Leukotriene B4 Receptor 2 Mediates the Production of G-CSF That Plays a Critical Role in Steroid-Resistant Neutrophilic Airway Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Mice
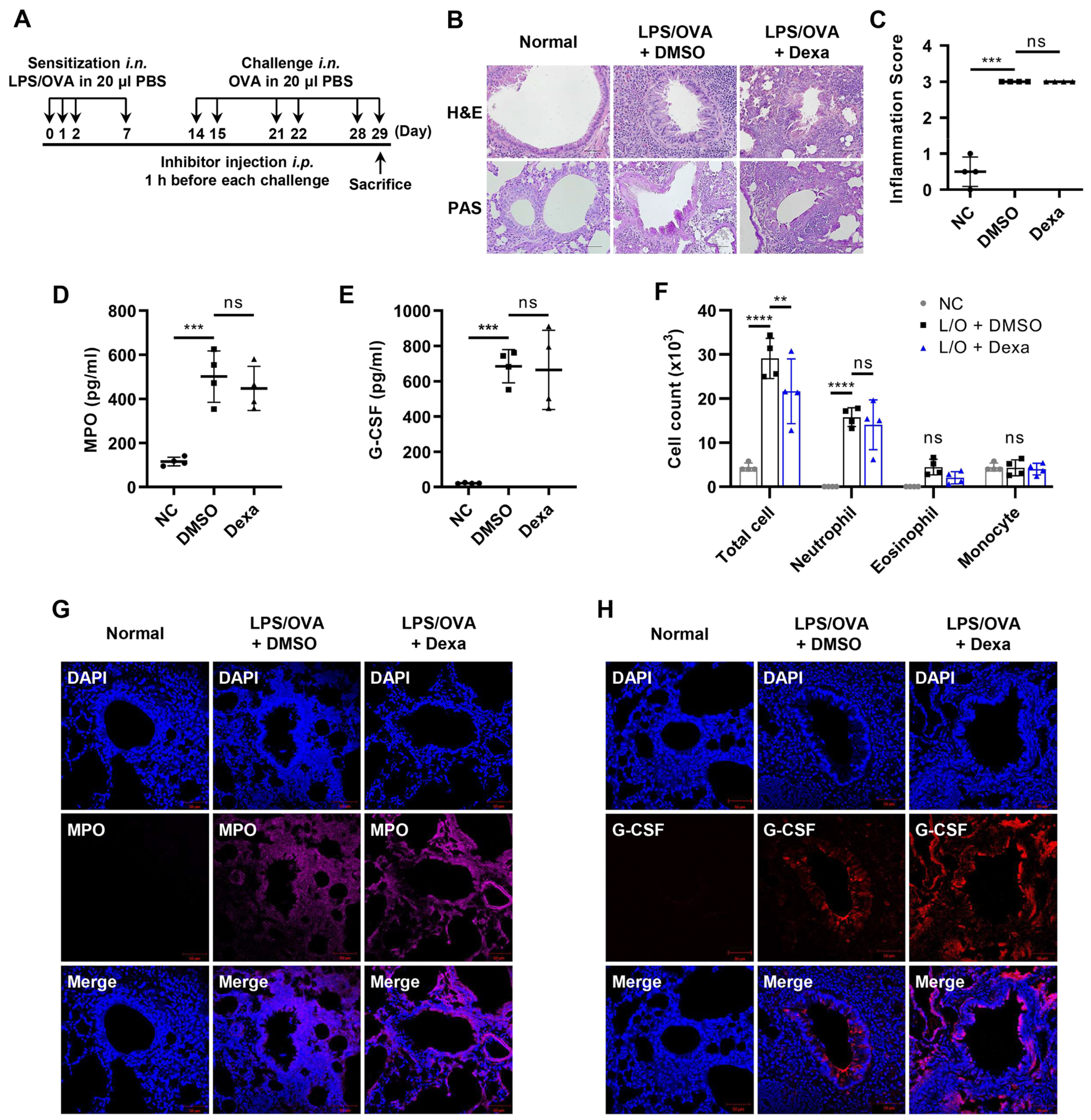
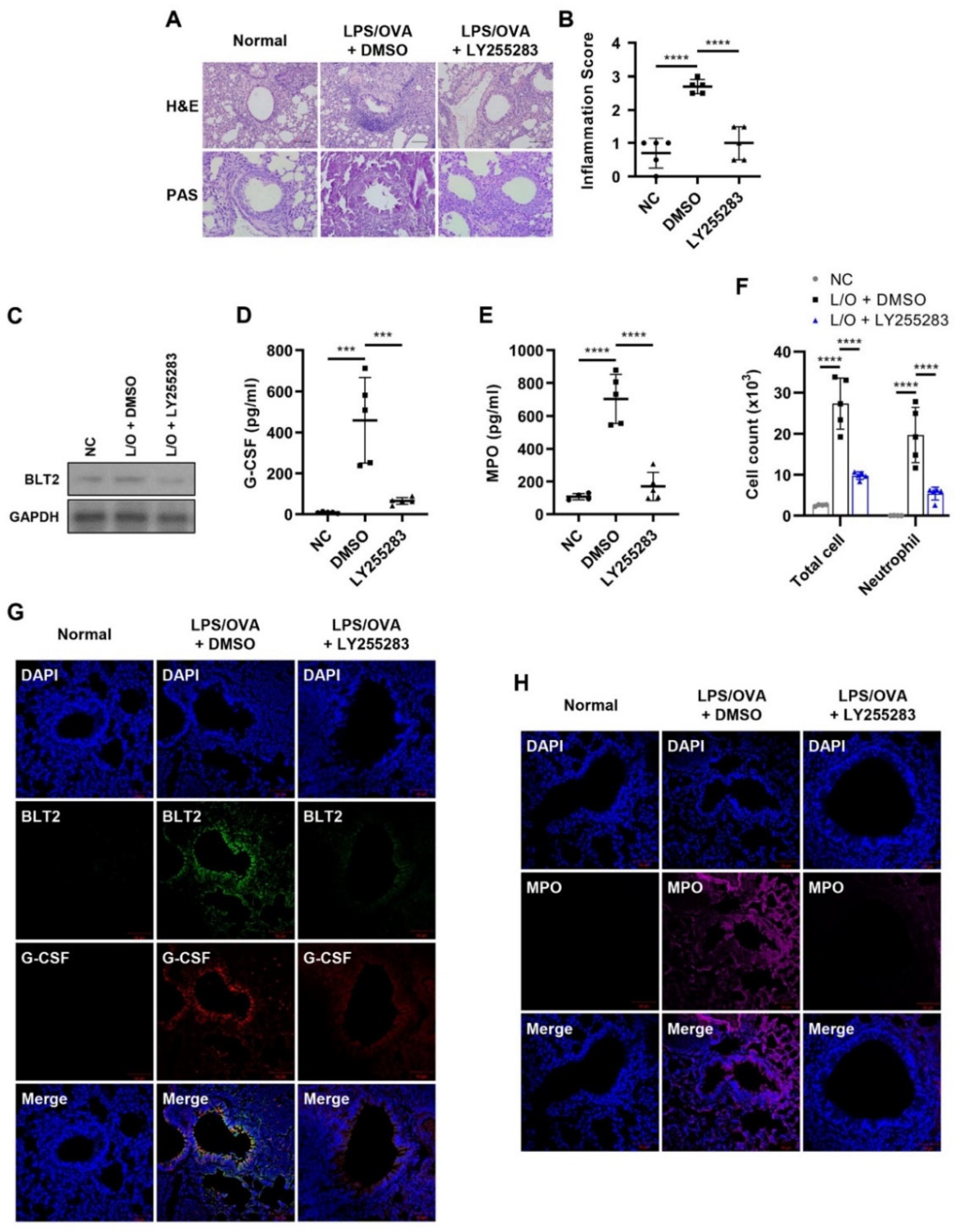
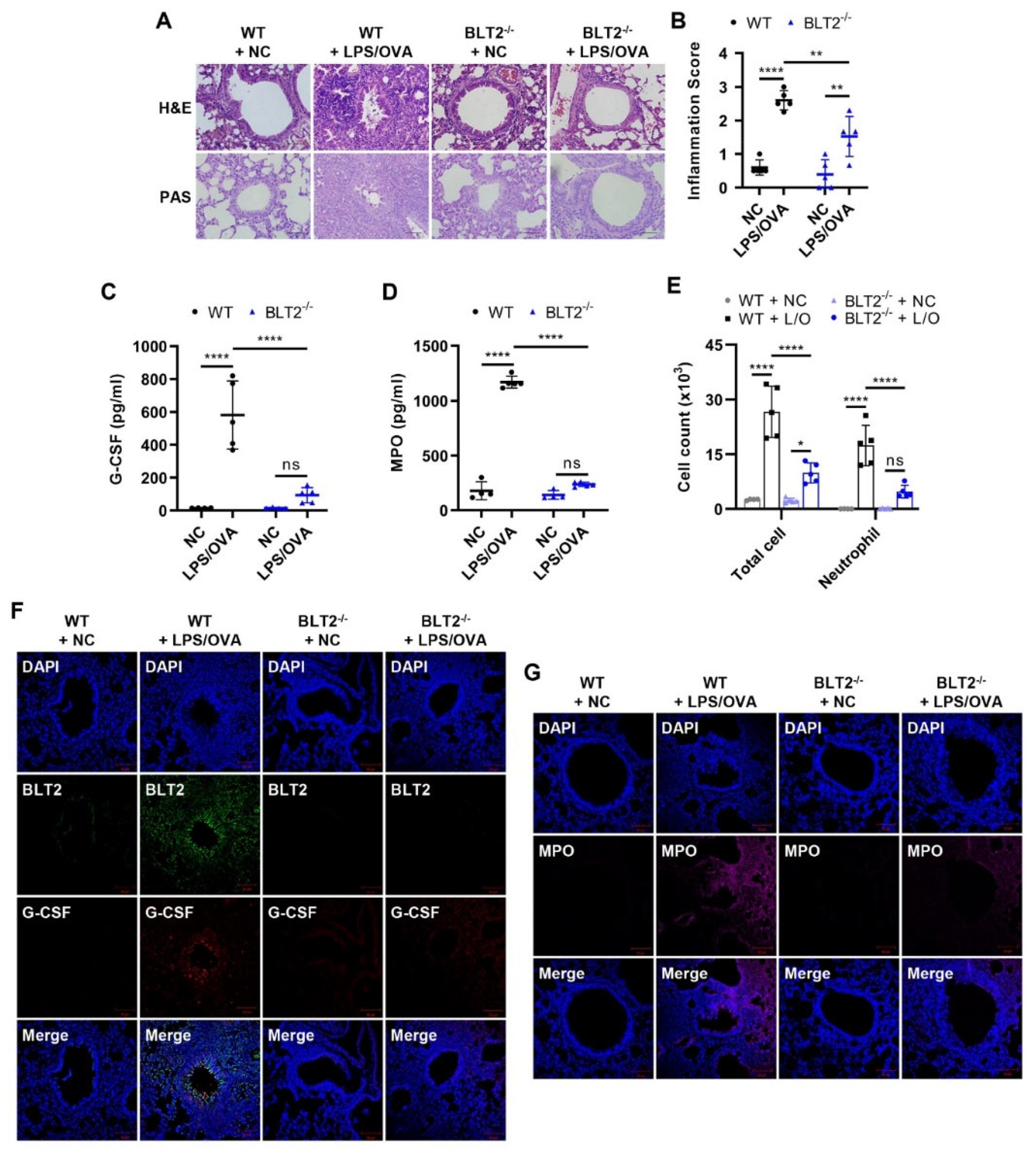
2.3. Animal Model for LPS/OVA-Induced Steroid-Resistant Neutrophilic Airway Inflammation
2.4. Western Blotting
2.5. Measurements of 12(S)-HETE, G-CSF, and Myeloperoxidase (MPO)
2.6. Bronchoalveolar Lavage Cell Counting
2.7. Histological Staining and Analysis of Lung Tissues
2.8. Statistical Analysis
3. Results
3.1. Elevated Levels of G-CSF in the Steroid-Resistant Neutrophilic Airway Inflammation Model
3.2. G-CSF Is Critical for Neutrophilic Airway Inflammation
3.3. BLT2 Mediates the Production of G-CSF in Neutrophilic Airway Inflammation
3.4. BLT2 Knockout Attenuates Both G-CSF Production and Neutrophilic Airway Inflammation
3.5. 12-LO Is Also Necessary for the Production of G-CSF and Contributes to Neutrophilic Airway Inflammation
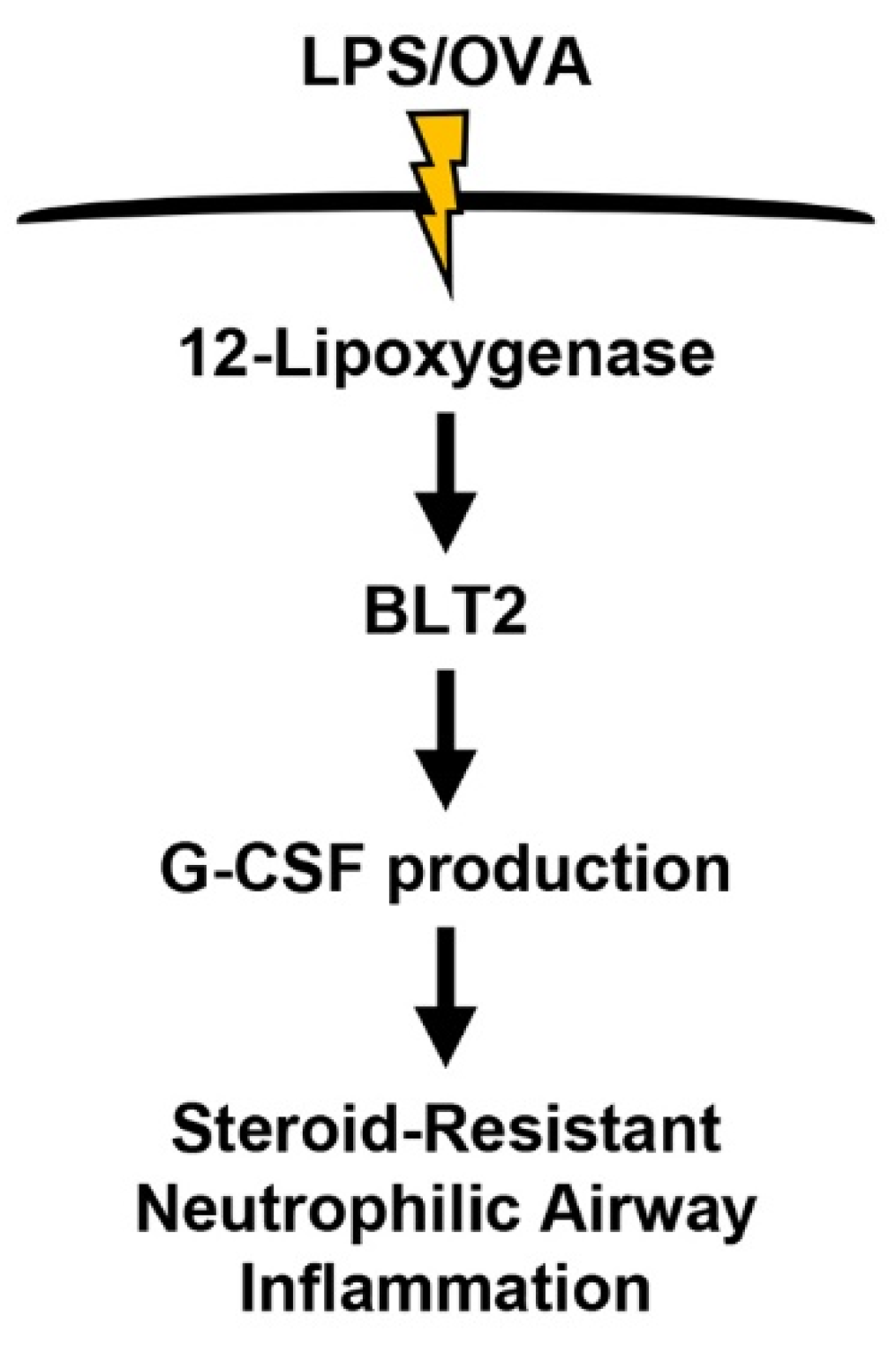
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shilovskiy, I.P.; Nikolskii, A.A.; Kurbacheva, O.M.; Khaitov, M.R. Modern View of Neutrophilic Asthma Molecular Mechanisms and Therapy. Biochemistry 2020, 85, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Z.; Wen, X.; Huang, G.; Nian, S.; Li, L.; Guo, X.; Ye, Y.; Yuan, Q. The onset, development and pathogenesis of severe neutrophilic asthma. Immunol. Cell Biol. 2022, 100, 144–159. [Google Scholar] [CrossRef]
