pharmacother. 2022, 151, 113107. [Google Scholar] [CrossRef] [PubMed]
- Andaluz-Ojeda, D.; Vidal-Cortes, P.; Sanz, Á.A.; Suberviola, B.; Carbajo, L.D.R.; Martín, L.N.; Silva, E.P.; Del Olmo, J.N.; Barberán, J.; Cusacovich, I. Immunomodulatory therapy for the management of critically ill patients with COVID-19: A narrative review. World J. Crit. Care Med. 2022, 11, 269–297. [Google Scholar] [CrossRef]
- Gallelli, L.; D’Agostino, B.; Marrocco, G.; De Rosa, G.; Filippelli, W.; Rossi, F.; Advenier, C. Role of tachykinins in the bronchoconstriction induced by HCl intraesophageal instillation in the rabbit. Life Sci. 2003, 72, 1135–1142. [Google Scholar] [CrossRef]
- D’Agostino, B.; Advenier, C.; De Palma, R.; Gallelli, L.; Marrocco, G.; Abbate, G.F.; Rossi, F. The involvement of sensory neuropeptides in airway hyper-responsiveness in rabbits sensitized and challenged to Parietaria judaica. Clin. Exp. Allergy 2002, 32, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.; Fröhlich-Nowoisky, J.; Oppitz, N.; Ackermann, M. Cinnamon and Hop Extracts as Potential Immunomodulators for Severe COVID-19 Cases. Front. Plant Sci. 2021, 12, 589783. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Front. Immunol. 2021, 12, 637553. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Purcell, L.A.; Snell, G.; Veesler, D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell 2021, 184, 3086–3108. [Google Scholar] [CrossRef]
- Boggiano, C.; Eisinger, R.W.; Lerner, A.M.; Anderson, J.M.; Woodcock, J.; Fauci, A.S.; Collins, F.S. Update on and future directions for use of anti-SARS-CoV-2 antibodies: National Institutes of Health Summit on Treatment and Prevention of COVID-19. Ann. Intern. Med. 2022, 175, 119–126. [Google Scholar] [CrossRef]
- Fiaschi, L.; Dragoni, F.; Schiaroli, E.; Bergna, A.; Rossetti, B.; Giammarino, F.; Biba, C.; Gidari, A.; Lai, A.; Nencioni, C.; et al. Efficacy of Licensed Monoclonal Antibodies and Antiviral Agents against the SARS-CoV-2 Omicron Sublineages BA.1 and BA.2. Viruses 2022, 14, 1374. [Google Scholar] [CrossRef]
- Yamasoba, D.; Kosugi, Y.; Kimura, I.; Fujita, S.; Uriu, K.; Ito, J.; Sato, K. Genotype to Phenotype Japan (G2P-Japan) Consortium. Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies. Lancet Infect. Dis. 2022, 22, 942–943. [Google Scholar] [CrossRef]
- Razonable, R.R.; O’Horo, J.C.; Challener, D.W.; Arndt, L.; Arndt, R.F.; Clune, C.G.; Culbertson, T.L.; Hall, S.T.; Heyliger, A.; Jackson, T.A.; et al. Curbing the Delta Surge: Clinical Outcomes After Treatment With Bamlanivimab-Etesevimab, Casirivimab-Imdevimab or Sotrovimab for Mild to Moderate Coronavirus Disease-2019. Mayo Clin. Proc. 2022, 97, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, R.; Philpot, L.M.; Bierle, D.M.; Anderson, R.J.; Arndt, L.L.; Arndt, R.F.; Culbertson, T.L.; Borgen, M.J.D.; Hanson, S.N.; Kennedy, B.D.; et al. Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab Among High-Risk Patients With Mild to Moderate Coronavirus Disease 2019. J. Infect. Dis. 2021, 224, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Hayek, S.; Ben-Shlomo, Y.; Dagan, N.; Reis, B.Y.; Barda, N.; Kepten, E.; Roitman, A.; Shapira, S.; Yaron, S.; Balicer, R.D.; et al. Effectiveness of REGEN-COV antibody combination in preventing severe COVID-19 outcomes. Nat. Commun. 2022, 13, 4480. [Google Scholar] [CrossRef]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; et al. Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2. N. Engl. J. Med. 2022, 386, 1475–1477. [Google Scholar] [CrossRef]
- Self, W.H.; Sandkovsky, U.; Reilly, C.S.; Vock, D.M.; Gottlieb, R.L.; Mack, M.; Golden, K.; Dishner, E.; Vekstein, A.; Ko, E.R.; et al. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): A randomised controlled trial. Lancet Infect. Dis. 2022, 22, 622–635. [Google Scholar] [CrossRef]
