Volatilomics as an Emerging Strategy to Determine Potential Biomarkers of Female Infertility: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Subject Sample Collection
2.3. Follicular Fluid Sample Collection
2.4. Follicular Fluid Preparation
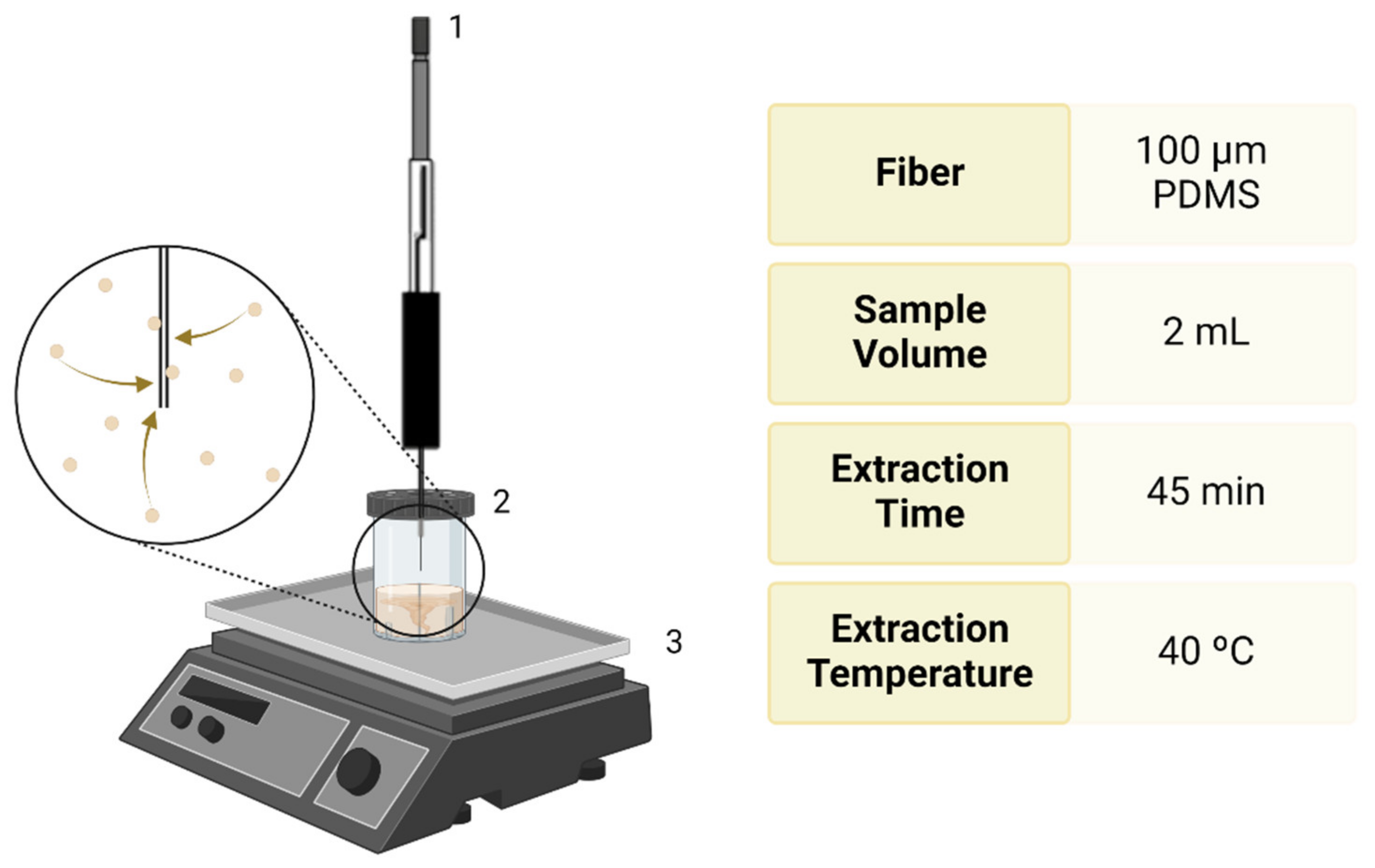
2.5. Extraction of Metabolites from the FF
2.6. Gas Chromatography–Mass Spectrometry (GC-MS) Conditions
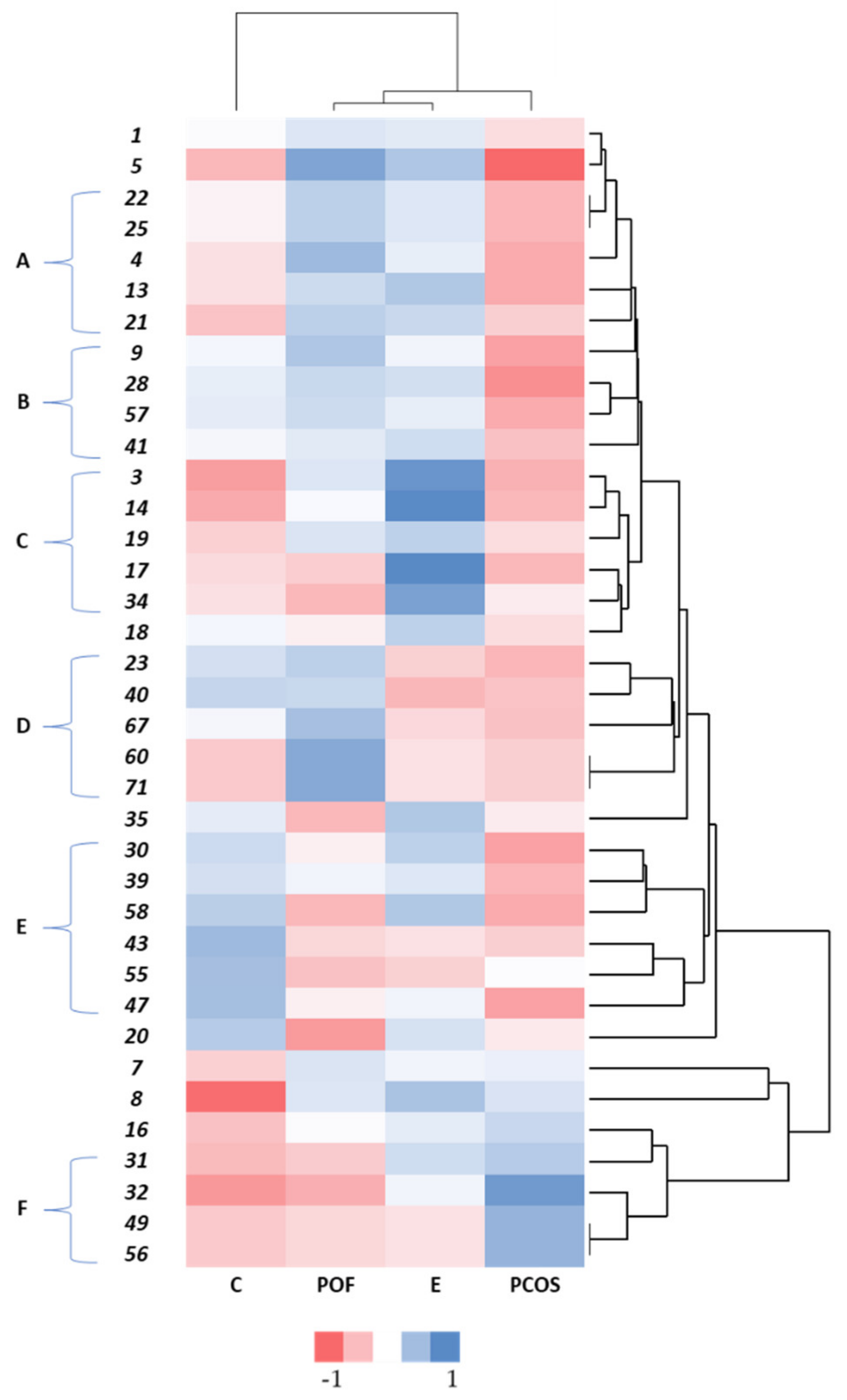
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, K.Y.; Patel, P.; Ramasamy, R. Consideration of gender differences in infertility evaluation. Curr. Opin. Urol. 2019, 29, 267–271. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; De Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The international glossary on infertility and fertility care, 2017. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef] [Green Version]
- Szamatowicz, M. Assisted reproductive technology in reproductive medicine—Possibilities and limitations. Ginekol. Pol. 2016, 87, 820–823. [Google Scholar] [CrossRef] [Green Version]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. Reply: International estimates on infertility prevalence and treatment seeking: Potential need and demand for medical care. Hum. Reprod. 2007, 24, 2380–2383. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J. Infertility: A primer for primary care providers. J. Am. Acad. Physician Assist. 2017, 30, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, T.J.; Vitrikas, K.R. Evaluation and treatment of infertility. Am. Fam. Physician 2015, 91, 308–314. [Google Scholar] [PubMed]
- Crawford, N.M.; Steiner, A.Z. Age-related infertility. Obstet. Gynecol. Clin. N. Am. 2015, 42, 15–25. [Google Scholar] [CrossRef]
- Gleicher, N.; Kushnir, V.A.; Weghofer, A.; Barad, D.H. The “graying” of infertility services: An impending revolution nobody is ready for. Reprod. Biol. Endocrinol. 2014, 12, 63. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.; Perestrelo, R.; Silva, P.; Capelinha, F.; Tomás, H.; Câmara, J.S. Volatomic pattern of breast cancer and cancer-free tissues as a powerful strategy to identify potential biomarkers. Analyst 2019, 144, 4153–4161. [Google Scholar] [CrossRef]
- Yu, L.; Liu, M.; Wang, Z.; Liu, T.; Liu, S.; Wang, B.; Pan, B.; Dong, X.; Guo, W. Correlation between steroid levels in follicular fluid and hormone synthesis related substances in its exosomes and embryo quality in patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2021, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Ran, H.; Chen, Y.; Ma, L. Lipidomics analysis of human follicular fluid form normal-weight patients with polycystic ovary syndrome: A pilot study. J. Ovarian Res. 2021, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pang, Y. Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites. Metabolites 2021, 11, 869. [Google Scholar] [CrossRef]
- Liu, R.; Bai, S.; Zheng, S.; Zhu, X.; Zhang, Y.; Xu, B.; Zhao, W. Identification of the Metabolomics Signature of Human Follicular Fluid from PCOS Women with Insulin Resistance. Dis. Markers 2022, 2022, 6877541. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R. PCOS: A diagnostic challenge. Reprod. Biomed. Online 2004, 8, 644–648. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Knauff, E.A.H.; Valkenburg, O.; Laven, J.S.; Eijkemans, M.J.; Fauser, B.C.J.M. PCOS according to the Rotterdam consensus criteria: Change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1210–1217. [Google Scholar] [CrossRef]
- Azziz, R.; Hincapie, L.A.; Knochenhauer, E.S.; Dewailly, D.; Fox, L.; Boots, L.R. Screening for 21-hydroxylase-deficient nonclassic adrenal hyperplasia among hyperandrogenic women: A prospective study. Fertil. Steril. 1999, 72, 915–925. [Google Scholar] [CrossRef]
- Azziz, R.; Tarlatzis, R.; Dunaif, A.; Ibanez, L.; Pugeat, M.; Taylor, A.; Fauser, C.J.M.; Medicine, R. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Cleeman, J.I. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Mohammad, M.B.; Seghinsara, A.M. Polycystic ovary syndrome (PCOS), diagnostic criteria, and AMH. Asian Pac. J. Cancer Prev. 2017, 18, 17–21. [Google Scholar] [CrossRef]
- de Zegher, F.; López-Bermejo, A.; Ibáñez, L. Central Obesity, Faster Maturation, and ‘PCOS’ in Girls. Trends Endocrinol. Metab. 2018, 29, 815–818. [Google Scholar] [CrossRef]