- Bruijnzeel, P.L.; Uddin, M.; Koenderman, L. Targeting neutrophilic inflammation in severe neutrophilic asthma: Can we target the disease-relevant neutrophil phenotype? J. Leukoc. Biol. 2015, 98, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Hastie, A.T.; Li, X.; Li, H.; Busse, W.W.; Jarjour, N.N.; Wenzel, S.E.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R.; et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J. Allergy Clin. Immunol. 2014, 133, 1557–1563 e1555. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Cook, A.D.; Tak, P.P. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat. Rev. Drug Discov. 2016, 16, 53–70. [Google Scholar] [CrossRef]
- Avalos, B.R. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood 1996, 88, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Dunn, A.; Ward, A. G-CSF: Function and modes of action (Review). Int. J. Mol. Med. 2002, 10, 3–10. [Google Scholar] [CrossRef]
- Panopoulos, A.D.; Watowich, S.S. Granulocyte colony-stimulating factor: Molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 2008, 42, 277–288. [Google Scholar] [CrossRef]
- Wang, H.; Aloe, C.; McQualter, J.; Papanicolaou, A.; Vlahos, R.; Wilson, N.; Bozinovski, S. G-CSFR antagonism reduces mucosal injury and airways fibrosis in a virus-dependent model of severe asthma. Br. J. Pharmacol. 2021, 178, 1869–1885. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, H.; Lee, S.; Kim, S.; Lee, J.U.; Choi, Y.; Park, H.W.; You, G.; Kang, H.; Lee, S.; et al. Airway G-CSF identifies neutrophilic inflammation and contributes to asthma progression. Eur. Respir. J. 2020, 55, 1900827. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; FitzPatrick, M.; Wilson, N.J.; Anthony, D.; Reading, P.C.; Satzke, C.; Dunne, E.M.; Licciardi, P.V.; Seow, H.J.; Nichol, K.; et al. CSF3R/CD114 mediates infection-dependent transition to severe asthma. J. Allergy Clin. Immunol. 2019, 143, 785–788 e786. [Google Scholar] [CrossRef]
