Long-Term Repeatable In Vivo Monitoring of Amyloid-β Plaques and Vessels in Alzheimer’s Disease Mouse Model with Combined TPEF/CARS Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
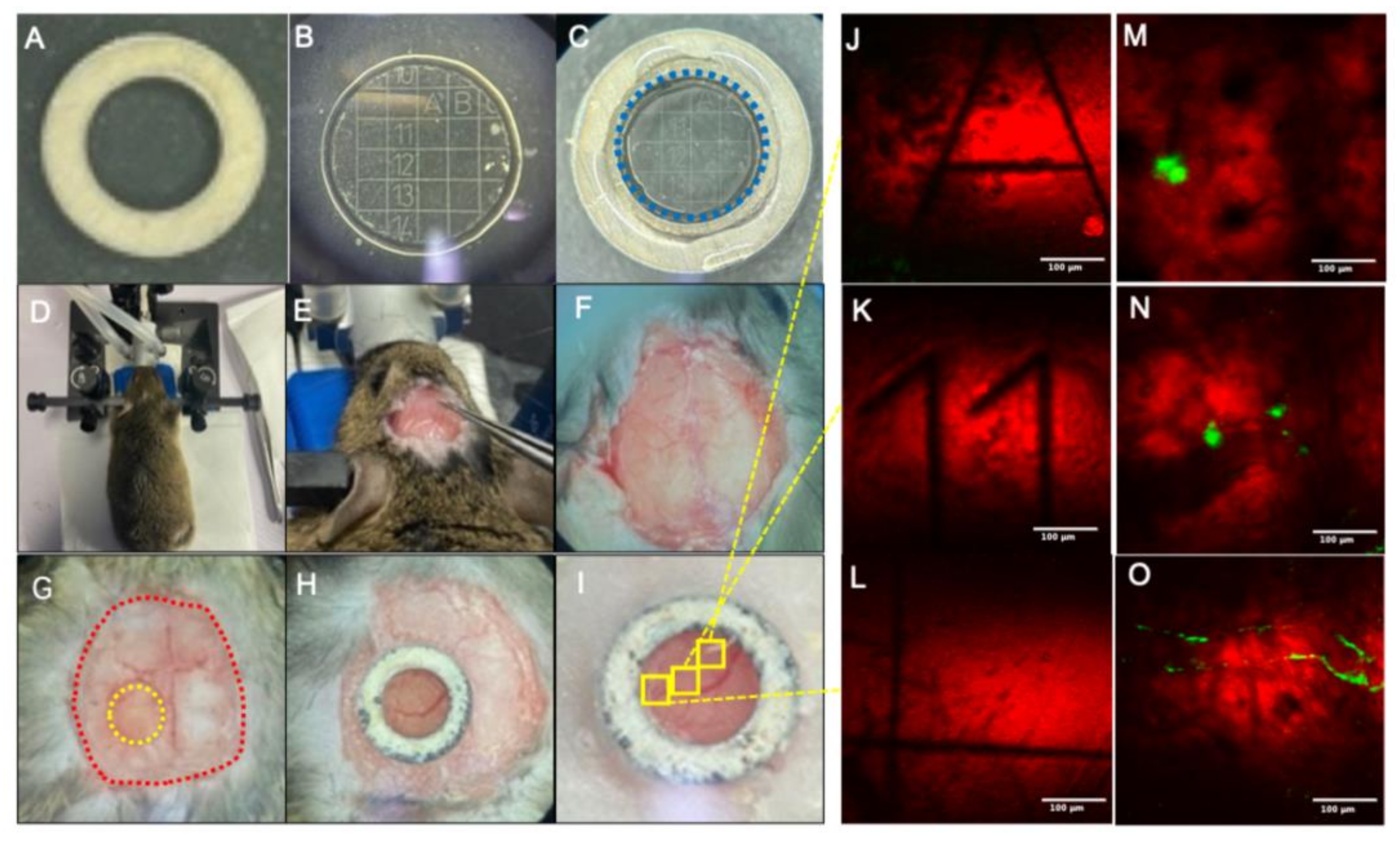
2.2. Mouse Cranial Window Fabrication
2.3. In Vivo TPEF/CARS Imaging
2.4. Image Analysis
3. Results
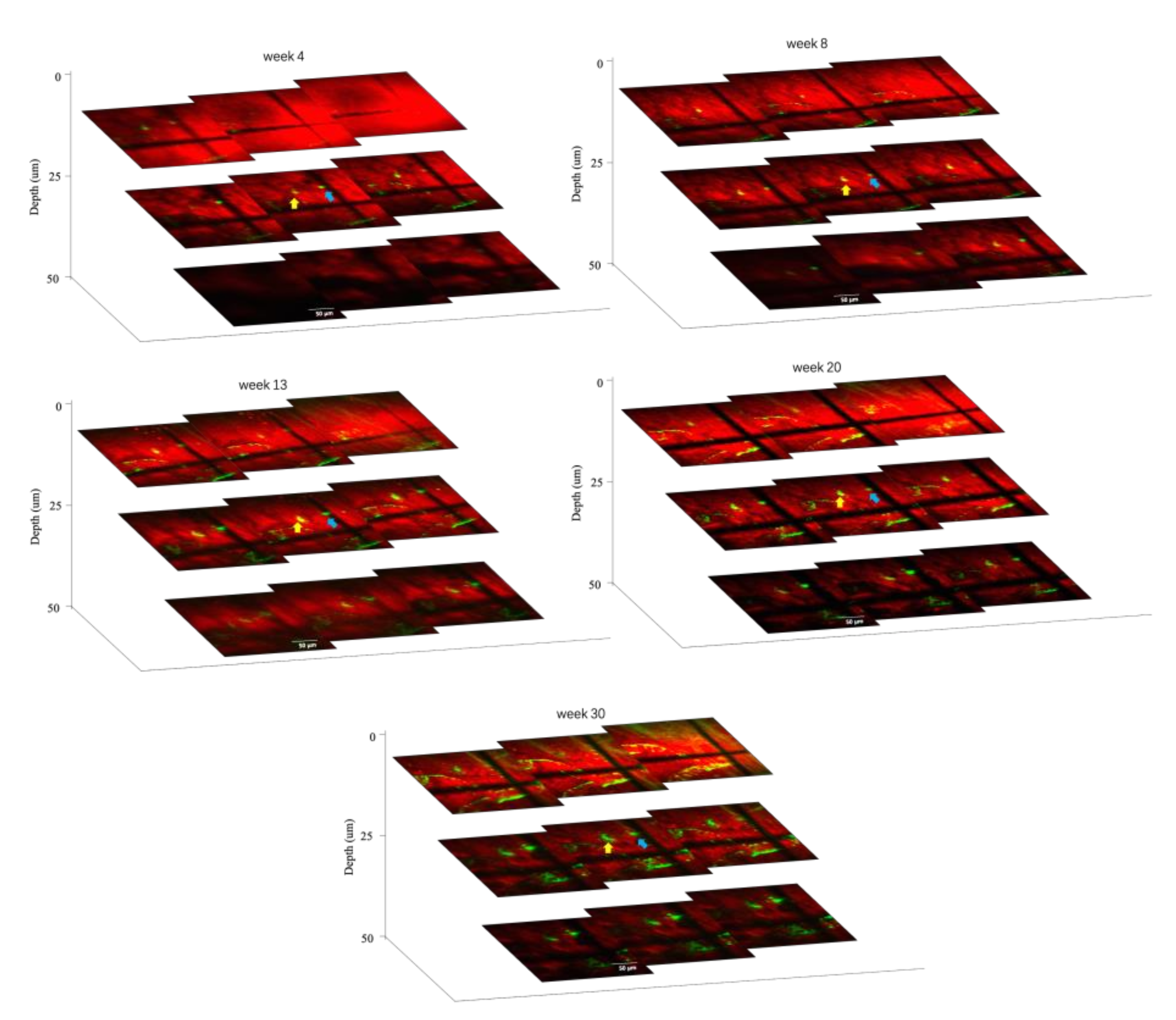
3.1. In Vivo Imaging of Aβ Plaques and Blood Vessels
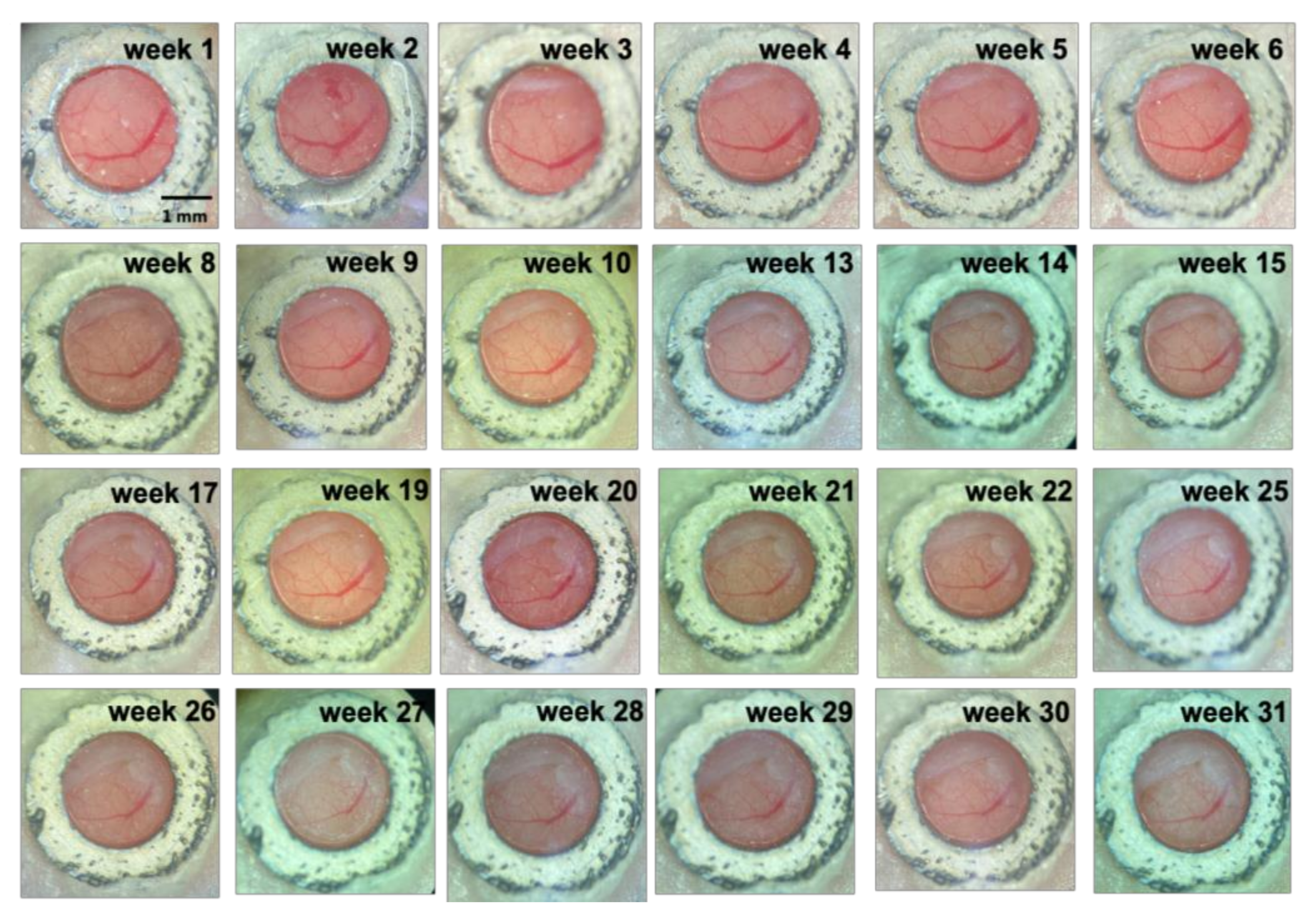
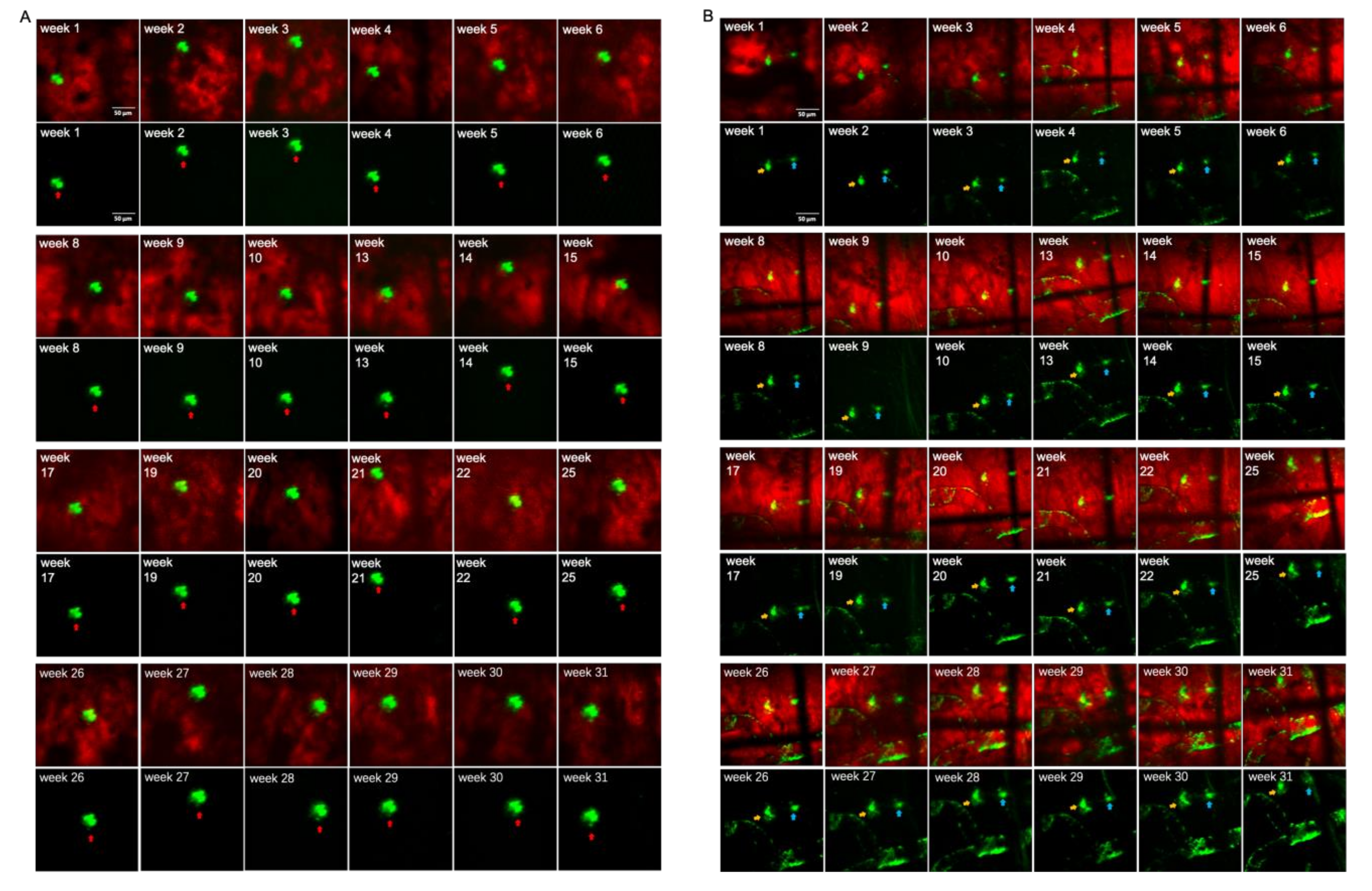
3.2. Long-Term Visualization of Amyloid Plaques
3.3. Visualization of CAA over Time
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, L.; Seifi, M.; Ma, R.; Mitchell, S.J.; Rudolph, U.; Viola, K.L.; Klein, W.L.; Lambert, J.J.; Swinny, J.D. Identification of intraneuronal amyloid beta oligomers in locus coeruleus neurons of Alzheimer’s patients and their potential impact on inhibitory neurotransmitter receptors and neuronal excitability. Neuropathol. Appl. Neurobiol. 2020, 47, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Kowall, N.W.; Beal, M.F.; Busciglio, J.; Duffy, L.K.; Yankner, B.A. An Invivo Model for the Neurodegenerative Effects of Beta-Amyloid and Protection by Substance-P. Proc. Natl. Acad. Sci. USA 1991, 88, 7247–7251. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. Medicine—The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, W.; Zhao, Y.; Song, T.; Xi, Y.; Han, G.; Jin, Y.; Song, M.; Bai, K.; Zhou, J.; et al. Lipoprotein-Inspired Nanoscavenger for the Three-Pronged Modulation of Microglia-Derived Neuroinflammation in Alzheimer’s Disease Therapy. Nano Lett. 2022, 22, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Bolmont, T.; Haiss, F.; Eicke, D.; Radde, R.; Mathis, C.A.; Klunk, W.E.; Kohsaka, S.; Jucker, M.; Calhoun, M.E. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 2008, 28, 4283–4292. [Google Scholar] [CrossRef]
- Meyer-Luehmann, M.; Spires-Jones, T.L.; Prada, C.; Garcia-Alloza, M.; de Calignon, A.; Rozkalne, A.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Bacskai, B.J.; Hyman, B.T. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 2008, 451, 720–724. [Google Scholar] [CrossRef]
- Yan, P.; Bero, A.W.; Cirrito, J.R.; Xiao, Q.L.; Hu, X.Y.; Wang, Y.; Gonzales, E.; Holtzman, D.M.; Lee, J.M. Characterizing the Appearance and Growth of Amyloid Plaques in APP/PS1 Mice. J. Neurosci. 2009, 29, 10706–10714. [Google Scholar] [CrossRef]
- Chen, C.; Liang, Z.; Zhou, B.; Li, X.; Lui, C.; Ip, N.Y.; Qu, J.Y. In Vivo Near-Infrared Two-Photon Imaging of Amyloid Plaques in Deep Brain of Alzheimer’s Disease Mouse Model. ACS Chem. Neurosci. 2018, 9, 3128–3136. [Google Scholar] [CrossRef]