- Ehrmann, D.A.; Barnes, R.B.; Rosenfield, R.L.; Cavaghan, M.K.; Imperial, J. Prevalence of Impaired Glucose Tolerance and Diabetes in Women with Polycystic Ovary Syndrome. Diabetes Care 1999, 22, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Dunaif, A.; Legro, R.S. Prevalence and Predictors of Risk for Type 2 Diabetes Mellitus and Impaired Glucose Tolerance in Polycystic Ovary Syndrome-Authors’ Response. J. Clin. Endocrinol. Metab. 1999, 84, 297–2976. [Google Scholar] [CrossRef]
- Dunaif, A.; Graf, M.; Mandeli, J.; Laumas, V.; Dobrjansky, A. Characterization of Groups of Hyperaiidrogenic Women with Acanthosis Nigricans, Impaired Glucose Tolerance, and/or Hyperinsulinemia. J. Clin. Endocrinol. Metab. 1987, 65, 499–507. [Google Scholar] [CrossRef]
- Robinson, S.; Kiddy, D.; Gelding, S.V.; Willis, D.; Niththyananthan, R.; Bush, A.; Johnston, D.G.; Franks, S. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin. Endocrinol. 1993, 39, 351–355. [Google Scholar] [CrossRef]
- Dahlgren, E.; Janson, P.O.; Johansson, S.; Lapidus, L.; Odén, A. Polycystic ovary syndrome and risk for myocardial infarction: Evaluated from a risk factor model based on a prospective population study of women. Acta Obstet. Gynecol. Scand. 1992, 71, 599–604. [Google Scholar] [CrossRef]
- Kiddy, D.S.; Hamilton-Fairley, D.; Bush, A.; Short, F.; Anyaoku, V.; Reed, M.J.; Franks, S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin. Endocrinol. 1992, 36, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Ledger, W.; Galletly, C.; Tomlinson, L.; Blaney, F.; Wang, X.; Norman, R.J. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum. Reprod. 1995, 10, 2705–2712. [Google Scholar] [CrossRef]
- Huber-Buchholz, M.M.; Carey, D.G.P.; Norman, R.J. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: Role of insulin sensitivity and luteinizing hormone. J. Clin. Endocrinol. Metab. 1999, 84, 1470–1474. [Google Scholar] [CrossRef]
- Morán, C.; Knochenhauer, E.; Boots, L.R.; Azziz, R. Adrenal androgen excess in hyperandrogenism: Relation to age and body mass. Fertil. Steril. 1999, 71, 671–674. [Google Scholar] [CrossRef]
- Pocate-Cheriet, K.; Santulli, P.; Kateb, F.; Bourdon, M.; Maignien, C.; Batteux, F.; Chouzenoux, S.; Patrat, C.; Wolf, J.P.; Bertho, G.; et al. The follicular fluid metabolome differs according to the endometriosis phenotype. Reprod. Biomed. Online 2020, 41, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- De Ziegler, D.; Gayet, V.; Aubriot, F.X.; Fauque, P.; Streuli, I.; Wolf, J.P.; De Mouzon, J.; Chapron, C. Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcomes. Fertil. Steril. 2010, 94, 2796–2799. [Google Scholar] [CrossRef] [PubMed]
- Mikhaleva, L.M.; Davydov, A.I.; Patsap, O.I.; Mikhaylenko, E.V.; Nikolenko, V.N.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Malignant Transformation and Associated Biomarkers of Ovarian Endometriosis: A Narrative Review. Adv. Ther. 2020, 37, 2580–2603. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, D.; Cosson, M.; Dufour, P. Is endometriosis an endometrial disease? Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 91, 113–125. [Google Scholar] [CrossRef]
- Prins, J.R.; Marissen, L.M.; Scherjon, S.A.; Hoek, A.; Cantineau, A.E.P. Is there an immune modulating role for follicular fluid in endometriosis? A narrative review. Reproduction 2020, 159, R45–R54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E.; Koninckx, P.; et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef]
- Sampson, J.A.; Novak, E. Peritoneal Endometriosis, Due to the Menstrual Dissemination of Endometrial Tissue into the Peritoneal Cavity. Am. J. Obstet. Gynecol. 1927, 15, 101–110. [Google Scholar] [CrossRef]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef]
- Adachi, M.; Nasu, K.; Tsuno, A.; Yuge, A.; Kawano, Y.; Narahara, H. Attachment to extracellular matrices is enhanced in human endometriotic stromal cells: A possible mechanism underlying the pathogenesis of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 85–88. [Google Scholar] [CrossRef]
- Jankowska, K. Premature ovarian failure. Przegląd Menopauzalny 2017, 16, 51–56. [Google Scholar] [CrossRef]
