Vitamin D in the Treatment of Oral Lichen Planus: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Literature Search and Identification of Studies
2.5. Study Selection
2.6. Outcome Parameters
2.7. Data Extraction
2.8. Risk of Bias Assessment
3. Results
3.1. Study Characteristics
3.2. Outcome Parameters
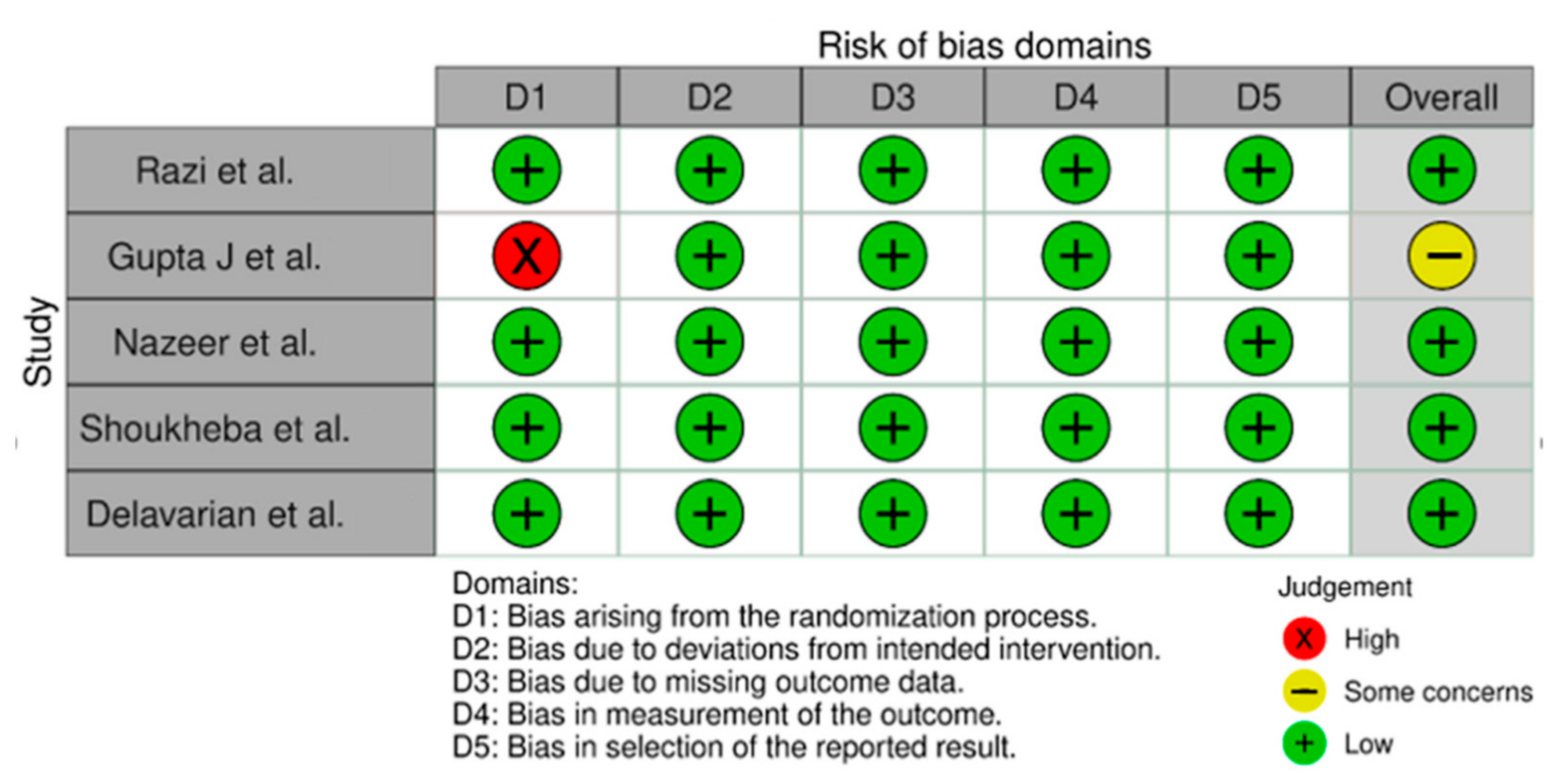
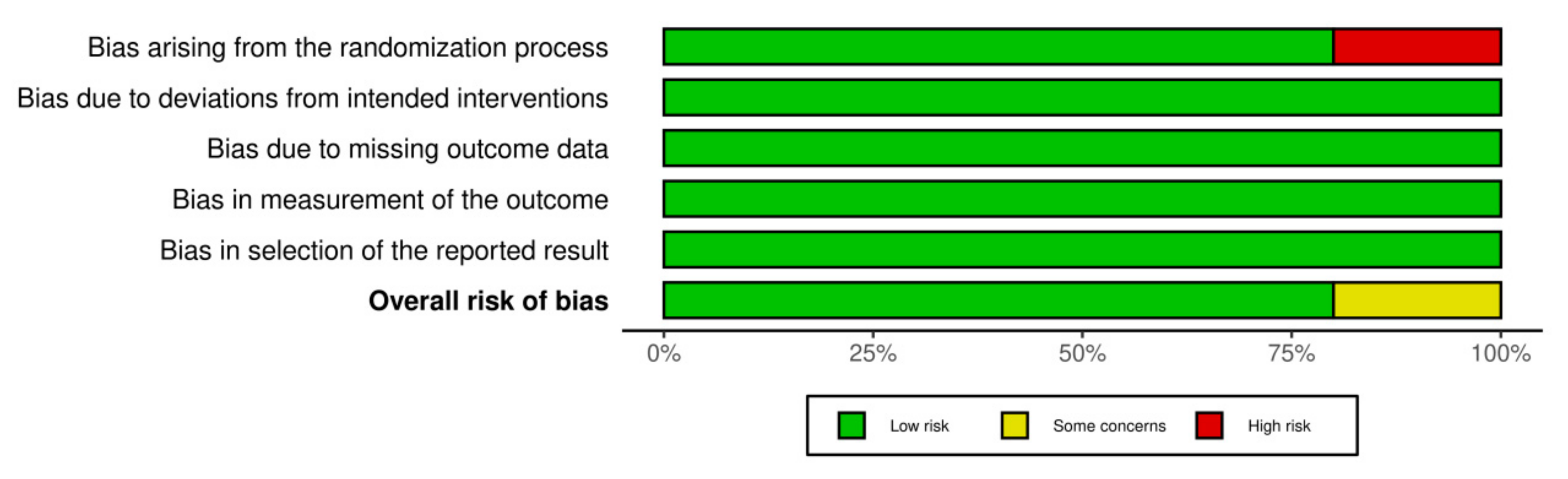
3.3. Assessment of Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farhi, D.; Dupin, N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, Part I: Facts and controversies. Clin. Dermatol. 2010, 28, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Walia, C.; Rallan, N.S.; Premkumar, A.; Roy, S. Clinical evaluation of efficacy of triamcinolone acetonide with tacrolimus in the management of oral lichen planus- A pilot prospective observational study. Contemp. Clin. Dent. 2022, 13, 236–241. Available online: https://www.contempclindent.org/text.asp?2022/13/3/236/356928 (accessed on 24 September 2022). [PubMed]
- Lavanya, N.; Jayanthi, P.; Rao, U.K.; Ranganathan, K. Oral lichen planus: An update on pathogenesis and treatment. J. Oral Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Alrashdan, M.S.; Cirillo, N.; McCullough, M. Oral lichen planus: A literature review and update. Arch. Dermatol. Res. 2016, 308, 539–551. [Google Scholar] [CrossRef]
- Li, C.; Tang, X.; Zheng, X.; Ge, S.; Wen, H.; Lin, X.; Chen, Z.; Lu, L. Global Prevalence and Incidence Estimates of Oral Lichen Planus: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 172–181. [Google Scholar] [CrossRef]
- Carbone, M.; Arduino, P.G.; Carrozzo, M.; Gandolfo, S.; Argiolas, M.R.; Bertolusso, G.; Conrotto, D.; Pentenero, M.; Broccoletti, R. Course of oral lichen planus: A retrospective study of 808 northern Italian patients. Oral Dis. 2009, 15, 235–243. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Scully, C.; Gil-Montoya, J.A. Oral lichen planus: Controversies surrounding malignant transformation. Oral Dis. 2008, 14, 229–243. [Google Scholar] [CrossRef]
- Idrees, M.; Kujan, O.; Shearston, K.; Farah, C.S. Oral lichen planus has a very low malignant transformation rate: A systemic review and meta-analysis using strict diagnostic and inclusion criteria. J. Oral Pathol. Med. 2021, 50, 287–298. [Google Scholar] [CrossRef]
- Radochová, V.; Ivančaková, R.K.; Heneberk, O.; Slezák, R. The Characteristics of Patients with Oral Lichen Planus and Malignant Transformation-A Retrospective Study of 271 Patients. Int J. Environ. Res. Public Health 2021, 18, 6525. [Google Scholar] [CrossRef]