- Chen, C.; Qin, Z.; He, S.; Wu, W.; Wang, Y.; Tam, K.F.; Ip, N.Y.; Qu, J.Y.; Luo, Q.; Ding, J.; et al. Adaptive optics two-photon microendoscopy for high-resolution and deep-brain imaging in vivo. Neural Imaging Sens. 2020, 11226, 1122606. [Google Scholar]
- Ding, J.; Luo, Q.; Qu, J.Y.; Ip, N.; Zhou, B.; Liang, Z.; Chen, C. Deep brain two-photon NIR fluorescence imaging for study of Alzheimer’s disease. Neural Imaging Sens. 2018, 10481, 104810A. [Google Scholar]
- Qin, Z.; He, S.; Yang, C.; Yung, J.S.; Chen, C.; Leung, C.K.; Liu, K.; Qu, J.Y. Adaptive optics two-photon microscopy enables near-diffraction-limited and functional retinal imaging in vivo. Light Sci. Appl. 2020, 9, 79. [Google Scholar] [CrossRef]
- Chen, W.W.; Lemieux, G.A.; Camp, C.H., Jr.; Chang, T.C.; Ashrafi, K.; Cicerone, M.T. Spectroscopic coherent Raman imaging of Caenorhabditis elegans reveals lipid particle diversity. Nat. Chem. Biol. 2020, 16, 1087–1095. [Google Scholar] [CrossRef]
- Evans, C.L.; Potma, E.O.; Puoris’haag, M.; Cote, D.; Lin, C.P.; Xie, X.S. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 16807–16812. [Google Scholar] [CrossRef]