- Coulam, C.B.; Adamson, S.C.; Annegers, J.F. Incidence of Premature Ovarian Failure. Obstet. Gynecol. 1986, 67, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Qing, X. Diagnosis of Idiopathic Premature Ovarian Failure by Color Doppler Ultrasound under the Intelligent Segmentation Algorithm. Hindawi Comput. Math. Methods Med. 2022, 2022, 2645607. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Dantas, A.P.; Miranda, J.; Crovetto, F.; Eixarch, E.; Rodriguez-Sureda, V.; Dominguez, C.; Casu, G.; Rovira, C.; Nadal, A.; et al. Premature placental aging in term small-for-gestational-age and growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2019, 53, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouatcheu, S.D.; Marko, J.; Tamura, D.; Khan, S.G.; Lee, C.R.; DiGiovanna, J.J.; Kraemer, K.H. Thyroid nodules in xeroderma pigmentosum patients: A feature of premature aging. J. Endocrinol. Investig. 2021, 44, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, H.; Lin, J.; Xiao, T.; Xu, R.; Fu, Y.; Zhang, Y.; Du, Y.; Cheng, J.; Jiang, H. Insulin impedes osteogenesis of BMSCs by inhibiting autophagy and promoting premature senescence via the TGF-β1 pathway. Aging 2020, 12, 2084–2100. [Google Scholar] [CrossRef]
- Camilleri, E. A Brief Overview On Premature Ovarian Failure. Malta Med. Stud. Assoc. 2022, 1–9. [Google Scholar]
- Pal, L.; Torrealday, S.; Kodaman, P. Premature Ovarian Insufficiency—An update on recent advances in understanding and management. F1000Research 2017, 6, 2069. [Google Scholar] [CrossRef]
- Pankiewicz, K.; Laudański, P.; Issat, T. The role of noncoding rna in the pathophysiology and treatment of premature ovarian insufficiency. Int. J. Mol. Sci. 2021, 22, 9336. [Google Scholar] [CrossRef]
- Popat, V.B.; Calis, K.A.; Kalantaridou, S.N.; Vanderhoof, V.H.; Koziol, D.; Troendle, J.F.; Reynolds, J.C.; Nelson, L.M. Bone mineral density in young women with primary ovarian insufficiency: Results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J. Clin. Endocrinol. Metab. 2014, 99, 3418–3426. [Google Scholar] [CrossRef] [Green Version]
- Brinca, A.T.; Ramalhinho, A.C.; Sousa, Â.; Oliani, A.H.; Breitenfeld, L.; Passarinha, L.A.; Gallardo, E. Follicular Fluid: A Powerful Tool for the Understanding and Diagnosis of Polycystic Ovary Syndrome. Biomedicines 2022, 10, 1254. [Google Scholar] [CrossRef]
- O’Gorman, A.; Wallace, M.; Cottell, E.; Gibney, M.J.; McAuliffe, F.M.; Wingfield, M.; Brennan, L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 2013, 146, 389–395. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, H.; Lian, F.; Zhang, X.; Pang, C.; Guo, Y.; Song, J.; Wang, A.; Shi, L.; Han, L. Human Follicular Fluid Metabolomics Study of Follicular Development and Oocyte Quality. Chromatographia 2017, 80, 901–909. [Google Scholar] [CrossRef]
- Luti, S.; Fiaschi, T.; Magherini, F.; Modesti, P.A.; Piomboni, P.; Governini, L.; Luddi, A.; Amoresano, A.; Illiano, A.; Pinto, G.; et al. Relationship between the metabolic and lipid profile in follicular fluid of women undergoing in vitro fertilization. Mol. Reprod. Dev. 2020, 87, 986–997. [Google Scholar] [CrossRef]
- Rajska, A.; Buszewska-Forajta, M.; Rachoń, D.; Markuszewski, M.J. Metabolomic insight into polycystic ovary syndrome—An overview. Int. J. Mol. Sci. 2020, 21, 4853. [Google Scholar] [CrossRef]
- McCartney, A.; Vignoli, A.; Biganzoli, L.; Love, R.; Tenori, L.; Luchinat, C.; Di Leo, A. Metabolomics in breast cancer: A decade in review. Cancer Treat. Rev. 2018, 67, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Koek, M.M.; Jellema, R.H.; van der Greef, J.; Tas, A.C.; Hankemeier, T. Quantitative metabolomics based on gas chromatography mass spectrometry: Status and perspectives. Metabolomics 2011, 7, 307–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wörheide, M.A.; Krumsiek, J.; Kastenmüller, G.; Arnold, M. Multi-omics integration in biomedical research—A metabolomics-centric review. Anal. Chim. Acta 2021, 1141, 144–162. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef]
- Longo, V.; Forleo, A.; Provenzano, S.P.; Coppola, L.; Zara, V.; Ferramosca, A.; Siciliano, P.; Capone, S. HS-SPME-GC-MS metabolomics approach for sperm quality evaluation by semen volatile organic compounds (VOCs) analysis. Biomed. Phys. Eng. Express 2018, 5, 015006. [Google Scholar] [CrossRef]
- Schmidt, K.; Podmore, I. Current Challenges in Volatile Organic Compounds Analysis as Potential Biomarkers of Cancer. J. Biomark. 2015, 2015, 981458. [Google Scholar] [CrossRef] [Green Version]
