The Need to Shift from Morphological to Structural Assessment for Carotid Plaque Vulnerability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and MRI
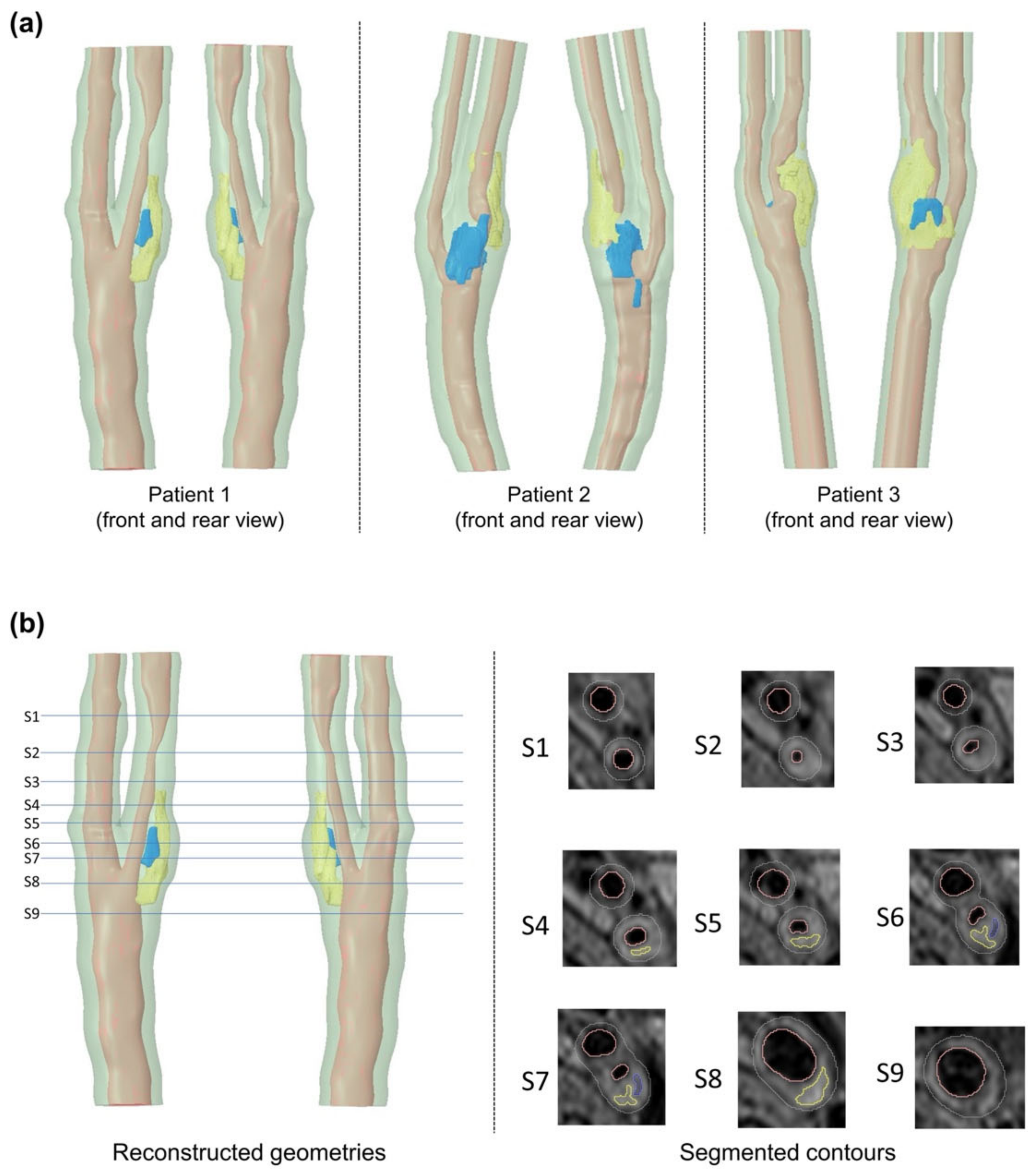