- Lim, R.S.; Suhalim, J.L.; Miyazaki-Anzai, S.; Miyazaki, M.; Levi, M.; Potma, E.O.; Tromberg, B.J. Identification of cholesterol crystals in plaques of atherosclerotic mice using hyperspectral CARS imaging. J. Lipid Res. 2011, 52, 2177–2186. [Google Scholar] [CrossRef]
- Paar, M.; Jungst, C.; Steiner, N.A.; Magnes, C.; Sinner, F.; Kolb, D.; Lass, A.; Zimmermann, R.; Zumbusch, A.; Kohlwein, S.D.; et al. Remodeling of Lipid Droplets during Lipolysis and Growth in Adipocytes. J. Biol. Chem. 2012, 287, 11164–11173. [Google Scholar] [CrossRef]
- Krafft, C.; Dietzek, B.; Popp, J.; Schmitt, M. Raman and coherent anti-Stokes Raman scattering microspectroscopy for biomedical applications. J. Biomed. Opt. 2012, 17, 040801. [Google Scholar] [CrossRef]
- Nan, X.; Potma, E.O.; Xie, X.S. Nonperturbative Chemical Imaging of Organelle Transport in Living Cells with Coherent Anti-Stokes Raman Scattering Microscopy. Biophys. J. 2006, 91, 728–735. [Google Scholar] [CrossRef]
- Cheng, J.X.; Jia, Y.K.; Zheng, G.; Xie, X.S. Laser-scanning coherent anti-Stokes Raman scattering microscopy and applications to cell biology. Biophys. J. 2002, 83, 502–509. [Google Scholar] [CrossRef]
- Evans, C.L.; Xie, X.S. Coherent anti-stokes Raman scattering microscopy: Chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 2008, 1, 883–909. [Google Scholar] [CrossRef]