- González-Moles, M.A.; Ruiz-Ávila, I.; González-Ruizd, L.; Ayéne, A.; Gil-Montoya, J.A.; Ramos-Garcíaa, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef]
- Giuliani, M.; Troiano, G.; Cordaro, M.; Corsalini, M.; Gioco, G.; Muzio, L.L.; Pignatelli, P.; Lajolo, C. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2019, 25, 693–709. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Ramos-García, P.; Warnakulasuriya, S. An appraisal of highest quality studies reporting malignant transformation of oral lichen planus based on a systematic review. Oral Dis. 2021, 27, 1908–1918. [Google Scholar] [CrossRef]
- Zotti, F.; Nocini, R.; Capocasale, G.; Bertossi, D.; Fior, A.; Peretti, M.; Manfrin, E.; Albanese, M. Oral Lichen Planus: Risk factors of malignant transformation and follow up. Ten years retrospective study. J. Clin. Exp. Dent. 2021, 13, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Gould, A.; Kurago, Z.; Fantasia, J.; Muller, S. Diagnosis of oral lichen planus: A position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 332–354. [Google Scholar] [CrossRef] [PubMed]
- Nuzzolo, P.; Celentano, A.; Bucc, P.; Adamo, D.; Ruoppo, E.; Leuci, S.; Mignogna, M.D. Lichen planus of the lips: An intermediate disease between the skin and mucosa? Retrospective clinical study and review of the literature. Int. J. Dermatol. 2016, 55, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Wiriyakijja, P.; Stephen Porter, S.; StefanoFedele, S.; Hodgson, T.; McMillan, R.; Shephard, M.; Ni-Riordain, R. Health-Related Quality of Life and Its Associated Predictors in Patients with Oral Lichen Planus: A Cross-Sectional Study. Int. Dent. J. 2021, 71, 140–152. [Google Scholar] [CrossRef]
- Hasan, S.; Ahmed, S.; Kiran, R.; Panigrahi, R.; Thachil, J.M.; Saeed, S. Oral lichen planus and associated comorbidities: An approach to holistic health. J. Family Med. Prim. Care. 2019, 8, 3504–3517. [Google Scholar] [CrossRef]
- Van der Meij, E.H.; Van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef]
- Rotaru, D.; Chisnoiu, R.; Picos, A.M.; Picos, A.; Chisnoiu, A. Treatment trends in oral lichen planus and oral lichenoid lesions. Exp. Ther Med. 2020, 20, 198. [Google Scholar] [CrossRef]
- Gupta, S.; Jawanda, M.K. Oral lichen planus: An update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J. Dermatol. 2015, 60, 222–229. Available online: https://www.e-ijd.org/text.asp?2015/60/3/222/156315 (accessed on 18 September 2022). [CrossRef]