- Keam, S.J. Tixagevimab + Cilgavimab: First Approval. Drugs 2022, 82, 1001–1010. [Google Scholar] [CrossRef]
- Hentzien, M.; Autran, B.; Piroth, L.; Yazdanpanah, Y.; Calmy, A. A monoclonal antibody stands out against omicron subvariants: A call to action for a wider access to bebtelovimab. Lancet Infect. Dis. 2022, 22, 1278. [Google Scholar] [CrossRef]
- Chavda, V.P.; Prajapati, R.; Lathigara, D.; Nagar, B.; Kukadiya, J.; Redwan, E.M.; Uversky, V.N.; Kher, M.N.; Patel, R. Therapeutic monoclonal antibodies for COVID-19 management: An update. Expert Opin. Biol. Ther. 2022, 22, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.V.; Piscoya, A.; Pasupuleti, V.; Phan, M.T.; Julakanti, S.; Khen, P.; Roman, Y.M.; Carranza-Tamayo, C.O.; Escobedo, A.A.; White, C.M. Beneficial and Harmful Effects of Monoclonal Antibodies for the Treatment and Prophylaxis of COVID-19: Systematic Review and Meta-Analysis. Am. J. Med. 2022, 135, 1349–1361.e18. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Sato, N.; Sakai, K.; Matsumoto, S.; Kaszynski, R.H.; Takemoto, M. Antiviral therapy for COVID-19: Derivation of optimal strategy based on past antiviral and favipiravir experiences. Pharmacol. Ther. 2022, 235, 108121. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Pang, Z.; Li, M.; Lou, F.; An, X.; Zhu, S.; Song, L.; Tong, Y.; Fan, H.; Fan, J. Molnupiravir and Its Antiviral Activity Against COVID-19. Front. Immunol. 2022, 13, 855496. [Google Scholar] [CrossRef] [PubMed]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R.; Henao-Restrepo, A.M.; Preziosi, M.P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernández García, C.; Kieny, M.P.; et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Schinazi, R.F.; Götte, M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021, 297, 100770. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Fé, L.X.S.G.M.; Cipolatti, E.P.; Pinto, M.C.C.; Branco, S.; Nogueira, F.C.S.; Ortiz, G.M.D.; Pinheiro, A.D.S.; Manoel, E.A. Enzymes in the time of COVID-19: An overview about the effects in the human body, enzyme market, and perspectives for new drugs. Med. Res. Rev. 2022, 42, 2126–2167. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Rubin, E.J.; Baden, L.R. The Potential of Intentional Drug Development. N. Engl. J. Med. 2022, 386, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Sagy, Y.W.; Hoshen, M.; Battat, E.; Lavie, G.; Sergienko, R.; Friger, M.; Waxman, J.G.; Dagan, N.; Balicer, R.; et al. Nirmatrelvir Use and Severe COVID-19 Outcomes during the Omicron Surge. N. Engl. J. Med. 2022, 387, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Charness, M.E.; Gupta, K.; Stack, G.; Strymish, J.; Adams, E.; Lindy, D.C.; Mohri, H.; Ho, D.D. Rebound of SARS-CoV-2 Infection after Nirmatrelvir–Ritonavir Treatment. N. Engl. J. Med. 2022, 387, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Carlin, A.F.; Clark, A.E.; Chaillon, A.; Garretson, A.F.; Bray, W.; Porrachia, M.; Santos, A.T.; Rana, T.M.; Smith, D.M. Virologic and Immunologic Characterization of COVID-19 Recrudescence after Nirmatrelvir/Ritonavir Treatment. Clin. Infect. Dis. 2022, ciac496. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Finch, N. New Perspectives on Antimicrobial Agents: Molnupiravir and Nirmatrelvir/Ritonavir for Treatment of COVID-19. Antimicrob. Agents Chemother. 2022, 66, e0240421. [Google Scholar] [CrossRef]
- FDA. FDA Updates on Paxlovid for Health Care Providers. 2022. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-updates-paxlovid-health-care-providers#:~:text=Yes.,Prescriber%20Patient%20Eligibility%20Screening%20Checklist (accessed on 12 September 2022).
