Estimation of Early Postmortem Interval from Long Noncoding RNA Gene Expression in the Incised Cutaneous Wound: An Experimental Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. Animal
2.3. Excisional Wound Model and Sampling
2.4. Histopathological Examination
2.5. Detection of Matrix Metalloproteases-9 (MMP-9) Expression
2.6. Detection of Long Noncoding Fatty Acid Oxidation (lncFAO) Gene Expression
2.6.1. Isolation of Total RNA
2.6.2. Reverse Transcription Reaction
2.6.3. Real Time-Polymerase Chain Reaction
3. Statistical Analysis
4. Results
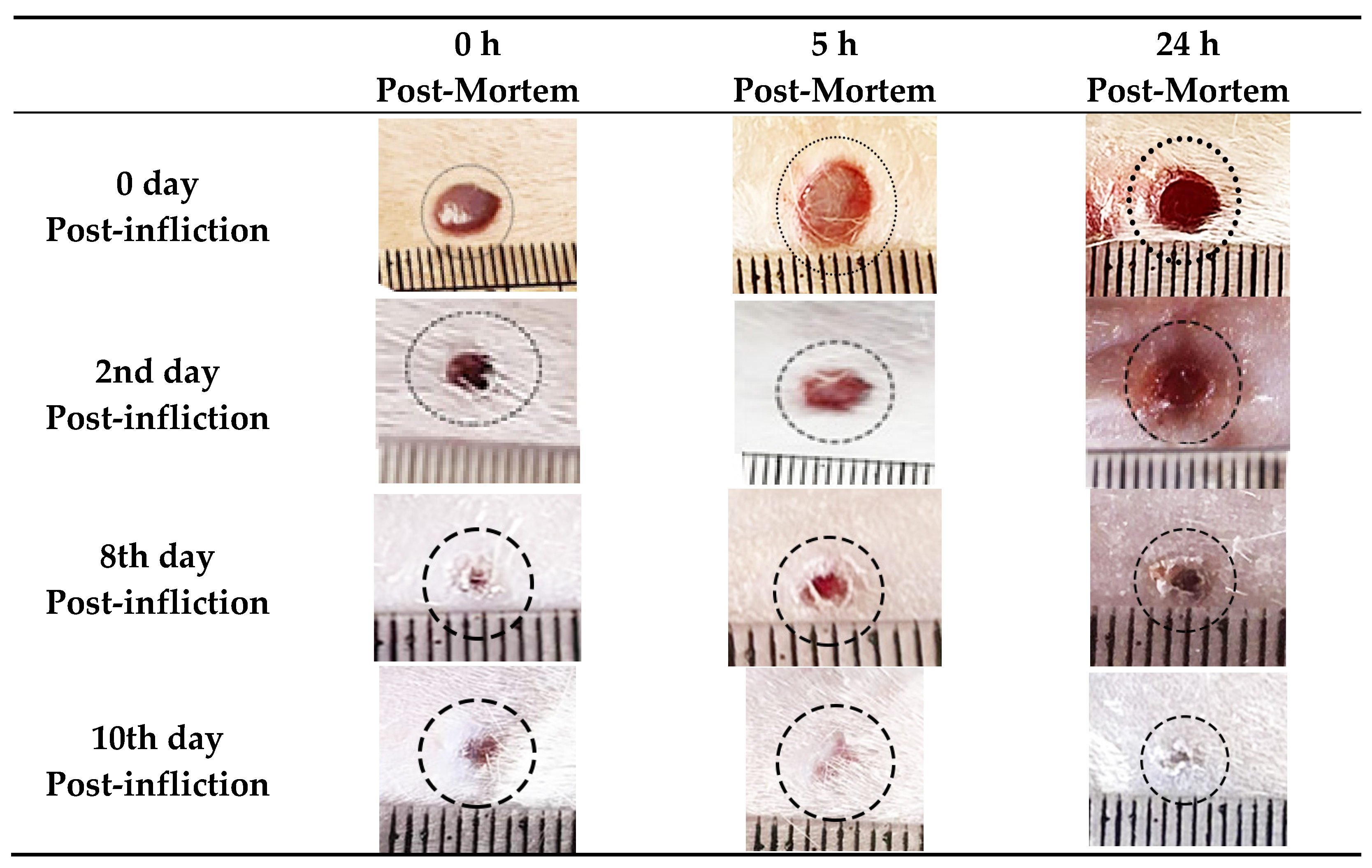
4.1. Morphological and Histopathological Findings
4.2. Immunohistochemical Study of Matrix Metalloproteases-9 (MMP-9) Expression
4.3. Long Noncoding Fatty Acid Oxidation (lncFAO) Gene Expression
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Khalaf, A.A.; Hassanen, E.I.; Zaki, A.R.; Tohamy, A.F.; Ibrahim, M.A. Histopathological, immunohistochemical, and molecular studies for determination of wound age and vitality in rats. Int. Wound J. 2019, 16, 1416–1425. [Google Scholar] [CrossRef]
- Luan, A.; Hu, M.S.; Leavitt, T.; Brett, E.A.; Wang, K.C.; Longaker, M.T.; Wan, D.C. Noncoding RNAs in wound healing: A new and vast frontier. Adv. Wound Care 2018, 7, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betz, P. Histological and enzyme histochemical parameters for the age estimation of human skin wounds. Int. J. Leg. Med. 1994, 107, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kuninaka, Y.; Nosaka, M.; Kimura, A.; Kawaguchi, T.; Hama, M.; Sakamoto, S.; Shinozaki, K.; Eisenmenger, W.; Kondo, T. Immunohistochemical analysis on MMP-2 and MMP-9 for wound age determination. Int. J. Leg. Med. 2015, 129, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Fujiu, K.; Yuki, R.; Oishi, Y.; Morioka, M.S.; Isagawa, T.; Matsuda, J.; Oshima, T.; Matsubara, T.; Sugita, J.; et al. A long noncoding RNA regulates inflammation resolution by mouse macrophages through fatty acid oxidation activation. Proc. Natl. Acad. Sci. USA 2020, 117, 14365–14375. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Breckwoldt, K.; Remmele, C.W.; Hartmann, D.; Dittrich, M.; Pfanne, A.; Just, A.; Xiao, K.; Kunz, M.; Müller, T.; et al. Development of long noncoding RNA-based strategies to modulate tissue vascularization. J. Am. Coll. Cardiol. 2015, 66, 2005–2015. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Long, W.; Li, Q.; Zhou, Q.; Wang, Y.; Wang, H.; Zhou, B.; Li, J. Distinct expression profiles of lncRNAs between regressive and mature scars. Cell. Physiol. Biochem. 2015, 35, 663–675. [Google Scholar] [CrossRef] [Green Version]