- Schlosser, B.J. Lichen planus and lichenoid reactions of the oral mucosa. Dermatol. Ther. 2010, 23, 251–267. [Google Scholar] [CrossRef]
- Didona, D.; Caposiena Caro, R.D.; Sequeira Santos, A.M.; Solimani, F.; Hertl, M. Therapeutic strategies for oral lichen planus: State of the art and new insights. Front. Med. 2022, 9, 997190. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, J.K.; Ezhilarasan, D.; Govindarajan, M.; Somasundaram, E. Therapeutic effectiveness of alternative medications in oral lichen planus: A systematic review. J. Oral Maxillofac. Pathol. 2020, 24, 344–351. [Google Scholar] [CrossRef]
- Sridharan, K.; Sivarama Krishnan, G. Interventions for oral lichen planus: A systematic review and network meta-analysis of randomized clinical trials. Aust. Dent. J. 2021, 66, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Goss, E.; Carrozzo, M.; Castellano, S.; Conrotto, D.; Broccoletti, R.; Gandolfo, S. Systemic and topical corticosteroid treatment of oral lichen planus: A comparative study with long-term follow-up. J. Oral Pathol. Med. 2003, 32, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Klein, B.A.; Al-Hadlaq, M.; Chirravur, P.; Bajonaid, A.; Xu, Y.; Intini, R.; Hussein, M.; Vacharotayangul, P.; Sroussi, H.; et al. Oral lichen planus: Comparative efficacy and treatment costs—A systematic review. BMC Oral Health 2022, 22, 161. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Bui, L.; Zhu, Z.; Hawkins, S.; Cortez-Resendiz, A.; Bellon, B. Vitamin D regulation of the immune system and its implications for COVID-19: A mini review. SAGE Open Med. 2021, 9, 20503121211014073. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Sirajudeen, S.; Shah, I.; Al-Menhali, A. A Narrative Role of Vitamin D and Its Receptor: With Current Evidence on the Gastric Tissues. Int. J. Mol. Sci. 2019, 20, 3832. [Google Scholar] [CrossRef] [PubMed]
- Van-Belle, T.L.; Gysemans, C.; Mathieu, C. Vitamin D in autoimmune, infectious and allergic diseases: A vital player? Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 617–632. [Google Scholar] [CrossRef]
- Mehmood, Z.H.; Papandreou, D. An updated mini review of vitamin D and obesity: Adipogenesis and inflammation state. Open Access Maced. J. Med. Sci. 2016, 4, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, M.A.; Manson, J.E.; Costenbader, K.H. Does vitamin D affect risk of developing autoimmune disease? A systematic review. Semin. Arthritis. Rheum. 2011, 40, 512–531. [Google Scholar] [CrossRef] [PubMed]