2.2. Segmentation and Reconstruction
2.3. Material Mapping Method
2.4. FSI Computational Model
2.5. Analysis of FSI Result
3. Results
3.1. Carotid Plaque Morphology
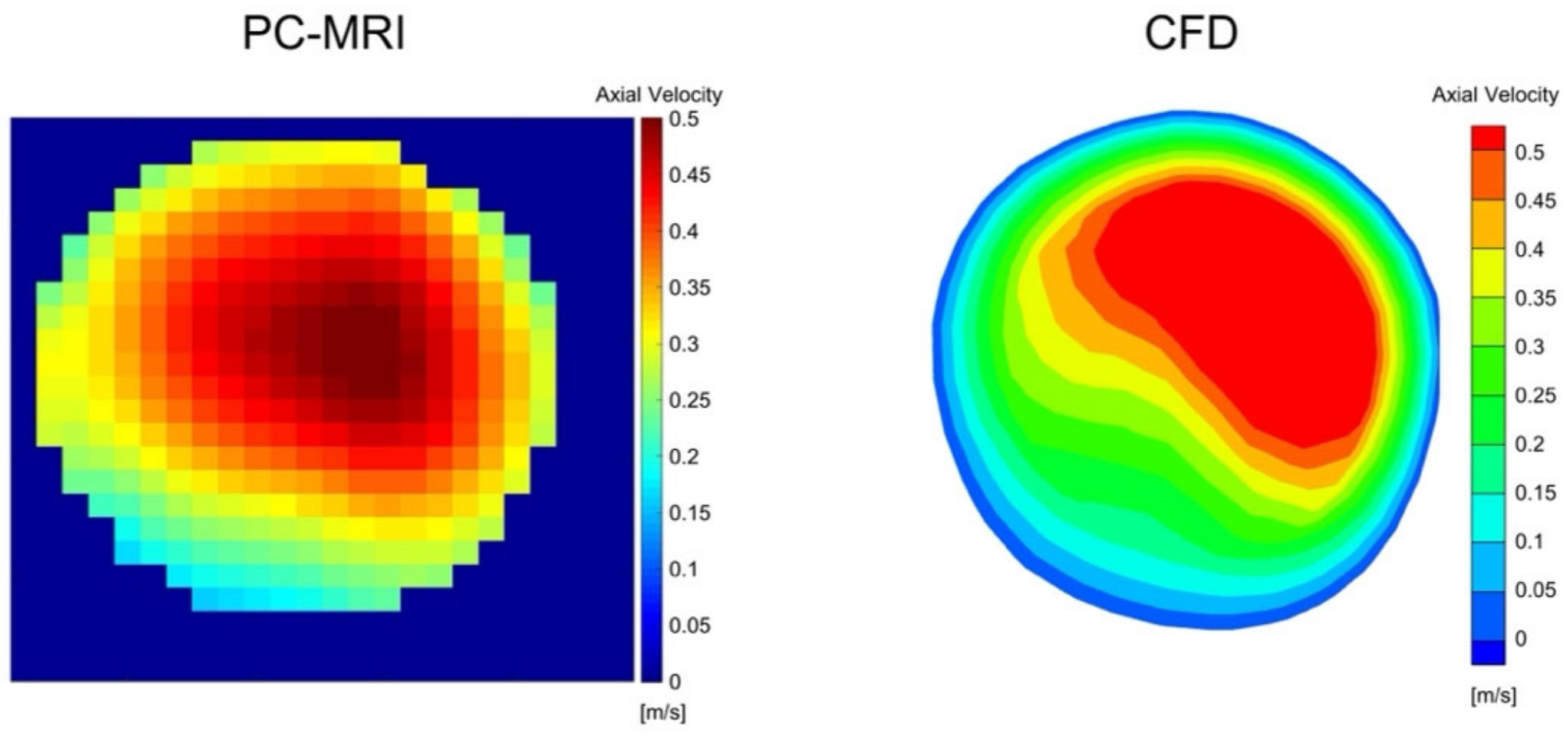
