Necroptosis Blockade Potentiates the Neuroprotective Effect of Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat Model
2.2. Histology
2.3. Immunohistochemistry
2.4. Magnetic Resonance Imaging (MRI) of the Rat Brain
2.5. Analysis of MRI Data
2.5.1. Measurement of the Stroke Volume
2.5.2. Region-of-Interest (ROI) Analysis of MRI Images
2.6. Data Analysis
3. Results
3.1. Necroptotic Markers Measurements in HI±Nec-1±HT
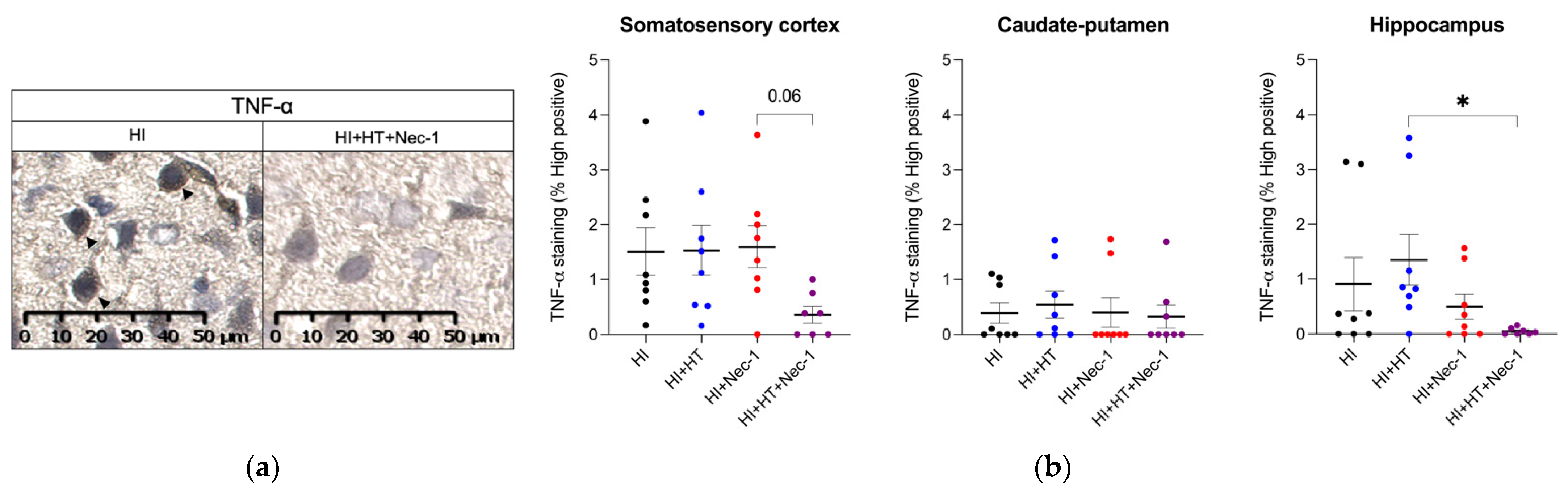
3.1.1. TNF-α Measurement in HI±Nec-1±HT
3.1.2. pRIPK3 Measurement in HI±Nec-1±HT
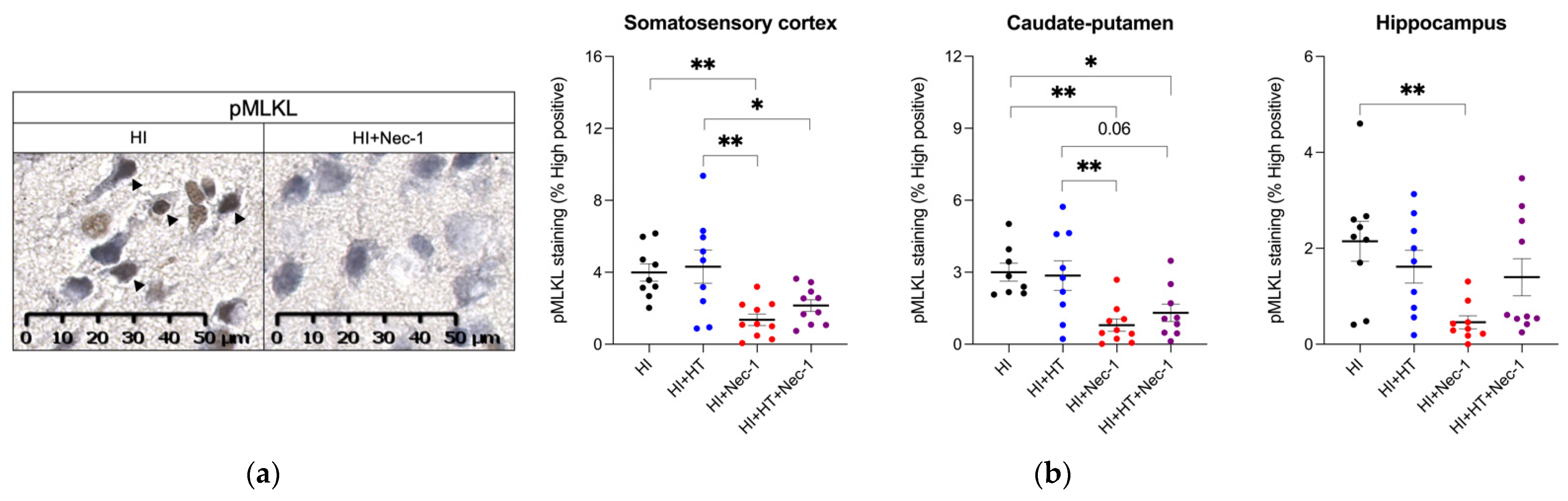
3.1.3. pMLKL Measurement in HI±Nec-1±HT
3.2. Effect of Nec-1+HT on HI-Induced Stroke
4. Discussion
Limitations and Future Directions

5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Biarge, M.; Diez-Sebastian, J.; Wusthoff, C.J.; Mercuri, E.; Cowan, F.M. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics 2013, 132, e952–e959. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; David Edwards, A.; Groenendaal, F. Perinatal brain damage: The term infant. Neurobiol. Dis. 2016, 92, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-ischemic encephalopathy: A review for the clinician. JAMA Pediatr. 2015, 169, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wachtel, E.V.; Verma, S.; Mally, P.V. Update on the current management of newborns with neonatal encephalopathy. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 100636. [Google Scholar] [CrossRef]
- Davidson, J.O.; Wassink, G.; van den Heuij, L.G.; Bennet, L.; Gunn, A.J. Therapeutic Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy - Where to from Here? Front. Neurol. 2015, 6, 198. [Google Scholar] [CrossRef]
- Wassink, G.; Davidson, J.O.; Dhillon, S.K.; Zhou, K.; Bennet, L.; Thoresen, M.; Gunn, A.J. Therapeutic Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy. Curr. Neurol. Neurosci. Rep. 2019, 19, 2. [Google Scholar] [CrossRef]
- Davidson, J.O.; Gonzalez, F.; Gressens, P.; Gunn, A.J. Newborn Brain Society Guidelines and Publications Committee Update on mechanisms of the pathophysiology of neonatal encephalopathy. Semin. Fetal Neonatal. Med. 2021, 26, 101267. [Google Scholar] [CrossRef]
- Rosado-de-Castro, P.H.; Mendez-Otero, R.; Pimentel-Coelho, P.M. Editorial: New Insights into the Pathophysiology and Treatment of Neonatal Hypoxic-Ischemic Encephalopathy. Front. Neurol. 2016, 7, 192. [Google Scholar] [CrossRef]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. TOBY Study Group Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014, 371, 140–149. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, M.-B.; Luo, H.-Y.; Shi, C.-H.; Xu, Y.