- El-Komy, M.H.; Samir, N.; Shaker, O.G. Estimation of vitamin D levels in patients with pemphigus vulgaris. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Minz, R.W.; Anand, S.; Parmar, N.V.; Kanwar, A.J. Vitamin D deficiency and lower TGF-β/IL-17 ratio in a North Indian cohort of pemphigus vulgaris. BMC Res. Notes 2014, 7, 536. [Google Scholar] [CrossRef]
- Marzano, A.V.; Trevisan, V.; Eller-Vainicher, C.; Cairoli, E.; Marchese, L.; Morelli, V. Evidence for vitamin D deficiency and increased prevalence of fractures in autoimmune bullous skin diseases. Br. J. Dermatol. 2012, 16, 688–691. [Google Scholar] [CrossRef]
- Marzano, A.V.; Trevisan, V.; Cairoli, E.; Eller-Vainicher, C.; Morelli, V.; Spada, A. Vitamin D and skeletal health in autoimmune bullous skin diseases: A case control study. Orphanet. J. Rare Dis. 2015, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Bayramgürler, D.; Apaydin, R.; Bilen, N. Limited benefit of topical calcipotriol in lichen planus treatment: A preliminary study. J. Dermatol. Treat. 2002, 13, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Theng, C.T.; Tan, S.H.; Gosh, C.L.; Suresh, S.; Wong, H.B.; Machin, D. Singapore Lichen Planus Study Group. A randomized controlled trial to compare calcipotriol with betamethasone valerate for the treatment of cutaneous lichen planus. J. Dermatolog. Treat. 2004, 15, 141–145. [Google Scholar] [CrossRef]
- Varma, R.B.; Valappila, N.J.; Pai, A.; Saddu, S.C.; Mathew, N. Oral lichen planus: Is vitamin d defi- ciency a predisposing factor? A case report. Int. J. Sci. Stud. 2014, 2, 230–232. [Google Scholar]
- Moreas, P.D.C.; Cintra, M.L.; Montalli, V.A.M.; Araujo, V.C.D.; Passador-Santos, F.; Napimoga, M.H.; Junqueira, J.L.C. Desquamative Gingivitis: Vitamin D deficiency? Oral Surg. Oral Med. Oral Patholo. Oral Radiol. 2020, 130, e140. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias Visualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn Meth. 2020, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Razi, A.; Mohiuddin, S.; Karim, A.A.; Iqbal, A. Vitamin D as an adjuvant therapy to cure oral lichen planus in peri-menopausal women. Pak. Oral Dent. J. 2018, 38, 399–403. [Google Scholar]
- Gupta, J.; Aggarwal, A.; Asadullah, M.; Khan, M.H.; Agrawal, N.; Khwaja, K.J. Vitamin D in the treatment of oral lichen planus: A pilot clinical study. J. Indian Acad Oral Med. Radiol. 2019, 31, 222–227. Available online: https://www.jiaomr.in/text.asp?2019/31/3/222/268283 (accessed on 15 September 2022).