- Wurpel, G.W.H.; Schins, J.M.; Müller, M. Chemical specificity in three-dimensional imaging with multiplex coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 2002, 27, 1093–1095. [Google Scholar] [CrossRef]
- Lee, B.R.; Joo, K.I.; Choi, E.S.; Jahng, J.; Kim, H.; Kim, E. Evans blue dye-enhanced imaging of the brain microvessels using spectral focusing coherent anti-Stokes Raman scattering microscopy. PLoS ONE 2017, 12, e0185519. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Zhang, Y.; Zhao, K.; Zhao, Y.J.; Liu, Y.Y.; Gong, H.; Luo, Q.M.; Zhu, D. Skull Optical Clearing Solution for Enhancing Ultrasonic and Photoacoustic Imaging. IEEE Trans. Med. Imaging 2016, 35, 1903–1906. [Google Scholar] [CrossRef] [PubMed]
- Dashti, S.R.; Baharvahdat, H.; Spetzler, R.F.; Sauvageau, E.; Chang, S.W.; Stiefel, M.F.; Park, M.S.; Bambakidis, N.C. Operative intracranial infection following craniotomy. Neurosurg. Focus 2008, 24, E10. [Google Scholar] [CrossRef] [PubMed]
- Hefendehl, J.K.; Wegenast-Braun, B.M.; Liebig, C.; Eicke, D.; Milford, D.; Calhoun, M.E.; Kohsaka, S.; Eichner, M.; Jucker, M. Long-term in vivo imaging of beta-amyloid plaque appearance and growth in a mouse model of cerebral beta-amyloidosis. J. Neurosci. 