Cardiovascular Magnetic Resonance Imaging-Based Right Atrial Strain Analysis of Cardiac Amyloidosis
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Hypertrophic Cardiomyopathy Population
2.3. Tricuspid Regurgitation Population
2.4. Healthy Control Subjects
2.5. Clinical Parameters
2.6. Cardiac MRI
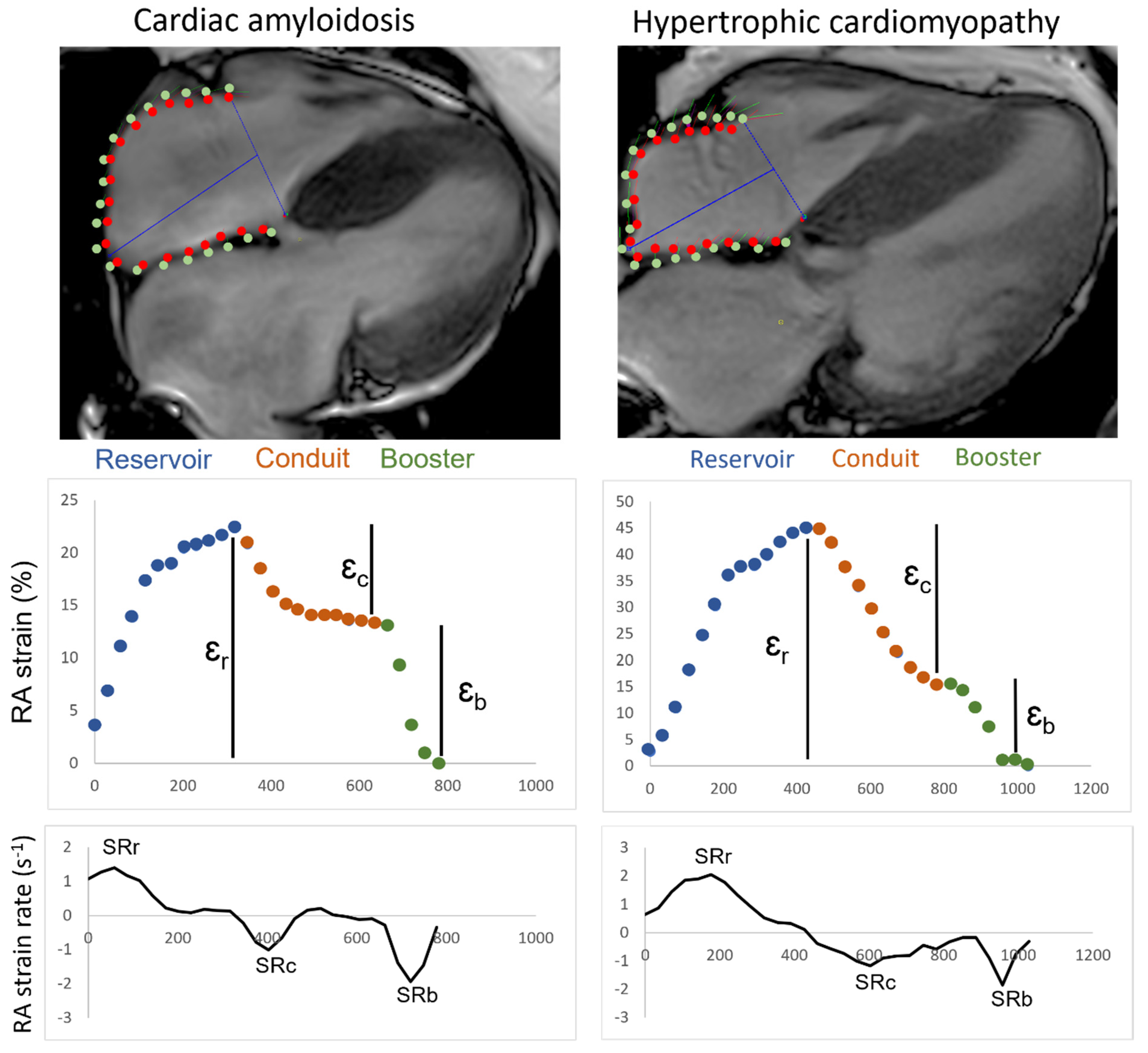
2.7. Strain Analysis
2.8. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Right Atrial Strain and Strain Rate
3.3. Sensitivity and Specificity of RAS and RASR for HCM and CA Patients
3.4. CA Patients with and without AF
3.5. Comparison of Subtypes ATTR and AL
3.6. Intra- and Interobserver Variation
4. Discussion
- I.
