How the Immune System Responds to Allergy Immunotherapy
Abstract
1. Introduction
2. Pathogenetic Mechanisms of Allergic Response
3. Alterations of Innate and Adaptive Immunity Induced by AIT
3.1. The Response of B-Cell Compartment to AIT
3.2. AIT-Induced Immunomodulation of T Cell Response
3.2.1. Immune-Deviation
3.2.2. Immune-Regulation
3.3. AIT-Induced Modulation of Innate Lymphoid Cells
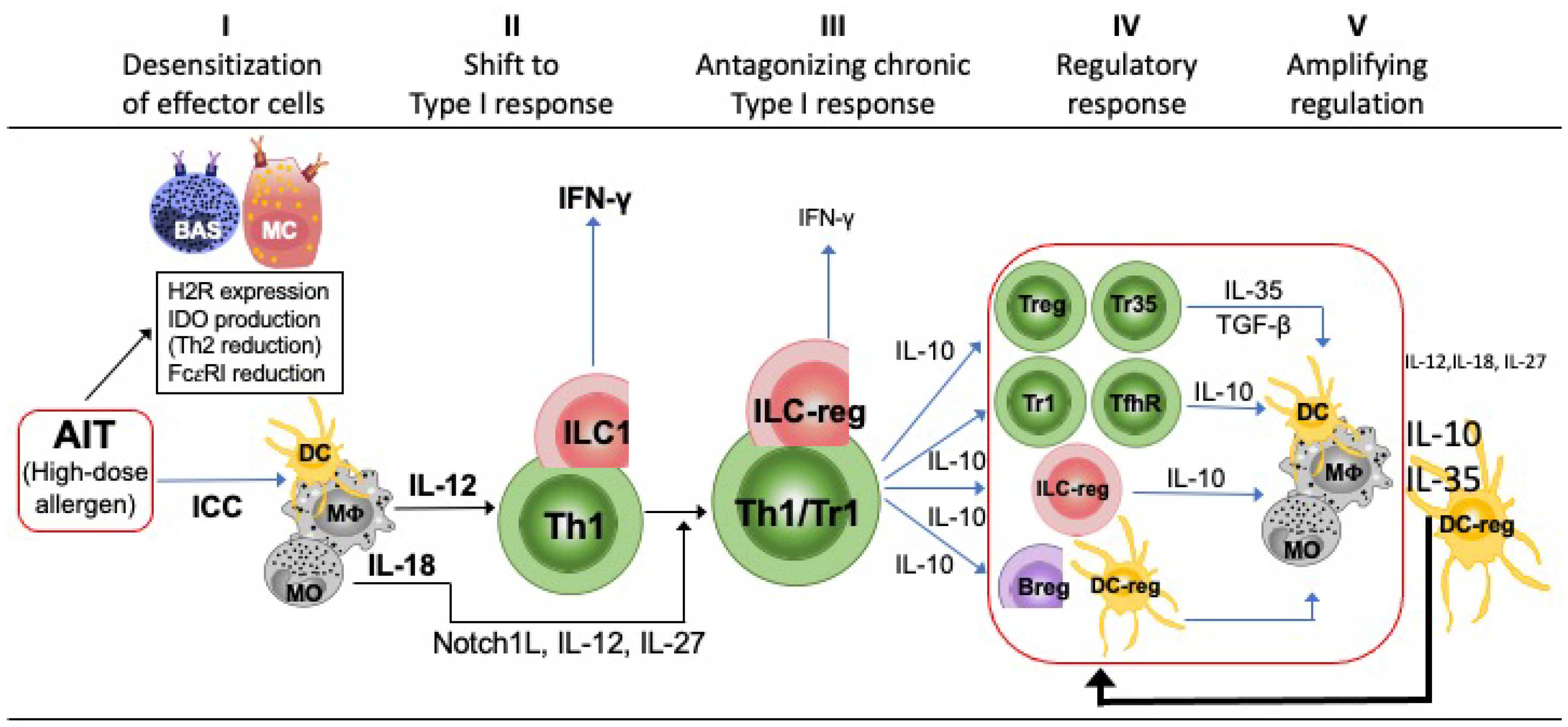
4. AIT-Induced Immune Deviation and Immune Regulation Are Two Related and Sequential Phases of the Chronic Stimulation with Allergen
5. Causes of Failure, Biomarkers of Efficacy, and New Strategies to Optimize AIT
5.1. Causes of AIT Failure
5.2. Biomarkers of Efficacy
5.3. Strategies to Optimize AIT
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Durham, S.R.; Varney, V.A.; Gaga, M.; Jacobson, M.R.; Varga, E.M.; Frew, A.J.; Kay, A.B. Grass pollen immunotherapy decreases the number of mast cells in the skin. Clin. Exp. Allergy 1999, 29, 1490–1496. [Google Scholar] [CrossRef]
- Ring, J.; Gutermuth, J. 100 years of hyposensitization: History of allergen-specific immunotherapy (ASIT). Allergy 2011, 66, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Maggi, E. T cell responses induced by allergen-specific immunotherapy. Clin. Exp. Immunol. 2010, 161, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Wraith, D.C.; Krishna, M.T. Peptide allergen-specific immunotherapy for allergic airway diseases—State of the art. Clin. Exp. Allergy 2021, 51, 751–769. [Google Scholar] [CrossRef]
- Passalacqua, G.; Canonica, G.W.; Bagnasco, D. Benefit of SLIT and SCIT for Allergic Rhinitis and Asthma. Curr. Allergy Asthma Rep. 2016, 16, 88. [Google Scholar] [CrossRef]
- Ozdemir, C.; Kucuksezer, U.C.; Akdis, M.; Akdis, C.A. Mechanisms of Aeroallergen Immunotherapy: Subcutaneous Immunotherapy and Sublingual Immunotherapy. Immunol. Allergy Clin. N. Am. 2016, 36, 71–86. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, Q.; He, C.; Chen, R.; Tang, Y.; Yan, S.; Luo, X.; Luo, R. Compliance, efficacy, and safety of subcutaneous and sublingual immunotherapy in children with allergic rhinitis. Pediatr. Allergy Immunol. 2021, 32, 86–91. [Google Scholar] [CrossRef]
- Tophof, M.A.; Hermanns, A.; Adelt, T.; Eberle, P.; Gronke, C.; Friedrichs, F.; Knecht, R.; Monter, E.; Schopfer, H.; Schwerk, N.; et al. Side effects during subcutaneous immunotherapy in children with allergic diseases. Pediatr. Allergy Immunol. 2018, 29, 267–274. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, D.; Huang, N.; Li, W.; Jiang, Q.; Wang, Y.; Wang, X.; Yang, L.; Zhu, R. Safety of house dust mite subcutaneous immunotherapy in preschool children with respiratory allergic diseases. Ital. J. Pediatr. 2021, 47, 101. [Google Scholar] [CrossRef]
- Pitsios, C.; Demoly, P.; Bilò, M.B.; Gerth van Wijk, R.; Pfaar, O.; Sturm, G.J.; Rodriguez del Rio, P.; Tsoumani, M.; Gawlik, R.; Paraskevopoulos, G.; et al. Clinical contraindications to allergen immunotherapy: An EAACI position paper. Allergy 2015, 70, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Taudorf, E.; Laursen, L.C.; Djurup, R.; Kappelgaard, E.; Pedersen, C.T.; Søsborg, M.; Wilkinson, P.; Weeke, B. Oral Administration of Grass Pollen to Hay Fever Patients. Allergy 1985, 40, 321–335. [Google Scholar] [CrossRef]
- Huang, H.-J.; Resch-Marat, Y.; Rodriguez-Dominguez, A.; Chen, K.-W.; Kiss, R.; Zieglmayer, P.; Zieglmayer, R.; Lemell, P.; Horak, F.; Valenta, F.; et al. Underestimation of house dust mite–specific IgE with extract-based ImmunoCAPs compared with molecular ImmunoCAPs. J. Allergy Clin. Immunol. 2018, 142, 1656–1659.e9. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Patel, C.; Burks, A.W. Immunotherapy approaches for peanut allergy. Expert Rev. Clin. Immunol. 2020, 16, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Rodríguez, R.; Batanero, E. The spectrum of olive pollen allergens. From structures to diagnosis and treatment. Methods 2014, 66, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Dorofeeva, Y.; Shilovskiy, I.; Tulaeva, I.; Focke-Tejkl, M.; Flicker, S.; Kudlay, D.; Khaitov, M.; Karsonova, A.; Riabova, K.; Karaulov, A.; et al. Past, present, and future of allergen immunotherapy vaccines. Allergy 2021, 76, 131–149. [Google Scholar] [CrossRef]
- Ma, T.; Wang, X.; Zhuang, Y.; Shi, H.; Ning, H.; Lan, T.; Zhang, T.; Kang, Z.; SiQin, B.; Yang, B.; et al. Prevalence and risk factors for allergic rhinitis in adults and children living in different grassland regions of Inner Mongolia. Allergy 2020, 75, 234–239. [Google Scholar] [CrossRef]
- Penagos, M.; Durham, S.R. Duration of allergen immunotherapy for inhalant allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 594–605. [Google Scholar] [CrossRef]
- Victor, J.R.; Fusaro, A.E.; da Silva Duarte, A.J.; Sato, M.N. Preconception maternal immunization to dust mite inhibits the type I hypersensitivity response of offspring. J. Allergy Clin. Immunol. 2003, 111, 269–277. [Google Scholar] [CrossRef]
- Barnes, P.J. Therapeutic approaches to asthma–chronic obstructive pulmonary disease overlap syndromes. J. Allergy Clin. Immunol. 2015, 136, 531–545. [Google Scholar] [CrossRef]
- Gregory, L.G.; Lloyd, C.M. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011, 32, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021, executive summary and rationale for key changes. Eur. Respir. J. 2022, 59, 2102730. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, L.O.; Ribeiro, A.M.; Lopes, F.D.; Tibério, I.D.; Tavares-de-Lima, W.; Prado, C. Different Phenotypes in Asthma: Clinical Findings and Experimental Animal Models. Clin. Rev. Allergy Immunol. 2022, 62, 240–263. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Corren, J. Asthma phenotypes and endotypes: An evolving paradigm for classification. Discov. Med. 2013, 15, 243–249. [Google Scholar]