- Nazeer, Z.; Singh, S.; Jayam, C.; Singh, R.; Md A Iqubal, M.A.; Revati Singh, R. Assessment of the Role of Vitamin D in the Treatment of Oral Lichen Planus. J. Contemp. Dent. Pract. 2020, 21, 390–395. [Google Scholar] [CrossRef]
- Shoukheba, M.Y. Adjunctive effect of vitamin D to local cortisone in treating oral lichen planus lesions in menopausal and post- menopausal Egyptian women. Egypt. Dent. J. 2020, 66, 2207–2215. [Google Scholar] [CrossRef]
- Delavarian, Z.; Dalirsani, Z.; Mousavi, Z.; Shakeri, M.T.; Rafatpanah, H.; Seif, F. Evaluation of the efficacy of vitamin D in the treatment of oral lichen planus: A double-blind randomized clinical trial. J. Oral Health Oral Epidemiol. 2021, 10, 107–115. [Google Scholar] [CrossRef]
- Speight, P.; Khurram, S.A.; Kujan, O. Oral potentially malignant disorders: Risk of progression to malignancy. Oral Surg Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Cheng, B.; Ondrey, F. Th1/Th2 cytokine ratio in tissue transudates from patients with oral lichen planus. Mediators Inflamm. 2007, 2007, 19854. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yu, F.; Yang, F.; Wang, J.; Chen, Q.; Wang, X.; Zhao, B.; Zhang, F. Experimental study on 1,25(OH)2 D3 amelioration of oral lichen planus through regulating NF-κB signaling pathway. Oral Dis. 2017, 23, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.; Hegarty, A.M.; Hodgson, T. Aetiology, diagnosis and treatment of oral lichen planus. Br. J. Hosp. Med. 2014, 75, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Bagan, J.; Compilato, D.; Paderni, C.; Campisi, G.; Panzarella, V.; Picciotti, M.; Lorenzini, G.; Di Fede, O. Topical therapies for oral lichen planus management and their efficacy: A narrative review. Curr Pharm Des. 2012, 18, 5470–5480. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Alanazi, R.; Alhajj, M.N.; Daer, A.; Hunaish, A.A.; Nabhan, A.B.; Al-Sosowa, A.A. Efficacy of topical hyaluronic acid for symptomatic oral lichen planus: A Systematic Review. J. Oral Res. 2021, 10, 1–7. [Google Scholar] [CrossRef]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A. Vitamin D and human health. Int, J. Mol. Sci. 2019, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micro nutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.B.; Anwar, M.K.; Shaker, O.G.; El-Sharkawy, D.A. Possible Relation between Vitamin D and Interleukin-17 in the Pathogenesis of Lichen Planus. Dermatology 2021, 237, 896–901. [Google Scholar] [CrossRef]
- Mohan, R.P.S.; Gupta, A.; Kamarthi, N.; Malik, S.; Goel, S.; Gupta, S. Incidence of Oral Lichen Planus in Peri-menopausal Women: A Cross-sectional Study in Western Uttar Pradesh Population. J. Midlife Health 2017, 8, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Katarzyna, C.; Monika, K.; Jakub, L.; Damian, M.; Jakub, P. The benefits of vitamin D3 supplementation for menopausal women-literature review. J. Educ. Health Sport 2021, 11, 47–51. [Google Scholar]
- Kotwal, S.D.; Bhat, A.N.; Yograj, S.; Kotwal, S. Evaluation of vitamin-D status in premenopausal and postmenopausal type-2 diabetic women and its relation to glycemic control. Int. J. Res. Med. Sci. 2020, 8, 1292–1298. [Google Scholar] [CrossRef]
- Cassol-Spanemberg, J.; Rivera-Campillo, M.E.; Otero-Rey, E.M.; Estrugo-Devesa, A.; Jané-Salas, E.; López-López, J. Oral lichen planus and its relationship with systemic diseases. A review of evidence. J. Clin. Exp. Dent. 2018, 10, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Oczko, M.; Zwyrtek, E.; Owczarek, J.E.; Szcześniak, D. Psychopathological profile and quality of life of patients with oral lichen planus. J. Appl. Oral Sci. 2018, 26, e20170146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mohan, R.P.S.; Kamarthi, N.; Malik, S.; Sumit Goel, S.; Gupta, S. Serum Vitamin D Level in Oral Lichen Planus Patients of North India- A Case-Control Study. J. Dermatol. Res. Ther. 2017, 1, 19–35. [Google Scholar] [CrossRef]