- Berenguer, C.V.; Pereira, F.; Pereira, J.A.M.; Câmara, J.S. Volatilomics: An Emerging and Promising Avenue for the Detection of Potential Prostate Cancer Biomarkers. Cancers 2022, 14, 3982. [Google Scholar] [CrossRef]
- Buljubasic, F.; Buchbauer, G. The scent of human diseases: A review on specific volatile organic compounds as diagnostic biomarkers. Flavour Fragr. J. 2015, 30, 5–25. [Google Scholar] [CrossRef]
- Longo, V.; Forleo, A.; Ferramosca, A.; Notari, T.; Pappalardo, S.; Siciliano, P.; Capone, S.; Montano, L. Blood, urine and semen Volatile Organic Compound (VOC) pattern analysis for assessing health environmental impact in highly polluted areas in Italy. Environ. Pollut. 2021, 286, 117410. [Google Scholar] [CrossRef] [PubMed]
- Di Lena, M.; Porcelli, F.; Altomare, D.F. Volatile organic compounds as new biomarkers for colorectal cancer: A review. Color. Dis. 2016, 18, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Trock, B.J. Application of metabolomics to prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 572–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Lee, W.-Y. Urinary metabolites for urological cancer detection: A review on the application of volatile organic compounds for cancers. Am. J. Clin. Exp. Urol. 2019, 7, 232–248. [Google Scholar] [PubMed]
- Spratlin, J.L.; Serkova, N.J.; Eckhardt, S.G. Clinical applications of metabolomics in oncology: A review. Clin. Cancer Res. 2009, 15, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, P.; Baker, J.; Boyd, M.T.; Coyle, S.; Probert, C.; Chapman, E.A. Optimisation of urine sample preparation for headspace-solid phase microextraction gas chromatography-mass spectrometry: Altering sample ph, sulphuric acid concentration and phase ratio. Metabolites 2020, 10, 482. [Google Scholar] [CrossRef]
- Silva, C.L.; Passos, M.; Câmara, J.S. Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers—A powerful strategy for breast cancer diagnosis. Talanta 2012, 89, 360–368. [Google Scholar] [CrossRef]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Amann, A.; Costello, B.D.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, J.; Kowalkowski, T.; Buszewski, B. Searching for selected VOCs in human breath samples as potential markers of lung cancer. Lung Cancer 2019, 135, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, S.; Miekisch, W.; Patricia, F.; Schubert, J.K. Breath analysis during one-lung ventilation in cancer patients. Eur. Respir. J. 2012, 40, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tienpont, B.; David, F.; Bicchi, C.; Sandra, P. High capacity headspace sorptive extraction. J. Microcolumn Sep. 2000, 12, 577–584. [Google Scholar] [CrossRef]
- Pugliese, G.; Trefz, P.; Brock, B.; Schubert, J.K.; Miekisch, W. Extending PTR based breath analysis to real-time monitoring of reactive volatile organic compounds. Analyst 2019, 144, 7359–7367. [Google Scholar] [CrossRef]
- Cavaco, C.; Pereira, J.A.M.; Taunk, K.; Taware, R.; Rapole, S.; Nagarajaram, H.; Câmara, J.S. Screening of salivary volatiles for putative breast cancer discrimination: An exploratory study involving geographically distant populations. Anal. Bioanal. Chem. 2018, 410, 4459–4468. [Google Scholar] [CrossRef]
- Cavaco, C.; Perestrelo, R.; Silva, C.; Aveiro, F.; Pereira, J.; Câmara, J.S. Establishment of the Saliva Volatomic Profi le as an Exploratory and Non-invasive Strategy to Find Potential Breast Cancer Biomarkers; Labmate Ltd.: Hertfordshire, UK, 2014. [Google Scholar]
- Streckfus, C.F.; Brown, R.E.; Bull, J.M. Proteomics, morphoproteomics, saliva and breast cancer: An emerging approach to guide the delivery of individualised thermal therapy, thermochemotherapy and monitor therapy response. Int. J. Hyperth. 2010, 26, 649–661. [Google Scholar] [CrossRef]
- Malathi, N.; Mythili, S.; Vasanthi, H.R. Salivary Diagnostics: A Brief Review. ISRN Dent. 2014, 2014, 158786. [Google Scholar] [CrossRef]
- Pereira, J.A.M.; Taware, R.; Porto-Figueira, P.; Rapole, S.; Câmara, J.S. The salivary volatome in breast cancer. In Precision Medicine for Investigators, Practitioners and Providers; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 301–307. [Google Scholar] [CrossRef]
- Bobkov, Y.V.; Walker, W.B.; Cattaneo, A.M. Altered functional properties of the codling moth Orco mutagenized in the intracellular loop-3. Sci. Rep. 2021, 11, 3893. [Google Scholar] [CrossRef]