- Lötvall, J.; Akdis, C.A.; Bacharier, L.B.; Bjermer, L.; Casale, T.B.; Custovic, A.; Lemanske Jr., R.F.; Wardlaw, A.J.; Wenzel, S.E.; Greenberger, P.A. Asthma endotypes: A new approach to classification of disease entities within the asthma syndrome. J. Allergy Clin. Immunol. 2011, 127, 355–360. [Google Scholar] [CrossRef]
- Wenzel, S. Severe asthma: From characteristics to phenotypes to endotypes. Clin. Exp. Allergy 2012, 42, 650–658. [Google Scholar] [CrossRef]
- Muraro, A.; Lemanske, R.F.; Hellings, P.W.; Akdis, C.A.; Bieber, T.; Casale, T.B.; Jutel, M.; Ong, P.Y.; Poulsen, L.K.; Schmid-Grendelmeier, P.; et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis—PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2016, 137, 1347–1358. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A. Endotypes of allergic diseases and asthma: An important step in building blocks for the future of precision medicine. Allergol. Int. 2016, 65, 243–252. [Google Scholar] [CrossRef]
- Gomulka, K.; Liebhart, J.; Jaskula, E.; Lange, A.; Medrala, W. The -2549 -2567 Del18 Polymorphism in VEGF and Irreversible Bronchoconstriction in Asthmatics. J. Investig. Allergol. Clin. Immunol. 2019, 29, 431–435. [Google Scholar] [CrossRef]
- Ober, C.; Nicolae, D.L. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat. Genet. 2011, 43, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.-F.; Rosenwasser, L.J. Unraveling the genetic basis of asthma and allergic diseases. Allergy Asthma Immunol. Res. 2010, 2, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Ntontsi, P.; Photiades, A.; Zervas, E.; Xanthou, G.; Samitas, K. Genetics and Epigenetics in Asthma. Int. J. Mol. Sci. 2021, 22, 2412. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, J.A. Genetics/epigenetics/allergy: The gun is loaded … but what pulls the trigger? Allergy Asthma Proc. 2019, 40, 76–83. [Google Scholar] [CrossRef]
- Bélanger, É.; Laprise, C. Could the Epigenetics of Eosinophils in Asthma and Allergy Solve Parts of the Puzzle? Int. J. Mol. Sci. 2021, 22, 8921. [Google Scholar] [CrossRef]
- Mazzone, R.; Zwergel, C.; Artico, M.; Taurone, S.; Ralli, M.; Greco, A.; Mai, A. The emerging role of epigenetics in human autoimmune disorders. Clin. Epigenetics 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Willis-Owen, S.A.G.; Laprise, C.; Wong, K.C.C.; Davies, G.A.; Hudson, T.J.; Binia, A.; Hopkin, J.M.; Yang, I.V.; Grundberg, E.; et al. An epigenome-wide association study of total serum immunoglobulin E concentration. Nature 2015, 520, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Cobos, F.A.; Schleich, F.; Sorbello, V.; Henket, M.; de Preter, K.; Bracke, K.R.; Conickx, G.; Mesnil, C.; Vandesompele, J.; et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J. Allergy Clin. Immunol. 2016, 137, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Chen, A.; Diaz, M.P.; Zirn, N.; Gupta, A.; Britto, C.; Sauler, M.; Yan, X.; Stewart, E.; Santerian, K.; et al. A Network of Sputum MicroRNAs Is Associated with Neutrophilic Airway Inflammation in Asthma. Am. J. Respir. Crit. Care Med. 2020, 202, 51–64. [Google Scholar] [CrossRef]
- Kabesch, M.; Tost, J. Recent findings in the genetics and epigenetics of asthma and allergy. Semin. Immunopathol. 2020, 42, 43–60. [Google Scholar] [CrossRef]
- Burrows, B.; Martinez, F.D.; Halonen, M.; Barbee, R.A.; Cline, M.G. Association of Asthma with Serum IgE Levels and Skin-Test Reactivity to Allergens. N. Engl. J. Med. 1989, 320, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Murrison, L.B.; Brandt, E.B.; Myers, J.B.; Hershey, G.K.K. Environmental exposures and mechanisms in allergy and asthma development. J. Clin. Investig. 2019, 129, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Landolina, N.; Gangwar, R.S.; Levi-Schaffer, F. Mast Cells’ Integrated Actions with Eosinophils and Fibroblasts in Allergic Inflammation. Adv. Immunol. 2015, 125, 41–85. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C. IL-33, an Alarmin of the IL-1 Family Involved in Allergic and Non Allergic Inflammation: Focus on the Mechanisms of Regulation of Its Activity. Cells 2021, 11, 107. [Google Scholar] [CrossRef]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.-C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef]
- Rahimi, R.A.; Nepal, K.; Cetinbas, M.; Sadreyev, R.I.; Luster, A.D. Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease. J. Exp. Med. 2020, 217, e20190865. [Google Scholar] [CrossRef]
- Gowthaman, U.; Chen, J.S.; Eisenbarth, S.C. Regulation of IgE by T follicular helper cells. J. Leukoc. Biol. 2020, 107, 409–418. [Google Scholar] [CrossRef]
- Nakamura, T. The roles of lipid mediators in type I hypersensitivity. J. Pharmacol. Sci. 2021, 147, 126–131. [Google Scholar] [CrossRef]
- Pastwińska, J.; Żelechowska, P.; Walczak-Drzewiecka, A.; Brzezińska-Błaszczyk, E.; Dastych, J. The Art of Mast Cell Adhesion. Cells 2020, 9, 2664. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M.; Kayagaki, N. Dying cells fan the flames of inflammation. Science 2021, 374, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Wambre, E.; Bajzik, V.; DeLong, J.H.; O’Brien, K.; Nguyen, Q.-A.; Speake, C.; Gersuk, V.H.; Deberg, H.A.; Whalen, E.; Ni, C.; et al. A phenotypically and functionally distinct human T H 2 cell subpopulation is associated with allergic disorders. Sci. Transl. Med. 2017, 9, eaam9171. [Google Scholar] [CrossRef] [PubMed]