- Niedecker, A.; Huhn, R.; Ritz-Timme, S.; Mayer, F. Complex challenges of estimating the age and vitality of muscle wounds: A study with matrix metalloproteinases and their inhibitors on animal and human tissue samples. Int. J. Leg. Med. 2021, 135, 1843–1853. [Google Scholar] [CrossRef]
- Ågren, M.S.; auf dem Keller, U. Matrix Metalloproteinases: How Much Can They Do? Int. J. Mol. Sci. 2020, 21, 2678. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Krejner, A.; Litwiniuk, M.; Grzela, T. Matrix metalloproteinases in the wound microenvironment: Therapeutic perspectives. Chronic Wound Care Manag. Res. 2016, 3, 29–39. [Google Scholar]
- Liang, Z.-H.; Pan, Y.-C.; Lin, S.-S.; Qiu, Z.-Y.; Zhang, Z. LncRNA MALAT1 promotes wound healing via regulating miR-141-3p/ZNF217 axis. Regen. Ther. 2020, 15, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zörnig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.-C.; Rui, B.-Y.; Wang, Q.-Y.; Zhou, D.; Zhang, Y.; Guo, S.-C. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018, 25, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, L.; Liechty, C.; Zgheib, C.; Hodges, M.M.; Liechty, K.W.; Xu, J. Long Noncoding RNA GAS5 Regulates Macrophage Polarization and Diabetic Wound Healing. J. Investig. Dermatol. 2020, 140, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Du, Q.; Bai, R.; Sun, J. Vitality and wound-age estimation in forensic pathology: Review and future prospects. Forensic Sci. Res. 2020, 5, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, P.G.; Munoz-Aguirre, M.; Reverter, F.; Sa Godinho, C.P.; Sousa, A.; Amadoz, A.; Sodaei, R.; Hidalgo, M.R.; Pervouchine, D.; Carbonell-Caballero, J.; et al. The effects of death and post-mortem cold ischemia on human tissue transcriptomes. Nat. Commun. 2018, 9, 490. [Google Scholar] [CrossRef] [Green Version]
- De Groot, C.J.; Jan-willem, M.T.; Dijkstra, C.D.; Van Der Valk, P. Postmortem delay effects on neuroglial cells and brain macrophages from Lewis rats with acute experimental allergic encephalomyelitis: An immunohistochemical and cytochemical study. J. Neuroimmunol. 1995, 59, 123–134. [Google Scholar] [CrossRef]
- Santos, G.A.; Vila, M.M.; Chaud, M.V.; Silva, W.L.; de Castro, A.G.; de Oliveira, J.M., Jr.; Tubino, M.; Balcao, V.M. Antimicrobial and antioxidant screening of curcumin and pyrocatechol in the prevention of biodiesel degradation: Oxidative stability. Biofuels 2016, 7, 581–592. [Google Scholar] [CrossRef] [Green Version]
- De Simone, S.; Giacani, E.; Bosco, M.A.; Vittorio, S.; Ferrara, M.; Bertozzi, G.; Cipolloni, L.; La Russa, R. The Role of miRNAs as New Molecular Biomarkers for Dating the Age of Wound Production: A Systematic Review. Front. Med. 2022, 8, 803067. [Google Scholar] [CrossRef]