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Volatile metabolomic signature of human breast cancer cell lines. Sci. Rep. 2017, 7, 43969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porto-Figueira, P.; Pereira, J.A.M.; Câmara, J.S. Exploring the potential of needle trap microextraction combined with chromatographic and statistical data to discriminate different types of cancer based on urinary volatomic biosignature. Anal. Chim. Acta 2018, 1023, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Salciccia, S.; Capriotti, A.L.; Laganà, A.; Fais, S.; Logozzi, M.; De Berardinis, E.; Busetto, G.M.; Di Pierro, G.B.; Ricciuti, G.P.; Del Giudice, F.; et al. Biomarkers in prostate cancer diagnosis: From current knowledge to the role of metabolomics and exosomes. Int. J. Mol. Sci. 2021, 22, 4367. [Google Scholar] [CrossRef]
- Živković Semren, T.; Brčić Karačonji, I.; Safner, T.; Brajenović, N.; Tariba Lovaković, B.; Pizent, A. Gas chromatographic-mass spectrometric analysis of urinary volatile organic metabolites: Optimization of the HS-SPME procedure and sample storage conditions. Talanta 2018, 176, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Araújo, A.M.; Pinto, J.; Jerónimo, C.; Henrique, R.; Bastos, M.D.L.; Carvalho, M.; Guedes De Pinho, P. Discrimination between the human prostate normal and cancer cell exometabolome by GC-MS. Sci. Rep. 2018, 8, 5539. [Google Scholar] [CrossRef] [Green Version]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Porto-Figueira, P.; Pereira, J.; Miekisch, W.; Câmara, J.S. Exploring the potential of NTME/GC-MS, in the establishment of urinary volatomic profiles. Lung cancer patients as case study. Sci. Rep. 2018, 8, 13113. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.R.; Pinto, J.; Amaro, F.; Bastos, M.d.L.; Carvalho, M.; Guedes De Pinho, P. Advances and perspectives in prostate cancer biomarker discovery in the last 5 years through tissue and urine metabolomics. Metabolites 2021, 11, 181. [Google Scholar] [CrossRef]
- Caramelo, D. Determination of Antipsychotic Drugs in Oral Fluid Samples Using Dried Saliva Spots Versão Final Após Defesa; Universidade da Beira Interior: Covilhã, Portugal, 2019. [Google Scholar]
- Eiceman, G.A. Instrumentation of Gas Chromatography. Encycl. Anal. Chem. 2000, 10671–10679. [Google Scholar] [CrossRef]
- Maurer, H.H. Role of gas chromatography-mass spectrometry with negative ion chemical ionization in clinical and forensic toxicology, doping control, and biomonitoring. Ther. Drug Monit. 2002, 24, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, J.; Masuram, S.; Richert, J.C. Gas chromatography-mass spectrometry-basic principles, instrumentation and selected applications for detection of organic compounds. Anal. Lett. 2007, 40, 1003–1012. [Google Scholar] [CrossRef]
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Implementing a central composite design for the optimization of solid phase microextraction to establish the urinary volatomic expression: A first approach for breast cancer. Metabolomics 2019, 15, 64. [Google Scholar] [CrossRef]
- Hart, R.J. Physiological aspects of female fertility: Role of the environment, modern lifestyle, and genetics. Physiol. Rev. 2016, 96, 873–909. [Google Scholar] [CrossRef] [PubMed]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Bang, D.Y.; Lee, I.K.; Lee, B.M. Toxicological characterization of phthalic acid. Toxicol. Res. 2011, 27, 191–203. [Google Scholar] [CrossRef]
- Przybylińska, P.A.; Wyszkowski, M. Environmental contamination with phthalates and its impact on living organisms. Ecol. Chem. Eng. S 2016, 23, 347–356. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hirata-Koizumi, M.; Ema, M. Potential adverse effects of phthalic acid esters on human health: A review of recent studies on reproduction. Regul. Toxicol. Pharmacol. 2008, 50, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Y.; Fang, Y.L.; Wang, Y.X.; Zeng, Q.; Guo, N.; Zhao, H.; Li, Y.F. Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod. Toxicol. 2016, 61, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Peterson, C.M.; Chen, Z.; Croughan, M.; Sundaram, R.; Stanford, J.; Varner, M.W.; Kennedy, A.; Giudice, L.; Fujimoto, V.Y.; et al. Bisphenol A and phthalates and endometriosis: The Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil. Steril. 2013, 100, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coldham, N.G.; Dave, M.; Sivapathasundaram, S.; McDonnell, D.P.; Connor, C.; Sauer, M.J. Evaluation of a recombinant yeast cell estrogen screening assay. Environ. Health Perspect. 1997, 105, 734–742. [Google Scholar] [CrossRef]