-M. Necroptosis in neurodegenerative diseases: A potential therapeutic target. Cell Death Dis. 2017, 8, e2905. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Phan, N.; Wang, Q.; Liu, B. Necroptosis in cardiovascular disease—a new therapeutic target. J. Mol. Cell Cardiol. 2018, 118, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Apaijai, N.; Chattipakorn, N.; Chattipakorn, S.C. The possible roles of necroptosis during cerebral ischemia and ischemia / reperfusion injury. Arch. Biochem. Biophys. 2020, 695, 108629. [Google Scholar] [CrossRef] [PubMed]
- Negroni, A.; Colantoni, E.; Cucchiara, S.; Stronati, L. Necroptosis in intestinal inflammation and cancer: New concepts and therapeutic perspectives. Biomolecules 2020, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yuan, J. Necroptosis in health and diseases. Semin. Cell Dev. Biol. 2014, 35, 14–23. [Google Scholar] [CrossRef]
- Chevin, M.; Sébire, G.; Deltenre, P.; Kadhim, H. Necroptotic neuronal cell death in amyotrophic lateral sclerosis: A relevant hypothesis with potential therapeutic implication? Med. Hypotheses 2020, 144, 110295. [Google Scholar] [CrossRef]
- Mifflin, L.; Ofengeim, D.; Yuan, J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat. Rev. Drug Discov. 2020, 19, 553–571. [Google Scholar] [CrossRef]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef]
- Moriwaki, K.; Chan, F.K.-M. RIP3: A molecular switch for necrosis and inflammation. Genes Dev. 2013, 27, 1640–1649. [Google Scholar] [CrossRef]
- Moriwaki, K.; Chan, F.K.M. Necrosis-dependent and independent signaling of the RIP kinases in inflammation. Cytokine Growth Factor Rev. 2014, 25, 167–174. [Google Scholar] [CrossRef]
- Mandal, P.; Berger, S.B.; Pillay, S.; Moriwaki, K.; Huang, C.; Guo, H.; Lich, J.D.; Finger, J.; Kasparcova, V.; Votta, B.; et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 2014, 56, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Giraud, A.; Guiraut, C.; Chevin, M.; Chabrier, S.; Sébire, G. Role of perinatal inflammation in neonatal arterial ischemic stroke. Front. Neurol. 2017, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Askalan, R.; Gabarin, N.; Armstrong, E.A.; Fang Liu, Y.; Couchman, D.; Yager, J.Y. Mechanisms of neurodegeneration after severe hypoxic-ischemic injury in the neonatal rat brain. Brain Res. 2015, 1629, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Valdez, R.; Martin, L.J.; Northington, F.J. Programmed Necrosis: A Prominent Mechanism of Cell Death following Neonatal Brain Injury. Neurol. Res. Int. 2012, 2012, 257563. [Google Scholar] [CrossRef] [PubMed]
- Northington, F.J.; Chavez-Valdez, R.; Martin, L.J. Neuronal cell death in neonatal hypoxia-ischemia. Ann. Neurol. 2011, 69, 743–758. [Google Scholar] [CrossRef]