- Bertschi, N.L.; Bazzini, C.; Schlapbach, C. The Concept of Pathogenic TH2 Cells: Collegium Internationale Allergologicum Update 2021. Int. Arch. Allergy. Immunol. 2021, 182, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Calise, J.; Garabatos, N.; Bajzik, V.; Farrington, M.; Robinson, D.; Jeong, D.; Londei, M.; Wambre, E. Optimal human pathogenic TH2 cell effector function requires local epithelial cytokine signaling. J. Allergy Clin. Immunol. 2021, 148, 867–875.e4. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Noguchi, E.; Imoto, Y.; Nanatsue, K.; Takeshita, K.; Shibasaki, M.; Arinami, T.; Fujieda, S. Upregulation of IL17RB during Natural Allergen Exposure in Patients with Seasonal Allergic Rhinitis. Allergol. Int. 2011, 60, 87–92. [Google Scholar] [CrossRef]
- Monian, B.; Tu, A.A.; Ruiter, B.; Morgan, D.M.; Petrossian, P.M.; Smith, N.P.; Gierahn, T.M.; Ginder, J.H.; Shreffler, W.G.; Love, J.C. Peanut oral immunotherapy differentially suppresses clonally distinct subsets of T helper cells. J. Clin. Investig. 2022, 132, e150634. [Google Scholar] [CrossRef]
- Matucci, A.; Bormioli, S.; Nencini, F.; Chiccoli, F.; Vivarelli, E.; Maggi, E.; Vultaggio, A. Asthma and Chronic Rhinosinusitis: How Similar Are They in Pathogenesis and Treatment Responses? Int. J. Mol. Sci. 2021, 22, 3340. [Google Scholar] [CrossRef]
- Joubert, P.; Hamid, Q. Role of airway smooth muscle in airway remodeling. J. Allergy Clin. Immunol. 2005, 116, 713–716. [Google Scholar] [CrossRef]
- Bartemes, K.R.; Kita, H. Roles of innate lymphoid cells (ILCs) in allergic diseases: The 10-year anniversary for ILC2s. J. Allergy Clin. Immunol. 2021, 147, 1531–1547. [Google Scholar] [CrossRef]
- Maggi, E.; Veneziani, I.; Moretta, L.; Cosmi, L.; Annunziato, F. Group 2 Innate Lymphoid Cells: A Double-Edged Sword in Cancer? Cancers 2020, 12, 3452. [Google Scholar] [CrossRef]
- Krabbendam, L.; Bernink, J.H.; Spits, H. Innate lymphoid cells: From helper to killer. Curr. Opin. Immunol. 2021, 68, 28–33. [Google Scholar] [CrossRef]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.J.; et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Dhariwal, J.; Cameron, A.; Trujillo-Torralbo, M.-B.; del Rosario, A.; Bakhsoliani, E.; Paulsen, M.; Jackson, D.J.; Edwards, M.R.; Rana, B.M.J.; Cousins, D.J.; et al. Mucosal Type 2 Innate Lymphoid Cells Are a Key Component of the Allergic Response to Aeroallergens. Am. J. Respir. Crit. Care Med. 2017, 195, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, L.; Liotta, F.; Maggi, L.; Annunziato, F. Role of Type 2 Innate Lymphoid Cells in Allergic Diseases. Curr. Allergy Asthma Rep. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.; Yang, L.; Shah, P.R.; Kobie, J.J.; Urban, J.F.; Mosmann, T.R. Amphiregulin, a T H 2 Cytokine Enhancing Resistance to Nematodes. Science 2006, 314, 1746. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Montaini, G.; Mazzoni, A.; Rossettini, B.; Capone, M.; Rossi, M.C.; Santarlasci, V.; Liotta, F.; Rossi, O.; Gallo, O.; et al. Human circulating group 2 innate lymphoid cells can express CD154 and promote IgE production. J. Allergy Clin. Immunol. 2017, 139, 964–976.e4. [Google Scholar] [CrossRef]
- Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S.; Annunziato, F. Th17 cells: New players in asthma pathogenesis. Allergy 2011, 66, 989–998. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Romagnani, S. Human and murine Th17. Curr. Opin. HIV AIDS 2010, 5, 114–119. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S. Main features of human T helper 17 cells. Ann. N. Y. Acad. Sci. 2013, 1284, 66–70. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S. Defining the human T helper 17 cell phenotype. Trends Immunol. 2012, 33, 505–512. [Google Scholar] [CrossRef]
- Jin, Y.; Deng, Z.; Cao, C.; Li, L. IL-17 polymorphisms and asthma risk: A meta-analysis of 11 single nucleotide polymorphisms. J. Asthma 2015, 52, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Hagau, N.; Slavcovici, A.; Gonganau, D.N.; Oltean, S.; Dirzu, D.S.; Brezoszki, E.S.; Maxim, M.; Ciuce, C.; Mlesnite, M.; Gavrus, R.L.; et al. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit. Care 2010, 14, R203. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, H.J.; Chang, Y.-J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17–producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Molet, S.; Hamid, Q.; Davoineb, F.; Nutku, E.; Tahaa, R.; Pagé, N.; Olivenstein, R.; Elias, J.; Chakir, J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001, 108, 430–438. [Google Scholar] [CrossRef]
- Zarobkiewicz, M.K.; Wawryk-Gawda, E.; Kowalska, W.; Janiszewska, M.; Bojarska-Junak, A. γδ T Lymphocytes in Asthma: A Complicated Picture. Arch. Immunol. Ther. Exp.(Warsz) 2021, 69, 4. [Google Scholar] [CrossRef]
- Schramm, C.M.; Puddington, L.; Yiamouyiannis, C.A.; Lingenheld, E.G.; Whiteley, H.E.; Wolyniec, W.W.; Noonan, T.C.; Thrall, R.S. Proinflammatory Roles of T-Cell Receptor (TCR) γδ and TCR αβ Lymphocytes in a Murine Model of Asthma. Am. J. Respir. Cell Mol. Biol. 2000, 22, 218–225. [Google Scholar] [CrossRef]
- Belkadi, A.; Dietrich, C.; Machavoine, F.; Victor, J.R.; Leite-de-Moraes, M. γδ T cells amplify Blomia tropicalis -induced allergic airway disease. Allergy 2019, 74, 395–398. [Google Scholar] [CrossRef]
- Chung, K.F. Precision medicine in asthma. Curr. Opin. Pulm. Med. 2018, 24, 4–10. [Google Scholar] [CrossRef]