- Okubo, T.; Suzuki, T.; Yokoyama, Y.; Kano, K.; Kano, I. Estimation of estrogenic and anti-estrogenic activities of some phthalate diesters and monoesters by MCF-7 cell proliferation assay in Vitro. Biol. Pharm. Bull. 2003, 26, 1219–1224. [Google Scholar] [CrossRef] [Green Version]
- Hannon, P.R.; Niermann, S.; Flaws, J.A. Acute Exposure to Di(2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice. Toxicol. Sci. 2016, 150, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.; Maronpot, R.; Heindel, J. DEHP suppresses estradioland ovuation in cycling rats. Toxicol. Appl. Pharmacol. 1994, 128, 216–223. [Google Scholar] [CrossRef]
- Craig, Z.R.; Singh, J.; Gupta, R.K.; Flaws, J.A. Co-treatment of mouse antral follicles with 17β-estradiol interferes with mono-2-ethylhexyl phthalate (MEHP)-induced atresia and altered apoptosis gene expression. Reprod. Toxicol. 2014, 45, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Bala, R.; Singh, V.; Rajender, S. Environment, Lifestyle, and Female Infertility. Reprod. Sci. 2021, 28, 617–638. [Google Scholar] [CrossRef]
- Silva, M.J.; Slakman, A.R.; Reidy, J.A.; Preau, J.L.; Herbert, A.R.; Samandar, E.; Needham, L.L.; Calafat, A.M. Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 805, 161–167. [Google Scholar] [CrossRef]
- da Costa, B.R.B.; De Martinis, B.S. Analysis of urinary VOCs using mass spectrometric methods to diagnose cancer: A review. Clin. Mass Spectrom. 2020, 18, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Upsona, K.; Sathyanarayana, S.; De Roosa, A.J.; Thompson, M.L.; Scholes, D.; Dills, R.; Holt, V.L. Phthalates and risk of endometriosis. NIH Public Access 2013, 126, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobellis, L.; Latini, G.; DeFelice, C.; Razzi, S.; Paris, I.; Ruggieri, F.; Mazzeo, P.; Petraglia, F. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum. Reprod. 2003, 18, 1512–1515. [Google Scholar] [CrossRef]
- Reddy, B.S.; Rozati, R.; Reddy, S.; Kodampur, S.; Reddy, P.; Reddy, R. High plasma concentrations of polychlorinated biphenyls and phthalate esters in women with endometriosis: A prospective case control study. Fertil. Steril. 2006, 85, 775–779. [Google Scholar] [CrossRef]
- Reddy, B.; Rozati, R.; Reddy, B.; Raman, N. Association of phthalate esters with endometriosis in Indian women. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 515–520. [Google Scholar] [CrossRef]
- Itoh, H.; Iwasaki, M.; Hanaoka, T.; Sasaki, H.; Tanaka, T.; Tsugane, S. Urinary phthalate monoesters and endometriosis in infertile Japanese women. Sci. Total Environ. 2009, 408, 37–42. [Google Scholar] [CrossRef]
- Weuve, J.; Hauser, R.; Calafat, A.M.; Missmer, S.A.; Wise, L.A. Association of exposure to phthalates with endometriosis and uterine leiomyomata: Findings from NHANES, 1999–2004. Environ. Health Perspect. 2010, 118, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Tsai, E.M.; Li, W.F.; Liao, P.C.; Chung, M.C.; Wang, Y.H.; Wang, S.L. Association between phthalate exposure and glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Hum. Reprod. 2010, 25, 986–994. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Chun, S.; Jang, J.Y.; Chae, H.D.; Kim, C.H.; Kang, B.M. Increased plasma levels of phthalate esters in women with advanced-stage endometriosis: A prospective case-control study. Fertil. Steril. 2011, 95, 357–359. [Google Scholar] [CrossRef]
- Vaglio, S.; Minicozzi, P.; Bonometti, E.; Mello, G.; Chiarelli, B. Volatile signals during pregnancy: A possible chemical basis for mother-infant recognition. J. Chem. Ecol. 2009, 35, 131–139. [Google Scholar] [CrossRef]
- Silva, C.L.; Passos, M.; Cmara, J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer 2011, 105, 1894–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordic Council of Ministers. Health Effects of Selected Chemicals; Nordic Council of Ministers: Copenhagen, Demark, 1992; Volume 4–5. [Google Scholar]
- Octadecanal|C18H36O—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/12533 (accessed on 28 September 2022).
- Tetradecanal|C14H28O—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/31291 (accessed on 28 September 2022).