2011, 31, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Radde, R.; Bolmont, T.; Kaeser, S.A.; Coomaraswamy, J.; Lindau, D.; Stoltze, L.; Calhoun, M.E.; Jaggi, F.; Wolburg, H.; Gengler, S.; et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006, 7, 940–946. [Google Scholar] [CrossRef]
- Li, S.W.; Luo, Z.Y.; Zhang, R.L.; Xu, H.; Zhou, T.; Liu, L.W.; Qu, J.L. Distinguishing Amyloid beta-Protein in a Mouse Model of Alzheimer’s Disease by Label-Free Vibrational Imaging. Biosensors 2021, 11, 365. [Google Scholar] [CrossRef]
- Christie, R.H.; Bacskai, B.J.; Zipfel, W.R.; Williams, R.M.; Kajdasz, S.T.; Webb, W.W.; Hyman, B.T. Growth arrest of individual senile plaques in a model of Alzheimer’s disease observed by in vivo multiphoton microscopy. J. Neurosci. 2001, 21, 858–864. [Google Scholar] [CrossRef]
- Love, S.; Miners, S.; Palmer, J.; Chalmers, K.; Kehoe, P. Insights into the pathogenesis and pathogenicity of cerebral amyloid angiopathy. Front. Biosci.-Landmark 2009, 14, 4778–4792. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.H.; Liao, Y.H.; Plogg, B.A.; Peng, W.G.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid beta. Sci. Transl. Med. 2012, 4, 147ra11. [Google Scholar] [CrossRef]
