Adenosine and Adenosine Receptors: Advances in Atrial Fibrillation
Abstract
1. Introduction
2. Adenosinergic System Signaling
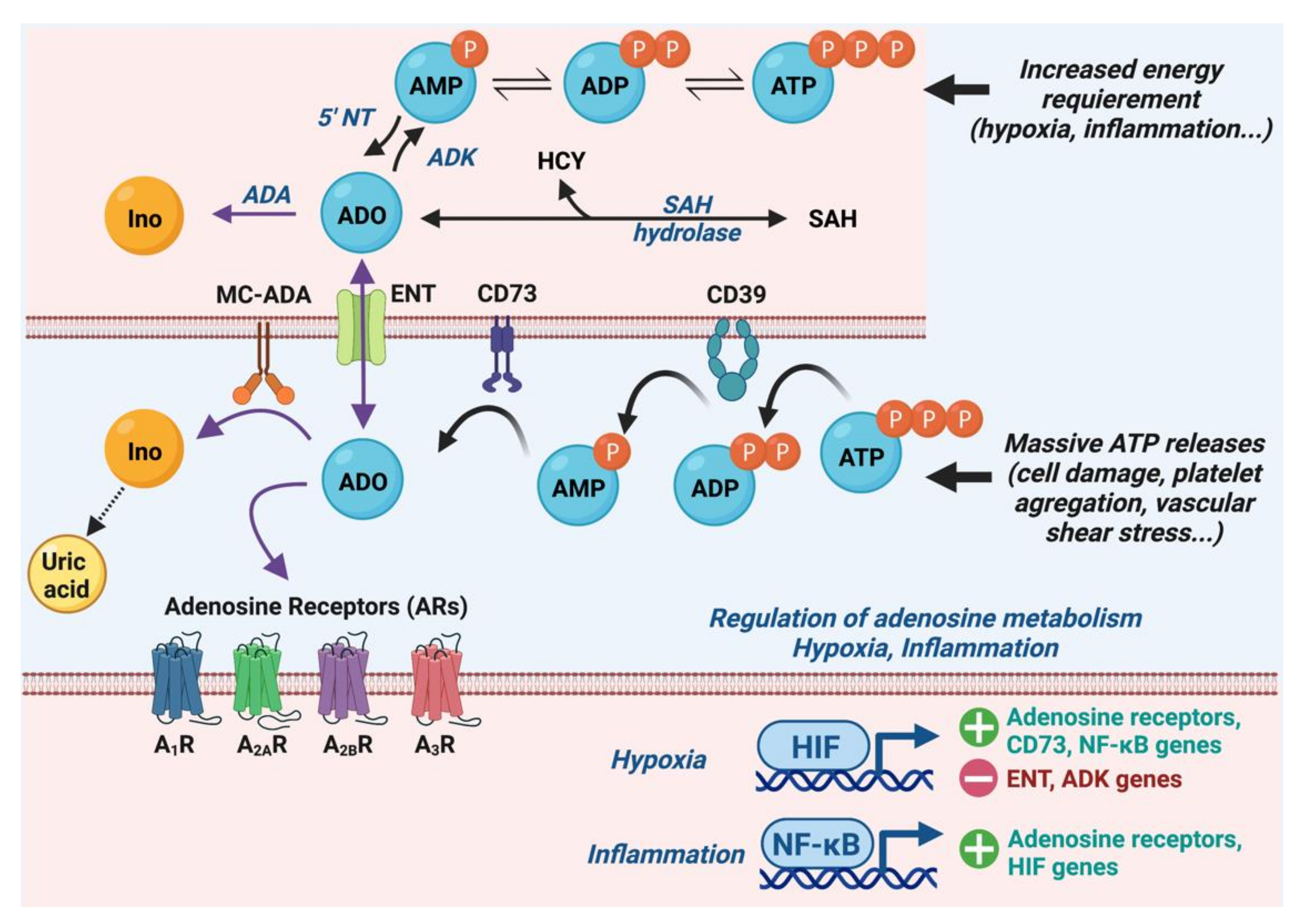
2.1. Metabolism of Adenosine
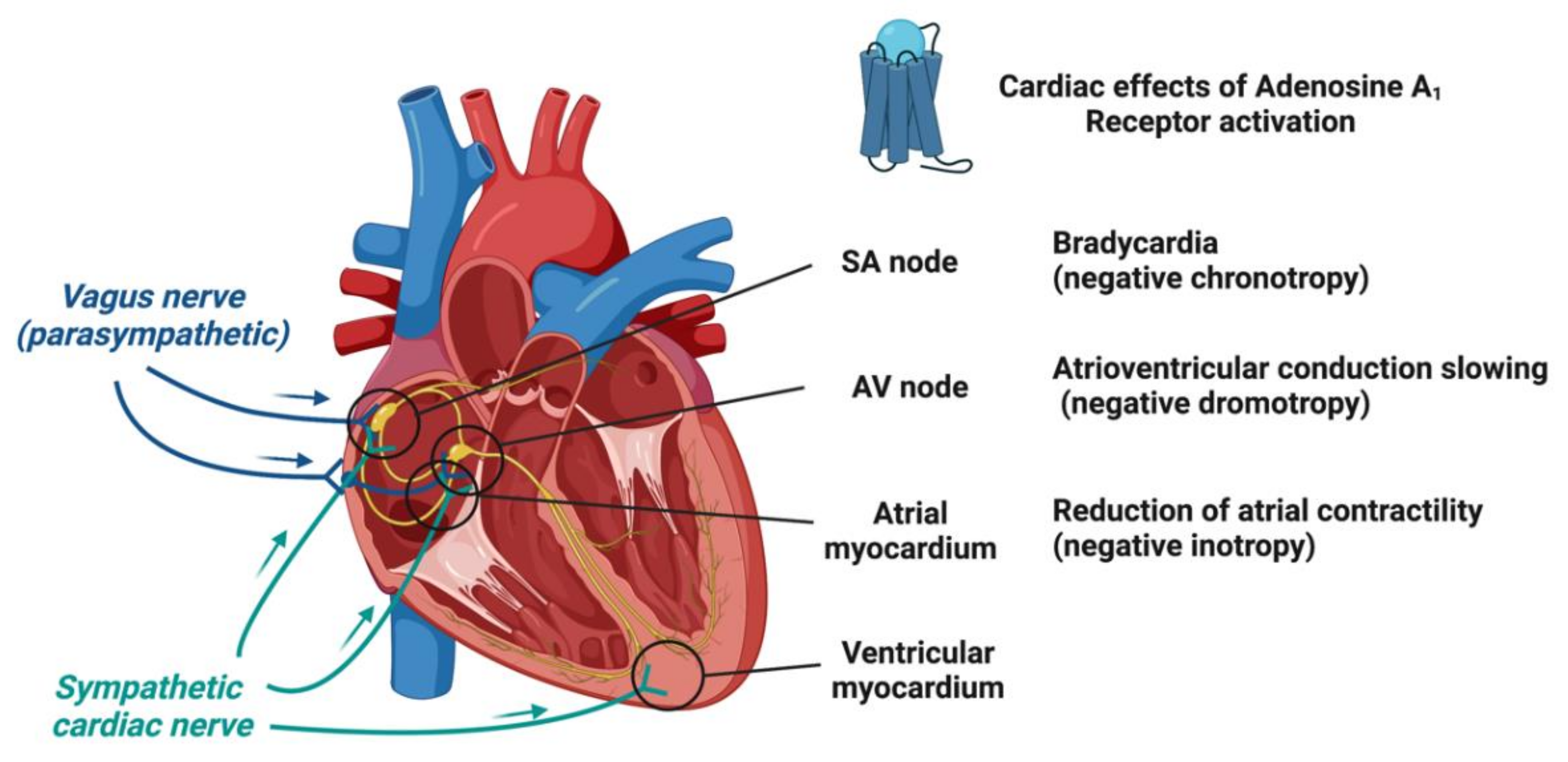
2.2. Adenosine Receptors and Their Cardiac Effects
2.3. Molecular and Ionic Bases of Adenosine Effects in Cardiac Electrophysiology
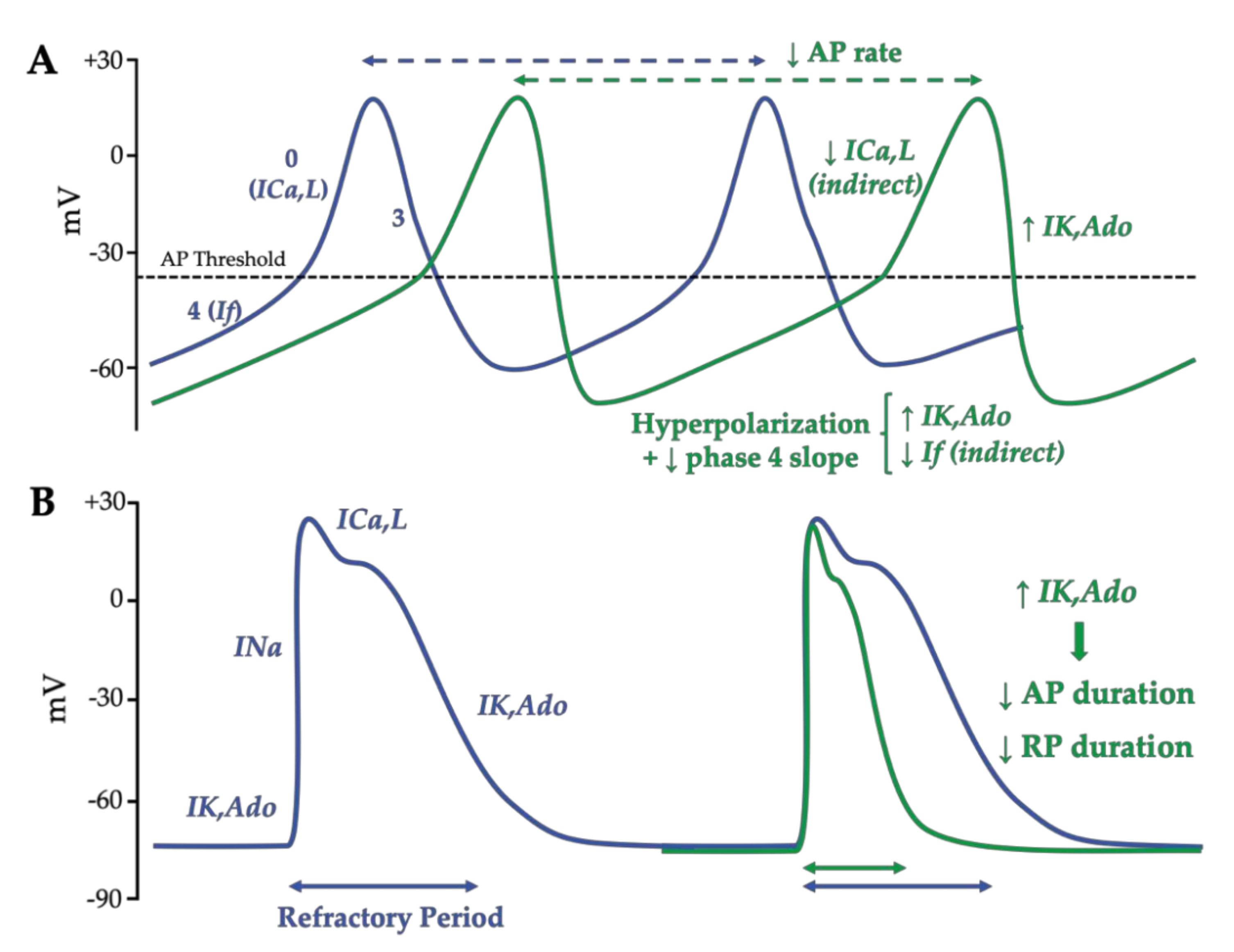
2.3.1. Effects of Adenosine on Ionic Currents
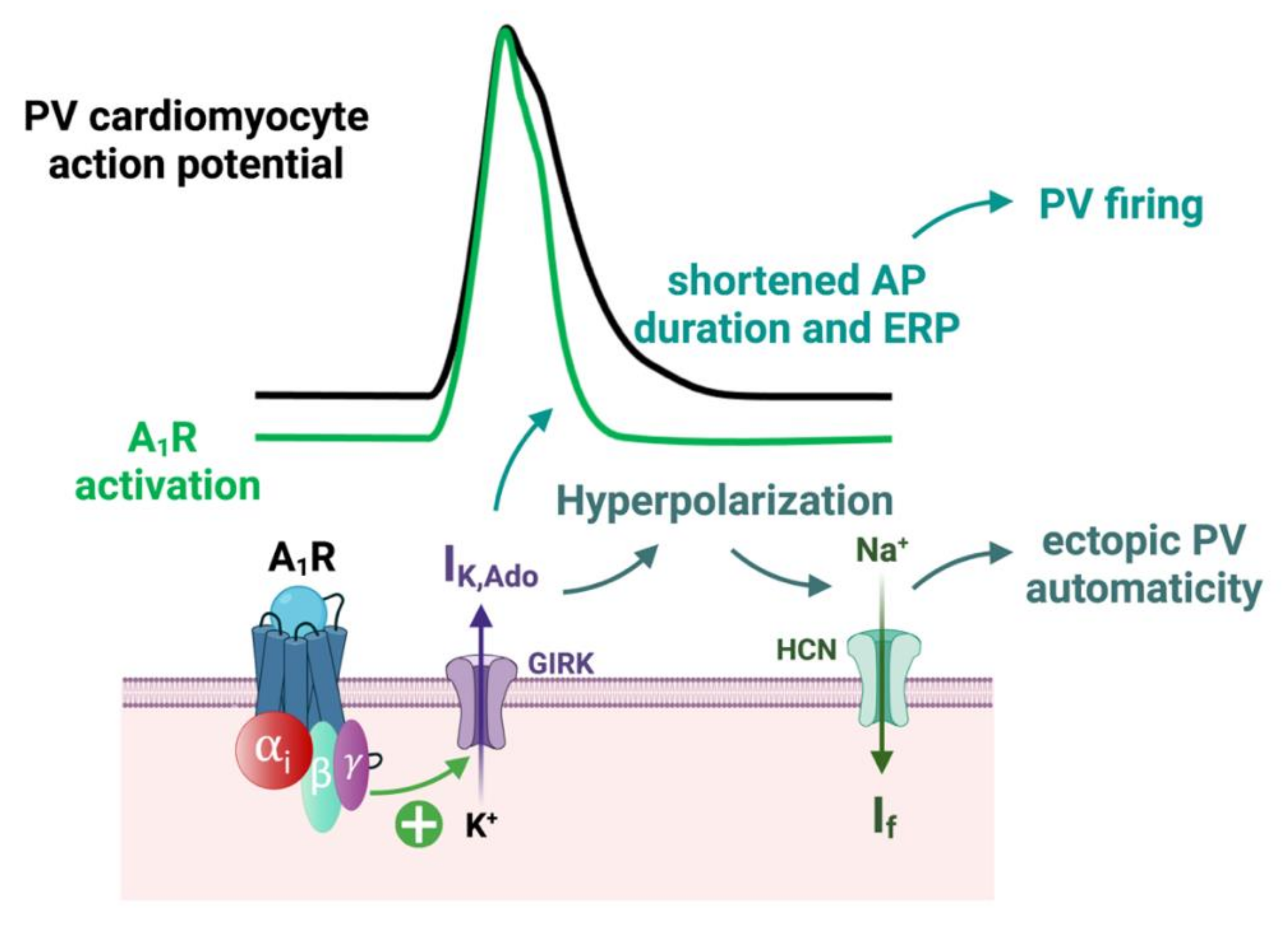
2.3.2. Adenosine Effects on the Action Potential in Nodal Cells and Working Cardiomyocytes
3. Pathophysiology of Atrial Fibrillation
3.1. Atrial Fibrillation Triggers
3.2. Mechanisms of AF Perpetuation
3.3. Substrate of Atrial Fibrillation and Atrial Cardiomyopathy
3.4. Pejorative Modulators of Atrial Fibrillation
4. Arrhythmogenic Effects of the Adenosinergic System
4.1. Adenosine Level and Expression of Adenosine Receptors in AF Patients
4.2. Implication of A1 Receptors in AF
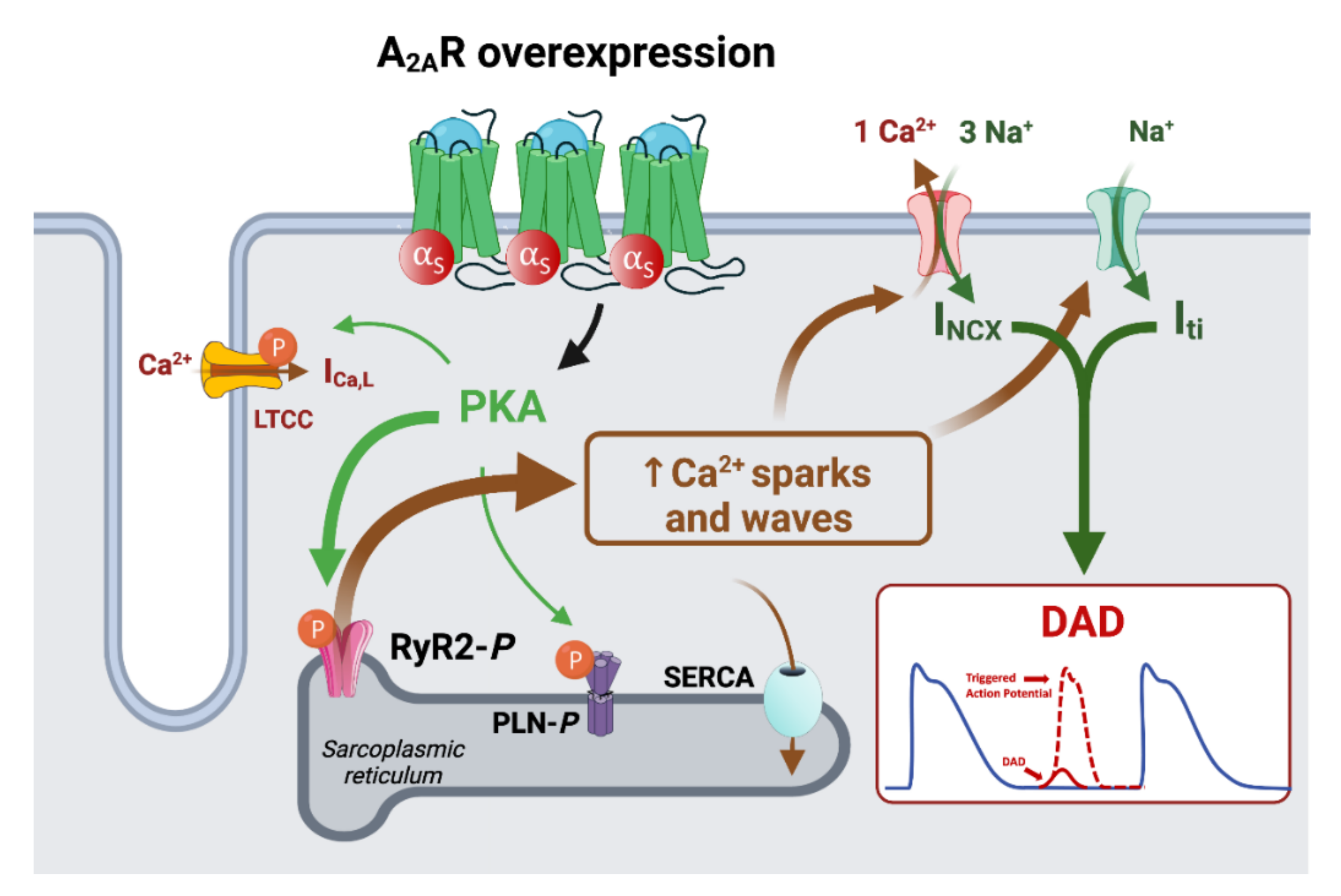
4.3. Implication of AR in the Remodeling of Calcium Handling
4.4. Modulation of Atrial Fibrosis by A2B Receptors
5. Association between Atrial Fibrillation Risk Factors and the Adenosinergic System
6. Conclusions
7. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Szentmiklosi, A.J.; Galajda, Z.; Cseppento, Á.; Gesztelyi, R.; Susán, Z.; Hegyi, B.; Nánási, P.P. The Janus Face of Adenosine: Antiarrhythmic and Proarrhythmic Actions. Curr. Pharm. Des. 2015, 21, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Guieu, R.; Deharo, J.-C.; Maille, B.; Crotti, L.; Torresani, E.; Brignole, M.; Parati, G. Adenosine and the Cardiovascular System: The Good and the Bad. J. Clin. Med. 2020, 9, E1366. [Google Scholar] [CrossRef] [PubMed]
- Drury, A.N.; Szent-Györgyi, A. The Physiological Activity of Adenine Compounds with Especial Reference to Their Action upon the Mammalian Heart. J. Physiol. 1929, 68, 213–237. [Google Scholar] [CrossRef]
- Burnstock G Purinergic Nerves. Pharmacol. Rev. 1972, 24, 509–581.