- Gauthier, M.; Ray, A.; Wenzel, S.E. Evolving Concepts of Asthma. Am. J. Respir. Crit. Care Med. 2015, 192, 660–668. [Google Scholar] [CrossRef]
- Puzzovio, P.G.; Levi-Schaffer, F. Latest Progresses in Allergic Diseases Biomarkers: Asthma and Atopic Dermatitis. Front. Pharmacol. 2021, 12, 747364. [Google Scholar] [CrossRef]
- Luce, S.; Batard, T.; Bordas-Le Floch, V.; le Gall, M.; Mascarell, L. Decrease in CD38 + TH2A cell frequencies following immunotherapy with house dust mite tablet correlates with humoral responses. Clin. Exp. Allergy 2021, 51, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Celebi Sözener, Z.; Mungan, D.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C. Tolerance mechanisms in allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S.; Biliotti, G.; Ricci, M. Depression of grass pollen-induced lymphocyte transformation by serum from hyposensitized patients. Clin. Exp. Immunol. 1975, 19, 83–91. [Google Scholar] [PubMed]
- Gardner, L.M.; O’Hehir, R.E.; Rolland, J.M. High Dose Allergen Stimulation of T Cells from House Dust Mite-Allergic Subjects Induces Expansion of IFN-γ+ T Cells, Apoptosis of CD4+IL-4+ T Cells and T Cell Anergy. Int. Arch. Allergy Immunol. 2004, 133, 1–13. [Google Scholar] [CrossRef]
- Antúnez, C.; Mayorga, C.; Corzo, J.L.; Jurado, A.; Torres, M.J. Two year follow-up of immunological response in mite-allergic children treated with sublingual immunotherapy. Comparison with subcutaneous administration. Pediatr. Allergy Immunol. 2008, 19, 210–218. [Google Scholar] [CrossRef]
- Akdis, C.A.; Blesken, T.; Akdis, M.; Wüthrich, B.; Blaser, K. Role of interleukin 10 in specific immunotherapy. J. Clin. Investig. 1998, 102, 98–106. [Google Scholar] [CrossRef]
- Guerra, F.; Carracedo, J.; Solana-Lara, R.; Sánchez-Guijo, P.; Ramírez, R. TH2 lymphocytes from atopic patients treated with immunotherapy undergo rapid apoptosis after culture with specific allergens. J. Allergy Clin. Immunol. 2001, 107, 647–653. [Google Scholar] [CrossRef]
- van der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martínez-Martínez, P.; Vermeulen, E.; Den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A.; et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef]
- Jutel, M.; Jaeger, L.; Suck, R.; Meyer, H.; Fiebig, H.; Cromwell, O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J. Allergy Clin. Immunol. 2005, 116, 608–613. [Google Scholar] [CrossRef]
- Meiler, F.; Klunker, S.; Zimmermann, M.; Akdis, C.A.; Akdis, M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy 2008, 63, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saiz, R.; Patil, S.U. The Multifaceted B Cell Response in Allergen Immunotherapy. Curr. Allergy Asthma Rep. 2018, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Abrahamsson, T.R.; Artacho, A.; Björkstén, B.; Collado, M.C.; Mira, A. and Jenmalm, M.C.Aberrant IgA responses to the gut microbiota during infancy precede asthma and allergy development. J. Allergy Clin. Immunol. 2017, 139, 1017–1025.e14. [Google Scholar] [CrossRef]
- Kukkonen, K.; Kuitunen, M.; Haahtela, T.; Korpela, R.; Poussa, T.; Savilahti, E. High intestinal IgA associates with reduced risk of IgE-associated allergic diseases. Pediatr. Allergy Immunol. 2010, 21, 67–73. [Google Scholar] [CrossRef]
- Kulis, M.; Saba, K.; Kim, E.H.; Bird, J.A.; Kamilaris, N.; Vickery, B.P.; Staats, H.; Burks, A.W. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J. Allergy Clin. Immunol. 2012, 129, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Pilette, C. Serum IgA response to grass pollen during allergen-injection immunotherapy for seasonal rhinitis*1. J. Allergy Clin. Immunol. 2004, 113, S105. [Google Scholar] [CrossRef]
- Shamji, M.H.; Larson, D.; Eifan, A.; Scadding, G.W.; Qin, T.; Lawson, K.; Sever, M.L.; Macfarlane, E.; Layhasi, J.A.; Wurtzen, P.A.; et al. Differential induction of allergen-specific IgA responses following timothy grass subcutaneous and sublingual immunotherapy. J. Allergy Clin. Immunol. 2021, 148, 1061–1071.e11. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Akdis, M. Advances in allergen immunotherapy: Aiming for complete tolerance to allergens. Sci. Transl. Med. 2015, 7, 280ps6. [Google Scholar] [CrossRef]
- van de Veen, W.; Stanic, B.; Wirz, O.F.; Jansen, K.; Globinska, A.; Akdis, M. Role of regulatory B cells in immune tolerance to allergens and beyond. J. Allergy Clin. Immunol. 2016, 138, 654–665. [Google Scholar] [CrossRef]
- Nouri-Aria, K.T.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Walker, S.M.; Wilcock, L.K.; Staple, S.Q.; Aalberse, R.C.; Till, S.J.; Durham, S.R. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J. Immunol. 2004, 172, 3252–3259. [Google Scholar] [CrossRef]
- Boonpiyathad, T.; Meyer, N.; Moniuszko, M.; Sokolowska, M.; Eljaszewicz, A.; Wirz, O.F.; Tomasiak-Lozowska, M.M.; Bodzenta-Lukaszyk, A.; Ruxrungtham, K.; van de Veen, W. High-dose bee venom exposure induces similar tolerogenic B-cell responses in allergic patients and healthy beekeepers. Allergy 2017, 72, 407–415. [Google Scholar] [CrossRef] [PubMed]
- van de Veen, W.; Stanic, B.; Yaman, G.; Wawrzyniak, M.; Söllner, S.; Akdis, D.G.; Ruckert, B.; Akdis, C.A.; Akdis, M. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J. Allergy Clin. Immunol. 2013, 131, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Noh, J.; Noh, G.; Choi, W.S.; Cho, S.; Lee, S.S. Allergen-specific transforming growth factor-β-producing CD19+CD5+ regulatory B-cell (Br3) responses in human late eczematous allergic reactions to cow’s milk. J. Interferon Cytokine Res. 2011, 31, 441–449. [Google Scholar] [CrossRef]