- Right atrial volumetric data significantly differ between CA patients and all other groups.
- II.
- RAS and RASR reflect significant impairment in CA patients with and without AF versus HCM and CTRL patients. However, RAS is insufficient for discrimination of CA from TR.
- III.
- Strain and volumetric data provide no discrimination between amyloidotic subtypes.
- IV.
- AF exacerbates the right atrial function in CA patients.
4.1. The Diagnostic Value of Right Atrial Volumetric Data
4.2. The Diagnostic Value of MRI-Based Strain Data
4.3. CA versus TR Patients
4.4. AL versus ATTR Subtypes
4.5. CA Patients with and without AF
4.6. Clinical Implications of RAS and RASR
4.7. Intra- and Interobserver Variation
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merlini, G.; Bellotti, V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 2003, 349, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Banypersad, S.M.; Moon, J.C.; Whelan, C.; Hawkins, P.N.; Wechalekar, A.D. Updates in cardiac amyloidosis: A review. J. Am. Heart Assoc. 2012, 1, e000364. [Google Scholar] [CrossRef]
- Rapezzi, C.; Merlini, G.; Quarta, C.C.; Riva, L.; Longhi, S.; Leone, O.; Salvi, F.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation 2009, 120, 1203–1212. [Google Scholar] [CrossRef]
- Kapoor, P.; Thenappan, T.; Singh, E.; Kumar, S.; Greipp, P.R. Cardiac amyloidosis: A practical approach to diagnosis and management. Am. J. Med. 2011, 124, 1006–1015. [Google Scholar] [CrossRef]
- Falk, R.H. Diagnosis and management of the cardiac amyloidoses. Circulation 2005, 112, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Dungu, J.N.; Valencia, O.; Pinney, J.H.; Gibbs, S.D.; Rowczenio, D.; Gilbertson, J.A.; Lachmann, H.; Wechalekar, A.; Gillmore, J.D.; Whelan, C.J. Cmr-based differentiation of al and attr cardiac amyloidosis. JACC Cardiovasc. Imaging 2014, 7, 133–142. [Google Scholar] [CrossRef]
- Giusca, S.; Steen, H.; Montenbruck, M.; Patel, A.R.; Pieske, B.; Erley, J.; Kelle, S.; Korosoglou, G. Multi-parametric assessment of left ventricular hypertrophy using late gadolinium enhancement, t1 mapping and strain-encoded cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2021, 23, 92. [Google Scholar] [CrossRef]
- Nochioka, K.; Quarta, C.C.; Claggett, B.; Roca, G.Q.; Rapezzi, C.; Falk, R.H.; Solomon, S.D. Left atrial structure and function in cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1128–1137. [Google Scholar] [CrossRef]
- Quarta, C.C.; Solomon, S.D.; Uraizee, I.; Kruger, J.; Longhi, S.; Ferlito, M.; Gagliardi, C.; Milandri, A.; Rapezzi, C.; Falk, R.H. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation 2014, 129, 1840–1849. [Google Scholar] [CrossRef]
- Higashi, H.; Inoue, K.; Inaba, S.; Nakao, Y.; Kinoshita, M.; Miyazaki, S.; Miyoshi, T.; Akazawa, Y.; Kawakami, H.; Uetani, T.; et al. Restricted left atrial dilatation can visually differentiate cardiac amyloidosis from hypertrophic cardiomyopathy. ESC Heart Fail. 2021, 8, 3198–3205. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yang, Y.; Wu, X.; Li, S.; Li, L.; Zhong, L.; Lin, Q.; Fei, H.; Liao, P.; Wang, W.; et al. Left atrial remodeling and the prognostic value of feature tracking derived left atrial strain in patients with light-chain amyloidosis: A cardiovascular magnetic resonance study. Int. J. Cardiovasc. Imaging 2022, 38, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Földeák, D.; Domsik, P.; Kalapos, A.; Kormányos, Á.; Borbényi, Z.; Forster, T. Right atrial deformation analysis in cardiac amyloidosis—Results from the three-dimensional speckle-tracking echocardiographic magyar-path study. Arq. Bras. Cardiol. 2018, 111, 384–391. [Google Scholar] [CrossRef]