- Fredholm, B.B. Adenosine--a Physiological or Pathophysiological Agent? J. Mol. Med. 2014, 92, 201–206. [Google Scholar] [CrossRef]
- Deussen, A.; Lloyd, H.G.; Schrader, J. Contribution of S-Adenosylhomocysteine to Cardiac Adenosine Formation. J. Mol. Cell. Cardiol. 1989, 21, 773–782. [Google Scholar] [CrossRef]
- Sumi, Y.; Woehrle, T.; Chen, Y.; Yao, Y.; Li, A.; Junger, W.G. Adrenergic Receptor Activation Involves ATP Release and Feedback through Purinergic Receptors. Am. J. Physiol. Cell Physiol. 2010, 299, C1118–C1126. [Google Scholar] [CrossRef]
- Le, G.Y.; Essackjee, H.C.; Ballard, H.J. Intracellular Adenosine Formation and Release by Freshly-Isolated Vascular Endothelial Cells from Rat Skeletal Muscle: Effects of Hypoxia and/or Acidosis. Biochem. Biophys. Res. Commun. 2014, 450, 93–98. [Google Scholar] [CrossRef]
- Colgan, S.P.; Eltzschig, H.K.; Eckle, T.; Thompson, L.F. Physiological Roles for Ecto-5’-Nucleotidase (CD73). Purinerg. Signal. 2006, 2, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Groppelli, A.; Brignole, M.; Chefrour, M.; Gastaldi, M.; El Oufir, F.; Deharo, J.C.; Parati, G.; Guieu, R. Adenosine Concentration in Patients With Neurally Mediated Syncope. Front. Cardiovasc. Med. 2022, 9, 900023. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, M.; Marlinge, M.; Kerbaul, F.; Resseguier, N.; Laine, M.; Cautella, J.; Cordier, C.; Colomb, B.; Kipson, N.; Thuny, F.; et al. Adenosine Plasma Level and A2A Receptor Expression in Patients With Cardiogenic Shock. Crit. Care Med. 2018, 46, e874–e880. [Google Scholar] [CrossRef]
- Grenz, A.; Homann, D.; Eltzschig, H.K. Extracellular Adenosine: A Safety Signal That Dampens Hypoxia-Induced Inflammation during Ischemia. Antioxid. Redox. Signal. 2011, 15, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Riegel, A.-K.; Eltzschig, H.K. Extracellular Nucleotide and Nucleoside Signaling in Vascular and Blood Disease. Blood 2014, 124, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide Signalling during Inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Möser, G.H.; Schrader, J.; Deussen, A. Turnover of Adenosine in Plasma of Human and Dog Blood. Am. J. Physiol. 1989, 256, C799–C806. [Google Scholar] [CrossRef]
- Klabunde, R.E. Dipyridamole Inhibition of Adenosine Metabolism in Human Blood. Eur. J. Pharmacol. 1983, 93, 21–26. [Google Scholar] [CrossRef]
- Pantely, G.A.; Bristow, J.D. Adenosine. Renewed Interest in an Old Drug. Circulation 1990, 82, 1854–1856. [Google Scholar] [CrossRef] [PubMed]
- Morello, S.; Ito, K.; Yamamura, S.; Lee, K.-Y.; Jazrawi, E.; Desouza, P.; Barnes, P.; Cicala, C.; Adcock, I.M. IL-1 Beta and TNF-Alpha Regulation of the Adenosine Receptor (A2A) Expression: Differential Requirement for NF-Kappa B Binding to the Proximal Promoter. J. Immunol. 2006, 177, 7173–7183. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, L.; Fromonot, J.; By, Y.; Durand-Gorde, J.-M.; Condo, J.; Kipson, N.; Guieu, R.; Fenouillet, E.; Ruf, J. NF-ΚB Enhances Hypoxia-Driven T-Cell Immunosuppression via Upregulation of Adenosine A2A Receptors. Cell. Signal. 2014, 26, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- St Hilaire, C.; Carroll, S.H.; Chen, H.; Ravid, K. Mechanisms of Induction of Adenosine Receptor Genes and Its Functional Significance. J. Cell. Physiol. 2009, 218, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bonello, S.; Zähringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Görlach, A. Reactive Oxygen Species Activate the HIF-1alpha Promoter via a Functional NFkappaB Site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen Sensing, Homeostasis, and Disease. N. Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, F.; Gaudry, M.; Ruf, J.; Guieu, R. Recent Advances in the Role of the Adenosinergic System in Coronary Artery Disease. Cardiovasc. Res. 2021, 117, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, M.; Vairo, D.; Marlinge, M.; Gaubert, M.; Guiol, C.; Mottola, G.; Gariboldi, V.; Deharo, P.; Sadrin, S.; Maixent, J.M.; et al. Adenosine and Its Receptors: An Expected Tool for the Diagnosis and Treatment of Coronary Artery and Ischemic Heart Diseases. Int. J. Mol. Sci. 2020, 21, E5321. [Google Scholar] [CrossRef]
- Le, T.-T.T.; Berg, N.K.; Harting, M.T.; Li, X.; Eltzschig, H.K.; Yuan, X. Purinergic Signaling in Pulmonary Inflammation. Front. Immunol. 2019, 10, 1633. [Google Scholar] [CrossRef]
- Pasquini, S.; Contri, C.; Borea, P.A.; Vincenzi, F.; Varani, K. Adenosine and Inflammation: Here, There and Everywhere. Int. J. Mol. Sci. 2021, 22, 7685. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Arslan, G.; Halldner, L.; Kull, B.; Schulte, G.; Wasserman, W. Structure and Function of Adenosine Receptors and Their Genes. Naunyn. Schmiedebergs. Arch. Pharmacol. 2000, 362, 364–374. [Google Scholar] [CrossRef]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and Classification of Adenosine Receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Headrick, J.P.; Ashton, K.J.; Rose’meyer, R.B.; Peart, J.N. Cardiovascular Adenosine Receptors: Expression, Actions and Interactions. Pharmacol. Ther. 2013, 140, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, P.C.; McIntosh, V.J.; Cao, F.X.; Lasley, R.D. Differential Effects of Adenosine A2a and A2b Receptors on Cardiac Contractility. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H2082–H2089. [Google Scholar] [CrossRef]
- Musser, B.; Morgan, M.E.; Leid, M.; Murray, T.F.; Linden, J.; Vestal, R.E. Species Comparison of Adenosine and Beta-Adrenoceptors in Mammalian Atrial and Ventricular Myocardium. Eur. J. Pharmacol. 1993, 246, 105–111. [Google Scholar] [CrossRef]
- Shryock, J.C.; Belardinelli, L. Adenosine and Adenosine Receptors in the Cardiovascular System: Biochemistry, Physiology, and Pharmacology. Am. J. Cardiol. 1997, 79, 2–10. [Google Scholar] [CrossRef]
- Schrader, J.; Baumann, G.; Gerlach, E. Adenosine as Inhibitor of Myocardial Effects of Catecholamines. Pflugers. Arch. 1977, 372, 29–35. [Google Scholar] [CrossRef]
- Dobson, J.G. Mechanism of Adenosine Inhibition of Catecholamine-Induced Responses in Heart. Circ. Res. 1983, 52, 151–160. [Google Scholar] [CrossRef]
- Wennmalm, M.; Fredholm, B.B.; Hedqvist, P. Adenosine as a Modulator of Sympathetic Nerve-Stimulation-Induced Release of Noradrenaline from the Isolated Rabbit Heart. Acta. Physiol. Scand. 1988, 132, 487–494. [Google Scholar] [CrossRef]
- Belardinelli, L.; Giles, W.R.; West, A. Ionic Mechanisms of Adenosine Actions in Pacemaker Cells from Rabbit Heart. J. Physiol. 1988, 405, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Norton, G.R.; Woodiwiss, A.J.; McGinn, R.J.; Lorbar, M.; Chung, E.S.; Honeyman, T.W.; Fenton, R.A.; Dobson, J.G.; Meyer, T.E. Adenosine A1 Receptor-Mediated Antiadrenergic Effects Are Modulated by A2a Receptor Activation in Rat Heart. Am. J. Physiol. 1999, 276, H341–H349. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Umemura, S.; Toya, Y.; Uchibori, T.; Kogi, K.; Takagi, N.; Ishii, M. Identification of Adenosine A2 Receptor-CAMP System in Human Aortic Endothelial Cells. Biochem. Biophys. Res. Commun. 1994, 199, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, V.; Vairo, D.; Guieu, R.; Marlingue, M.; Ravis, E.; Lagier, D.; Mari, A.; Thery, E.; Collart, F.; Gaudry, M.; et al. Expressions of Adenosine A2A Receptors in Coronary Arteries and Peripheral Blood Mononuclear Cells Are Correlated in Coronary Artery Disease Patients. Int. J. Cardiol. 2017, 230, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Marala, R.B.; Mustafa, S.J. Immunological Characterization of Adenosine A2A Receptors in Human and Porcine Cardiovascular Tissues. J. Pharmacol. Exp. Ther. 1998, 286, 1051–1057. [Google Scholar]
