Association between Obesity, Race or Ethnicity, and Luminal Subtypes of Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Molecular and Clinical Subtyping
2.3. Statistical Methods
3. Results
3.1. Characteristics of Patients with Luminal A vs. Luminal B Breast Cancer
3.2. Effect of Race on Luminal B Breast Cancer
3.3. Predicting the Risk of Luminal B vs. Luminal A Cancer Using Demographic Variables
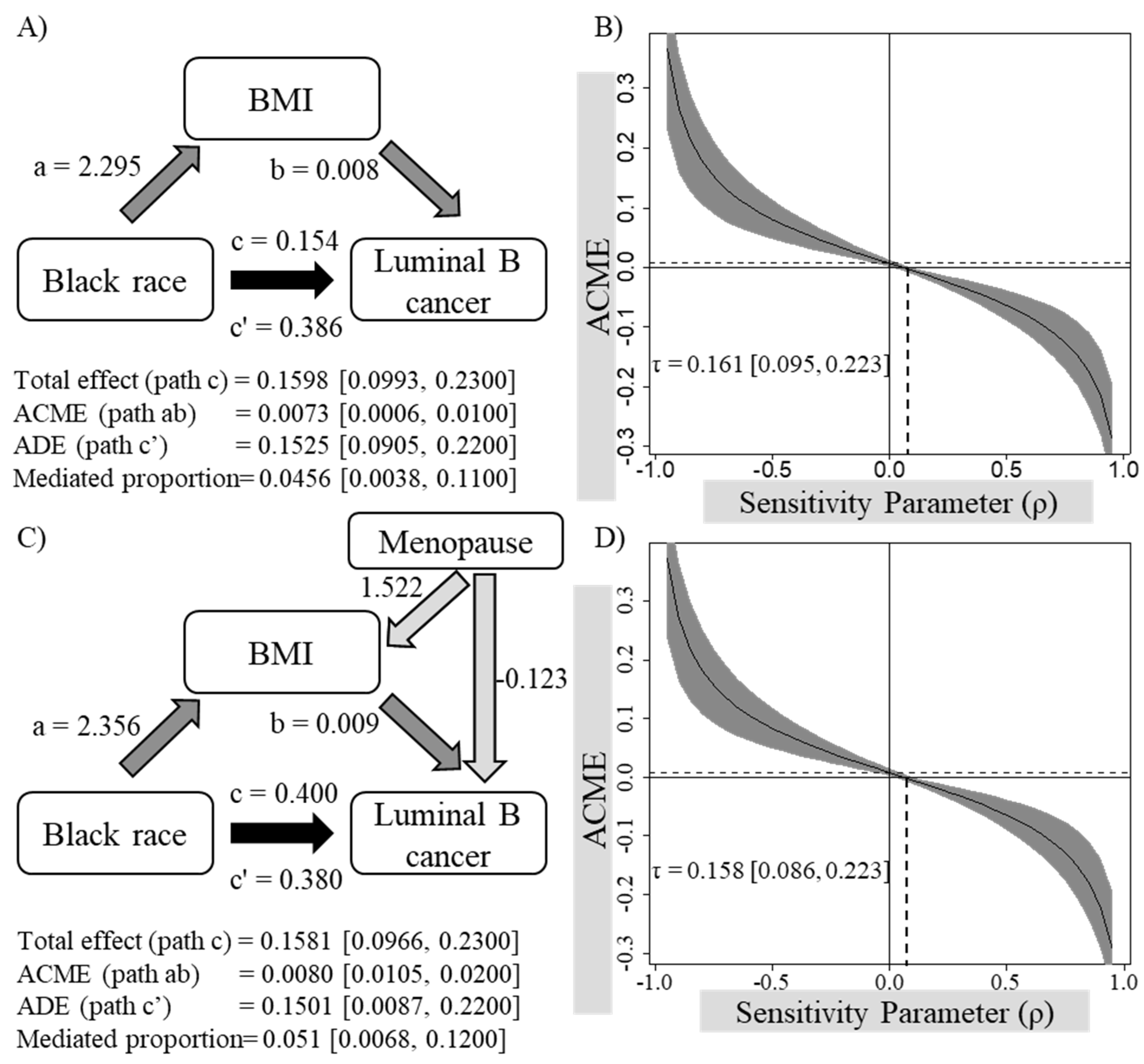
3.4. Analyses Examining Whether BMI Mediates the Association between Black/African American Race and Luminal B Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Althuis, M.D.; Fergenbaum, J.H.; Garcia-Closas, M.; Brinton, L.A.; Madigan, M.P.; Sherman, M.E. Etiology of hormone receptor–defined breast cancer: A systematic review of the literature. Cancer Epidemiol. Prev. Biomark. 2004, 13, 1558–1568. [Google Scholar] [CrossRef]
- Anastasiadi, Z.; Lianos, G.D.; Ignatiadou, E.; Harissis, H.V.; Mitsis, M. Breast cancer in young women: An overview. Updat. Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Makki, J.; Myint, O.; Wynn, A.A.; Samsudin, A.T.; Daisy Vanitha, J. Expression distribution of cancer stem cells, epithelial to mesenchymal transition, and telomerase activity in breast cancer and their association with clinicopathologic characteristics. Clin. Med. Insights: Pathol. 2015, 8, CPath-S19615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5· 24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Munsell, M.F.; Sprague, B.L.; Berry, D.A.; Chisholm, G.; Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev. 2014, 36, 114–136. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer: Targets Ther. 2019, 11, 151. [Google Scholar] [CrossRef] [Green Version]
- Jerônimo, A.F.d.A.; Freitas, Â.G.Q.; Weller, M. Risk factors of breast cancer and knowledge about the disease: An integrative revision of Latin American studies. Cienc. Saude Coletiva 2017, 22, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Albrektsen, G.; Heuch, I.; Hansen, S.; Kvåle, G. Breast cancer risk by age at birth, time since birth and time intervals between births: Exploring interaction effects. Br. J. Cancer 2005, 92, 167–175. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Noubissi, F.; Lowe, L.; Fonseca, D.D.; Alo, R.A.; Payton, M.; Tchounwou, P.B. Health and racial disparity in breast cancer. Breast Cancer Metastasis Drug Resist. 2019, 1152, 31–49. [Google Scholar]
- Dai, X.; Li, T.; Bai, Z.; Yang, Y.; Liu, X.; Zhan, J.; Shi, B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015, 5, 2929. [Google Scholar] [PubMed]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J. Cancer 2016, 7, 1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160. [Google Scholar] [CrossRef]