- Papanicolaou, A.; Wang, H.; Satzke, C.; Vlahos, R.; Wilson, N.; Bozinovski, S. Novel Therapies for Pneumonia-Associated Severe Asthma Phenotypes. Trends Mol. Med. 2020, 26, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Sanak, M. Eicosanoid Mediators in the Airway Inflammation of Asthmatic Patients: What is New? Allergy Asthma Immunol. Res. 2016, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Peebles, R.S., Jr. Prostaglandins in asthma and allergic diseases. Pharmacol. Ther. 2019, 193, 1–19. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Leishangthem, G.D.; Singh, S.; Dinda, A.K.; Biswal, S.; Agrawal, A.; Ghosh, B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013, 3, 1540. [Google Scholar] [CrossRef]
- Tager, A.M.; Luster, A.D. BLT1 and BLT2: The leukotriene B(4) receptors. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 123–134. [Google Scholar] [CrossRef]
- Yokomizo, T.; Izumi, T.; Chang, K.; Takuwa, Y.; Shimizu, T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 1997, 387, 620–624. [Google Scholar] [CrossRef]
- Islam, S.A.; Thomas, S.Y.; Hess, C.; Medoff, B.D.; Means, T.K.; Brander, C.; Lilly, C.M.; Tager, A.M.; Luster, A.D. The leukotriene B4 lipid chemoattractant receptor BLT1 defines antigen-primed T cells in humans. Blood 2006, 107, 444–453. [Google Scholar] [CrossRef][Green Version]
- Kim, N.D.; Chou, R.C.; Seung, E.; Tager, A.M.; Luster, A.D. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J. Exp. Med. 2006, 203, 829–835. [Google Scholar] [CrossRef]
- Pace, E.; Ferraro, M.; Di Vincenzo, S.; Bruno, A.; Giarratano, A.; Scafidi, V.; Lipari, L.; Di Benedetto, D.V.; Sciarrino, S.; Gjomarkaj, M. Cigarette smoke increases BLT2 receptor functions in bronchial epithelial cells: In vitro and ex vivo evidence. Immunology 2013, 139, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Lundeen, K.A.; Sun, B.; Karlsson, L.; Fourie, A.M. Leukotriene B4 receptors BLT1 and BLT2: Expression and function in human and murine mast cells. J. Immunol. 