- Piboonniyom, S.-O.; Treister, N.; Pitiphat, W.; Woo, S.-B. Scoring system for monitoring oral lichenoid lesions: A preliminary study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Van der Waal, I. Disease scoring systems for oral lichen planus; a critical appraisal. Med. Oral Patol. Oral Y Cir. Bucal. 2015, 20, e199–e204. [Google Scholar] [CrossRef]
- Lodi, G.; Carrozzo, M.; Furness, S.; Thongprasom, K. Interventions for treating oral lichen planus: A systematic review. Br. J. Dermatol. 2012, 166, 938–947. [Google Scholar] [CrossRef]
- Zakrzewska, J.M.; Chan, E.S.; Thornhill, M.H. A systematic review of placebo-controlled randomized clinical trials of treatments used in oral lichen planus. Br. J. Dermatol. 2005, 153, 336–341. [Google Scholar] [CrossRef]
- Thongprasom, K.; Luangjarmekorn, L.; Sererat, T.; Taweesap, W. Relative efficacy of fluocinolone acetonide compared with triamcinolone acetonide in treatment of oral lichen planus. J. Oral Pathol. Med. 1992, 21, 456–458. [Google Scholar] [CrossRef]
- Elsabagh, H.H.; Moussa, E.; Mahmoud, S.A.; Elsaka, R.O.; Abdelrahman, H. Efficacy of Melatonin in prevention of radiation-induced oral mucositis: A randomized clinical trial. Oral Dis. 2020, 26, 566–572. [Google Scholar] [CrossRef]
- Chainani-Wu, N.; Silverman, J.S.; Reingold, A.; Bostrom, A.; Lozada-Nur, F.; Weintraub, J. Validation of instruments to measure the symptoms and signs of oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodon 2008, 105, 51–58. [Google Scholar] [CrossRef]
- Escudier, M.; Ahmed, N.; Shirlaw, P.; Setterfield, J.; Tappuni, A.; Black, M.M.; Challacombe, S.J. A scoring system for mucosal disease severity with special reference to oral lichen planus. Br. J. Dermatol. 2007, 157, 765–770. [Google Scholar] [CrossRef]
- Kaliakatsou, F.; Hodgson, T.; Lewsey, J.; Hegarty, A.; Murphy, A.; Porter, S. Management of recalcitrant ulcerative oral lichen planus with topical tacrolimus. J. Am. Acad. Dermatol. 2002, 46, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid. Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- Nair, P.; Venkatesh, B.; Center, J.R. Vitamin D deficiency and supplementation in critical illness-the known knowns and known unknowns. Crit. Care 2018, 22, 276. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.A.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Fuleihan, G.E.H.; Josse, R.G.; Lips, P.; Morales-Torres, J.; Yoshimura, N.; et al. IOF position statement: Vitamin D recommendations for older adults. Osteopor. Int. 2010, 21, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Choi, Y. The microbiology of oral lichen planus: Is microbial infection the cause of oral lichen planus? Mol. Oral Microbiol. 2018, 33, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, K.; Zhang, B.; Tu, Q.; Yao, Y.; Cui, B.; Ren, B.; He, J.; Shenm, X.; Joy, D.; et al. Salivary mycobiome dysbiosis and its potential impact on bacteriome shifts and host immunity in oral lichen planus. Int. J. Oral Sci. 2019, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Du, G.H.; Wang, Y.F.; Chen, J.J.; Deng, Y.W.; Han, X.Z.; Tang, G.Y. Potential association between Fusobacterium nucleatum enrichment on oral mucosal surface and oral lichen planus. Oral Dis. 2020, 26, 122–130. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home oral care of periodontal patients using antimicrobial gel with postbiotics, lactoferrin, and aloe barbadensis leaf juice powder vs. conventional chlorhexidine gel: A split-mouth randomized clinical trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef] [PubMed]

| Sn | Author (s)/Year/Country | Type of Study | Age/Sex/Follow Up | Sample Size | Oral Lichen Planus (OLP) Diagnosis | Treatment Plan | Test of Significance | Outcome | Conclusions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Razi et al., 2018 [43] Pakistan | Randomized controlled Clinical Trial | 35–45 years/Peri-menopausal females; 4 weeks follow up | 100 | Clinical diagnosis | OLP patients with vitamin D serum levels below 30 ng/mL were divided into 2 groups: | Paired Sample T-test | Visual analog scale (VAS) score | Patients receiving standard therapy + Vitamin D supplementation (Group II) exhibited amelioration in the clinical appearance of the lesion between week 1 and week 4. | |||||||