- Preston, S.D.; Steart, P.V.; Wilkinson, A.; Nicoll, J.A.R.; Weller, R.O. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: Defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol. Appl. Neurobiol. 2003, 29, 106–117. [Google Scholar] [CrossRef]
- Chow, N.; Bell, R.D.; Deane, R.; Streb, J.W.; Chen, J.Y.; Brooks, A.; Van Nostrand, W.; Miano, J.M.; Zlokovic, B.V. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc. Natl. Acad. Sci. USA 2007, 104, 823–828. [Google Scholar] [CrossRef]


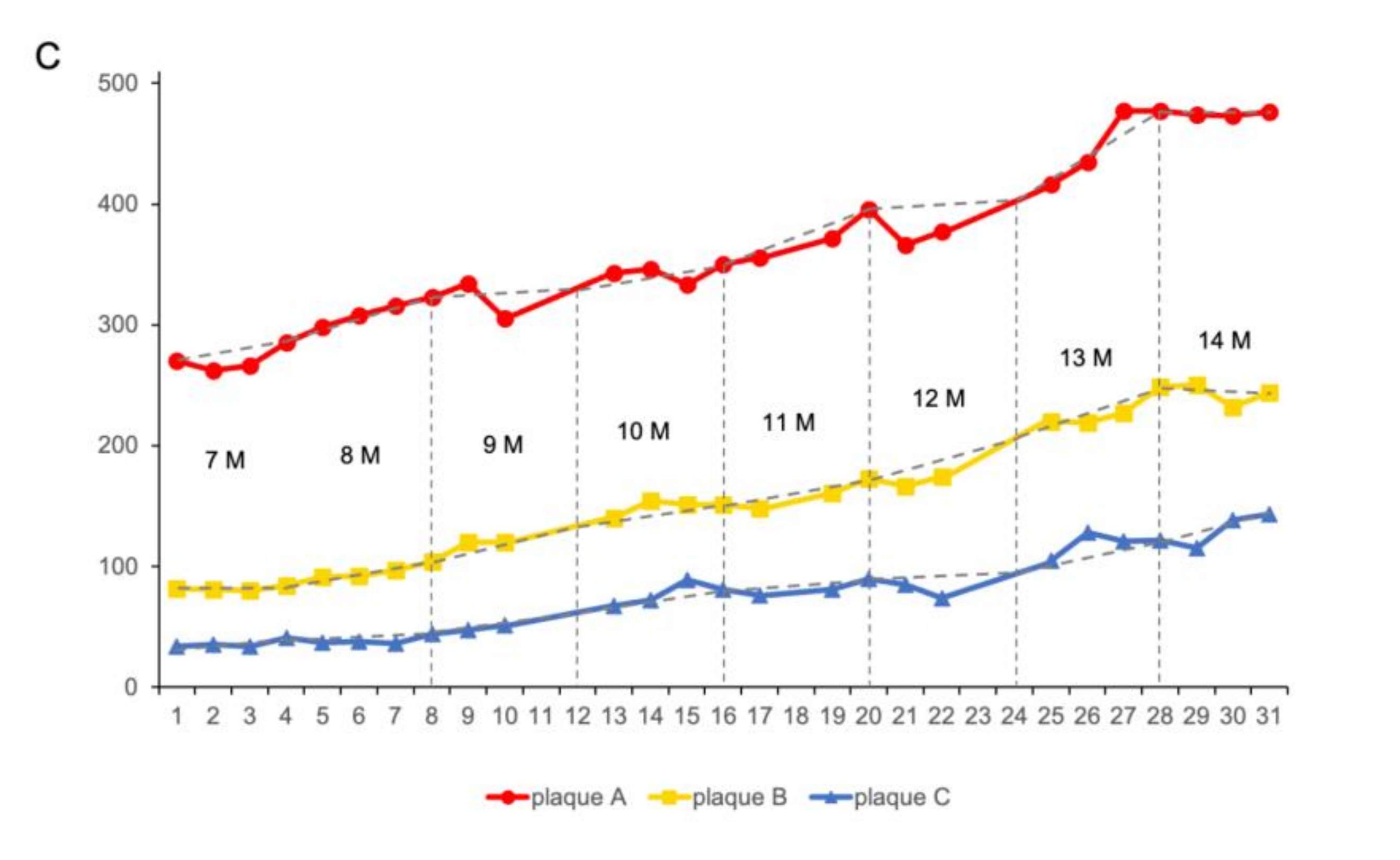

| Growth Rate%/Month | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| plaque A | 5.56 | 8.39 | 6.19 | 20.41 | 11.24 | 9.29 | 14.66 | 0.43 |
| plaque B | 2.44 | 14.28 | 12.91 | 7.86 | 16.89 | 26.51 | 13.18 | −6.40 |
| plaque C | 2.94 | 5.41 | 27.65 | 20.9 | 34.21 | 23.53 | 16.19 | 24.35 |
| 8 Months | 14 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | |
| 1 | 16.90 | 14.04 | 9.03 | 19.24 | 22.56 | 27.91 | 12.75 | 32.46 |
| 2 | 22.12 | 14.78 | 19.73 | 21.38 | 40.54 | 35.91 | 34.43 | 40.61 |
| 3 | 5.55 | 14.24 | 4.04 | 3.55 | 22.30 | 22.50 | 15.97 | 8.19 |
| 4 | N/A | N/A | 9.45 | 9.78 | N/A | N/A | 29.31 | 18.55 |
| 5 | N/A | N/A | N/A | 20.63 | N/A | N/A | N/A | 36.