- Hove-Madsen, L.; Prat-Vidal, C.; Llach, A.; Ciruela, F.; Casadó, V.; Lluis, C.; Bayes-Genis, A.; Cinca, J.; Franco, R. Adenosine A2A Receptors Are Expressed in Human Atrial Myocytes and Modulate Spontaneous Sarcoplasmic Reticulum Calcium Release. Cardiovasc. Res. 2006, 72, 292–302. [Google Scholar] [CrossRef]
- Maille, B.; Fromonot, J.; Guiol, C.; Marlinge, M.; Baptiste, F.; Lim, S.; Colombani, C.; Chaptal, M.C.; Chefrour, M.; Gastaldi, M.; et al. A2 Adenosine Receptor Subtypes Overproduction in Atria of Perioperative Atrial Fibrillation Patients Undergoing Cardiac Surgery: A Pilot Study. Front. Cardiovasc. Med. 2021, 8, 761164. [Google Scholar] [CrossRef]
- Boknik, P.; Eskandar, J.; Hofmann, B.; Zimmermann, N.; Neumann, J.; Gergs, U. Role of Cardiac A2A Receptors Under Normal and Pathophysiological Conditions. Front. Pharmacol. 2020, 11, 627838. [Google Scholar] [CrossRef]
- Zhan, E.; McIntosh, V.J.; Lasley, R.D. Adenosine A₂A and A₂B Receptors Are Both Required for Adenosine A₁ Receptor-Mediated Cardioprotection. Am. J. Physiol Heart Circ. Physiol. 2011, 301, H1183–H1189. [Google Scholar] [CrossRef]
- Mustafa, S.J.; Morrison, R.R.; Teng, B.; Pelleg, A. Adenosine Receptors and the Heart: Role in Regulation of Coronary Blood Flow and Cardiac Electrophysiology. Handb. Exp. Pharmacol. 2009, 193, 161–188. [Google Scholar] [CrossRef]
- Dubey, R.K.; Gillespie, D.G.; Jackson, E.K. Adenosine Inhibits Collagen and Protein Synthesis in Cardiac Fibroblasts: Role of A2B Receptors. Hypertension 1998, 31, 943–948. [Google Scholar] [CrossRef]
- Ho, M.-F.; Low, L.M.; Rose’Meyer, R.B. Pharmacology of the Adenosine A3 Receptor in the Vasculature and Essential Hypertension. PLoS ONE 2016, 11, e0150021. [Google Scholar] [CrossRef]
- Hussain, A.; Gharanei, A.M.; Nagra, A.S.; Maddock, H.L. Caspase Inhibition via A3 Adenosine Receptors: A New Cardioprotective Mechanism against Myocardial Infarction. Cardiovasc. Drugs Ther. 2014, 28, 19–32. [Google Scholar] [CrossRef]
- West, G.A.; Belardinelli, L. Sinus Slowing and Pacemaker Shift Caused by Adenosine in Rabbit SA Node. Pflugers. Arch. 1985, 403, 66–74. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M. Ionic Basis of the Electrophysiological Actions of Adenosine on Cardiomyocytes. FASEB J. 1995, 9, 359–365. [Google Scholar] [CrossRef]
- Heller, L.J.; Olsson, R.A. Inhibition of Rat Ventricular Automaticity by Adenosine. Am. J. Physiol. 1985, 248, H907–H913. [Google Scholar] [CrossRef]
- Ledent, C.; Vaugeois, J.M.; Schiffmann, S.N.; Pedrazzini, T.; El Yacoubi, M.; Vanderhaeghen, J.J.; Costentin, J.; Heath, J.K.; Vassart, G.; Parmentier, M. Aggressiveness, Hypoalgesia and High Blood Pressure in Mice Lacking the Adenosine A2a Receptor. Nature 1997, 388, 674–678. [Google Scholar] [CrossRef]
- Yang, J.-N.; Chen, J.-F.; Fredholm, B.B. Physiological Roles of A1 and A2A Adenosine Receptors in Regulating Heart Rate, Body Temperature, and Locomotion as Revealed Using Knockout Mice and Caffeine. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1141–H1149. [Google Scholar] [CrossRef]
- Chan, T.O.; Funakoshi, H.; Song, J.; Zhang, X.-Q.; Wang, J.; Chung, P.H.; DeGeorge, B.R.; Li, X.; Zhang, J.; Herrmann, D.E.; et al. Cardiac-Restricted Overexpression of the A2A-Adenosine Receptor in FVB Mice Transiently Increases Contractile Performance and Rescues the Heart Failure Phenotype in Mice Overexpressing the A(1)-Adenosine Receptor. Clin. Transl. Sci. 2008, 1, 126–133. [Google Scholar] [CrossRef]
- Lerman, B.B.; Markowitz, S.M.; Cheung, J.W.; Liu, C.F.; Thomas, G.; Ip, J.E. Supraventricular Tachycardia. Circ. Arrhythmia Elec. 2018, 11, e006953. [Google Scholar] [CrossRef]
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.-P.; et al. 2019 ESC Guidelines for the Management of Patients with Supraventricular TachycardiaThe Task Force for the Management of Patients with Supraventricular Tachycardia of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 655–720. [Google Scholar] [CrossRef] [PubMed]
- Lokhandwala, M.F. Inhibition of Cardiac Sympathetic Neurotransmission by Adenosine. Eur. J. Pharmacol. 1979, 60, 353–357. [Google Scholar] [CrossRef]
- DiFrancesco, D.; Tortora, P. Direct Activation of Cardiac Pacemaker Channels by Intracellular Cyclic AMP. Nature 1991, 351, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Kurachi, Y.; Nakajima, T.; Sugimoto, T. On the Mechanism of Activation of Muscarinic K+ Channels by Adenosine in Isolated Atrial Cells: Involvement of GTP-Binding Proteins. Pflügers. Arch. 1986, 407, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, B.; Skibsbye, L.; Olesen, S.-P.; Grunnet, M.; Jespersen, T. GIRK Channel Activation via Adenosine or Muscarinic Receptors Has Similar Effects on Rat Atrial Electrophysiology. J. Cardiovasc. Pharmacol. 2013, 62, 192–198. [Google Scholar] [CrossRef]
- Pfaffinger, P.J.; Martin, J.M.; Hunter, D.D.; Nathanson, N.M.; Hille, B. GTP-Binding Proteins Couple Cardiac Muscarinic Receptors to a K Channel. Nature 1985, 317, 536–538. [Google Scholar] [CrossRef]
- Lopatin, A.N.; Nichols, C.G. Inward Rectifiers in the Heart: An Update on I(K1). J. Mol. Cell. Cardiol. 2001, 33, 625–638. [Google Scholar] [CrossRef]
- Anumonwo, J.M.B.; Lopatin, A.N. Cardiac Strong Inward Rectifier Potassium Channels. J. Mol. Cell. Cardiol. 2010, 48, 45–54. [Google Scholar] [CrossRef]
- Guo, D.; Ramu, Y.; Klem, A.M.; Lu, Z. Mechanism of Rectification in Inward-Rectifier K+ Channels. J. Gen. Physiol. 2003, 121, 261–276. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Gordon, E.A.; Wickman, K.; Velimirović, B.; Krapivinsky, L.; Clapham, D.E. The G-Protein-Gated Atrial K+ Channel IKAch Is a Heteromultimer of Two Inwardly Rectifying K+-Channel Proteins. Nature 1995, 374, 135–141. [Google Scholar] [CrossRef]
- Long, V.P.; Bonilla, I.M.; Baine, S.; Glynn, P.; Kumar, S.; Schober, K.; Mowrey, K.; Weiss, R.; Lee, N.Y.; Mohler, P.J.; et al. Chronic Heart Failure Increases Negative Chronotropic Effects of Adenosine in Canine Sinoatrial Cells via A1R Stimulation and GIRK-Mediated IKado. Life Sci. 2020, 240, 117068. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Csepe, T.A.; Hansen, B.J.; Sul, L.V.; Kalyanasundaram, A.; Zakharkin, S.O.; Zhao, J.; Guha, A.; Van Wagoner, D.R.; Kilic, A.; et al. Adenosine-Induced Atrial Fibrillation: Localized Reentrant Drivers in Lateral Right Atria Due to Heterogeneous Expression of Adenosine A1 Receptors and GIRK4 Subunits in the Human Heart. Circulation 2016, 134, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, E.L.; Narayan, P.; Mentzer, R.M.; Lasley, R.D. Cardiac Myocyte Adenosine A2a Receptor Activation Fails to Alter CAMP or Contractility: Role of Receptor Localization. Am. J. Physiol. Heart. Circ. Physiol. 2002, 282, H1035–H1040. [Google Scholar] [CrossRef]