| Group | Week 1 | Week 4 | ||||||||||||||
| I | 5.04 ± 2.20 | 1.80 ± 0.40 | ||||||||||||||
| II | 1.80 ± 0.40 | 0.80 ± 0.40 | ||||||||||||||
| Group | Vitamin D supplement | Steroids | Size of Lesion | |||||||||||||
| Group | Week 1 | Week 4 | ||||||||||||||
| I | *** | I | 1.80 ± 1.40 | 1.48 ± 0.74 | ||||||||||||
| II | *** | *** | II | 1.80 ± 1.40 | 0.80 ± 0.40 | |||||||||||
| 2. | Gupta J et al., 2019 [44] India | Observational study | All age groups/Both genders, 12 weeks follow up | 106 | Clinical Diagnosis based on typical bilateral white interlacing Whickham’s striae, burning sensations and intolerance to spices. However, doubtful cases (Gingival desquamation/inconspicuous reticular pattern) were biopsied for a confirmatory OLP diagnosis. | OLP patients were divided into 3 groups based on Vitamin D levels, and history of Stress. | Fischer’s Exact test | VAS (Pain score) | (1) Patients treated with vitamin D supplementation (Group II and III) reported statistically significant amelioration in OLP symptoms. (2) Patients treated with vitamin D supplements and psychological Counseling (Group III) reported a marked diminution in the burning sensations. | |||||||
| Group | Counseling | Vit D | Steroids | Group | 0–4 | >4 | ||||||||||
| I | 66.70% | 33.30% | ||||||||||||||
| II | 73.90% | 26.10% | ||||||||||||||
| III | 93.30% | 6.70% | ||||||||||||||
| I | *** | *** | Size of Lesion | |||||||||||||
| II | *** | *** | Group | 0–2 | 3–5 | |||||||||||
| I | 46.70% | 53.30% | ||||||||||||||
| III | *** | *** | *** | II | 86.90% | 13.10% | ||||||||||
| III | 86.70% | 13.30% | ||||||||||||||
| 3 | Nazeer et al., 2020 [45] India | Observational study | 35–45 years/Both genders; 4 and 15 weeks follow up | 450 | Clinical Diagnosis based on typical bilateral white interlacing Whickham’s striae, burning sensations and intolerance to spices. However, doubtful cases (Gingival desquamation/inconspicuous reticular pattern) were biopsied for a confirmatory OLP diagnosis. | OLP patients were divided into 3 groups based on their serum Vitamin D levels and history of stress. | ANOVA test | VAS (Pain score) | (1) Patients treated with vitamin D supplementation reported a statistically significant amelioration in subjective symptoms (Group I and II). | |||||||
| Group | Counseling | Vit D | Steroids | Group | 0–4 | >4 | ||||||||||
| I | 54.70% | 45.30% | ||||||||||||||
| II | 64.70% | 35.30% | ||||||||||||||
| I | *** | *** | *** | III | 33.30% | 66.70% | ||||||||||
| Size of Lesion | ||||||||||||||||
| II | *** | *** | Group | 0–2 | 3–5 | |||||||||||
| I | 86.70% | 13.30% | ||||||||||||||
| III | *** | II | 56.70% | 43.30% | ||||||||||||
| III | 46.70% | 53.30% | ||||||||||||||
| 4 | Shoukheba et al., 2020 [46] Egypt | Randomized controlled Clinical Trial | 45–65 years/Post-menopausal females; 2,4,6 weeks follow up | 30 | Clinical diagnosis | OLP patients with serum Vitamin D levels below 30 ng/mL were randomly divided into 2 groups. | Paired Sample T Test | VAS (Pain score) | (1) A statistically significant reduction in pain scores (VAS) compared to the baseline data was observed with both groups. (2) At 6 weeks of follow up Group II receiving Vitamin D supplementation showed a 100% reduction in lesion size. | |||||||
| Group | Week 2 | Week 4 | Week 6 | |||||||||||||
| I | 2.8 ± 0.67 | 1.73 ± 0.70 | 2.8 ± 0.63 | |||||||||||||
| II | 2.13 ± 0.91 | 1.33 ± 0.70 | 1.86 ± 0.51 | |||||||||||||
| Group | Week 2 | Week 4 | Week 6 | |||||||||||||
| Group | Vitamin D supplement | Steroids | Size of Lesion | |||||||||||||
| Group | 0–2 | 3–5 | ||||||||||||||
| I | *** | I | 46% | 54% | ||||||||||||
| II | *** | *** | II | 100% | 0% | |||||||||||
| 5. | Delavarian et al., 2021 [47] Iran | Randomized double-blind, placebo-controlled clinical trial | 22–70 years old/both genders (mostly females) | 28 | Clinical and histopathological Diagnosis based on World Health Organization (WHO) modified criteria. | Based on OLP diagnosis and vitamin D levels less than 30 ng/mL, 28 patients were divided into 2 groups. Group I (Intervention group; n = 13) and Group II (Control group; n = 15) | Paired Sample T Test | VAS (Pain score) | A significant decrease in the severity of lesions was observed in the intervention group (p = 0.043). | |||||||