- Jain, C.C.; Newman, D.B.; Geske, J.B. Mitral valve disease in hypertrophic cardiomyopathy: Evaluation and management. Curr. Cardiol. Rep. 2019, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Prihadi, E.A.; Delgado, V.; Leon, M.B.; Enriquez-Sarano, M.; Topilsky, Y.; Bax, J.J. Morphologic types of tricuspid regurgitation: Characteristics and prognostic implications. JACC Cardiovasc. Imaging 2019, 12, 491–499. [Google Scholar] [CrossRef]
- Truong, V.T.; Palmer, C.; Young, M.; Wolking, S.; Ngo, T.N.M.; Sheets, B.; Hausfeld, C.; Ornella, A.; Taylor, M.D.; Zareba, K.M.; et al. Right atrial deformation using cardiovascular magnetic resonance myocardial feature tracking compared with two-dimensional speckle tracking echocardiography in healthy volunteers. Sci. Rep. 2020, 10, 5237. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2015, 132, 1570–1579. [Google Scholar] [CrossRef]
- Kwong, R.Y.; Heydari, B.; Abbasi, S.; Steel, K.; Al-Mallah, M.; Wu, H.; Falk, R.H. Characterization of cardiac amyloidosis by atrial late gadolinium enhancement using contrast-enhanced cardiac magnetic resonance imaging and correlation with left atrial conduit and contractile function. Am. J. Cardiol. 2015, 116, 622–629. [Google Scholar] [CrossRef]
- Mohty, D.; Pradel, S.; Magne, J.; Fadel, B.; Boulogne, C.; Petitalot, V.; Raboukhi, S.; Darodes, N.; Damy, T.; Aboyans, V.; et al. Prevalence and prognostic impact of left-sided valve thickening in systemic light-chain amyloidosis. Clin. Res. Cardiol. 2017, 106, 331–340. [Google Scholar] [CrossRef]
- Mints, Y.Y.; Doros, G.; Berk, J.L.; Connors, L.H.; Ruberg, F.L. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: A systematic review and clinical experience. ESC Heart Fail. 2018, 5, 772–779. [Google Scholar] [CrossRef]
- Sanchis, K.; Cariou, E.; Colombat, M.; Ribes, D.; Huart, A.; Cintas, P.; Fournier, P.; Rollin, A.; Carrié, D.; Galinier, M.; et al. Atrial fibrillation and subtype of atrial fibrillation in cardiac amyloidosis: Clinical and echocardiographic features, impact on mortality. Amyloid 2019, 26, 128–138. [Google Scholar] [CrossRef]
- Elliot, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Limongelli, G.; Mahrholdt, H.; Lafont, A.; et al. 2014 esc guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the european society of cardiology (esc). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 aha/acc guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2020, 76, e159–e240. [Google Scholar] [CrossRef]