- Gao, J.J.; Swain, S.M. Luminal a breast cancer and molecular assays: A review. Oncol. 2018, 23, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, B.; Bedard, P.L. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011, 13, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Moustaid-Moussa, N.; Claycombe, K.J. Immunity as a link between obesity and insulin resistance. Mol. Asp. Med. 2012, 33, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Menikdiwela, K.R.; Tôrres Guimarães, J.P.; Ramalingam, L.; Kalupahana, N.S.; Dufour, J.M.; Washburn, R.L.; Moustaid-Moussa, N. Mechanisms linking endoplasmic reticulum (ER) stress and microRNAs to adipose tissue dysfunction in obesity. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 455–481. [Google Scholar] [CrossRef]
- Ramalingam, L.; Menikdiwela, K.; LeMieux, M.; Dufour, J.M.; Kaur, G.; Kalupahana, N.; Moustaid-Moussa, N. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1106–1114. [Google Scholar] [CrossRef]
- Rasha, F.; Ramalingam, L.; Gollahon, L.; Rahman, R.L.; Rahman, S.M.; Menikdiwela, K.; Moustaid-Moussa, N. Mechanisms linking the renin-angiotensin system, obesity, and breast cancer. Endocr. Relat. Cancer 2019, 26, R653–R672. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.-Y.; Brain, E.; Causeret, S.; DeLorenzi, M. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krijgsman, O.; Roepman, P.; Zwart, W.; Carroll, J.S.; Tian, S.; de Snoo, F.A.; Bender, R.A.; Bernards, R.; Glas, A.M. A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res. Treat. 2012, 133, 37–47. [Google Scholar] [CrossRef]
- Sobel, M.E. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol. Methodol. 1982, 13, 290–312. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Personal. Soc. Psychol. 1986, 51, 1173. [Google Scholar] [CrossRef]
- Imai, K.; Keele, L.; Yamamoto, T. Identification, inference and sensitivity analysis for causal mediation effects. Stat. Sci. 2010, 25, 51–71. [Google Scholar] [CrossRef] [Green Version]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.-M. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl. Cancer Inst. 2015, 107, djv048. [Google Scholar] [CrossRef]
- Troester, M.A.; Sun, X.; Allott, E.H.; Geradts, J.; Cohen, S.M.; Tse, C.-K.; Kirk, E.L.; Thorne, L.B.; Mathews, M.; Li, Y. Racial differences in PAM50 subtypes in the Carolina Breast Cancer Study. JNCI: J. Natl. Cancer Inst. 2018, 110, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef] [Green Version]