- Byard, R.W.; Saukko, P. Bernard Knight: Knight’s Forensic Pathology, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Sharma, A.; Dikshit, P.; Aggrawal, A.; Mandal, A. A postmortem study of histopathological findings to determine the age of abrasion and laceration. J. Forensic Med. Toxicol. 2010, 27, 43–46. [Google Scholar]
- Hu, M.; Wu, Y.; Yang, C.; Wang, X.; Wang, W.; Zhou, L.; Zeng, T.; Zhou, J.; Lao, G.; Yan, L.; et al. Novel long noncoding RNA lnc-URIDS delays diabetic wound healing by targeting Plod1. Diabetes 2020, 69, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care. 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Sharma, C.; Dobson, G.P.; Davenport, L.M.; Morris, J.L.; Letson, H.L. The role of matrix metalloproteinase-9 and its inhibitor TIMP-1 in burn injury: A systematic review. Int. J. Burn. Trauma 2021, 11, 275. [Google Scholar]
- Tarlton, J.; Vickery, C.; Leaper, D.; Bailey, A. Postsurgical wound progression monitored by temporal changes in the expression of matrix metalloproteinase-9. Br. J. Dermatol. 1997, 137, 506–516. [Google Scholar] [CrossRef]
- Reiss, M.J.; Han, Y.P.; Garcia, E.; Goldberg, M.; Yu, H.; Garner, W.L. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery 2010, 147, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Mirza, R.; Koh, T.J. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011, 56, 256–264. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997, 4, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Almulhim, A.M.; Menezes, R.G. Evaluation of Postmortem Changes; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Wei, W.; Michu, Q.; Wenjuan, D.; Jianrong, W.; Zhibing, H.; Ming, Y.; Bo, J.; Xia, L. Histological changes in human skin 32 days after death and the potential forensic significance. Sci. Rep. 2020, 10, 18753. [Google Scholar] [CrossRef]
- Dolfi, B.; Gallerand, A.; Haschemi, A.; Guinamard, R.R.; Ivanov, S. Macrophage metabolic regulation in atherosclerotic plaque. Atherosclerosis 2021, 334, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Mounier, R.; Horvath, A.; Cuvellier, S.; Dumont, F.; Poliska, S.; Ardjoune, H.; Juban, G.; Nagy, L.; Chazaud, B. Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J. Immunol. 2016, 196, 4771–4782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, P.; He, L.; Zimmerman, M.; Yang, G.; Köster, S.; Ouimet, M.; Wang, H.; Moore, K.J.; Dartois, V.; Schilling, J.D.; et al. Inhibition of fatty acid oxidation promotes macrophage control of mycobacterium tuberculosis. mBio 2020, 11, e01139-20. [Google Scholar] [CrossRef]