- Yang, X.-S.; Yi, T.-L.; Zhang, S.; Xu, Z.-W.; Yu, Z.-Q.; Sun, H.-T.; Yang, C.; Tu, Y.; Cheng, S.-X. Hypoxia-inducible factor-1 alpha is involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci. Rep. 2017, 7, 5818. [Google Scholar] [CrossRef]
- Ryan, F.; Khodagholi, F.; Dargahi, L.; Minai-Tehrani, D.; Ahmadiani, A. Temporal Pattern and Crosstalk of Necroptosis Markers with Autophagy and Apoptosis Associated Proteins in Ischemic Hippocampus. Neurotox. Res. 2018, 34, 79–92. [Google Scholar] [CrossRef]
- Hribljan, V.; Lisjak, D.; Petrović, D.J.; Mitrečić, D. Necroptosis is one of the modalities of cell death accompanying ischemic brain stroke: From pathogenesis to therapeutic possibilities. Croat. Med. J. 2019, 60, 121–126. [Google Scholar] [CrossRef]
- Han, F.; Guan, X.; Guo, W.; Lu, B. Therapeutic potential of a TrkB agonistic antibody for ischemic brain injury. Neurobiol. Dis. 2019, 127, 570–581. [Google Scholar] [CrossRef]
- Chavez-Valdez, R.; Martin, L.J.; Flock, D.L.; Northington, F.J. Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience 2012, 219, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Northington, F.J.; Chavez-Valdez, R.; Graham, E.M.; Razdan, S.; Gauda, E.B.; Martin, L.J. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J. Cereb. Blood Flow Metab. 2011, 31, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Shi, J.; Tang, Y.; Zhao, F.; Li, S.; Meng, J.; Tang, J.; Lin, X.; Peng, X.; Mu, D. MLKL inhibition attenuates hypoxia-ischemia induced neuronal damage in developing brain. Exp. Neurol. 2016, 279, 223–231. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Chevin, M.; Guiraut, C.; Maurice-Gelinas, C.; Deslauriers, J.; Grignon, S.; Sébire, G. Neuroprotective effects of hypothermia in inflammatory-sensitized hypoxic-ischemic encephalopathy. Int. J. Dev. Neurosci. 2016, 55, 1–8. [Google Scholar] [CrossRef]
- Savard, A.; Brochu, M.-E.; Chevin, M.; Guiraut, C.; Grbic, D.; Sébire, G. Neuronal self-injury mediated by IL-1β and MMP-9 in a cerebral palsy model of severe neonatal encephalopathy induced by immune activation plus hypoxia-ischemia. J. Neuroinflamm. 2015, 12, 111. [Google Scholar] [CrossRef]
- Chevin, M.; Chelabi, K.; Chabrier, S.; Sébire, G. Added value of interleukin-1 blockade to hypothermia in the treatment of neonatal encephalopathy. Am. J. Obstet. Gynecol. 2020, 223, 458–460. [Google Scholar] [CrossRef]
- Brochu, M.-E.; Girard, S.; Lavoie, K.; Sébire, G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. J. Neuroinflamm. 2011, 8, 55. [Google Scholar] [CrossRef]