- Reid, S.; Haddad, D.; Tezak, A.; Weidner, A.; Wang, X.; Mautz, B.; Moore, J.; Cadiz, S.; Zhu, Y.; Zheng, W. Impact of molecular subtype and race on HR+, HER2− breast cancer survival. Breast Cancer Res. Treat. 2021, 189, 845–852. [Google Scholar] [CrossRef]
- Acheampong, T.; Kehm, R.D.; Terry, M.B.; Argov, E.L.; Tehranifar, P. Incidence trends of breast cancer molecular subtypes by age and race/ethnicity in the US from 2010 to 2016. JAMA Netw. Open 2020, 3, e2013226. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.A.; Keegan, T.H.; Yang, J.; Press, D.J.; Kurian, A.W.; Patel, A.H.; Lacey Jr, J.V. Age-specific incidence of breast cancer subtypes: Understanding the black–white crossover. J. Natl. Cancer Inst. 2012, 104, 1094–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, B.; Han, Y.; Lian, M.; Colditz, G.A.; Weber, J.D.; Ma, C.; Liu, Y. Evaluation of racial/ethnic differences in treatment and mortality among women with triple-negative breast cancer. JAMA Oncol. 2021, 7, 1016–1023. [Google Scholar] [CrossRef]
- Eley, J.W.; Hill, H.A.; Chen, V.W.; Austin, D.F.; Wesley, M.N.; Muss, H.B.; Greenberg, R.S.; Coates, R.J.; Correa, P.; Redmond, C.K. Racial differences in survival from breast cancer: Results of the National Cancer Institute Black/White Cancer Survival Study. Jama 1994, 272, 947–954. [Google Scholar] [CrossRef]
- Stapleton, S.M.; Oseni, T.O.; Bababekov, Y.J.; Hung, Y.-C.; Chang, D.C. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018, 153, 594–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.K.; Wiggins, C.L.; Nibbe, A.M.; Storlie, C.B.; Prossnitz, E.R.; Royce, M.; Lomo, L.C.; Hill, D.A. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. NPJ Breast Cancer 2019, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Saxena, N.K.; Sharma, D. Metastasis suppression by adiponectin: LKB1 rises up to the challenge. Cell Adhes. Migr. 2010, 4, 358–362. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, Y.; Funahashi, T.; Tanaka, S.; Taguchi, T.; Tamaki, Y.; Shimomura, I.; Noguchi, S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int. J. Cancer 2006, 118, 1414–1419. [Google Scholar] [CrossRef]
- Dieudonne, M.-N.; Bussiere, M.; Dos Santos, E.; Leneveu, M.-C.; Giudicelli, Y.; Pecquery, R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 2006, 345, 271–279. [Google Scholar] [CrossRef]