- Jutel, M.; Pichler, W.J.; Skrbic, D.; Urwyler, A.; Dahinden, C.; Müller, U.R. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-gamma secretion in specific allergen-stimulated T cell cultures. J. Immunol. 1995, 154, 4187–4194. [Google Scholar]
- Maggi, E.; Parronchi, P.; Manetti, R.; Simonelli, C.; Piccinni, M.P.; Rugiu, F.S.; De Carli, M.; Ricci, M.; Romagnani, S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J. Immunol. 1992, 148, 2142–2147. [Google Scholar] [PubMed]
- Manetti, R.; Annunziato, F.; Tomasevic, L.; Giannò, V.; Parronchi, P.; Romagnani, S.; Maggi, E. Polyinosinic acid: Polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-α and interleukin-12. Eur. J. Immunol. 1995, 25, 2656–2660. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Manetti, R.; Brugnolo, F.; Parronchi, P.; Maggi, E.; Nagata, K.; Romagnani, S. Reversal of human allergen-specific CRTH2+ TH2 cells by IL-12 or the PS-DSP30 oligodeoxynucleotide. J. Allergy Clin. Immunol. 2001, 108, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Filì, L.; Ferri, S.; Guarna, F.; Sampognaro, S.; Manuelli, C.; Liotta, F.; Cosmi, L.; Matucci, M.; Vultaggio, A.; Annunziato, F. Redirection of allergen-specific TH2 responses by a modified adenine through Toll-like receptor 7 interaction and IL-12/IFN release. J. Allergy Clin. Immunol. 2006, 118, 511–517. [Google Scholar] [CrossRef]
- Mazzoni, A.; Santarlasci, V.; Maggi, L.; Capone, M.; Rossi, M.C.; Querci, V.; De Palma, R.; Chang, H.-D.; Thiel, A.; Cimaz, R. Demethylation of the RORC2 and IL17A in Human CD4 + T Lymphocytes Defines Th17 Origin of Nonclassic Th1 Cells. J. Immunol. 2015, 194, 3116–3126. [Google Scholar] [CrossRef]
- Wisniewski, J.A.; Muehling, L.M.; Eccles, J.D.; Capaldo, B.J.; Agrawal, R.; Shirley, D.-A.; Patrie, J.T.; Workman, L.J.; Schuyler, A.J.; Lawrence, M.G.; et al. TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status. J. Allergy Clin. Immunol. 2018, 141, 2048–2060.e13. [Google Scholar] [CrossRef]
- Giri, S.; Lal, G. Differentiation and functional plasticity of gamma-delta (γδ) T cells under homeostatic and disease conditions. Mol. Immunol. 2021, 136, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Borger, J.G.; Lau, M.; Hibbs, M.L. The Influence of Innate Lymphoid Cells and Unconventional T Cells in Chronic Inflammatory Lung Disease. Front. Immunol. 2019, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.M.; Golebski, K.; Spits, H. Plasticity of innate lymphoid cell subsets. Nat. Rev. Immunol. 2020, 20, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Krabbendam, L.; Bal, S.M.; Spits, H.; Golebski, K. New insights into the function, development, and plasticity of type 2 innate lymphoid cells. Immunol. Rev. 2018, 286, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kozutsumi, D.; Tsunematsu, M.; Yamaji, T.; Murakami, R.; Yokoyama, M.; Kino, K. Cry-Consensus Peptide, a Novel Peptide for Immunotherapy of Japanese Cedar Pollinosis, Induces Th1-Predominant Response in Cry j 1-Sensitized B10.S Mice. Biol. Pharm. Bull. 2006, 29, 2506–2509. [Google Scholar] [CrossRef]
- Ciprandi, G.; Sormani, M.P.; Filaci, G.; Fenoglio, D. Carry-over effect on IFN-gamma production induced by allergen-specific immunotherapy. Int. Immunopharmacol. 2008, 8, 1622–1625. [Google Scholar] [CrossRef]
- Trichot, C.; Faucheux, L.; Karpf, L.; Grandclaudon, M.; Pattarini, L.; Bagot, M.; Mahevas, T.; Jachiet, M.; Saussine, A.; Bouaziz, J.-D.; et al. TH cell diversity and response to dupilumab in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 756–759. [Google Scholar] [CrossRef]
- Durham, S.R.; Ying, S.; Varney, V.A.; Jacobson, M.R.; Sudderick, R.M.; Mackay, I.S.; Kay, A.B.; Hamid, Q.A. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-γ. J. Allergy Clin. Immunol. 1996, 97, 1356–1365. [Google Scholar] [CrossRef]
- Wachholz, P.A.; Nouri-Aria, K.T.; Wilson, D.R.; Walker, S.M.; Verhoef, A.; Till, S.J.; Durham, S.R. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1: Th2 cytokine ratios. Immunology 2002, 105, 56–62. [Google Scholar] [CrossRef]
- Nouri-Aria, K.T.; Pilette, C.; Jacobson, M.R.; Watanabe, H.; Durham, S.R. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: Effect of immunotherapy. J. Allergy Clin. Immunol. 2005, 116, 73–79. [Google Scholar] [CrossRef]
- Hamid, Q.; Schotman, E.; Jacobson, M.; Walker, S.; Durham, S. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J. Allergy Clin. Immunol. 1997, 99, 254–260. [Google Scholar] [CrossRef]
- Alexander, C.; Tarzi, M.; Larche, M.; Kay, A.B. The effect of Fel d 1-derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy 2005, 60, 1269–1274. [Google Scholar] [CrossRef]
- Moote, W.; Kim, H.; Ellis, A.K. Allergen-specific immunotherapy. Allergy Asthma Clin. Immunol. 2018, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Larché, M. Immunoregulation by targeting T cells in the treatment of allergy and asthma. Curr. Opin. Immunol. 2006, 18, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Grindebacke, H.; Wing, K.; Andersson, A.-C.; Suri-Payer, E.; Rak, S.; Rudin, A. Defective suppression of Th2 cytokines by CD4+CD25+ regulatory T cells in birch allergics during birch pollen season. Clin. Exp. Allergy 2004, 34, 1364–1372. [Google Scholar] [CrossRef]
- Francis, J.N.; Till, S.J.; Durham, S.R. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003, 111, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- del Prete, G.; de Carli, M.; Almerigogna, F.; Giudizi, M.G.; Biagiotti, R.; Romagnani, S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J. Immunol. 1993, 150, 353–360. [Google Scholar]