- Sun, J.-H.; Zhu, X.-Y.; Li, S.-Q.; Dong, T.-N.; Du, Q.-X. Measuring temporal expression, systematic response, and post-mortem stability to assess potential markers for estimating wound age: An example of Fosl1 in contused skeletal muscle. Aust. J. Forensic Sci. 2019, 51, 158–170. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Geiß, C.; Salas, E.; Guevara-Coto, J.; Régnier-Vigouroux, A.; Mora-Rodríguez, R.A. Multistability in Macrophage Activation Pathways and Metabolic Implications. Cells 2022, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Pi, L.; Fang, B.; Meng, X.; Qian, L. LncRNA XIST accelerates burn wound healing by promoting M2 macrophage polarization through targeting IL-33 via miR-19b. Cell Death Discov. 2022, 8, 220. [Google Scholar] [CrossRef] [PubMed]




| Post-Wounding Time | Congestion | Edema | Hemorrhage | Cellular Infiltration | Fibroblast Proliferation | Angiogenesis | Epithelization | Collagen |
|---|---|---|---|---|---|---|---|---|
| 0 day | - | + | - | - | - | - | - | - |
| 2nd day | ++ | ++ | + | ++ | + | - | - | - |
| 4th day | +++ | +++ | ++ | +++ | ++ | + | +/++ | - |
| 8th day | ++ | ++ | + | +/++ | ++ | ++ | ++/+++ | ++ |
| 10th day | - | - | - | - | - | - | +++ | +++ |
| Postmortem | 0 h | 5 h | 24 h | |
|---|---|---|---|---|
| Post-Wounding | ||||
| 0 day | 0.9 ± 0.2 | 0.95 ± 0.3 | 0.92 ± 0.3 | |
| 2nd day | 1.95 ± 0.7 * | 1.8 ± 0.8 * | 1.6 ± 0.5 *,# | |
| 4th day | 2.8 ± 0.8 * | 2.6 ± 0.3 * | 2.2 ± 0.2 *,# | |
| 8th day | 5.45 ± 0.5 * | 3.61 ± 1 *,# | 2 ± 0.7 *,# | |
| 10th day | 10 ± 2.5 * | 4.2 ± 1 *,# | 2.4 ± 0.8 *,# | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.M.; Ibrahim, S.F.; Elrewieny, N.M.; Elyamany, A.M.; Khalil, W.K.B.; Shalby, A.B.; Khater, S.A. Estimation of Early Postmortem Interval from Long Noncoding RNA Gene Expression in the Incised Cutaneous Wound: An Experimental Study. Biomedicines 2022, 10, 2919. https://doi.org/10.3390/biomedicines10112919
Ali MM, Ibrahim SF, Elrewieny NM, Elyamany AM, Khalil WKB, Shalby AB, Khater SA. Estimation of Early Postmortem Interval from Long Noncoding RNA Gene Expression in the Incised Cutaneous Wound: An Experimental Study. Biomedicines. 2022; 10(11):2919. https://doi.org/10.3390/biomedicines10112919
Chicago/Turabian StyleAli, Mona M., Samah F. Ibrahim, Noha M. Elrewieny, Aya M. Elyamany, Wagdy K. B. Khalil, Aziza B. Shalby, and Sarah A. Khater. 2022. "Estimation of Early Postmortem Interval from Long Noncoding RNA Gene Expression in the Incised Cutaneous Wound: An Experimental Study" Biomedicines 10, no. 11: 2919. https://doi.org/10.3390/biomedicines10112919
APA StyleAli, M. M., Ibrahim, S. F., Elrewieny, N. M., Elyamany, A. M., Khalil, W. K. B., Shalby, A. B., & Khater, S. A. (2022). Estimation of Early Postmortem Interval from Long Noncoding RNA Gene Expression in the Incised Cutaneous Wound: An Experimental Study. Biomedicines, 10(11), 2919. https://doi.org/10.3390/biomedicines10112919