- Mertens, C.; Darnell, J.E., Jr. SnapShot: JAK-STAT signaling. Cell 2007, 131, 612. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Plenge, R. JAK and STAT Signaling Molecules in Immunoregulation and Immune-Mediated Disease. Immunity 2012, 36, 542–550. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef]
- Bronte, V.; Ugel, S.; Tinazzi, E.; Vella, A.; De Sanctis, F.; Canè, S.; Batani, V.; Trovato, R.; Fiore, A.; Petrova, V.; et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J. Clin. Investig. 2020, 130, 6409–6416. [Google Scholar] [CrossRef]
- Cantini, F.; Niccoli, L.; Nannini, C.; Matarrese, D.; Di Natale, M.E.; Lotti, P.; Aquilini, D.; Landini, G.; Cimolato, B.; Di Pietro, M.A.; et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; Pellegrini, R.D.C.; et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- REMAP-CAP Investigators; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; Van Bentum-Puijk, W.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- Golan, Y.; Campos, J.A.S.; Woolson, R.; Cilla, D.; Hanabergh, R.; Gonzales-Rojas, Y.; Lopez, R.; Finberg, R.; Balboni, A. Favipiravir in patients with early mild-to-moderate COVID-19: A randomized controlled trial. Clin. Infect. Dis. 2022, ciac712. [Google Scholar] [CrossRef] [PubMed]
- Özlüşen, B.; Kozan, Ş.; Akcan, R.E.; Kalender, M.; Yaprak, D.; Peltek, İ.B.; Keske, Ş.; Gönen, M.; Ergönül, Ö. Effectiveness of favipiravir in COVID-19: A live systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2575–2583. [Google Scholar] [CrossRef]
- Shannon, A.; Fattorini, V.; Sama, B.; Selisko, B.; Feracci, M.; Falcou, C.; Gauffre, P.; El Kazzi, P.; Delpal, A.; Decroly, E.; et al. A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase. Nat. Commun. 2022, 13, 621. [Google Scholar] [CrossRef]
- Shimizu, R.; Sonoyama, T.; Fukuhara, T.; Kuwata, A.; Matsuo, Y.; Kubota, R. Safety, Tolerability, and Pharmacokinetics of the Novel Antiviral Agent Ensitrelvir Fumaric Acid, a SARS-CoV-2 3CL Protease Inhibitor, in Healthy Adults. Antimicrob. Agents Chemother. 2022, 66, e0063222. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, J.; Zou, L.; Jiang, T.; Wang, G.; Chen, L.; Huang, L.; Meng, F.; Huang, L.; Wang, N.; et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020, 146, 137–146.e3. [Google Scholar] [CrossRef]
- Guimarães, P.O.; Quirk, D.; Furtado, R.H.; Maia, L.N.; Saraiva, J.F.; Antunes, M.O.; Filho, R.K.; Junior, V.M.; Soeiro, A.M.; Tognon, A.P.; et al. Tofacitinib in Patients Hospitalized with COVID-19 Pneumonia. N. Engl. J. Med. 2021, 385, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.-F.; Bardin, M.C.; Fulgencio, J.; Mogelnicki, D.; Bréchot, C. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. eClinicalMedicine 2022, 45, 101310. [Google Scholar] [CrossRef] [PubMed]
- Rejdak, K.; Fiedor, P.; Bonek, R.; Goch, A.; Gala-Błądzińska, A.; Chełstowski, W.; Łukasiak, J.; Kiciak, S.; Dąbrowski, P.; Dec, M.; et al. The use of amantadine in the prevention of progression and treatment of COVID-19 symptoms in patients infected with the SARS-CoV-2 virus (COV-PREVENT): Study rationale and design. Contemp. Clin. Trials 2022, 116, 106755. [Google Scholar] [CrossRef]
- Drayman, N.; DeMarco, J.K.; Jones, K.A.; Azizi, S.-A.; Froggatt, H.M.; Tan, K.; Maltseva, N.I.; Chen, S.; Nicolaescu, V.; Dvorkin, S.; et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science 2021, 373, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Murakami, M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity 2020, 52, 731–733. [Google Scholar] [CrossRef]
- Karolyi, M.; Pawelka, E.; Omid, S.; Koenig, F.; Kauer, V.; Rumpf, B.; Hoepler, W.; Kuran, A.; Laferl, H.; Seitz, T.; et al. Camostat Mesylate Versus Lopinavir/Ritonavir in Hospitalized Patients with COVID-19—Results From a Randomized, Controlled, Open Label, Platform Trial (ACOVACT). Front. Pharmacol. 2022, 13, 870493. [Google Scholar] [CrossRef]