- Song, J.; Xiang, S.; Yang, Y.; Sun, Z. Assessment of follicular fluid metabolomics of polycystic ovary syndrome in kidney yang deficiency syndrome. Eur. J. Integr. Med. 2019, 30, 100944. [Google Scholar] [CrossRef]
- Prates, E.G.; Alves, S.P.; Marques, C.C.; Baptista, M.C.; Horta, A.E.M.; Bessa, R.J.B.; Pereira, R.M. Fatty acid composition of porcine cumulus oocyte complexes (COC) during maturation: Effect of the lipid modulators trans-10, cis-12 conjugated linoleic acid (t10,c12 CLA) and forskolin. Vitr. Cell. Dev. Biol.-Anim. 2013, 49, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Tiper, I.V.; East, J.E.; Subrahmanyam, P.B.; Webb, T.J. Sphingosine 1-phosphate signaling impacts lymphocyte migration, inflammation and infection. Pathog. Dis. 2016, 74, ftw063. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, F.B.; Montani, D.A.; Pilau, E.J.; Gozzo, F.C.; Fraietta, R.; Turco, E.G. Lo Ovarian environment aging: Follicular fluid lipidomic and related metabolic pathways. J. Assist. Reprod. Genet. 2018, 35, 1385–1393. [Google Scholar] [CrossRef]
- Cordeiro, F.B.; Cataldi, T.R.; de Souza, B.Z.; Rochetti, R.C.; Fraietta, R.; Labate, C.A.; Lo Turco, E.G. Hyper response to ovarian stimulation affects the follicular fluid metabolomic profile of women undergoing IVF similarly to polycystic ovary syndrome. Metabolomics 2018, 14, 51. [Google Scholar] [CrossRef]
- Lucki, N.C.; Sewer, M.B. The interplay between bioactive sphingolipids and steroid hormones. Steroids 2010, 75, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Andrieu-Abadie, N.; Levade, T. Sphingomyelin hydrolysis during apoptosis. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2002, 1585, 126–134. [Google Scholar] [CrossRef]
- Schmitt, B.G.; Jensen, E.; Laufersweiler, M.C.; Rose, J.L. Threshold of Toxicological Concern: Extending the chemical space by inclusion of a highly curated dataset for organosilicon compounds. Regul. Toxicol. Pharmacol. 2021, 127, 105074. [Google Scholar] [CrossRef]
- Neumann, F.; Diallo, F.A.; Hasan, S.H.; Schenck, B.; Traore, I. The Influence of Pharmaceutical Compounds on Male Fertility. Andrologia 1976, 8, 203–235. [Google Scholar] [CrossRef]
- Kala, S.V.; Lykissa, E.D.; Lebovitz, R.M. Detection and Characterization of Poly(dimethylsiloxane)s in Biological Tissues by GC/AED and GC/MS. Anal. Chem. 1997, 69, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Cyclomethicone, Cyclotetrasiloxane, Cyclopentasiloxane, Cyclohexasiloxane, and Cycloheptasiloxane. Int. J. Toxicol. 2011, 30, 149S–227S. [Google Scholar] [CrossRef] [PubMed]
- Etteieb, S.; Cherif, S.; Kawachi, A.; Han, J.; Elayni, F.; Tarhouni, J.; Isoda, H. Combining Biological and Chemical Screenings to Assess Cytotoxicity of Emerging Contaminants in Discharges into Surface Water. Water Air Soil Pollut. 2016, 227, 341. [Google Scholar] [CrossRef]
- Marianna, S.; Alessia, P.; Susan, C.; Francesca, C.; Angela, S.; Francesca, C.; Antonella, N.; Patrizia, I.; Nicola, C.; Emilio, C. Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol. Biosyst. 2017, 13, 1213–1222. [Google Scholar] [CrossRef]
- Karaer, A.; Tuncay, G.; Mumcu, A.; Dogan, B. Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Syst. Biol. Reprod. Med. 2019, 65, 39–47. [Google Scholar] [CrossRef]
- Sun, Z.; Song, J.; Zhang, X.; Wang, A.; Guo, Y.; Yang, Y.; Wang, X.; Xu, K.; Deng, J. Novel SWATHTM technology for follicular fluid metabolomics in patients with endometriosis. Pharmazie 2018, 73, 218–323. [Google Scholar] [CrossRef]
- Montiel, M.C.; Máximo, F.; Serrano-Arnaldos, M.; Ortega-Requena, S.; Murcia, M.D.; Bastida, J. Biocatalytic solutions to cyclomethicones problem in cosmetics. Eng. Life Sci. 2019, 19, 370–388. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, A.; Fagan, J. Toxins in cosmetics. What Consumers and Government can do to get Safer Cosmetic Products on the Market. Available online: https://rucore.libraries.rutgers.edu/rutgers-lib/54763/PDF/1/ (accessed on 28 September 2022).
- Sirotkin, A.V.; Harrath, A.H. Influence of oil-related environmental pollutants on female reproduction. Reprod. Toxicol. 2017, 71, 142–145. [Google Scholar] [CrossRef]
- Sharma, R.P.; Schuhmacher, M.; Kumar, V. Review on crosstalk and common mechanisms of endocrine disruptors: Scaffolding to improve PBPK/PD model of EDC mixture. Environ. Int. 2017, 99, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.M.; Park, E.K.; LeeAn, S.Y.; Ha, M.; Kim, E.J.; Kwon, H.; Hong, Y.C.; Jeong, W.C.; Hur, J.; Cheong, H.K.; et al. BTEX exposure and its health effects in pregnant women following the Hebei Spirit oil spill. J. Prev. Med. Public Health 2009, 42, 96–103. [Google Scholar] [CrossRef]
- Suaidi, N.A.; Alshawsh, M.A.; Hoe, S.Z.; Mokhtar, M.H.; Zin, S.R.M. Toxicological Effects of Technical Xylene Mixtures on the Female Reproductive System: A Systematic Review. Toxics 2022, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Mizutare, T.; Makishima, M.; Suzuki, N.; Fujimoto, N.; Igarashi, K.; Ohta, S. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: Their structures and estrogenic potency. Toxicol. Sci. 2004, 78, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Rehan, M.; Ahmad, E.; Sheikh, I.A.; Abuzenadah, A.M.; Damanhouri, G.A.; Bajouh, O.S.; Al Basri, S.F.; Assiri, M.M.; Beg, M.A. Androgen and progesterone receptors are targets for bisphenol a (BPA), 4-methyl-2,4-bis-(p-hydroxyphenyl)pent-1-ene-A potent metabolite of BPA, and 4-tert-octylphenol: A computational insight. PLoS ONE 2015, 10, e0138438. [Google Scholar] [CrossRef] [Green Version]