- Boknik, P.; Drzewiecki, K.; Eskandar, J.; Gergs, U.; Grote-Wessels, S.; Fabritz, L.; Kirchhof, P.; Müller, F.U.; Stümpel, F.; Schmitz, W.; et al. Phenotyping of Mice with Heart Specific Overexpression of A2A-Adenosine Receptors: Evidence for Cardioprotective Effects of A2A-Adenosine Receptors. Front. Pharmacol. 2018, 9, 13. [Google Scholar] [CrossRef]
- Woodiwiss, A.J.; Honeyman, T.W.; Fenton, R.A.; Dobson, J.G. Adenosine A2a-Receptor Activation Enhances Cardiomyocyte Shortening via Ca2+-Independent and -Dependent Mechanisms. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H1434–H1441. [Google Scholar] [CrossRef]
- Llach, A.; Molina, C.E.; Prat-Vidal, C.; Fernandes, J.; Casadó, V.; Ciruela, F.; Lluís, C.; Franco, R.; Cinca, J.; Hove-Madsen, L. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur. Heart J. 2011, 32, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Brandenburg, S.; Pawlowitz, J.; Steckmeister, V.; Subramanian, H.; Uhlenkamp, D.; Scardigli, M.; Mushtaq, M.; Amlaz, S.I.; Kohl, T.; Wegener, J.W.; et al. A Junctional CAMP Compartment Regulates Rapid Ca2+ Signaling in Atrial Myocytes. J. Mol. Cell. Cardiol. 2022, 165, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Christ, T.; Boknik, P.; Wöhrl, S.; Wettwer, E.; Graf, E.M.; Bosch, R.F.; Knaut, M.; Schmitz, W.; Ravens, U.; Dobrev, D. L-Type Ca2+ Current Downregulation in Chronic Human Atrial Fibrillation Is Associated With Increased Activity of Protein Phosphatases. Circulation 2004, 110, 2651–2657. [Google Scholar] [CrossRef]
- Bokník, P.; Unkel, C.; Kirchhefer, U.; Kleideiter, U.; Klein-Wiele, O.; Knapp, J.; Linck, B.; Lüss, H.; Ulrich Müller, F.; Schmitz, W.; et al. Regional Expression of Phospholamban in the Human Heart. Cardiovasc. Res. 1999, 43, 67–76. [Google Scholar] [CrossRef]
- Boknik, P.; Drzewiecki, K.; Eskandar, J.; Gergs, U.; Hofmann, B.; Treede, H.; Grote-Wessels, S.; Fabritz, L.; Kirchhof, P.; Fortmüller, L.; et al. Evidence for Arrhythmogenic Effects of A2A-Adenosine Receptors. Front. Pharmacol. 2019, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, J.; Mandveno, A.; Potter, J.D. Cardiac Troponin I Phosphorylation Increases the Rate of Cardiac Muscle Relaxation. Circ. Res. 1995, 76, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Cardiac Excitation–Contraction Coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Llach, A.; Molina, C.E.; Fernandes, J.; Padró, J.; Cinca, J.; Hove-Madsen, L. Sarcoplasmic Reticulum and L-Type Ca2+ Channel Activity Regulate the Beat-to-Beat Stability of Calcium Handling in Human Atrial Myocytes. J. Physiol. 2011, 589, 3247–3262. [Google Scholar] [CrossRef]
- Dhamoon, A.S.; Pandit, S.V.; Sarmast, F.; Parisian, K.R.; Guha, P.; Li, Y.; Bagwe, S.; Taffet, S.M.; Anumonwo, J.M.B. Unique Kir2.x Properties Determine Regional and Species Differences in the Cardiac Inward Rectifier K+ Current. Circ. Res. 2004, 94, 1332–1339. [Google Scholar] [CrossRef]
- Chandler, N.J.; Greener, I.D.; Tellez, J.O.; Inada, S.; Musa, H.; Molenaar, P.; Difrancesco, D.; Baruscotti, M.; Longhi, R.; Anderson, R.H.; et al. Molecular Architecture of the Human Sinus Node: Insights into the Function of the Cardiac Pacemaker. Circulation 2009, 119, 1562–1575. [Google Scholar] [CrossRef]
- MacDonald, E.A.; Rose, R.A.; Quinn, T.A. Neurohumoral Control of Sinoatrial Node Activity and Heart Rate: Insight From Experimental Models and Findings From Humans. Front. Physiol. 2020, 11, 170. [Google Scholar] [CrossRef]
- Kléber, A.G.; Rudy, Y. Basic Mechanisms of Cardiac Impulse Propagation and Associated Arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef]
- Amos, G.J.; Wettwer, E.; Metzger, F.; Li, Q.; Himmel, H.M.; Ravens, U. Differences between Outward Currents of Human Atrial and Subepicardial Ventricular Myocytes. J. Physiol. 1996, 491, 31–50. [Google Scholar] [CrossRef]
- Belardinelli, L.; Isenberg, G. Isolated Atrial Myocytes: Adenosine and Acetylcholine Increase Potassium Conductance. Am. J. Physiol. 1983, 244, H734–H737. [Google Scholar] [CrossRef]
- Engelstein, E.D.; Lippman, N.; Stein, K.M.; Lerman, B.B. Mechanism-Specific Effects of Adenosine on Atrial Tachycardia. Circulation 1994, 89, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.J.; Kane, K.A.; Rankin, A.C. Ionic Basis of a Differential Effect of Adenosine on Refractoriness in Rabbit AV Nodal and Atrial Isolated Myocytes. Cardiovasc. Res. 1999, 43, 974–984. [Google Scholar] [CrossRef]
- Jame, S.; Barnes, G. Stroke and Thromboembolism Prevention in Atrial Fibrillation. Heart 2020, 106, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, M.A.; Fudim, M.; DeVore, A.D.; Piccini, J.P. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Failure 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; Yu, H.T.; Kim, T.-H.; Jang, E.; Sung, J.-H.; Pak, H.-N.; Lee, M.-Y.; Lee, M.-H.; Lip, G.Y.H.; et al. Risk of Dementia in Stroke-Free Patients Diagnosed with Atrial Fibrillation: Data from a Population-Based Cohort. Eur. Heart J. 2019, 40, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.; Fan, D.; Fang, M.C.; Singer, D.E.; Witt, D.M.; Schmelzer, J.R.; Williams, M.S.; Gurwitz, J.H.; Sung, S.H.; Go, A.S. Anxiety, Depression, and Adverse Clinical Outcomes in Patients With Atrial Fibrillation Starting Warfarin: Cardiovascular Research Network WAVE Study. J. Am. Heart Assoc. 2018, 7, e007814. [Google Scholar] [CrossRef]
- Son, Y.-J.; Baek, K.-H.; Lee, S.J.; Seo, E.J. Health-Related Quality of Life and Associated Factors in Patients with Atrial Fibrillation: An Integrative Literature Review. Int. J. Environ. Res. Public Health 2019, 16, 3042. [Google Scholar] [CrossRef]
- Cheniti, G.; Vlachos, K.; Pambrun, T.; Hooks, D.; Frontera, A.; Takigawa, M.; Bourier, F.; Kitamura, T.; Lam, A.; Martin, C.; et al. Atrial Fibrillation Mechanisms and Implications for Catheter Ablation. Front. Physiol. 2018, 9, 1458. [Google Scholar] [CrossRef]
- Wijesurendra, R.S.; Casadei, B. Mechanisms of Atrial Fibrillation. Heart 2019, 105, 1860–1867. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Lin, W.-S.; Tai, C.-T.; Hsieh, M.-H.; Tsai, C.-F.; Lin, Y.-K.; Tsao, H.-M.; Huang, J.-L.; Yu, W.-C.; Yang, S.-P.; Ding, Y.-A.; et al. Catheter Ablation of Paroxysmal Atrial Fibrillation Initiated by Non–Pulmonary Vein Ectopy. Circulation 2003, 107, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Tai, C.-T.; Hsieh, M.-H.; Tsao, H.-M.; Lin, Y.-J.; Chang, S.-L.; Huang, J.-L.; Lee, K.-T.; Chen, Y.-J.; Cheng, J.-J.; et al. Predictors of Non-Pulmonary Vein Ectopic Beats Initiating Paroxysmal Atrial Fibrillation: Implication for Catheter Ablation. J. Am. Coll. Cardiol. 2005, 46, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; Marchlinski, F.E. Techniques for the Provocation, Localization, and Ablation of Non–Pulmonary Vein Triggers for Atrial Fibrillation. Heart Rhythm 2017, 14, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Arentz, T.; Haegeli, L.; Sanders, P.; Weber, R.; Neumann, F.J.; Kalusche, D.; Haïssaguerre, M. High-Density Mapping of Spontaneous Pulmonary Vein Activity Initiating Atrial Fibrillation in Humans. J. Cardiovasc. Electrophysiol. 2007, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Cha, T.-J.; Zhang, L.; Chartier, D.; Melnyk, P.; Hohnloser, S.H.; Nattel, S. Cellular Electrophysiology of Canine Pulmonary Vein Cardiomyocytes: Action Potential and Ionic Current Properties. J. Physiol. 2003, 551, 801–813. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial Remodeling and Atrial Fibrillation: Recent Advances and Translational Perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Greer-Short, A.; Musa, H.; Alsina, K.M.; Ni, L.; Word, T.A.; Reynolds, J.O.; Gratz, D.; Lane, C.; El-Refaey, M.; Unudurthi, S.; et al. Calmodulin Kinase II Regulates Atrial Myocyte Late Sodium Current, Calcium Handling, and Atrial Arrhythmia. Heart Rhythm 2020, 17, 503–511. [Google Scholar] [CrossRef]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced Sarcoplasmic Reticulum Ca2+ Leak and Increased Na+-Ca2+ Exchanger Function Underlie Delayed Afterdepolarizations in Patients With Chronic Atrial Fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef]