- Radulovic, S.; Jacobson, M.R.; Durham, S.R.; Nouri-Aria, K.T. Grass pollen immunotherapy induces Foxp3-expressing CD4+CD25+ cells in the nasal mucosa. J. Allergy Clin. Immunol. 2008, 121, 1467–1472.e1. [Google Scholar] [CrossRef]
- Ying, S.; Humbert, M.; Meng, Q.; Pfister, R.; Menz, G.; Gould, H.J.; Kay, A.B.; Durham, S.R. Local expression of ϵ germline gene transcripts and RNA for the ϵ heavy chain of IgE in the bronchial mucosa in atopic and nonatopic asthma. J. Allergy Clin. Immunol. 2001, 107, 686–692. [Google Scholar] [CrossRef]
- James, L.K.; Durham, S.R. Update on mechanisms of allergen injection immunotherapy. Clin. Exp. Allergy 2008, 38, 1074–1088. [Google Scholar] [CrossRef]
- Charrad, R.; Berraïes, A.; Hamdi, B.; Ammar, J.; Hamzaoui, K.; Hamzaoui, A. Anti-inflammatory activity of IL-37 in asthmatic children: Correlation with inflammatory cytokines TNF-α, IL-β, IL-6 and IL-17A. Immunobiology 2016, 221, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Lunding, L.P.; Zissler, U.M.; Vock, C.; Webering, S.; Ehlers, J.C.; Orinska, Z.; Chaker, A.; Schmidt-Weber, C.B.; Lang, N.J.; et al. IL-37 regulates allergic inflammation by counterbalancing pro-inflammatory IL-1 and IL-33. Allergy 2022, 77, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Niedbala, W.; Wei, X.-Q.; Cai, B.; Hueber, A.J.; Leung, B.P.; McInnes, I.B.; Liew, F.Y. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol. 2007, 37, 3021–3029. [Google Scholar] [CrossRef]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Chaturvedi, V.; Henderson, A.L.; Giacomin, P.R.; Guy, C.; Bankoti, J.; Finkelstein, D.; Forbes, K.; Workman, C.J.; Brown, S.A.; et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010, 11, 1093–1101. [Google Scholar] [CrossRef]
- Whitehead, G.S.; Wilson, R.H.; Nakano, K.; Burch, L.H.; Nakano, H.; Cook, D.N. IL-35 production by inducible costimulator (ICOS)-positive regulatory T cells reverses established IL-17-dependent allergic airways disease. J. Allergy Clin. Immunol. 2012, 129, e1–e5. [Google Scholar] [CrossRef]
- Shamji, M.H.; Layhadi, J.A.; Achkova, D.; Kouser, L.; Perera-Webb, A.; Couto-Francisco, N.C.; Parkin, R.V.; Matsuoka, T.; Scadding, G.; Ashton-Rickardt, P.G.; et al. Role of IL-35 in sublingual allergen immunotherapy. J. Allergy Clin. Immunol. 2019, 143, 1131–1142.e4. [Google Scholar] [CrossRef]
- Mitthamsiri, W.; Pradubpongsa, P.; Sangasapaviliya, A.; Boonpiyathad, T. Decreased CRTH2 Expression and Response to Allergen Re-stimulation on Innate Lymphoid Cells in Patients With Allergen-Specific Immunotherapy. Allergy Asthma Immunol. Res. 2018, 10, 662–674. [Google Scholar] [CrossRef]
- Golebski, K.; Layhadi, J.A.; Sahiner, U.; Steveling-Klein, E.H.; Lenormand, M.M.; Li, R.C.Y.; Bal, S.M.; Heesters, B.A.; Vilà-Nadal, G.; Hunewald, O.; et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 2021, 54, 291–307.e7. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Duffy, M.; Brady, M.T.; McKiernan, S.; Hall, W.; Hegarty, J.; Curry, M.; Mills, K.H.G. CD4 T Helper Type 1 and Regulatory T Cells Induced against the Same Epitopes on the Core Protein in Hepatitis C Virus–Infected Persons. J. Infect. Dis. 2002, 185, 720–727. [Google Scholar] [CrossRef]
- Parronchi, P.; Sampognaro, S.; Annunziato, F.; Brugnolo, F.; Radbruch, A.; di Modugno, F.; Ruffilli, A.; Romagnani, S.; Maggi, E. Influence of both TCR repertoire and severity of the atopic status on the cytokine secretion profile of Parietaria officinalis -specific T cells. Eur. J. Immunol. 1998, 28, 37–46. [Google Scholar] [CrossRef]
- Reefer, A.J.; Carneiro, R.M.; Custis, N.J.; Platts-Mills, T.A.E.; Sung, S.-S.J.; Hammer, J.; Woodfolk, J.A. A Role for IL-10-Mediated HLA-DR7-Restricted T Cell-Dependent Events in Development of the Modified Th2 Response to Cat Allergen. J. Immunol. 2004, 172, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Plewako, H.; Wosińska, K.; Arvidsson, M.; Björkander, J.; Håkansson, L.; Rak, S. Production of interleukin-12 by monocytes and interferon-γ by natural killer cells in allergic patients during rush immunotherapy. Ann. Allergy Asthma Immunol. 2006, 97, 464–468. [Google Scholar] [CrossRef]
- Qian, C.; An, H.; Yu, Y.; Liu, S.; Cao, X. TLR agonists induce regulatory dendritic cells to recruit Th1 cells via preferential IP-10 secretion and inhibit Th1 proliferation. Blood 2007, 109, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Bozza, S.; Zelante, T.; Moretti, S.; Bonifazi, P.; DeLuca, A.; D’Angelo, C.; Giovannini, G.; Garlanda, C.; Boon, L.; Bistoni, F.; et al. Lack of Toll IL-1R8 Exacerbates Th17 Cell Responses in Fungal Infection. J. Immunol. 2008, 180, 4022–4031. [Google Scholar] [CrossRef]
- Mamessier, E.; Birnbaum, J.; Dupuy, P.; Vervloet, D.; Magnan, A. Ultra-rush venom immunotherapy induces differential T cell activation and regulatory patterns according to the severity of allergy. Clin. Exp. Allergy 2006, 36, 704–713. [Google Scholar] [CrossRef]
- Bohle, B.; Kinaciyan, T.; Gerstmayr, M.; Radakovics, A.; Jahn-Schmid, B.; Ebner, C. Sublingual immunotherapy induces IL-10–producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J. Allergy Clin. Immunol. 2007, 120, 707–713. [Google Scholar] [CrossRef]
- O’Hehir, R.E.; Gardner, L.M.; de Leon, M.P.; Hales, B.J.; Biondo, M.; Douglass, J.A.; Rolland, J.M.; Sandrini, A. House Dust Mite Sublingual Immunotherapy. Am. J. Respir. Crit. Care Med. 2009, 180, 936–947. [Google Scholar] [CrossRef]