- Chevin, M.; Chabrier, S.; Dinomais, M.; Bedell, B.J.; Sébire, G. Benefits of hypothermia in neonatal arterial ischemic strokes: A preclinical study. Int. J. Dev. Neurosci. 2020, 80, 257–266. [Google Scholar] [CrossRef]
- Su, X.; Wang, H.; Kang, D.; Zhu, J.; Sun, Q.; Li, T.; Ding, K. Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway. Neurochem. Res. 2015, 40, 643–650. [Google Scholar] [CrossRef]
- Degterev, A.; Linkermann, A. Generation of small molecules to interfere with regulated necrosis. Cell Mol. Life Sci. 2016, 73, 2251–2267. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Ofengeim, D.; Yuan, J. Targeting RIPK1 for the treatment of human diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 9714–9722. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Pierce, L.; Ciardiello, A.J.; Vannucci, S.J. Neonatal encephalopathy: Pre-clinical studies in neuroprotection. Biochem. Soc. Trans. 2014, 42, 564–568. [Google Scholar] [CrossRef]
- Towfighi, J.; Mauger, D.; Vannucci, R.C.; Vannucci, S.J. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia–ischemia: A light microscopic study. Dev. Brain Res. 1997, 100, 149–160. [Google Scholar] [CrossRef]
- Chevin, M.; Guiraut, C.; Sébire, G. Effect of hypothermia on interleukin-1 receptor antagonist pharmacodynamics in inflammatory-sensitized hypoxic-ischemic encephalopathy of term newborns. J. Neuroinflamm. 2018, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Shi, L.; Wang, Z.; Zhou, J.; Manaenko, A.; Reis, C.; Chen, S.; Zhang, J. RIP1-RIP3-DRP1 pathway regulates NLRP3 inflammasome activation following subarachnoid hemorrhage. Exp. Neurol. 2017, 295, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Degterev, A.; Jagtap, P.; Xing, X.; Choi, S.; Denu, R.; Yuan, J.; Cuny, G.D. Structure-activity relationship study of novel necroptosis inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5039–5044. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Buckley, E.M.; Patel, S.D.; Miller, B.F.; Franceschini, M.A.; Vannucci, S.J. In vivo Monitoring of Cerebral Hemodynamics in the Immature Rat: Effects of Hypoxia-Ischemia and Hypothermia. Dev. Neurosci. 2015, 37, 407–416. [Google Scholar] [CrossRef]
- Allard, M.-J.; Giraud, A.; Segura, M.; Sebire, G. Sex-specific maternofetal innate immune responses triggered by group B Streptococci. Sci. Rep. 2019, 9, 8587. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.R.; Gundersen, J.K.; Falck, M.; Maes, E.; Osredkar, D.; Løberg, E.M.; Sabir, H.; Walløe, L.; Thoresen, M. Variability and sex-dependence of hypothermic neuroprotection in a rat model of neonatal hypoxic-ischaemic brain injury: A single laboratory meta-analysis. Sci. Rep. 2020, 10, 10833. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; van Bel, F.; van der Kooij, M.A.; Heijnen, C.J.; Groenendaal, F. Hypothermia and erythropoietin for neuroprotection after neonatal brain damage. Pediatr. Res. 2013, 73, 18–23. [Google Scholar] [CrossRef]