- Apaydın, B.; Çınar, G.; Cihan-Üstündağ, G. Small-molecule Antiviral Agents in Ongoing Clinical Trials for COVID-19. Curr. Drug Targets 2021, 22, 1986–2005. [Google Scholar] [CrossRef]
- Singh, D.; Bogus, M.; Moskalenko, V.; Lord, R.; Moran, E.J.; Crater, G.D.; Bourdet, D.L.; Pfeifer, N.D.; Woo, J.; Kaufman, E.; et al. A phase 2 multiple ascending dose study of the inhaled pan-JAK inhibitor nezulcitinib (TD-0903) in severe COVID-19. Eur. Respir. J. 2021, 58, 2100673. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A. A systematic review and meta-analysis of the association between the neutrophil, lymphocyte, and platelet count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio and COVID-19 progression and mortality. Expert Rev. Clin. Immunol. 2022, 18, 1187–1202. [Google Scholar] [CrossRef]
- Forouzani-Haghighi, B.; Rezvani, A.; Vazin, A. Immune Targeted Therapies for COVID-19 Infection: A Narrative Review. Iran. J. Med. Sci. 2022, 47, 291–299. [Google Scholar] [CrossRef]
- Bonam, S.R.; Hu, H.; Bayry, J. Role of the PD-1 and PD-L1 axis in COVID-19. Future Microbiol. 2022, 17, 985–988. [Google Scholar] [CrossRef]
- El Bairi, K.; Trapani, D.; Petrillo, A.; Le Page, C.; Zbakh, H.; Daniele, B.; Belbaraka, R.; Curigliano, G.; Afqir, S. Repurposing anticancer drugs for the management of COVID-19. Eur. J. Cancer 2020, 141, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.W.; Joshi, I.; Leal, L.; Ooi, E.E. Immune Immunomodulation in Coronavirus Disease 2019 (COVID-19): Strategic Considerations for Personalized Therapeutic Intervention. Clin. Infect. Dis. 2022, 74, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedzka-Rystwej, P.; Majchrzak, A.; Aksak-Wąs, B.; Serwin, K.; Czajkowski, Z.; Grywalska, E.; Korona-Głowniak, I.; Roliński, J.; Parczewski, M. Programmed Cell Death-1/Programmed Cell Death-1 Ligand as Prognostic Markers of Coronavirus Disease 2019 Severity. Cells 2022, 11, 1978. [Google Scholar] [CrossRef]
- Shapira, T.; Monreal, I.A.; Dion, S.P.; Buchholz, D.W.; Imbiakha, B.; Olmstead, A.D.; Jager, M.; Désilets, A.; Gao, G.; Martins, M.; et al. A TMPRSS2 inhibitor acts as a pan-SARS-CoV-2 prophylactic and therapeutic. Nature 2022, 605, 340–348. [Google Scholar] [CrossRef]
- Zhao, M.-M.; Yang, W.-L.; Yang, F.-Y.; Zhang, L.; Huang, W.-J.; Hou, W.; Fan, C.-F.; Jin, R.-H.; Feng, Y.-M.; Wang, Y.-C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target. Ther. 2021, 6, 134. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Drelich, A.; Chenna, B.C.; Mellott, D.M.; Taylor, Z.W.; Tat, V.; Garcia, C.Z.; Katzfuss, A.; Tseng, C.-T.K.; et al. Self-Masked Aldehyde Inhibitors of Human Cathepsin L Are Potent Anti-CoV-2 Agents. Front. Chem. 2022, 10, 867928. [Google Scholar] [CrossRef]
- Ma, X.R.; Alugubelli, Y.R.; Ma, Y.; Vatansever, E.C.; Scott, D.A.; Qiao, Y.; Yu, G.; Xu, S.; Liu, W.R. MPI8 is Potent against SARS-CoV-2 by Inhibiting Dually and Selectively the SARS-CoV-2 Main Protease and the Host Cathepsin, L. ChemMedChem 2022, 17, e202100456. [Google Scholar] [CrossRef]
- Gomes, C.P.; Fernandes, D.E.; Casimiro, F.; Da Mata, G.F.; Passos, M.T.; Varela, P.; Mastroianni-Kirsztajn, G.; Pesquero, J.B. Cathepsin L in COVID-19: From Pharmacological Evidences to Genetics. Front. Cell. Infect. Microbiol. 2020, 10, 589505. [Google Scholar] [CrossRef]
- Smieszek, S.P.; Przychodzen, B.P.; Polymeropoulos, M.H. Amantadine disrupts lysosomal gene expression: A hypothesis for COVID19 treatment. Int. J. Antimicrob. Agents 2020, 55, 106004. [Google Scholar] [CrossRef]