- Jones, S.M.; Pons, L.; Roberts, J.L.; Scurlock, A.M.; Perry, T.T.; Kulis, M.; Shreffler, W.G.; Steele, P.; Henry, K.A.; Adair, M.; et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J. Allergy Clin. Immunol. 2009, 124, 292–300.e97. [Google Scholar] [CrossRef]
- Cosmi, L.; Santarlasci, V.; Angeli, R.; Liotta, F.; Maggi, L.; Frosali, F.; Rossi, O.; Falagiani, P.; Riva, G.; Romagnani, S.; et al. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulates allergen-specific immunoglobulin E and increases both interferon-gamma- and interleukin-10-production. Clin. Exp. Allergy 2006, 36, 261–272. [Google Scholar] [CrossRef]
- Häringer, B.; Lozza, L.; Steckel, B.; Geginat, J. Identification and characterization of IL-10/IFN-γ–producing effector-like T cells with regulatory function in human blood. J. Exp. Med. 2009, 206, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Pot, C.; Jin, H.; Awasthi, A.; Liu, S.M.; Lai, C.-Y.; Madan, R.; Sharpe, A.H.; Karp, C.L.; Miaw, S.-C. Cutting Edge: IL-27 Induces the Transcription Factor c-Maf, Cytokine IL-21, and the Costimulatory Receptor ICOS that Coordinately Act Together to Promote Differentiation of IL-10-Producing Tr1 Cells. J. Immunol. 2009, 183, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lee, J.S.; Gartlan, K.H.; Schuster, I.S.; Comerford, I.; Varelias, A.; Ullah, A.; Vuckovic, S.; Koyama, M.; Kuns, R.D.; et al. Eomesodermin promotes the development of type 1 regulatory T (TR 1) cells. Sci. Immunol. 2017, 2, eaah7152. [Google Scholar] [CrossRef] [PubMed]
- Gruarin, P.; Maglie, S.; Simone, M.; Häringer, B.; Vasco, C.; Ranzani, V.; Bosotti, R.; Noddings, J.S.; Larghi, P.; Facciotti, F.; et al. Eomesodermin controls a unique differentiation program in human IL-10 and IFN-γ coproducing regulatory T cells. Eur. J. Immunol. 2019, 49, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Mascanfroni, I.D.; Takenaka, M.C.; Yeste, A.; Patel, B.; Wu, Y.; Kenison, J.E.; Siddiqui, S.; Basso, A.S.; Otterbein, L.E.; Pardoll, D.M.; et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med. 2015, 21, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S.; Bacchetta, R.; Battaglia, M.; Gagliani, N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity 2018, 49, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Alfen, J.S.; Larghi, P.; Facciotti, F.; Gagliani, N.; Bosotti, R.; Paroni, M.; Maglie, S.; Gruarin, P.; Vasco, C.M.; Ranzani, V.; et al. Intestinal IFN-γ–producing type 1 regulatory T cells coexpress CCR5 and programmed cell death protein 1 and downregulate IL-10 in the inflamed guts of patients with inflammatory bowel disease. J. Allergy Clin. Immunol. 2018, 142, 1537–1547.e8. [Google Scholar] [CrossRef]
- Bonnal, R.J.P.; Rossetti, G.; Lugli, E.; de Simone, M.; Gruarin, P.; Brummelman, J.; Dufruca, L.; Passaro, M.; Bason, R.; Gervasoni, F.; et al. Clonally expanded EOMES+ Tr1-like cells in primary and metastatic tumors are associated with disease progression. Nat. Immunol. 2021, 22, 735–745. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med. 2007, 204, 239–243. [Google Scholar] [CrossRef]
- Rutz, S.; Janke, M.; Kassner, N.; Hohnstein, T.; Krueger, M.; Scheffold, A. Notch regulates IL-10 production by T helper 1 cells. Proc. Natl. Acad. Sci. USA 2008, 105, 3497–3502. [Google Scholar] [CrossRef]
- Jankovic, D.; Kullberg, M.C.; Feng, C.G.; Goldszmid, R.S.; Collazo, C.M.; Wilson, M.; Wynn, T.A.; Kamanaka, M.; Flavell, R.A.; Sher, A. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 2007, 204, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.F.; Oukka, M.; Kuchroo, V.J.; Sacks, D. CD4+CD25−Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 2007, 204, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, S.; Nencini, F.; Grosso, F.; Dies, L.; Bormioli, S.; Cammelli, D.; Maggi, E.; Matucci, A.; Vultaggio, A.; ABIRISK Consortium. T Cell Response to Infliximab in Exposed Patients: A Longitudinal Analysis. Front. Immunol. 2019, 9, 3113. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, A.; Vivarelli, E.; Virgili, G.; Lucenteforte, E.; Bartoloni, A.; Nozzoli, C.; Morettini, A.; Berni, A.; Malandrino, D.; Rossi, O.; et al. Prompt Predicting of Early Clinical Deterioration of Moderate-to-Severe COVID-19 Patients: Usefulness of a Combined Score Using IL-6 in a Preliminary Study. J. Allergy Clin. Immunol. Pract. 2020, 8, 2575–2581.e2. [Google Scholar] [CrossRef]
- Vultaggio, A.; Nencini, F.; Bormioli, S.; Silvestri, E.; Dies, L.; Vivarelli, E.; Maggi, E.; Matucci, A. Drug-specific Treg cells are induced during desensitization procedure for rituximab and tocilizumab in patients with anaphylaxis. Sci. Rep. 2021, 11, 12558. [Google Scholar] [CrossRef]
- Vultaggio, A.; Matucci, A.; Nencini, F.; Bormioli, S.; Vivarelli, E.; Maggi, E. Mechanisms of Drug Desensitization: Not Only Mast Cells. Front. Pharmacol. 2020, 11, 590991. [Google Scholar] [CrossRef]
- Klimek, L.; Pfaar, O.; Hamelmann, E.; Kleine-Tebbe, J.; Taube, C.; Wagenmann, M.; Werfel, T.; Brehler, R.; Novak, N.; Mulleneisen, N.; et al. COVID-19 vaccination and allergen immunotherapy (AIT) - A position paper of the German Society for Applied Allergology (AeDA) and the German Society for Allergology and Clinical Immunology (DGAKI). Allergol. Select. 2021, 5, 251–259. [Google Scholar] [CrossRef]
- Shamji, M.H.; Kappen, J.H.; Akdis, M.; Jensen-Jarolim, E.; Knol, E.F.; Kleine-Tebbe, J.; Bohle, B.; Chaker, A.M.; Till, S.J.; Valenta, R.; et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: An EAACI Position Paper. Allergy 2017, 72, 1156–1173. [Google Scholar] [CrossRef]