2006, 177, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Yokomizo, T. Biological functions of 12(S)-hydroxyheptadecatrienoic acid as a ligand of leukotriene B4 receptor 2. Inflamm. Regen. 2018, 38, 29. [Google Scholar] [CrossRef]
- Cho, K.J.; Seo, J.M.; Shin, Y.; Yoo, M.H.; Park, C.S.; Lee, S.H.; Chang, Y.S.; Cho, S.H.; Kim, J.H. Blockade of airway inflammation and hyperresponsiveness by inhibition of BLT2, a low-affinity leukotriene B4 receptor. Am. J. Respir. Cell Mol. Biol. 2010, 42, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ro, M.; Lee, A.J.; Kim, J.H. 5-/12-Lipoxygenase-linked cascade contributes to the IL-33-induced synthesis of IL-13 in mast cells, thus promoting asthma development. Allergy 2018, 73, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Seo, J.M.; Lee, M.G.; Kim, J.H. BLT2 Is upregulated in allergen-stimulated mast cells and mediates the synthesis of Th2 cytokines. J. Immunol. 2010, 185, 6329–6337. [Google Scholar] [CrossRef]
- Lee, A.J.; Ro, M.; Kim, J.H. Leukotriene B4 Receptor 2 Is Critical for the Synthesis of Vascular Endothelial Growth Factor in Allergen-Stimulated Mast Cells. J. Immunol. 2016, 197, 2069–2078. [Google Scholar] [CrossRef]
- Park, D.; Kwak, D.W.; Kim, J.H. Leukotriene B4 receptors contribute to house dust mite-induced eosinophilic airway inflammation via TH2 cytokine production. BMB Rep. 2021, 54, 182–187. [Google Scholar] [CrossRef]
- Lee, A.J.; Ro, M.; Cho, K.J.; Kim, J.H. Lipopolysaccharide/TLR4 Stimulates IL-13 Production through a MyD88-BLT2-Linked Cascade in Mast Cells, Potentially Contributing to the Allergic Response. J. Immunol. 2017, 199, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Ro, M.; Kwon, S.Y.; Kim, J.H. Leukotriene B4 receptors mediate the production of IL-17, thus contributing to neutrophil-dominant asthmatic airway inflammation. Allergy 2019, 74, 1797–1799. [Google Scholar] [CrossRef]