- Tedesco, F.; Calugi, L.; Lenci, E.; Trabocchi, A. Peptidomimetic Small-Molecule Inhibitors of 3CLPro Activity and Spike–ACE2 Interaction: Toward Dual-Action Molecules against Coronavirus Infections. J. Org. Chem. 2022, 87, 12041–12051. [Google Scholar] [CrossRef] [PubMed]
- Boozari, M.; Hosseinzadeh, H. Natural products for COVID -19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother. Res. 2021, 35, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wen, H.; Qin, Y.; Wu, S.; Zhang, G.; Wu, C.-I.; Cai, Q. Homo-harringtonine, highly effective against coronaviruses, is safe in treating COVID-19 by nebulization. Sci. China Life Sci. 2022, 65, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, B.L.; de Oliveira, N.C.; Ritter, M.R.; Tonin, F.S.; Melo, E.B.; Sanches, A.C.C.; Fernandez-Llimos, F.; Petruco, M.V.; de Mello, J.C.P.; Chierrito, D.; et al. The naturally-derived alkaloids as a potential treatment for COVID -19: A scoping review. Phytother. Res. 2022, 36, 2686–2709. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Cossette, M.; Guertin, M.C.; Bouabdallaoui, N.; Dubé, M.P.; Boivin, G. Colcorona study group. Predictive risk factors for hospitalization and response to colchicine in patients with COVID-19. Int. J. Infect. Dis. 2022, 116, 387–390. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Bouabdallaoui, N.; L’Allier, P.L.; Gaudet, D.; Shah, B.; Pillinger, M.H.; Lopez-Sendon, J.; da Luz, P.; Verret, L.; Audet, S.; et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): A phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir. Med. 2021, 9, 924–932. [Google Scholar] [CrossRef]
- Fan, S.; Zhen, Q.; Chen, C.; Wang, W.; Wu, Q.; Ma, H.; Zhang, C.; Zhang, L.; Lu, B.; Ge, H.; et al. Clinical efficacy of low-dose emetine for patients with COVID-19: A real-world study. J. Bio-X Res. 2021, 4, 53–59. [Google Scholar] [CrossRef]
- Javed, H.; Meeran, M.F.N.; Jha, N.K.; Ojha, S. Carvacrol, a Plant Metabolite Targeting Viral Protease (Mpro) and ACE2 in Host Cells Can Be a Possible Candidate for COVID-19. Front. Plant Sci. 2021, 11, 601335. [Google Scholar] [CrossRef]
- Nazıroğlu, M. A novel antagonist of TRPM2 and TRPV4 channels: Carvacrol. Metab. Brain Dis. 2022, 37, 711–728. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Turning the Tide: Natural Products and Natural-Product-Inspired Chemicals as Potential Counters to SARS-CoV-2 Infection. Front. Pharmacol. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ye, J.; Jia, M.; Li, X.; Wei, S.; Li, N. The common regulatory pathway of COVID-19 and multiple inflammatory diseases and the molecular mechanism of cepharanthine in the treatment of COVID-19. Front. Pharmacol. 2022, 13, 960267. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Guarner-Lans, V.; Soria-Castro, E.; Pech, L.M.; Pérez-Torres, I. Is Antioxidant Therapy a Useful Complementary Measure for COVID-19 Treatment? An Algorithm for Its Application. Medicina 2020, 56, 386. [Google Scholar] [CrossRef] [PubMed]
- Kuck, J.L.; Bastarache, J.A.; Shaver, C.M.; Fessel, J.P.; Dikalov, S.I.; May, J.M.; Ware, L.B. Ascorbic acid attenuates endothelial permeability triggered by cell-free hemoglobin. Biochem. Biophys. Res. Commun. 2018, 495, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, G.; Coppola, A.; Izzo, R.; Annunziata, A.; Bernardo, M.; Lombardi, A.; Trimarco, V.; Santulli, G.; Trimarco, B. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: A randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. eClinicalMedicine 2021, 40, 101125. [Google Scholar] [CrossRef]
- Izzo, R.; Trimarco, V.; Mone, P.; Aloè, T.; Marzani, M.C.; Diana, A.; Fazio, G.; Mallardo, M.; Maniscalco, M.; Marazzi, G.; et al. Combining L-Arginine with vitamin C improves long-COVID symptoms: The LINCOLN Survey. Pharmacol. Res. 2022, 183, 106360. [Google Scholar] [CrossRef]