- Smith, A.L.; Rosenkrantz, T.S.; Fitch, R.H. Effects of Sex and Mild Intrainsult Hypothermia on Neuropathology and Neural Reorganization following Neonatal Hypoxic Ischemic Brain Injury in Rats. Neural Plast. 2016, 2016, 2585230. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Verstegen, M.M.A.; Mezzanotte, L.; de Jonge, J.; Löwik, C.W.G.M.; van der Laan, L.J.W. Necroptotic cell death in liver transplantation and underlying diseases: Mechanisms and clinical perspective. Liver Transpl. 2019, 25, 1091–1104. [Google Scholar] [CrossRef]
- Speir, M.; Lawlor, K.E. RIP-roaring inflammation: RIPK1 and RIPK3 driven NLRP3 inflammasome activation and autoinflammatory disease. Semin. Cell Dev. Biol. 2021, 109, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ofengeim, D.; Najafov, A.; Das, S.; Saberi, S.; Li, Y.; Hitomi, J.; Zhu, H.; Chen, H.; Mayo, L.; et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 2016, 353, 603–608. [Google Scholar] [CrossRef]
- Chevin, M.; Sébire, G. Necroptosis in ALS: A hot topic in-progress. Cell Death Discov. 2021, 7, 79. [Google Scholar] [CrossRef]
- Ofengeim, D.; Ito, Y.; Najafov, A.; Zhang, Y.; Shan, B.; DeWitt, J.P.; Ye, J.; Zhang, X.; Chang, A.; Vakifahmetoglu-Norberg, H.; et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015, 10, 1836–1849. [Google Scholar] [CrossRef]
- Ofengeim, D.; Mazzitelli, S.; Ito, Y.; DeWitt, J.P.; Mifflin, L.; Zou, C.; Das, S.; Adiconis, X.; Chen, H.; Zhu, H.; et al. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, E8788–E8797. [Google Scholar] [CrossRef]
- Iannielli, A.; Bido, S.; Folladori, L.; Segnali, A.; Cancellieri, C.; Maresca, A.; Massimino, L.; Rubio, A.; Morabito, G.; Caporali, L.; et al. Pharmacological Inhibition of Necroptosis Protects from Dopaminergic Neuronal Cell Death in Parkinson’s Disease Models. Cell Rep. 2018, 22, 2066–2079. [Google Scholar] [CrossRef] [PubMed]
- Weisel, K.; Scott, N.E.; Tompson, D.J.; Votta, B.J.; Madhavan, S.; Povey, K.; Wolstenholme, A.; Simeoni, M.; Rudo, T.; Richards-Peterson, L.; et al. Randomized clinical study of safety, pharmacokinetics, and pharmacodynamics of RIPK1 inhibitor GSK2982772 in healthy volunteers. Pharmacol. Res. Perspect. 2017, 5, e00365. [Google Scholar] [CrossRef] [PubMed]