- Kwak, D.W.; Park, D.; Kim, J.H. Leukotriene B4 receptors play critical roles in house dust mites-induced neutrophilic airway inflammation and IL-17 production. Biochem. Biophys. Res. Commun. 2021, 534, 646–652. [Google Scholar] [CrossRef]
- Kwak, D.W.; Park, D.; Kim, J.H. Leukotriene B4 Receptors Are Necessary for the Stimulation of NLRP3 Inflammasome and IL-1beta Synthesis in Neutrophil-Dominant Asthmatic Airway Inflammation. Biomedicines 2021, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Oh, S.Y.; Jeon, S.G.; Park, H.W.; Lee, S.Y.; Chun, E.Y.; Bang, B.; Lee, H.S.; Oh, M.H.; Kim, Y.S.; et al. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J. Immunol. 2007, 178, 5375–5382. [Google Scholar] [CrossRef]
- Mo, Y.; Chen, J.; Humphrey, D.M., Jr.; Fodah, R.A.; Warawa, J.M.; Hoyle, G.W. Abnormal epithelial structure and chronic lung inflammation after repair of chlorine-induced airway injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L168–L178. [Google Scholar] [CrossRef]
- Lawlor, K.E.; Campbell, I.K.; Metcalf, D.; O’Donnell, K.; van Nieuwenhuijze, A.; Roberts, A.W.; Wicks, I.P. Critical role for granulocyte colony-stimulating factor in inflammatory arthritis. Proc. Natl. Acad. Sci. USA 2004, 101, 11398–11403. [Google Scholar] [CrossRef] [PubMed]
- Tsantikos, E.; Lau, M.; Castelino, C.M.; Maxwell, M.J.; Passey, S.L.; Hansen, M.J.; McGregor, N.E.; Sims, N.A.; Steinfort, D.P.; Irving, L.B.; et al. Granulocyte-CSF links destructive inflammation and comorbidities in obstructive lung disease. J. Clin. Investig. 2018, 128, 2406–2418. [Google Scholar] [CrossRef]