- Li, H.; Yuan, S.; Wei, X.; Sun, H. Metal-based strategies for the fight against COVID-19. Chem. Commun. 2022, 58, 7466–7482. [Google Scholar] [CrossRef]
- Rayman, M.P.; Taylor, E.W.; Zhang, J. The relevance of selenium to viral disease with special reference to SARS-CoV-2 and COVID-19. Proc. Nutr. Soc. 2022, 1–12. [Google Scholar] [CrossRef]
- Weglarz-Tomczak, E.; Tomczak, J.M.; Talma, M.; Burda-Grabowska, M.; Giurg, M.; Brul, S. Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Sci. Rep. 2021, 11, 3640. [Google Scholar] [CrossRef]
- Naseef, P.P.; Elayadeth-Meethal, M.; Salim, K.M.; Anjana, A.; Muhas, C.; Vajid, K.A.; Kuruniyan, M.S. Therapeutic potential of induced iron depletion using iron chelators in COVID-19. Saudi J. Biol. Sci. 2022, 29, 1947–1956. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Deferiprone: A Forty-Year-Old Multi-Targeting Drug with Possible Activity against COVID-19 and Diseases of Similar Symptomatology. Int. J. Mol. Sci. 2022, 23, 6735. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.; Aali, M.; Zhou, J.; Holbein, B. Comparison of Treatment Effects of Different Iron Chelators in Experimental Models of Sepsis. Life 2021, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Liparulo, A.; Esposito, R.; Santonocito, D.; Muñoz-Ramírez, A.; Spaziano, G.; Bruno, F.; Xiao, J.; Puglia, C.; Filosa, R.; Berrino, L.; et al. Formulation and Characterization of Solid Lipid Nanoparticles Loading RF22-c, a Potent and Selective 5-LO Inhibitor, in a Monocrotaline-Induced Model of Pulmonary Hypertension. Front. Pharmacol. 2020, 11, 83. [Google Scholar] [CrossRef]
- Mirra, D.; Spaziano, G.; Esposito, R.; Santonocito, D.; Filosa, R.; Roviezzo, F.; Malgieri, G.; D’Abrosca, G.; Iovino, P.; Gallelli, L.; et al. Formulation of Solid Lipid Nanoparticles Loaded with Nociceptin/Orphanin FQ (N/OFQ) and Characterization in a Murine Model of Airway Hyperresponsiveness. Pharmaceuticals 2022, 15, 1210. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, B.; Orlotti, D.; Calò, G.; Sullo, N.; Russo, M.; Guerrini, R.; De Nardo, M.; Mazzeo, F.; Candeletti, S.; Rossi, F. Nociceptin Modulates Bronchoconstriction Induced by Sensory Nerve Activation in Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2010, 42, 250–254. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, B.; Marrocco, G.; De Nardo, M.; Calò, G.; Guerrini, R.; Gallelli, L.; Advenier, C.; Rossi, F. Activation of the nociceptin/orphanin FQ receptor reduces bronchoconstriction and microvascular leakage in a rabbit model of gastroesophageal reflux. Br. J. Pharmacol. 2005, 144, 813–820. [Google Scholar] [CrossRef]
- Rouget, C.; Cui, Y.Y.; D’Agostino, B.; Faisy, C.; Naline, E.; Bardou, M.; Advenier, C. Nociceptin inhibits airway microvascular leakage induced by HCl intra-oesophageal instillation. Br. J. Pharmacol. 2004, 141, 1077–1183. [Google Scholar] [CrossRef]
- Tartaglione, G.; Spaziano, G.; Sgambato, M.; Russo, T.P.; Liparulo, A.; Esposito, R.; Mirra, S.; Filosa, R.; Roviezzo, F.; Polverino, F.; et al. Nociceptin/Orphanin Fq in inflammation and remodeling of the small airways in experimental model of airway hyperresponsiveness. Physiol. Rep. 2018, 6, e13906. [Google Scholar] [CrossRef]
- Esposito, R.; Spaziano, G.; Giannattasio, D.; Ferrigno, F.; Liparulo, A.; Rossi, A.; Roviezzo, F.; Sessa, M.; Falciani, M.; Berrino, L.; et al. Montelukast Improves Symptoms and Lung Function in Asthmatic Women Compared With Men. Front. Pharmacol. 2019, 10, 1094. [Google Scholar] [CrossRef]
- D’Agostino, B.; Sgambato, M.; Esposito, R.; Spaziano, G. N/OFQ-NOP System and Airways. Handb. Exp. Pharmacol. 2019, 254, 313–322. [Google Scholar] [CrossRef]