- Valdes, E.W.; Barth, P.; Piran, M.; Laser, K.T.; Burchert, W.; Körperich, H. Left-ventricular reference myocardial strain assessed by cardiovascular magnetic resonance feature tracking and fsenc-impact of temporal resolution and cardiac muscle mass. Front. Cardiovasc. Med. 2021, 8, 764496. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the eacvi/ase/industry task force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Grothues, F.; Smith, G.C.; Moon, J.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.V.; Pan, J.; Rai, S.N.; Galandiuk, S. Roc-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery 2016, 159, 1638–1645. [Google Scholar] [CrossRef]
- Petersen, S.E.; Aung, N.; Sanghvi, M.M.; Zemrak, F.; Fung, K.; Paiva, J.M.; Francis, J.M.; Khanji, M.Y.; Lukaschuk, E.; Lee, A.M.; et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (cmr) in caucasians from the uk biobank population cohort. J. Cardiovasc. Magn. Reson. 2017, 19, 18. [Google Scholar] [CrossRef]
- Cappelli, F.; Perfetto, F.; Martone, R.; Di Mario, C. Cardiac amyloidosis in patients undergoing tavr: Why we need to think about it. Cardiovasc. Revascularization Med. 2021, 22, 109–114. [Google Scholar] [CrossRef]
- Teixeira, R.; Monteiro, R.; Garcia, J.; Baptista, R.; Ribeiro, M.; Cardim, N.; Goncalves, L. The relationship between tricuspid regurgitation severity and right atrial mechanics: A speckle tracking echocardiography study. Int. J. Cardiovasc. Imaging 2015, 31, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.A.; Jain, M.; Pimentel, D.R.; Wang, B.; Connors, L.H.; Skinner, M.; Apstein, C.S.; Liao, R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ. Res. 2004, 94, 1008–1010. [Google Scholar] [CrossRef]
- Palmer, C.; Truong, V.T.; Slivnick, J.A.; Wolking, S.; Coleman, P.; Mazur, W.; Zareba, K.M. Atrial function and geometry differences in transthyretin versus immunoglobulin light chain amyloidosis: A cardiac magnetic resonance study. Sci. Rep. 2022, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Damy, T.; FontAna, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2018, 39, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef]
- Py, A.; Schaaf, M.; Duhamel, S.; Si-Mohamed, S.; Daher, J.; Altman, M.; de Breyne, B.; Mechtouff, L.; Placide, J.; Chauveau, S.; et al. Atrial premature activity detected after an ischaemic stroke unveils atrial myopathy. Arch. Cardiovasc. Dis. 2020, 113, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.Y.; Buckert, D.; Ma, G.S.; Rasche, V. Quantitative assessment of left and right atrial strains using cardiovascular magnetic resonance based tissue tracking. Front. Cardiovasc. Med. 2021, 8, 690240. [Google Scholar] [CrossRef]


| CTRL | HCM | CA | TR | Comparison | Global Test | Post Hoc Test | |
|---|---|---|---|---|---|---|---|
| N | 47 | 20 | 41 | 31 | |||
| Males (%) | 25 (53%) | 10 (50%) | 32 (78%) | 16 (52%) | |||
| Atrial fibrillation (%) | - | 1 (5.0) | 17 (41.5) | 29 (93.5) | |||
| Arterial hypertension (%) | - | 10 (50.0) | 32 (78.0) | 24 (77.4) | |||
| Coronary artery disease (%) | - | 2 (10.0) | 13 (31.7) | 9 (29.0) | |||
| COPD (%) | - | 1 (5.0) | 3 (7.3) | 3 (9.7) | |||
| Diabetes mellitus (%) | - | 3 (15.0) | 1 (2.4) | 8 (25.8) | |||
| Stroke (%) | - | 1 (5.0) | 5 (12.2) | 4 (12.9) | |||
| NYHA (%) | - | ||||||
| I | 1 (5.0) | 0 (0) | 0 (0) | ||||
| II | 8 (40.0) | 6 (14.6) | 8 (25.8) | ||||
| III | 11 (55.0) | 33 (80.5) | 23 (74.2) | ||||
| IV | 0 (0) | 2 (4.9) | 0 (0) | ||||