- Heijman, J.; Guichard, J.-B.; Dobrev, D.; Nattel, S. Translational Challenges in Atrial Fibrillation. Circ. Res. 2018, 122, 752–773. [Google Scholar] [CrossRef]
- Reinhardt, F.; Beneke, K.; Pavlidou, N.G.; Conradi, L.; Reichenspurner, H.; Hove-Madsen, L.; Molina, C.E. Abnormal Calcium Handling in Atrial Fibrillation Is Linked to Changes in Cyclic AMP Dependent Signaling. Cells 2021, 10, 3042. [Google Scholar] [CrossRef]
- Chou, C.-C.; Nihei, M.; Zhou, S.; Tan, A.; Kawase, A.; Macias, E.S.; Fishbein, M.C.; Lin, S.-F.; Chen, P.-S. Intracellular Calcium Dynamics and Anisotropic Reentry in Isolated Canine Pulmonary Veins and Left Atrium. Circulation 2005, 111, 2889–2897. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Garfinkel, A.; Chen, P.S.; Weiss, J.N. Mechanisms of Discordant Alternans and Induction of Reentry in Simulated Cardiac Tissue. Circulation 2000, 102, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Provost, J.; Costet, A.; Wan, E.; Gambhir, A.; Whang, W.; Garan, H.; Konofagou, E.E. Assessing the Atrial Electromechanical Coupling during Atrial Focal Tachycardia, Flutter, and Fibrillation Using Electromechanical Wave Imaging in Humans. Comput. Biol. Med. 2015, 65, 161–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roney, C.H.; Bayer, J.D.; Cochet, H.; Meo, M.; Dubois, R.; Jaïs, P.; Vigmond, E.J. Variability in Pulmonary Vein Electrophysiology and Fibrosis Determines Arrhythmia Susceptibility and Dynamics. PLOS Comput. Biol. 2018, 14, e1006166. [Google Scholar] [CrossRef] [PubMed]
- Hocini, M.; Ho, S.Y.; Kawara, T.; Linnenbank, A.C.; Potse, M.; Shah, D.; Jaïs, P.; Janse, M.J.; Haïssaguerre, M.; De Bakker, J.M.T. Electrical Conduction in Canine Pulmonary Veins: Electrophysiological and Anatomic Correlation. Circulation 2002, 105, 2442–2448. [Google Scholar] [CrossRef]
- Lehnart, S.E.; Wehrens, X.H.T.; Laitinen, P.J.; Reiken, S.R.; Deng, S.-X.; Cheng, Z.; Landry, D.W.; Kontula, K.; Swan, H.; Marks, A.R. Sudden Death in Familial Polymorphic Ventricular Tachycardia Associated with Calcium Release Channel (Ryanodine Receptor). Leak. Circulation 2004, 109, 3208–3214. [Google Scholar] [CrossRef]
- Burashnikov, A.; Antzelevitch, C. Reinduction of Atrial Fibrillation Immediately after Termination of the Arrhythmia Is Mediated by Late Phase 3 Early Afterdepolarization-Induced Triggered Activity. Circulation 2003, 107, 2355–2360. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Shah, A.J.; Cochet, H.; Hocini, M.; Dubois, R.; Efimov, I.; Vigmond, E.; Bernus, O.; Trayanova, N. Intermittent Drivers Anchoring to Structural Heterogeneities as a Major Pathophysiological Mechanism of Human Persistent Atrial Fibrillation. J. Physiol. 2016, 594, 2387–2398. [Google Scholar] [CrossRef]
- Qu, Z. Critical Mass Hypothesis Revisited: Role of Dynamical Wave Stability in Spontaneous Termination of Cardiac Fibrillation. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H255–H263. [Google Scholar] [CrossRef]
- Moe, G.K.; Rheinboldt, W.C.; Abildskov, J.A. A COMPUTER MODEL OF ATRIAL FIBRILLATION. Am. Heart J. 1964, 67, 200–220. [Google Scholar] [CrossRef]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological Mechanisms of Atrial Fibrillation: A Translational Appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.; Shalhoub, J.; Ng, F.S.; Krahn, A.D.; Laksman, Z.; Andrade, J.G.; Deyell, M.W.; Kanagaratnam, P.; Sikkel, M.B. Size Matters in Atrial Fibrillation: The Underestimated Importance of Reduction of Contiguous Electrical Mass Underlying the Effectiveness of Catheter Ablation. EP Europace 2021, 23, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; O’Neill, L.; Roney, C.H.; Julia, J.; Metzner, A.; Reißmann, B.; Mukherjee, R.K.; Sim, I.; Whitaker, J.; Wright, M.; et al. Left Atrial Effective Conducting Size Predicts Atrial Fibrillation Vulnerability in Persistent but Not Paroxysmal Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2019, 30, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.J.; Csepe, T.A.; Zhao, J.; Ignozzi, A.J.; Hummel, J.D.; Fedorov, V.V. Maintenance of Atrial Fibrillation: Are Reentrant Drivers With Spatial Stability the Key? Circ. Arrhythm. Electrophysiol. 2016, 9, e004398. [Google Scholar] [CrossRef]
- Lim, H.S.; Hocini, M.; Dubois, R.; Denis, A.; Derval, N.; Zellerhoff, S.; Yamashita, S.; Berte, B.; Mahida, S.; Komatsu, Y.; et al. Complexity and Distribution of Drivers in Relation to Duration of Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 69, 1257–1269. [Google Scholar] [CrossRef]
- Frontera, A.; Pagani, S.; Limite, L.R.; Peirone, A.; Fioravanti, F.; Enache, B.; Cuellar, S.J.; Vlachos, K.; Meyer, C.; Montesano, G.; et al. Slow Conduction Corridors and Pivot Sites Characterize the Electrical Remodeling in Atrial Fibrillation. JACC Cli. Electrophysiol. 2022, 8, 561–577. [Google Scholar] [CrossRef]
- Allessie, M.A.; Bonke, F.I.; Schopman, F.J. Circus Movement in Rabbit Atrial Muscle as a Mechanism of Tachycardia. III. The “Leading Circle” Concept: A New Model of Circus Movement in Cardiac Tissue without the Involvement of an Anatomical Obstacle. Circ. Res. 1977, 41, 9–18. [Google Scholar] [CrossRef]
- Nattel, S.; Xiong, F.; Aguilar, M. Demystifying Rotors and Their Place in Clinical Translation of Atrial Fibrillation Mechanisms. Nat. Rev. Cardiol. 2017, 14, 509–520. [Google Scholar] [CrossRef]
- Pandit, S.V.; Berenfeld, O.; Anumonwo, J.M.B.; Zaritski, R.M.; Kneller, J.; Nattel, S.; Jalife, J. Ionic Determinants of Functional Reentry in a 2-D Model of Human Atrial Cells during Simulated Chronic Atrial Fibrillation. Biophys. J. 2005, 88, 3806–3821. [Google Scholar] [CrossRef]
- Pope, M.T.B.; Kuklik, P.; Briosa, E.; Gala, A.; Leo, M.; Mahmoudi, M.; Paisey, J.; Betts, T.R. Impact of Adenosine on Wavefront Propagation in Persistent Atrial Fibrillation: Insights From Global Noncontact Charge Density Mapping of the Left Atrium. J. Am. Heart Assoc. 2022, 11, e021166. [Google Scholar] [CrossRef]
- Voigt, N.; Trausch, A.; Knaut, M.; Matschke, K.; Varró, A.; Van Wagoner, D.R.; Nattel, S.; Ravens, U.; Dobrev, D. Left-to-Right Atrial Inward Rectifier Potassium Current Gradients in Patients with Paroxysmal versus Chronic Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Skibsbye, L.; Jespersen, T.; Christ, T.; Maleckar, M.M.; van den Brink, J.; Tavi, P.; Koivumäki, J.T. Refractoriness in Human Atria: Time and Voltage Dependence of Sodium Channel Availability. J. Mol. Cell. Cardiol. 2016, 101, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Maille, B.; Das, M.; Hussein, A.; Shaw, M.; Chaturvedi, V.; Williams, E.; Morgan, M.; Ronayne, C.; Snowdon, R.L.; Gupta, D. Reverse Electrical and Structural Remodeling of the Left Atrium Occurs Early after Pulmonary Vein Isolation for Persistent Atrial Fibrillation. J. Interv. Card. Electrophysiol. 2020, 58, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE Expert Consensus on Atrial Cardiomyopathies: Definition, Characterization, and Clinical Implication. EP Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef] [PubMed]