- Rumble, J.M.; Huber, A.K.; Krishnamoorthy, G.; Srinivasan, A.; Giles, D.A.; Zhang, X.; Wang, L.; Segal, B.M. Neutrophil-related factors as biomarkers in EAE and MS. J. Exp. Med. 2015, 212, 23–35. [Google Scholar] [CrossRef] [PubMed]
- He, J.Q.; Shumansky, K.; Connett, J.E.; Anthonisen, N.R.; Pare, P.D.; Sandford, A.J. Association of genetic variations in the CSF2 and CSF3 genes with lung function in smoking-induced COPD. Eur. Respir. J. 2008, 32, 25–34. [Google Scholar] [CrossRef]
- Steinke, J.W.; Lawrence, M.G.; Teague, W.G.; Braciale, T.J.; Patrie, J.T.; Borish, L. Bronchoalveolar lavage cytokine patterns in children with severe neutrophilic and paucigranulocytic asthma. J. Allergy Clin. Immunol. 2021, 147, 686–693. [Google Scholar] [CrossRef]
- Ouyang, S.; Liu, C.; Xiao, J.; Chen, X.; Lui, A.C.; Li, X. Targeting IL-17A/glucocorticoid synergy to CSF3 expression in neutrophilic airway diseases. JCI Insight 2020, 5, e132836. [Google Scholar] [CrossRef]
- Agache, I.; Ciobanu, C.; Agache, C.; Anghel, M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir. Med. 2010, 104, 1131–1137. [Google Scholar] [CrossRef]
- Sun, Y.C.; Zhou, Q.T.; Yao, W.Z. Sputum interleukin-17 is increased and associated with airway neutrophilia in patients with severe asthma. Chin. Med. J. (Engl.) 2005, 118, 953–956. [Google Scholar] [PubMed]
- Chakir, J.; Shannon, J.; Molet, S.; Fukakusa, M.; Elias, J.; Laviolette, M.; Boulet, L.P.; Hamid, Q. Airway remodeling-associated mediators in moderate to severe asthma: Effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 2003, 111, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Phipps, S.; Baines, K.J.; Oreo, K.M.; Gunawardhana, L.; Gibson, P.G. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur. Respir. J. 2014, 43, 1067–1076. [Google Scholar] [CrossRef]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome-mediated, IL-1beta-Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, D.C.; Peebles, R.S., Jr. Th17-mediated inflammation in asthma. Curr. Opin. Immunol. 2013, 25, 755–760. [Google Scholar] [CrossRef]