- Kletkiewicz, H.; Klimiuk, M.; Woźniak, A.; Mila-Kierzenkowska, C.; Dokladny, K.; Rogalska, J. How to Improve the Antioxidant Defense in Asphyxiated Newborns—Lessons from Animal Models. Antioxidants 2020, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.Y.; Asselin, J. Effect of mild hypothermia on cerebral energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. Stroke 1996, 27, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Wisnowski, J.L.; Wu, T.-W.; Reitman, A.J.; McLean, C.; Friedlich, P.; Vanderbilt, D.; Ho, E.; Nelson, M.D.; Panigrahy, A.; Blüml, S. The effects of therapeutic hypothermia on cerebral metabolism in neonates with hypoxic-ischemic encephalopathy: An in vivo 1H-MR spectroscopy study. J. Cereb. Blood Flow Metab. 2016, 36, 1075–1086. [Google Scholar] [CrossRef]
- Tovar-y-Romo, L.B.; Penagos-Puig, A.; Ramírez-Jarquín, J.O. Endogenous recovery after brain damage: Molecular mechanisms that balance neuronal life/death fate. J. Neurochem. 2016, 136, 13–27. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Sridharan, H.; Huang, C.; Mandal, P.; Upton, J.W.; Gough, P.J.; Sehon, C.A.; Marquis, R.W.; Bertin, J.; Mocarski, E.S. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013, 288, 31268–31279. [Google Scholar] [CrossRef]
- Walsh, C.M. Grand challenges in cell death and survival: Apoptosis vs. necroptosis. Front. Cell Dev. Biol. 2014, 2, 3. [Google Scholar] [CrossRef]
- Feltham, R.; Vince, J.E.; Lawlor, K.E. Caspase-8: Not so silently deadly. Clin. Transl. Immunol. 2017, 6, e124. [Google Scholar] [CrossRef]
- Kang, T.-B.; Yang, S.-H.; Toth, B.; Kovalenko, A.; Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 2013, 38, 27–40. [Google Scholar] [CrossRef]
- Wegner, K.W.; Saleh, D.; Degterev, A. Complex pathologic roles of RIPK1 and RIPK3: Moving beyond necroptosis. Trends Pharmacol. Sci. 2017, 38, 202–225. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevin, M.; Chabrier, S.; Allard, M.-J.; Sébire, G. Necroptosis Blockade Potentiates the Neuroprotective Effect of Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy. Biomedicines 2022, 10, 2913. https://doi.org/10.3390/biomedicines10112913
Chevin M, Chabrier S, Allard M-J, Sébire G. Necroptosis Blockade Potentiates the Neuroprotective Effect of Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy. Biomedicines. 2022; 10(11):2913. https://doi.org/10.3390/biomedicines10112913
Chicago/Turabian StyleChevin, Mathilde, Stéphane Chabrier, Marie-Julie Allard, and Guillaume Sébire. 2022. "Necroptosis Blockade Potentiates the Neuroprotective Effect of Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy" Biomedicines 10, no. 11: 2913. https://doi.org/10.3390/biomedicines10112913
APA StyleChevin, M., Chabrier, S., Allard, M.-J., & Sébire, G. (2022). Necroptosis Blockade Potentiates the Neuroprotective Effect of Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy. Biomedicines, 10(11), 2913. https://doi.org/10.3390/biomedicines10112913