| Age (yrs) b | 55.9 ± 10.4 a | 63.9 ± 7.4 | 79 ± 14 a | 82 ± 4 a | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TI CA-TR | p < 0.001 | p = 0.007 p < 0.001 p < 0.001 p < 0.001 p < 0.001 p = 0.072 |
| Weight (kg) c | 74.8 ± 12.8 | 83.6 ± 81.5 | 76.3 ± 75 | 71.2 ± 11.1 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p = 0.014 | p = 0.064 p = 0.950 p = 0.645 p = 0.175 p = 0.008 p = 0.369 |
| Height (cm) c | 172.7 ± 10.7 | 171.7 ± 9.2 | 173.4 ± 10.3 | 165.9 ± 9.9 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TI CA-TR | p = 0.015 | p = 0.985 p = 0.984 p = 0.029 p = 0.922 p = 0.209 p = 0.013 |
| BSA (m²) c | 1.9 ± 0.2 | 2 ± 0.2 | 1.9 ± 0.2 | 1.8 ± 0.2 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p = 0.027 | p = 0.441 p = 0.985 p = 0.216 p = 0.624 p = 0.019 p = 0.125 |
| BMI (kg/m²) d | 25.0 ± 2.7 | 28.3 ± 3.9 | 24.6 ± 3.9 a | 25.8 ± 3.3 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p = 0.002 | p < 0.001 p = 0.916 p = 0.224 p < 0.001 p = 0.026 p = 0.197 |
| HR (bpm) d | 64.9 ± 9.7 | 63.1 ± 7.8 | 74.0 ± 14.8 | 71.5 ± 10.7 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p < 0.001 | p = 0.436 p = 0.001 p = 0.008 p = 0.001 p = 0.004 p = 0.671 |
| RA Vmaxi (mL/m²) b | 41.5 ± 9.8 | 40.2 ± 6.8 | 48.0 ± 17.0 a | 119.4 ± 41.6 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p < 0.001 | p = 0.929 p = 0.014 p < 0.001 p = 0.004 p < 0.001 p < 0.001 |
| RA Vmini (mL/m²) b | 21.0 ± 7.0 a | 14.5 ± 3.9 | 35.0 ± 14.5 a | 99.4 ± 36.9 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p < 0.001 | all p < 0.001 |
| RA SVi (mL/m²) b | 19.2 ± 7.3 | 25.7 ± 6.9 | 13.0 ± 12.0 a | 18.8 ± 16.5 a | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p < 0.001 | p = 0.007 p = 0.164 p = 0.986 p < 0.001 p = 0.146 p = 0.325 |
| RA EF (%) d | 48.39 ± 17.0 a | 63.4 ± 9.8 | 30.0 ± 20.0 a | 14.3 ± 11.0 a | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p < 0.001 | p = 0.002 p = 0.002 p < 0.001 p < 0.001 p < 0.001 p < 0.001 |
| RV EDVi (mL/m²) b | 76.4 ± 12.7 | 70.4 ± 11.9 | 87.6 ± 20.2 | 102.1 ± 27.1 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p < 0.001 | p = 0.253 p = 0.014 p < 0.001 p < 0.001 p < 0.001 p = 0.074 |
| RV ESVi (mL/m²) b | 30.4 ± 8.2 | 30.9 ± 10.4 | 55.4 ± 18.1 | 50.6 ± 17.1 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p < 0.001 | p = 0.997 p < 0.001 p < 0.001 p < 0.001 p < 0.001 p = 0.661 |
| RV SVi (mL/m²) d | 46.1 ± 6.8 | 39.5 ± 11.7 | 32.0 ± 25.0 a | 51.5 ± 15.6 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p < 0.001 | p = 0.023 p < 0.001 p = 0.270 p = 0.117 p = 0.003 p < 0.001 |
| RV EF (%) b | 60.7 ± 5.7 | 65.0 ± 13.0 a | 36.4 ± 13.4 | 50.5 ± 8.4 | CTRL-HCM CTRL-CA CTRL-TR HCM-CA HCM-TR CA-TR | p < 0.001 | p = 0.952 p < 0.001 p < 0.001 p < 0.001 p < 0.001 p < 0.001 |
| Cut-Off Value | AUC | Quality | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Right atrial strain | |||||
| Reservoir (%) | 27.2 | 0.850 (0.749 to 0.951) | good | 90.7 | 75.0 |
| Conduit (%) | 12.2 | 0.793 (0.664 to 0.922) | fair | 81.4 | 75.0 |
| Booster (%) | 7.4 | 0.804 (0.697 to 0.911) | good | 62.8 | 90.0 |
| Right atrial strain rate | |||||
| Reservoir (s−1) | 0.85 | 0.829 (0.730 to 0.928) | good | 60.5 | 95.0 |
| Conduit (s−1) | −0.75 | 0.759 (0.615 to 0.903) | fair | 81.4 | 65.0 |
| Booster (s−1) | −1.45 | 0.862 (0.761 to 0.962) | good | 79.1 | 90.0 |
| SR | AF | p-Value | |