- Wei, Q.; Liao, J.; Jiang, M.; Liu, J.; Liang, X.; Nong, G. Relationship between Th17-mediated immunity and airway inflammation in childhood neutrophilic asthma. Allergy Asthma Clin. Immunol. 2021, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Negewo, N.A.; Baines, K.J. Role of the NLRP3 inflammasome in asthma: Relationship with neutrophilic inflammation, obesity, and therapeutic options. J. Allergy Clin. Immunol. 2021, 147, 2060–2062. [Google Scholar] [CrossRef]
- Gelfand, E.W. Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma. Semin. Immunol. 2017, 33, 44–51. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, D.-W.; Park, D.; Kim, J.-H. Leukotriene B4 Receptor 2 Mediates the Production of G-CSF That Plays a Critical Role in Steroid-Resistant Neutrophilic Airway Inflammation. Biomedicines 2022, 10, 2979. https://doi.org/10.3390/biomedicines10112979
Kwak D-W, Park D, Kim J-H. Leukotriene B4 Receptor 2 Mediates the Production of G-CSF That Plays a Critical Role in Steroid-Resistant Neutrophilic Airway Inflammation. Biomedicines. 2022; 10(11):2979. https://doi.org/10.3390/biomedicines10112979
Chicago/Turabian StyleKwak, Dong-Wook, Donghwan Park, and Jae-Hong Kim. 2022. "Leukotriene B4 Receptor 2 Mediates the Production of G-CSF That Plays a Critical Role in Steroid-Resistant Neutrophilic Airway Inflammation" Biomedicines 10, no. 11: 2979. https://doi.org/10.3390/biomedicines10112979
APA StyleKwak, D.-W., Park, D., & Kim, J.-H. (2022). Leukotriene B4 Receptor 2 Mediates the Production of G-CSF That Plays a Critical Role in Steroid-Resistant Neutrophilic Airway Inflammation. Biomedicines, 10(11), 2979. https://doi.org/10.3390/biomedicines10112979