- Okuda, K.; Takiguchi, M.; Yoshihara, S. In vivo estrogenic potential of 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, an active metabolite of bisphenol A, in uterus of ovariectomized rat. Toxicol. Lett. 2010, 197, 7–11. [Google Scholar] [CrossRef]
- Hirao-Suzuki, M.; Nagase, K.; Suemori, T.; Tsutsumi, K.; Shigemori, E.; Tanaka, M.; Takiguchi, M.; Sugihara, N.; Yoshihara, S.; Takeda, S. 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP) Targets Estrogen Receptor β, to Evoke the Resistance of Human Breast Cancer MCF-7 Cells to G-1, an Agonist for G Protein-Coupled Estrogen Receptor 1. Biol. Pharm. Bull. 2021, 44, 1524–1529. [Google Scholar] [CrossRef]
- Hirao-Suzuki, M.; Takeda, S.; Okuda, K.; Takiguchi, M.; Yoshihara, S. Repeated Exposure to 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP), an Active Metabolite of Bisphenol A, Aggressively Stimulates Breast Cancer Cell Growth in an Estrogen Receptor β (ERβ)–Dependent Manner. Mol. Pharmacol. 2019, 95, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.H.; Su, C.C.; Lee, K.I.; Chen, Y.W. Effects of Bisphenol A Metabolite 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene on Lung Function and Type 2 Pulmonary Alveolar Epithelial Cell Growth. Sci. Rep. 2016, 6, 39254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.C.; Yang, C.Y.; Su, C.C.; Fang, K.M.; Yen, C.C.; Lin, C.T.; Liu, J.M.; Lee, K.I.; Chen, Y.W.; Liu, S.H.; et al. 4-Methyl-2,4-Bis(4-Hydroxyphenyl)Pent-1-Ene, a Major Active Metabolite of Bisphenol a, Triggers Pancreatic Β-Cell Death Via a Jnk/Ampkα Activation-Regulated Endoplasmic Reticulum Stress-Mediated Apoptotic Pathway. Int. J. Mol. Sci. 2021, 22, 4379. [Google Scholar] [CrossRef] [PubMed]
- Hou, E.; Zhao, Y.; Hang, J.; Qiao, J. Metabolomics and correlation network analysis of follicular fluid reveals associations between l-tryptophan, l-tyrosine and polycystic ovary syndrome. Biomed. Chromatogr. 2020, 35, e4993. [Google Scholar] [CrossRef]
- Yang, X.; Wu, R.; Qi, D.; Fu, L.; Song, T.; Wang, Y.; Bian, Y.; Shi, Y. Profile of Bile Acid Metabolomics in the Follicular Fluid of PCOS Patients. Metabolites 2021, 11, 845. [Google Scholar] [CrossRef]
- Gongadashetti, K.; Gupta, P.; Dada, R.; Malhotra, N. Follicular fluid oxidative stress biomarkers and art outcomes in PCOS women undergoing in vitro fertilization: A cross-sectional study. Int. J. Reprod. Biomed. 2021, 19, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, T.; Song, J.; Deng, J.; Sun, Z. Study on follicular fluid metabolomics components at different ages based on lipid metabolism. Reprod. Biol. Endocrinol. 2020, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yin, T.L.; Chen, Y.; Li, Y.; Yin, L.; Ding, J.; Yang, J.; Feng, H.L. Follicular dynamics of glycerophospholipid and sphingolipid metabolisms in polycystic ovary syndrome patients. J. Steroid Biochem. Mol. Biol. 2018, 185, 142–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Yin, T.L.; Yang, J.; Xiong, C.L. Follicular metabolic changes and effects on oocyte quality in polycystic ovary syndrome patients. Oncotarget 2017, 8, 80472–80480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naessen, T.; Kushnir, M.M.; Chaika, A.; Nosenko, J.; Mogilevkina, I.; Rockwood, A.L.; Carlstrom, K.; Bergquist, J.; Kirilovas, D. Steroid profiles in ovarian follicular fluid in women with and without polycystic ovary syndrome, analyzed by liquid chromatography-tandem mass spectrometry. Fertil. Steril. 2010, 94, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, W.; Zhou, C.; Zhou, Y.; Liu, X.; Ding, G.; Hu, Y.; Pan, J.; Sheng, J.; Jin, L.; et al. Steroid metabolome profiling of follicular fluid in normo- and hyperandrogenic women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2021, 206, 105806. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, N.; Amato, J.; Pagano, B.; Pagano, A.; D’Oriano, L.; Pelliccia, S.; Giustiniano, M.; Brancaccio, D.; Merlino, F.; Novellino, E.; et al. 1H NMR-based metabolomics study on follicular fluid from patients with Polycystic Ovary Syndrome Nunzia. Biochim. Clin. 2018, 42, 26–31. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinca, A.T.; Anjos, O.; Alves, M.M.C.; Sousa, Â.; Oliani, A.H.; Breitenfeld, L.; Passarinha, L.A.; Ramalhinho, A.C.; Gallardo, E. Volatilomics as an Emerging Strategy to Determine Potential Biomarkers of Female Infertility: A Pilot Study. Biomedicines 2022, 10, 2852. https://doi.org/10.3390/biomedicines10112852
Brinca AT, Anjos O, Alves MMC, Sousa Â, Oliani AH, Breitenfeld L, Passarinha LA, Ramalhinho AC, Gallardo E. Volatilomics as an Emerging Strategy to Determine Potential Biomarkers of Female Infertility: A Pilot Study. Biomedicines. 2022; 10(11):2852. https://doi.org/10.3390/biomedicines10112852
Chicago/Turabian StyleBrinca, Ana Teresa, Ofélia Anjos, Maria Manuel Casteleiro Alves, Ângela Sousa, António Hélio Oliani, Luiza Breitenfeld, Luís A. Passarinha, Ana Cristina Ramalhinho, and Eugenia Gallardo. 2022. "Volatilomics as an Emerging Strategy to Determine Potential Biomarkers of Female Infertility: A Pilot Study" Biomedicines 10, no. 11: 2852. https://doi.org/10.3390/biomedicines10112852
APA StyleBrinca, A. T., Anjos, O., Alves, M. M. C., Sousa, Â., Oliani, A. H., Breitenfeld, L., Passarinha, L. A., Ramalhinho, A. C., & Gallardo, E. (2022). Volatilomics as an Emerging Strategy to Determine Potential Biomarkers of Female Infertility: A Pilot Study. Biomedicines, 10(11), 2852. https://doi.org/10.3390/biomedicines10112852