|---|---|---|---|
| N (%) | 24 (58.5) | 17 (41.4) | |
| Arterial hypertension (%) | 16 (66.7) | 16 (94.1) | p = 0.039 |
| NTproBNP (ng/L) | 2551 ± 3766 | 3465 ± 5938 | p = 0.223 |
| Coronary artery disease (%) | 4 (16.7) | 9 (52.9) | p = 0.015 |
| COPD (%) | 1 (4.2) | 2 (11.8) | p = 0.363 |
| Diabetes mellitus (%) | 0 | 1 (5.9) | p = 0.235 |
| Stroke (%) | 4 (16.7) | 1 (5.9) | p = 0.304 |
| NYHA (%) | |||
| I | 0 | 0 | |
| II | 4 (16.7) | 2 (11.8) | |
| III | 20 (83.3) | 13 (76.5) | |
| IV | 0 | 2 (11.8) | |
| Right atrial strain | |||
| Reservoir (%) b | 14.0 {12.6} | 6.1 {7.0} | p = 0.007 |
| Conduit (%) b | 6.0 {10.6} | 4.3 {4.5} | p = 0.109 |
| Booster (%) b | 7.4 {10.7} | 1.7 {4.4} | p = 0.059 |
| Right atrial strain rate | |||
| Reservoir (s−1) b | 0.90 {1.25} | 0.40 {0.75} | p = 0.008 |
| Conduit (s−1) b | −0.50 {0.35} | −0.40 {0.30} | p = 0.089 |
| Booster (s−1) b | −1.20 {0.80} | −0.60 {0.65} | p = 0.013 |
| CA | HCM | p-Value | |
|---|---|---|---|
| CA Without AF | |||
| Right atrial strain | |||
| Reservoir (%) b | 16.1 {14.0} | 34.0 {18.8} | p = 0.001 |
| Conduit (%) b | 8.7 {10.8} | 23.0 {14.1} | p = 0.004 |
| Booster (%) b | 8.0 {12.3} | 11.6 {10.1} | p = 0.027 |
| Right atrial strain rate | |||
| Reservoir (s−1) b | 1.20 {1.18} | 2.00 {1.40} | p = 0.007 |
| Conduit (s−1) b | −0.60 {0.45} | −1.30 {1.10} | p = 0.039 |
| Booster (s−1) b | −1.15 {0.85} | −1.90 {0.80} | p < 0.001 |
| CA With AF † | |||
| Right atrial strain | |||
| Reservoir (%) b | 7.0 {6.3} | 34.0 {18.8} | p < 0.001 |
| Conduit (%) b | 3.7 {4.8} | 23.0 {14.1} | p < 0.001 |
| Booster (%) b | 2.3 {4.8} | 11.6 {10.1} | p < 0.001 |
| Right atrial strain rate | |||
| Reservoir (s−1) b | 0.40 {0.65} | 2.00 {1.40} | p < 0.001 |
| Conduit (s−1) b | −0.30 {0.40} | −1.30 {1.10} | p = 0.001 |
| Booster (s−1) b | −0.60 {0.90} | −1.90 {0.80} | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckstein, J.; Sciacca, V.; Körperich, H.; Paluszkiewicz, L.; Valdés, E.W.; Burchert, W.; Gerçek, M.; Farr, M.; Sommer, P.; Sohns, C.; et al. Cardiovascular Magnetic Resonance Imaging-Based Right Atrial Strain Analysis of Cardiac Amyloidosis. Biomedicines 2022, 10, 3004. https://doi.org/10.3390/biomedicines10123004
Eckstein J, Sciacca V, Körperich H, Paluszkiewicz L, Valdés EW, Burchert W, Gerçek M, Farr M, Sommer P, Sohns C, et al. Cardiovascular Magnetic Resonance Imaging-Based Right Atrial Strain Analysis of Cardiac Amyloidosis. Biomedicines. 2022; 10(12):3004. https://doi.org/10.3390/biomedicines10123004
Chicago/Turabian StyleEckstein, Jan, Vanessa Sciacca, Hermann Körperich, Lech Paluszkiewicz, Elena Weise Valdés, Wolfgang Burchert, Muhammed Gerçek, Martin Farr, Philipp Sommer, Christian Sohns, and et al. 2022. "Cardiovascular Magnetic Resonance Imaging-Based Right Atrial Strain Analysis of Cardiac Amyloidosis" Biomedicines 10, no. 12: 3004. https://doi.org/10.3390/biomedicines10123004
APA StyleEckstein, J., Sciacca, V., Körperich, H., Paluszkiewicz, L., Valdés, E. W., Burchert, W., Gerçek, M., Farr, M., Sommer, P., Sohns, C., & Piran, M. (2022). Cardiovascular Magnetic Resonance Imaging-Based Right Atrial Strain Analysis of Cardiac Amyloidosis. Biomedicines, 10(12), 3004. https://doi.org/10.3390/biomedicines10123004