- Passalacqua, G.; Bagnasco, D.; Ferrando, M.; Heffler, E.; Puggioni, F.; Canonica, G.W. Current insights in allergen immunotherapy. Ann. Allergy Asthma Immunol. 2018, 120, 152–154. [Google Scholar] [CrossRef]
- Larche, M.; Hickey, P.; Hebert, J.; Hafner, R. Safety and Tolerability of Escalating Doses of House Dust Mite- Peptide Antigen Desensitization (HDM-PAD). J. Allergy Clin. Immunol. 2013, 131, AB37. [Google Scholar] [CrossRef]
- Spertini, F.; DellaCorte, G.; Kettner, A.; de Blay, F.; Jacobsen, L.; Jutel, M.; Worm, M.; Charlon, V.; Reymond, C. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1–derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: Results of a phase IIb study. J. Allergy Clin. Immunol. 2016, 138, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zhernov, Y.; Curin, M.; Khaitov, M.; Karaulov, A.; Valenta, R. Recombinant allergens for immunotherapy: State of the art. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Pfaar, O.; Worm, M. New opportunities for allergen immunotherapy using synthetic peptide immuno-regulatory epitopes (SPIREs). Expert Rev. Clin. Immunol. 2016, 12, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Campana, R.; Focke-Tejkl, M.; Niederberger, V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future. J. Allergy Clin. Immunol. 2016, 137, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, C.; Montagni, M.; Ridolo, E. The efficiency of peptide immunotherapy for respiratory allergy. Expert Rev. Clin. Pharmacol. 2016, 9, 831–837. [Google Scholar] [CrossRef]
- Linhart, B.; Valenta, R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine 2012, 30, 4328–4335. [Google Scholar] [CrossRef]
- Pauli, G.; Larsen, T.H.; Rak, S.; Horak, F.; Pastorello, E.; Valenta, R.; Purohit, A.; Arvidsson, M.; Kavina, A.; Schroeder, J.W.; et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2008, 122, 951–960. [Google Scholar] [CrossRef]
- Valenta, R.; Campana, R.; Niederberger, V. Recombinant allergy vaccines based on allergen-derived B cell epitopes. Immunol. Lett. 2017, 189, 19–26. [Google Scholar] [CrossRef]
- Jennings, G.T.; Bachmann, M.F. The coming of age of virus-like particle vaccines. Biol. Chem. 2008, 389, 521–536. [Google Scholar] [CrossRef]
- Mohsen, O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus–like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Kundig, T.; Senti, G.; Schnetzler, G.; Wolf, C.; Prinzvavricka, B.; Fulurija, A.; Henneke, F.; Sladko, K.; Jennings, G.T.; Bachmann, M.F. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J. Allergy Clin. Immunol. 2006, 117, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Creticos, P.S.; Schroeder, J.T.; Hamilton, R.G.; Balcer-Whaley, S.L.; Khattignavong, A.P.; Lindblad, R.; Li, H.; Coffman, R.; Seyfert, V.; Eiden, J.J.; et al. Immunotherapy with a Ragweed–Toll-Like Receptor 9 Agonist Vaccine for Allergic Rhinitis. N. Engl. J. Med. 2006, 355, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Senti, G.; Johansen, P.; Haug, S.; Bull, C.; Gottschaller, C.; Müller, P.; Pfister, T.; Maurer, P.; Bachmann, M.F.; Graf, N.; et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: A phase I/IIa clinical trial. Clin. Exp. Allergy 2009, 39, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, S.; Nencini, F.; Filì, L.; Occhiato, E.G.; Romagnani, S.; Parronchi, P.; Maggi, E.; Vultaggio, A. Dermatophagoides pteronyssinus group 2 allergen bound to 8-OH modified adenine reduces the Th2-mediated airway inflammation without inducing a Th17 response and autoimmunity. Mol. Immunol. 2016, 77, 60–70. [Google Scholar] [CrossRef]
- Nencini, F.; Pratesi, S.; Petroni, G.; Filì, L.; Cardilicchia, E.; Casini, A.; Occhiato, E.G.; Calosi, L.; Bani, D.; Romagnani, S.; et al. Treatment with 8-OH-modified adenine (TLR7 ligand)-allergen conjugates decreases T helper type 2-oriented murine airway inflammation. Immunology 2015, 145, 570–582. [Google Scholar] [CrossRef]
| HVA | AR | BA | FA | LR | SR (Which Require Epinephrine Treatment) | Ref | |
|---|---|---|---|---|---|---|---|
| SCIT | X | X | X | Erythema, pruritus, and swelling at the injection site | Low frequencies | [7,9,10] | |
| SLIT | X | X | Oropharyngeal pruritus, swelling, or both; throat irritation; nausea/vomiting; diarrhea; abdominal discomfort; heartburn; and uvular edema | Uncommon | [8,9,10] | ||
| OIT | X | Oral pruritus, abdominal discomfort, and rashes | Common during home dosing | [14] | |||
| ILIT | X | Local swelling at the injection site | Uncommon | [14] | |||
| EPIT | X | X | Local eczematous reactions | Uncommon | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veneziani, I.; Landolina, N.; Ricci, B.; Rossi, O.; Moretta, L.; Maggi, E. How the Immune System Responds to Allergy Immunotherapy. Biomedicines 2022, 10, 2825. https://doi.org/10.3390/biomedicines10112825
Veneziani I, Landolina N, Ricci B, Rossi O, Moretta L, Maggi E. How the Immune System Responds to Allergy Immunotherapy. Biomedicines. 2022; 10(11):2825. https://doi.org/10.3390/biomedicines10112825
Chicago/Turabian StyleVeneziani, Irene, Nadine Landolina, Biancamaria Ricci, Oliviero Rossi, Lorenzo Moretta, and Enrico Maggi. 2022. "How the Immune System Responds to Allergy Immunotherapy" Biomedicines 10, no. 11: 2825. https://doi.org/10.3390/biomedicines10112825
APA StyleVeneziani, I., Landolina, N., Ricci, B., Rossi, O., Moretta, L., & Maggi, E. (2022). How the Immune System Responds to Allergy Immunotherapy. Biomedicines, 10(11), 2825. https://doi.org/10.3390/biomedicines10112825