- Maille, B.; Marlinge, M.; Vairo, D.; Mottola, G.; Koutbi, L.; Deharo, P.; Gastaldi, M.; Gaudry, M.; Guiol, C.; Bottone, S.; et al. Adenosine Plasma Level in Patients with Paroxysmal or Persistent Atrial Fibrillation and Normal Heart during Ablation Procedure and/or Cardioversion. Purinergic Signal. 2019, 15, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.-M.; Dzeja, P.P.; Shen, W.K.; Jahangir, A.; Hart, C.Y.T.; Terzic, A.; Redfield, M.M. Failing Atrial Myocardium: Energetic Deficits Accompany Structural Remodeling and Electrical Instability. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1313–H1320. [Google Scholar] [CrossRef]
- Godoy-Marín, H.; Duroux, R.; Jacobson, K.A.; Soler, C.; Colino-Lage, H.; Jiménez-Sábado, V.; Montiel, J.; Hove-Madsen, L.; Ciruela, F. Adenosine A2A Receptors Are Upregulated in Peripheral Blood Mononuclear Cells from Atrial Fibrillation Patients. Int. J. Mol. Sci. 2021, 22, 3467. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Wang, W.; Dai, Y.; Ning, C.; Luo, R.; Sun, K.; Glover, L.; Grenz, A.; Sun, H.; et al. Elevated Ecto-5’-Nucleotidase-Mediated Increased Renal Adenosine Signaling via A2B Adenosine Receptor Contributes to Chronic Hypertension. Circ. Res. 2013, 112, 1466–1478. [Google Scholar] [CrossRef]
- Silhol, F.; Marlinge, M.; Guiol, C.; Chefrour, M.; Mace, P.; Criado, C.; Kipson, N.; Vaisse, B.; Vairo, D.; Sarlon, G.; et al. Characterization of Adenosine A2 Receptors in Peripheral Blood Mononuclear Cells of Patients with Fibromuscular Dysplasia. Hypertens. Res. 2020, 43, 466–469. [Google Scholar] [CrossRef]
- Franceschi, F.; Deharo, J.-C.; Giorgi, R.; By, Y.; Monserrat, C.; Condo, J.; Ibrahim, Z.; Saadjian, A.; Guieu, R. Peripheral Plasma Adenosine Release in Patients with Chronic Heart Failure. Heart 2009, 95, 651–655. [Google Scholar] [CrossRef]
- Linz, D.; Elliott, A.D.; Hohl, M.; Malik, V.; Schotten, U.; Dobrev, D.; Nattel, S.; Böhm, M.; Floras, J.; Lau, D.H.; et al. Role of Autonomic Nervous System in Atrial Fibrillation. Int. J. Cardiol. 2019, 287, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Saadjian, A.Y.; Lévy, S.; Franceschi, F.; Zouher, I.; Paganelli, F.; Guieu, R.P. Role of Endogenous Adenosine as a Modulator of Syncope Induced during Tilt Testing. Circulation 2002, 106, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Tebbenjohanns, J.; Schumacher, B.; Pfeiffer, D.; Jung, W.; Lüderitz, B. Dose and Rate-Dependent Effects of Adenosine on Atrial Action Potential Duration in Humans. J. Interv. Card. Electrophysiol. 1997, 1, 33–37. [Google Scholar] [CrossRef]
- Strickberger, S.A.; Man, K.C.; Daoud, E.G.; Goyal, R.; Brinkman, K.; Knight, B.P.; Weiss, R.; Bahu, M.; Morady, F. Adenosine-Induced Atrial Arrhythmia: A Prospective Analysis. Ann. Intern. Med. 1997, 127, 417–422. [Google Scholar] [CrossRef]
- Soattin, L.; Lubberding, A.F.; Bentzen, B.H.; Christ, T.; Jespersen, T. Inhibition of Adenosine Pathway Alters Atrial Electrophysiology and Prevents Atrial Fibrillation. Front. Physiol. 2020, 11, 493. [Google Scholar] [CrossRef]
- Datino, T.; Macle, L.; Qi, X.-Y.; Maguy, A.; Comtois, P.; Chartier, D.; Guerra, P.G.; Arenal, A.; Fernández-Avilés, F.; Nattel, S. Mechanisms by Which Adenosine Restores Conduction in Dormant Canine Pulmonary Veins. Circulation 2010, 121, 963–972. [Google Scholar] [CrossRef]
- Gupta, T.; Kaur, M.; Sahni, D. Identification of Novel Pulmonary Vein Nodes as Generators of Ectopic Arrhythmic Foci for Atrial Fibrillation: An Immunohistochemical Proof. Surg. Radiol. Anat. 2022, 44, 129–136. [Google Scholar] [CrossRef]
- Li, J.-Y.; Wang, H.-J.; Xu, B.; Wang, X.-P.; Fu, Y.-C.; Chen, M.-Y.; Zhang, D.-X.; Liu, Y.; Xue, Q.; Li, Y. Hyperpolarization Activated Cation Current (If) in Cardiac Myocytes from Pulmonary Vein Sleeves in the Canine with Atrial Fibrillation. J. Geriatr. Cardiol. 2012, 9, 366–374. [Google Scholar] [CrossRef]
- Chan, C.-S.; Lin, Y.-K.; Chen, Y.-C.; Lu, Y.-Y.; Chen, S.-A.; Chen, Y.-J. Heart Failure Differentially Modulates Natural (Sinoatrial Node) and Ectopic (Pulmonary Veins) Pacemakers: Mechanism and Therapeutic Implication for Atrial Fibrillation. Int. J. Mol. Sci. 2019, 20, 3224. [Google Scholar] [CrossRef]
- Sinno, H.; Derakhchan, K.; Libersan, D.; Merhi, Y.; Leung, T.K.; Nattel, S. Atrial Ischemia Promotes Atrial Fibrillation in Dogs. Circulation 2003, 107, 1930–1936. [Google Scholar] [CrossRef]
- Ponti, R.D.; Marazzato, J.; Bagliani, G.; Leonelli, F.M.; Padeletti, L. Sick Sinus Syndrome. Cardiac Electrophysiol. Clin. 2018, 10, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Hansen, B.J.; Fedorenko, O.; Csepe, T.A.; Kalyanasundaram, A.; Li, N.; Hage, L.T.; Glukhov, A.V.; Billman, G.E.; Weiss, R.; et al. Upregulation of Adenosine A1 Receptors Facilitates Sinoatrial Node Dysfunction in Chronic Canine Heart Failure by Exacerbating Nodal Conduction Abnormalities Revealed by Novel Dual-Sided Intramural Optical Mapping. Circulation 2014, 130, 315–324. [Google Scholar] [CrossRef]
- Alboni, P.; Menozzi, C.; Brignole, M.; Paparella, N.; Gaggioli, G.; Lolli, G.; Cappato, R. Effects of Permanent Pacemaker and Oral Theophylline in Sick Sinus Syndrome the THEOPACE Study: A Randomized Controlled Trial. Circulation 1997, 96, 260–266. [Google Scholar] [CrossRef]
- Van Wagoner, D.R.; Pond, A.L.; Lamorgese, M.; Rossie, S.S.; McCarthy, P.M.; Nerbonne, J.M. Atrial L-Type Ca2+ Currents and Human Atrial Fibrillation. Circ. Res. 1999, 85, 428–436. [Google Scholar] [CrossRef]
- Visentin, S.; Wu, S.N.; Belardinelli, L. Adenosine-Induced Changes in Atrial Action Potential: Contribution of Ca and K Currents. Am. J. Physiol. 1990, 258, H1070–H1078. [Google Scholar] [CrossRef]
- Molina, C.E.; Llach, A.; Herraiz-Martínez, A.; Tarifa, C.; Barriga, M.; Wiegerinck, R.F.; Fernandes, J.; Cabello, N.; Vallmitjana, A.; Benitéz, R.; et al. Prevention of Adenosine A2A Receptor Activation Diminishes Beat-to-Beat Alternation in Human Atrial Myocytes. Basic. Res. Cardiol. 2016, 111, 5. [Google Scholar] [CrossRef]
- Vecchio, E.A.; White, P.J.; May, L.T. Targeting Adenosine Receptors for the Treatment of Cardiac Fibrosis. Front. Pharmacol. 2017, 8, 243. [Google Scholar] [CrossRef]
- Vecchio, E.A.; White, P.J.; May, L.T. The Adenosine A2B G Protein-Coupled Receptor: Recent Advances and Therapeutic Implications. Pharmacol. Ther. 2019, 198, 20–33. [Google Scholar] [CrossRef]
- Toldo, S.; Zhong, H.; Mezzaroma, E.; Van Tassell, B.W.; Kannan, H.; Zeng, D.; Belardinelli, L.; Voelkel, N.F.; Abbate, A. GS-6201, a Selective Blocker of the A2B Adenosine Receptor, Attenuates Cardiac Remodeling after Acute Myocardial Infarction in the Mouse. J. Pharmacol. Exp. Ther. 2012, 343, 587–595. [Google Scholar] [CrossRef]
- Zhong, H.; Belardinelli, L.; Zeng, D. Pro-Fibrotic Role of the A2B Adenosine Receptor in Human Cardiac Fibroblasts. J. Card. Fail. 2011, 17, S65. [Google Scholar] [CrossRef]