- Surmacz, E. Leptin and adiponectin: Emerging therapeutic targets in breast cancer. J. Mammary Gland Biol. Neoplasia 2013, 18, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Turkoz, F.P.; Solak, M.; Petekkaya, I.; Keskin, O.; Kertmen, N.; Sarici, F.; Arik, Z.; Babacan, T.; Ozisik, Y.; Altundag, K. Association between common risk factors and molecular subtypes in breast cancer patients. Breast 2013, 22, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, M.M.; Press, M.F.; Haile, R.W.; Lynch, C.F.; Glaser, S.L.; Schildkraut, J.; Gammon, M.D.; Douglas Thompson, W.; Bernstein, J.L. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res. Treat. 2011, 130, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouckaert, O.; Van Asten, K.; Laenen, A.; Soubry, A.; Smeets, A.; Nevelstreen, I.; Vergote, I.; Wildiers, H.; Paridaens, R.; Van Limbergen, E. Body mass index, age at breast cancer diagnosis, and breast cancer subtype: A cross-sectional study. Breast Cancer Res. Treat. 2018, 168, 189–196. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Colby, S.; Ortman, J.M. Projections of the Size and Composition of the US Population: 2014 to 2060; US Census Bureau: Washington, DC, USA, 2015.
| Luminal A | Luminal B | Δ [95% CI]/Proportion ‡ | t/χ2 ‡ | df | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Mean/N † | SD/% † | Mean/N † | SD/% † | |||||
| BMI | 29.86 | 7.10 | 30.55 | 7.52 | −0.69 [−1.21, −0.17] | −2.583 | 2890.6 | 0.010 |
| Age | 61.43 | 11.12 | 60.18 | 12.51 | 1.25 [0.42, 2.08] | 2.950 | 2922.2 | 0.003 |
| Menopause | 1340 | 0.82 | 1057 | 0.79 | 0.56 | 4.982 | 1 | 0.026 |
| Race | 25.254 | 7 | <0.001 | |||||
| Black/African American | 112 | 0.06 | 165 | 0.10 | 0.40 | |||
| Asian | 38 | 0.02 | 37 | 0.02 | 0.51 | |||
| Latin American | 93 | 0.05 | 73 | 0.05 | 0.56 | |||
| Native American | 4 | 0.00 | 3 | 0.00 | 0.57 | |||
| Pacific Islander | 3 | 0.00 | 3 | 0.00 | 0.50 | |||
| Other | 35 | 0.02 | 30 | 0.02 | 0.54 | |||
| Unknown | 253 | 0.13 | 210 | 0.13 | 0.55 | |||
| White | 1390 | 0.72 | 1089 | 0.68 | 0.56 | |||
| Model | Variable | β | SE | t-Statistic | p-Value |
|---|---|---|---|---|---|
| 1 | Intercept | 0.361 | 0.038 | 9.411 | <0.001 |
| BMI | 0.003 | 0.001 | 2.595 | 0.010 | |
| 2 | Intercept | 0.590 | 0.046 | 12.729 | <0.001 |
| Age | −0.002 | 0.001 | −2.983 | 0.003 | |
| 3 | Intercept | 0.494 | 0.021 | 23.690 | <0.001 |
| Menopause | −0.053 | 0.023 | −2.280 | 0.023 | |
| 4 | Intercept | 0.596 | 0.030 | 19.957 | <0.001 |
| Asian | −0.102 | 0.065 | −1.583 | 0.114 | |
| Latin American | −0.156 | 0.049 | −3.198 | 0.001 | |
| Native American | −0.167 | 0.190 | −0.879 | 0.380 | |
| Pacific Islander | −0.096 | 0.205 | −0.467 | 0.641 | |
| Other | −0.134 | 0.068 | −1.959 | 0.050 | |
| Unknown | −0.142 | 0.038 | −3.766 | <0.001 | |
| White | −0.156 | 0.031 | −4.969 | <0.001 | |
| 5 | Intercept (All other races) | 0.441 | 0.009 | 47.053 | <0.001 |