- Vartak, R.; Patil, S.M.; Saraswat, A.; Patki, M.; Kunda, N.K.; Patel, K. Aerosolized nanoliposomal carrier of remdesivir: An effective alternative for COVID-19 treatment in vitro. Nanomedicine 2021, 16, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.; Tawfeek, H.M.; Abdelfattah, A.; Batiha, G.E.-S.; Hetta, H.F. Recent updates in COVID-19 with emphasis on inhalation therapeutics: Nanostructured and targeting systems. J. Drug Deliv. Sci. Technol. 2021, 63, 102435. [Google Scholar] [CrossRef] [PubMed]
- Erelel, M.; Kaskal, M.; Akbal-Dagistan, O.; Issever, H.; Dagistanli, A.S.; Balkanci, H.; Oguz, M.S.; Qarayeva, A.; Culha, M.; Erturk, A.; et al. Early Effects of Low Molecular Weight Heparin Therapy with Soft-Mist Inhaler for COVID-19-Induced Hypoxemia: A Phase IIb Trial. Pharmaceutics 2021, 13, 1768. [Google Scholar] [CrossRef]
- van Haren, F.M.; Richardson, A.; Yoon, H.; Artigas, A.; Laffey, J.G.; Dixon, B.; Smith, R.; Vilaseca, A.B.; Barbera, R.A.; Ismail, T.I.; et al. INHALEd nebulised unfractionated HEParin for the treatment of hospitalised patients with COVID-19 (INHALE-HEP): Protocol and statistical analysis plan for an investigator-initiated international metatrial of randomised studies. Br. J. Clin. Pharmacol. 2021, 87, 3075–3091. [Google Scholar] [CrossRef]
- Van Haren, F.M.P.; Page, C.; Laffey, J.G.; Artigas, A.; Camprubi-Rimblas, M.; Nunes, Q.; Smith, R.; Shute, J.; Carroll, M.; Tree, J.; et al. Nebulised heparin as a treatment for COVID-19: Scientific rationale and a call for randomised evidence. Crit. Care 2020, 24, 454. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Huang, B.; Zhao, L.; Deng, Y.; Ren, J.; Tan, W. Novaferon Effectively Inhibits Ancestral SARS-CoV-2 and Omicron Variant inVitro, 2022. China CDC Wkly. 2022, 4, 509–512. [Google Scholar] [CrossRef] [PubMed]
- NIH. Phase 3 Inhaled Novaferon Study in Hospitalized Patients with Moderate to Severe COVID-19 (NOVATION-1). Available online: https://clinicaltrials.gov/ct2/show/NCT04669015 (accessed on 17 September 2022).
| Drug | Target | NCT | Clinical Status | Study Design | Treatment | Primary Outcome |
|---|---|---|---|---|---|---|
| Favipiravir | RdRp | NCT04600895 | Phase III Completed | Double-blinded, randomized study | Favipiravir vs. Placebo | Time (0–28 days) to sustained clinical recovery. The endpoint will be positive when the subject has reached sustained alleviation of symptoms. |
| AT-527 | RdRp/NiRAN | NCT04889040 | Phase III Terminated | Multicenter, randomized, double-blind study | AT-527 vs. Placebo | Time (0–29 days) to alleviation or improvement of COVID-19 symptoms, evaluated by COVID-19 Symptom Diary. |
| S-217622 (Ensitrelvir) | 3C-like protease | NCT05305547 | Phase III Recruiting | Multicenter, randomized, double-blind, 24-week study | S-217622 vs. placebo | Median time (0–29 days) to sustained symptom resolution. |
| Amantadine | Cathepsin L Agonism of Sigma-1 receptors Modulation of neurotransmitters | NCT04854759 | Phase III Recruiting | Multicenter randomized, double-blind, non-commercial study | Amantadine Hydrochloride vs. Placebo | Development of clinical deterioration (0–15 days), defined as dyspnoea, physical examination, doctor’s assessment; Clinical deterioration occurs, defined as drop in O2 saturation and achievement of ≥4 points on the WHO scale. |
| Amantadine | Cathepsin L Agonism of Sigma-1 receptorsModulation of neurotransmitters | NCT04894617 | Phase III Recruiting | Randomized, double-blinded, placebo-controlled, single center study | Amantadine vs. Lactose monohydrate | Clinical status on day 14 according to 8 point ordinal scale for clinical improvement. |