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Xu, H.; Samanta, S.; Zhang, R.; Luo, G.; Wang, Y.; Liu, L.; Weng, X.; He, J.; Liao, C.; et al. Long-Term Repeatable In Vivo Monitoring of Amyloid-β Plaques and Vessels in Alzheimer’s Disease Mouse Model with Combined TPEF/CARS Microscopy. Biomedicines 2022, 10, 2949. https://doi.org/10.3390/biomedicines10112949
Luo Z, Xu H, Samanta S, Zhang R, Luo G, Wang Y, Liu L, Weng X, He J, Liao C, et al. Long-Term Repeatable In Vivo Monitoring of Amyloid-β Plaques and Vessels in Alzheimer’s Disease Mouse Model with Combined TPEF/CARS Microscopy. Biomedicines. 2022; 10(11):2949. https://doi.org/10.3390/biomedicines10112949
Chicago/Turabian StyleLuo, Ziyi, Hao Xu, Soham Samanta, Renlong Zhang, Guoquan Luo, Yiming Wang, Liwei Liu, Xiaoyu Weng, Jun He, Changrui Liao, and et al. 2022. "Long-Term Repeatable In Vivo Monitoring of Amyloid-β Plaques and Vessels in Alzheimer’s Disease Mouse Model with Combined TPEF/CARS Microscopy" Biomedicines 10, no. 11: 2949. https://doi.org/10.3390/biomedicines10112949
APA StyleLuo, Z., Xu, H., Samanta, S., Zhang, R., Luo, G., Wang, Y., Liu, L., Weng, X., He, J., Liao, C., Wang, Y., Guo, B., & Qu, J. (2022). Long-Term Repeatable In Vivo Monitoring of Amyloid-β Plaques and Vessels in Alzheimer’s Disease Mouse Model with Combined TPEF/CARS Microscopy. Biomedicines, 10(11), 2949. https://doi.org/10.3390/biomedicines10112949