| Black/African American | 0.154 | 0.031 | 4.936 | <0.001 | |
| 6 | Intercept | −0.511 | 0.181 | −2.816 | 0.005 |
| BMI | 0.015 | 0.005 | 0.648 | 0.008 | |
| Black/African American | 0.600 | 0.139 | 4.333 | <0.001 | |
| Menopause | −0.218 | 0.099 | −2.194 | 0.028 |
| Step | Dependent Variable | Predictor | β | SE | t-Statistic | p-Value |
|---|---|---|---|---|---|---|
| Mediation analysis without a covariate | ||||||
| 1 | Luminal B breast cancer | Intercept | 0.441 | 0.009 | 47.053 | <0.001 |
| Black/African American | 0.154 | 0.031 | 4.936 | <0.001 | ||
| 2 | BMI | Intercept | 29.988 | 0.144 | 208.187 | <0.001 |
| Black/African American | 2.295 | 0.482 | 4.764 | <0.001 | ||
| 3 | Luminal B breast cancer | Intercept | −0.396 | 0.105 | −3.783 | <0.001 |
| Black/African American | 0.386 | 0.857 | 4.501 | <0.001 | ||
| BMI | 0.008 | 0.003 | 2.399 | 0.016 | ||
| Mediation analysis including menopausal state as a covariate | ||||||
| 1 | Luminal B breast cancer | Intercept | −0.064 | 0.056 | −1.140 | 0.255 |
| Black/African American | 0.400 | 0.085 | 4.681 | <0.001 | ||
| Menopause | −0.109 | 0.062 | −1.780 | 0.075 | ||
| 2 | BMI | Intercept | 28.751 | 0.318 | 90.522 | <0.001 |
| Black/African American | 2.356 | 0.480 | 4.905 | <0.001 | ||
| Menopause | 1.522 | 0.349 | 4.364 | <0.001 | ||
| 3 | Luminal B breast cancer | Intercept | −0.314 | 0.113 | −2.787 | 0.005 |
| Black/African American | 0.380 | 0.086 | 4.429 | <0.001 | ||
| BMI | 0.009 | 0.003 | 2.558 | 0.011 | ||
| Menopause | −0.123 | 0.062 | −1.990 | 0.047 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menikdiwela, K.R.; Kahathuduwa, C.; Bolner, M.L.; Rahman, R.L.; Moustaid-Moussa, N. Association between Obesity, Race or Ethnicity, and Luminal Subtypes of Breast Cancer. Biomedicines 2022, 10, 2931. https://doi.org/10.3390/biomedicines10112931
Menikdiwela KR, Kahathuduwa C, Bolner ML, Rahman RL, Moustaid-Moussa N. Association between Obesity, Race or Ethnicity, and Luminal Subtypes of Breast Cancer. Biomedicines. 2022; 10(11):2931. https://doi.org/10.3390/biomedicines10112931
Chicago/Turabian StyleMenikdiwela, Kalhara R., Chanaka Kahathuduwa, Michelle L. Bolner, Rakhshanda Layeequr Rahman, and Naima Moustaid-Moussa. 2022. "Association between Obesity, Race or Ethnicity, and Luminal Subtypes of Breast Cancer" Biomedicines 10, no. 11: 2931. https://doi.org/10.3390/biomedicines10112931
APA StyleMenikdiwela, K. R., Kahathuduwa, C., Bolner, M. L., Rahman, R. L., & Moustaid-Moussa, N. (2022). Association between Obesity, Race or Ethnicity, and Luminal Subtypes of Breast Cancer. Biomedicines, 10(11), 2931. https://doi.org/10.3390/biomedicines10112931