| Amantadine | Cathepsin L Agonism of Sigma-1 receptors Modulation of neurotransmitters | NCT04952519 | Phase III Recruiting | Randomized, parallel assignment, triple-blinded study | Amantadine vs. Placebo | Time to recovery (defined as the first day during the 28 day clinical follow-up during which the patient’s clinical condition is graded 1, 2 or 3 on an eight-point “Normal Symptom Score”) |
| Amantadine | Cathepsin L Agonism of Sigma-1 receptorsModulation of neurotransmitters | NCT05504057 | Phase IV Recruiting | Observational, case–control, Retrospective study | Amantadine vs. Antihistamine | Rate of hospital admissions (time frame: from March 2020) |
| Ruxolitinib | JAK1/JAK2 | NCT04377620 | Phase III Terminated | Randomized, double-blind, multicenter study | Ruxolitinib vs Placebo | Overall mortality (percentage of participants who have died due to any cause during a time frame from 0 day to day 29). |
| Tofacitinib | JAK2 | NCT04469114 | Phase III Completed | Multicenter, randomized, double-blinded, parallel-design study | Tofacitinib: vs. Placebo | Death or respiratory failure * until day 28 |
| Nitazoxanide | - inhibition of inflammatory cytokines - induction of interferon-stimulated viral gene expression. | NCT04486313 | Phase III Completed | Multicenter, randomized, double-blinded study | Nitazoxanide vs. Placebo | Reducing the Time to Sustained Response (0–21 days) |
| Masitinib | Mpro | NCT05047783 | Phase II Recruiting | Randomized, double-blinded, placebo-controlled study | Masitinib Mesylate Vs. Palcebo | SARS-Cov-2 viral load at day 10 (time-weighted average change from baseline in viral shedding) |
| Camostat mesylate | TMPRSS2 | NCT04351724 | Phase II/III Recruiting | Randomized, controlled, multicenter, open-label basket study | Camostat mesylate vs. Lopinavir/Ritonavir | Clinical improvement defined as time from randomization to an improvement of at least one category measured on a seven-category ordinal scale (proposed by WHO). |
| BLD-2660 | calpain (CAPN) 1, 2, and 9 | NCT04334460 | Phase II | Randomized, double-blinded study | BLD-2660 vs. Placebo | Time to Recovery (0–28 days) defined by no longer requiring oxygen support or hospital discharge, whichever occurs first. |
| Nezulcitinib | pan-JAK inhibitor | NCT04402866 | Phase II Completed | Randomized, double-blinded, parallel-group, multicenter study | Nezulcitinib vs. Placebo | Number of Respiratory Failure-free Days (RFDs) from randomization to day 28. An RFD was defined as a day that a participant was alive and did not require the use of any respiratory support. |
| Pembrolizumab/Tocilizumab | PD-1/IL-6 | NCT04335305 | Phase II Terminated | Randomized, controlled, open-label study | Pembrolizumab/Tocilizumab vs. SOC | Percentage of patients with normalization of SpO2 ≥ 96% on room air (time frame from treatment initiation to day 14) assessed by hospital records. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, R.; Mirra, D.; Sportiello, L.; Spaziano, G.; D’Agostino, B. Overview of Antiviral Drug Therapy for COVID-19: Where Do We Stand? Biomedicines 2022, 10, 2815. https://doi.org/10.3390/biomedicines10112815
Esposito R, Mirra D, Sportiello L, Spaziano G, D’Agostino B. Overview of Antiviral Drug Therapy for COVID-19: Where Do We Stand? Biomedicines. 2022; 10(11):2815. https://doi.org/10.3390/biomedicines10112815
Chicago/Turabian StyleEsposito, Renata, Davida Mirra, Liberata Sportiello, Giuseppe Spaziano, and Bruno D’Agostino. 2022. "Overview of Antiviral Drug Therapy for COVID-19: Where Do We Stand?" Biomedicines 10, no. 11: 2815. https://doi.org/10.3390/biomedicines10112815
APA StyleEsposito, R., Mirra, D., Sportiello, L., Spaziano, G., & D’Agostino, B. (2022). Overview of Antiviral Drug Therapy for COVID-19: Where Do We Stand? Biomedicines, 10(11), 2815. https://doi.org/10.3390/biomedicines10112815