- Lu, D.; Insel, P.A. Hydrolysis of Extracellular ATP by Ectonucleoside Triphosphate Diphosphohydrolase (ENTPD) Establishes the Set Point for Fibrotic Activity of Cardiac Fibroblasts. J. Biol. Chem. 2013, 288, 19040–19049. [Google Scholar] [CrossRef]
- Phosri, S.; Bunrukchai, K.; Parichatikanond, W.; Sato, V.H.; Mangmool, S. Epac Is Required for Exogenous and Endogenous Stimulation of Adenosine A2B Receptor for Inhibition of Angiotensin II-Induced Collagen Synthesis and Myofibroblast Differentiation. Purinergic Signal. 2018, 14, 141–156. [Google Scholar] [CrossRef]
- Chen, Y.; Epperson, S.; Makhsudova, L.; Ito, B.; Suarez, J.; Dillmann, W.; Villarreal, F. Functional Effects of Enhancing or Silencing Adenosine A2b Receptors in Cardiac Fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2478–H2486. [Google Scholar] [CrossRef][Green Version]
- Wakeno, M.; Minamino, T.; Seguchi, O.; Okazaki, H.; Tsukamoto, O.; Okada, K.; Hirata, A.; Fujita, M.; Asanuma, H.; Kim, J.; et al. Long-Term Stimulation of Adenosine A2b Receptors Begun After Myocardial Infarction Prevents Cardiac Remodeling in Rats. Circulation 2006, 114, 1923–1932. [Google Scholar] [CrossRef]
- Maas, J.E.; Wan, T.C.; Figler, R.A.; Gross, G.J.; Auchampach, J.A. Evidence That the Acute Phase of Ischemic Preconditioning Does Not Require Signaling by the A2B Adenosine Receptor. J. Mol. Cell. Cardiol. 2010, 49, 886–893. [Google Scholar] [CrossRef]
- Feng, W.; Song, Y.; Chen, C.; Lu, Z.Z.; Zhang, Y. Stimulation of Adenosine A2B Receptors Induces Interleukin-6 Secretion in Cardiac Fibroblasts via the PKC-Delta-P38 Signalling Pathway. Br. J. Pharmacol. 2010, 159, 1598–1607. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, H.; Everett, T.H.; Wilson, E.; Chang, R.; Zeng, D.; Belardinelli, L.; Olgin, J.E. Blockade of A2B Adenosine Receptor Reduces Left Ventricular Dysfunction and Ventricular Arrhythmias 1 Week after Myocardial Infarction in the Rat Model. Heart Rhythm 2014, 11, 101–109. [Google Scholar] [CrossRef]
- Sassi, Y.; Ahles, A.; Truong, D.-J.J.; Baqi, Y.; Lee, S.-Y.; Husse, B.; Hulot, J.-S.; Foinquinos, A.; Thum, T.; Müller, C.E.; et al. Cardiac Myocyte–Secreted CAMP Exerts Paracrine Action via Adenosine Receptor Activation. J. Clin. Invesitig. 2014, 124, 5385–5397. [Google Scholar] [CrossRef]
- Perez-Aso, M.; Fernandez, P.; Mediero, A.; Chan, E.S.; Cronstein, B.N. Adenosine 2A Receptor Promotes Collagen Production by Human Fibroblasts via Pathways Involving Cyclic AMP and AKT but Independent of Smad2/3. FASEB J. 2014, 28, 802–812. [Google Scholar] [CrossRef]
- Epperson, S.A.; Brunton, L.L.; Ramirez-Sanchez, I.; Villarreal, F. Adenosine Receptors and Second Messenger Signaling Pathways in Rat Cardiac Fibroblasts. Am. J. Physiol. Cell. Physiol. 2009, 296, C1171–C1177. [Google Scholar] [CrossRef]
- Wragg, E.S.; Pannucci, P.; Hill, S.J.; Woolard, J.; Cooper, S.L. Involvement of β-Adrenoceptors in the Cardiovascular Responses Induced by Selective Adenosine A2A and A2B Receptor Agonists. Pharmacol. Res. Perspect. 2022, 10, e00975. [Google Scholar] [CrossRef]
- Lee, S.W.; Anderson, A.; Guzman, P.A.; Nakano, A.; Tolkacheva, E.G.; Wickman, K. Atrial GIRK Channels Mediate the Effects of Vagus Nerve Stimulation on Heart Rate Dynamics and Arrhythmogenesis. Front. Physiol. 2018, 9, 943. [Google Scholar] [CrossRef]
- Javed, S.; Gupta, D.; Lip, G.Y.H. Obesity and Atrial Fibrillation: Making Inroads through Fat. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 59–67. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Wong, G.R.; Lee, G.; Voskoboinik, A.; Kee, K.; Goldin, J.; Watts, T.; Linz, D.; Wirth, D.; Parameswaran, R.; et al. Sleep Apnoea Has a Dose-Dependent Effect on Atrial Remodelling in Paroxysmal but Not Persistent Atrial Fibrillation: A High-Density Mapping Study. EP Europace 2021, 23, 691–700. [Google Scholar] [CrossRef]
- Sidhu, K.; Tang, A. Modifiable Risk Factors in Atrial Fibrillation: The Role of Alcohol, Obesity, and Sleep Apnea. Can. J. Cardiol. 2017, 33, 947–949. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, M.; Xu, M.; Zhang, Y.; Xu, J.; Huang, Y.; Li, X.; Yao, G.; Sui, W.; Zhang, M.; et al. Left Ventricular and Atrial Remodelling in Hypertensive Patients Using Thresholds from International Guidelines and EMINCA Data. Eur. Heart J. Cardiovasc. Imag. 2022, 23, 166–174. [Google Scholar] [CrossRef]
- Heijman, J.; Kirchner, D.; Kunze, F.; Chrétien, E.M.; Michel-Reher, M.B.; Voigt, N.; Knaut, M.; Michel, M.C.; Ravens, U.; Dobrev, D. Muscarinic Type-1 Receptors Contribute to IK,ACh in Human Atrial Cardiomyocytes and Are Upregulated in Patients with Chronic Atrial Fibrillation. Int. J. Cardiol. 2018, 255, 61–68. [Google Scholar] [CrossRef]
- Dobrev, D.; Friedrich, A.; Voigt, N.; Jost, N.; Wettwer, E.; Christ, T.; Knaut, M.; Ravens, U. The G Protein-Gated Potassium Current I(K,ACh) Is Constitutively Active in Patients with Chronic Atrial Fibrillation. Circulation 2005, 112, 3697–3706. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Fedorov, V.V.; Beloshapko, G.G.; Glukhov, A.V.; Yushmanova, A.V.; Rosenshtraukh, L.V. Roles of Adrenergic and Cholinergic Stimulation in Spontaneous Atrial Fibrillation in Dogs. J. Am. Coll. Cardiol. 2004, 43, 483–490. [Google Scholar] [CrossRef]
- Huang, J.L.; Wen, Z.C.; Lee, W.L.; Chang, M.S.; Chen, S.A. Changes of Autonomic Tone before the Onset of Paroxysmal Atrial Fibrillation. Int. J. Cardiol. 1998, 66, 275–283. [Google Scholar] [CrossRef]
- Tan, A.Y.; Zhou, S.; Ogawa, M.; Song, J.; Chu, M.; Li, H.; Fishbein, M.C.; Lin, S.-F.; Chen, L.S.; Chen, P.-S. Neural Mechanisms of Paroxysmal Atrial Fibrillation and Paroxysmal Atrial Tachycardia in Ambulatory Canines. Circulation 2008, 118, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Deharo, J.-C.; Guieu, R.; Mechulan, A.; Peyrouse, E.; Kipson, N.; Ruf, J.; Gerolami, V.; Devoto, G.; Marrè, V.; Brignole, M. Syncope Without Prodromes in Patients with Normal Heart and Normal Electrocardiogram. J. Am. Coll. Cardiol. 2013, 62, 1075–1080. [Google Scholar] [CrossRef] [PubMed][Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maille, B.; Lalevée, N.; Marlinge, M.; Vahdat, J.; Mottola, G.; Degioanni, C.; De Maria, L.; Klein, V.; Thuny, F.; Franceschi, F.; et al. Adenosine and Adenosine Receptors: Advances in Atrial Fibrillation. Biomedicines 2022, 10, 2963. https://doi.org/10.3390/biomedicines10112963
Maille B, Lalevée N, Marlinge M, Vahdat J, Mottola G, Degioanni C, De Maria L, Klein V, Thuny F, Franceschi F, et al. Adenosine and Adenosine Receptors: Advances in Atrial Fibrillation. Biomedicines. 2022; 10(11):2963. https://doi.org/10.3390/biomedicines10112963
Chicago/Turabian StyleMaille, Baptiste, Nathalie Lalevée, Marion Marlinge, Juliette Vahdat, Giovanna Mottola, Clara Degioanni, Lucille De Maria, Victor Klein, Franck Thuny, Frédéric Franceschi, and et al. 2022. "Adenosine and Adenosine Receptors: Advances in Atrial Fibrillation" Biomedicines 10, no. 11: 2963. https://doi.org/10.3390/biomedicines10112963
APA StyleMaille, B., Lalevée, N., Marlinge, M., Vahdat, J., Mottola, G., Degioanni, C., De Maria, L., Klein, V., Thuny, F., Franceschi, F., Deharo, J.-C., Guieu, R., & Fromonot, J. (2022). Adenosine and Adenosine Receptors: Advances in Atrial Fibrillation. Biomedicines, 10(11), 2963. https://doi.org/10.3390/biomedicines10112963