| Group | Week 2 | Week 4 | Week 6 | Week 8 | ||||||||||||
| I | 7.38 ± 3.25 | 4.13 ± 2.64 | 2.75 ± 2.43 | 2.13 ± 2.33 | ||||||||||||
| II | 1.21 ± 1.67 | 0.93 ± 1.32 | 1.29 ± 2.16 | 1.64 ± 2.31 | ||||||||||||
| Group | Vitamin D supplement | Steroids | Lactose | Size of Lesion | ||||||||||||
| Group | Week 2 | Week 4 | Week 6 | Week 8 | ||||||||||||
| I | *** | *** | I | 3.63 ± 0.92 | 3.63 ± 0.74 | 3.38 ± 0.52 | 3.50 ± 0.93 | |||||||||
| II | *** | *** | II | 3.14 ± 0.54 | 3.07 ± 0.92 | 3.07 ± 0.62 | 3.14 ± 0.86 | |||||||||
| Salient Property | Mechanism | |
|---|---|---|
| 1. | Anti-inflammatory and Immunomodulatory | Vitamin D also induces antimicrobial peptide expression like defensins β2 and β4 and cathelicidin antimicrobial peptide (CAMP) by keratinocytes, macrophages, monocytes, epithelial, pulmonary, gastric, and corneal cells, thus, augmenting chemotaxis, autophagy, phagolysosomal immune cell fusion, and strengthening the physical barrier functioning. These anti-microbial properties boost the body’s defense mechanism against microbial infections. Vitamin D modulates both the adaptive and innate immune response. The calcitriol metabolite of vitamin D interacts with nuclear vitamin D receptors (nVDR) present on immune cells (B and T lymphocytes), neutrophils, monocytes, and dendritic cells (DC). Calcitriol exhibit a downregulatory effect on the cell-mediated (Th1) immune responses by suppressing the release of type 1 proinflammatory cytokines (such as IL-6, IL-8, IL-12, IL-17, IL-21, IFN-γ, TNF-α, and IL-9). However, it upregulates the humoral (Th2) response by facilitating the production of type 2 anti-inflammatory cytokines (such as IL-4, IL-5, and IL-10). |
| 2. | Keratinocyte proliferation and differentiation | Vitamin D has a regulatory effect on keratinocyte proliferation and differentiation. Calcitriol inhibits B cell differentiation and proliferation and promotes apoptosis. Vitamin D/analogs may facilitate the restoration of the normal epidermal cytokeratin profile, thus, further attributing to its therapeutic potential in lichen planus. |
| 3. | Adrenal cortisol regulation | Increased episodes of anxiety, depression, and psychic ailments have been associated with OLP patients. Chronic stress attributed as the predominant predisposing factor for acute flare-ups in OLP triggers increased adrenal cortisol production and causes reduced expression of vitamin D receptors. This vicious cycle eventually results in decreased uptake/activation of vitamin D, thus, affirming the possible corroboration between psychological factors, vitamin D deficiency, and OLP. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, S.; Choudhury, P.; Ahmad, S.A.; Alam, T.; Panigrahi, R.; Aziz, S.; Kaleem, S.M.; Priyadarshini, S.R.; Sahoo, P.K.; Hasan, S. Vitamin D in the Treatment of Oral Lichen Planus: A Systematic Review. Biomedicines 2022, 10, 2964. https://doi.org/10.3390/biomedicines10112964
Saeed S, Choudhury P, Ahmad SA, Alam T, Panigrahi R, Aziz S, Kaleem SM, Priyadarshini SR, Sahoo PK, Hasan S. Vitamin D in the Treatment of Oral Lichen Planus: A Systematic Review. Biomedicines. 2022; 10(11):2964. https://doi.org/10.3390/biomedicines10112964
Chicago/Turabian StyleSaeed, Shazina, Priyadarshini Choudhury, Syed Ansar Ahmad, Tanveer Alam, Rajat Panigrahi, Shahid Aziz, Sultan Mohammed Kaleem, Smita R. Priyadarshini, Pradyumna Ku Sahoo, and Shamimul Hasan. 2022. "Vitamin D in the Treatment of Oral Lichen Planus: A Systematic Review" Biomedicines 10, no. 11: 2964. https://doi.org/10.3390/biomedicines10112964
APA StyleSaeed, S., Choudhury, P., Ahmad, S. A., Alam, T., Panigrahi, R., Aziz, S., Kaleem, S. M., Priyadarshini, S. R., Sahoo, P. K., & Hasan, S. (2022). Vitamin D in the Treatment of Oral Lichen Planus: A Systematic Review. Biomedicines, 10(11), 2964. https://doi.org/10.3390/biomedicines10112964