3.2. PC-MRI and CFD Comparison
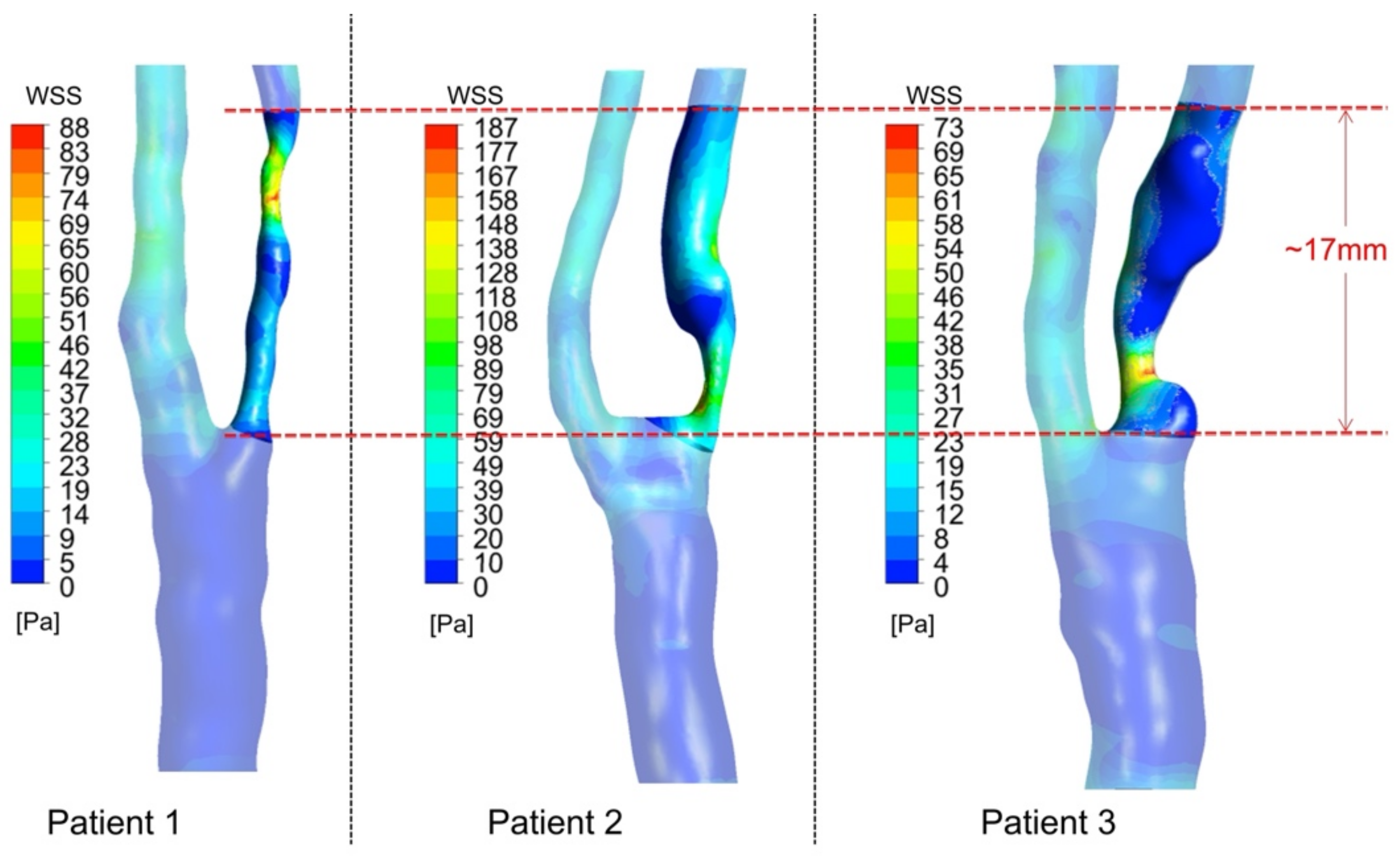
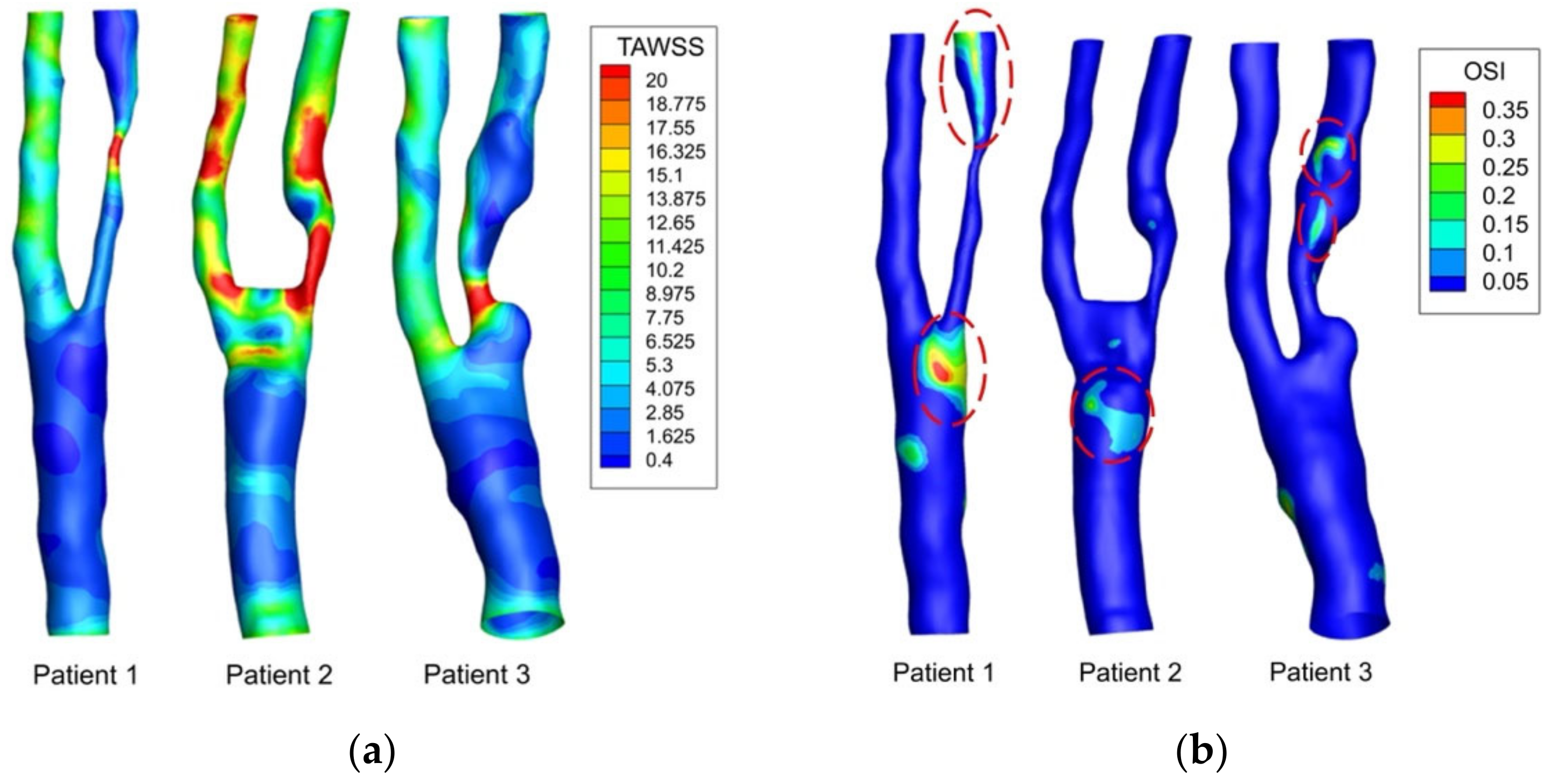
3.3. WSS-Based Descriptors
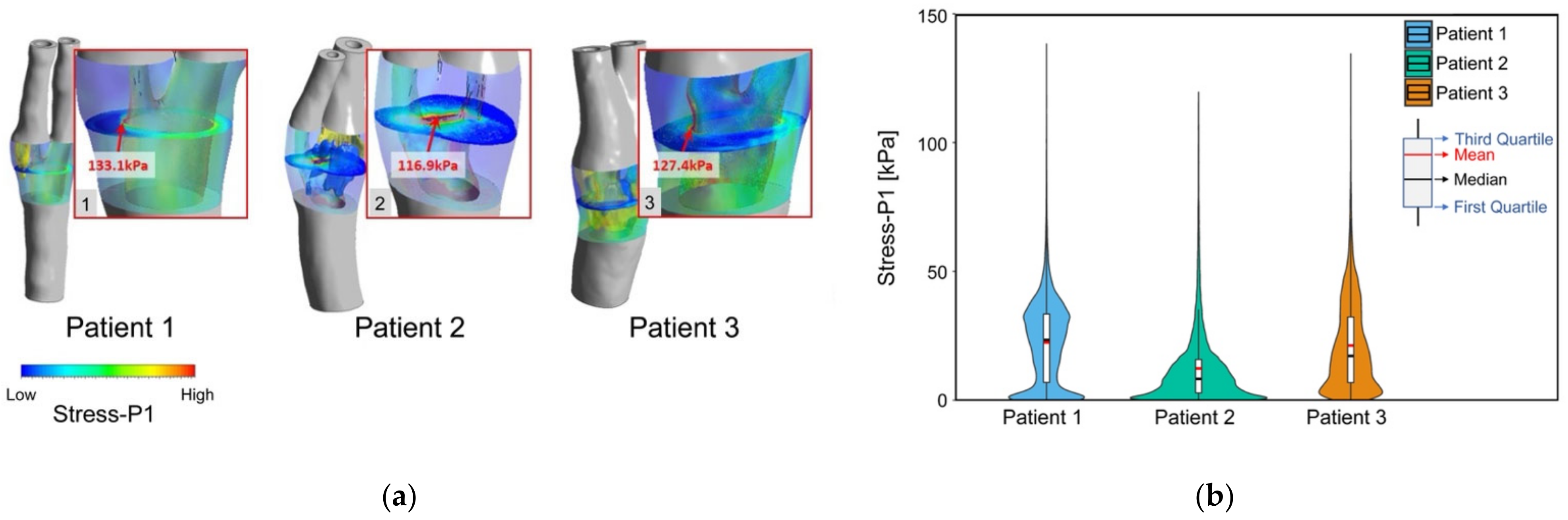
3.4. Plaque Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lusby, R.J.; Woodcock, J.P.; MacHleder, H.I.; Ferrell, L.D.; Jeans, W.D.; Skidmore, R.; Sheddon, E.J.; Baird, R.N. Transient Ischaemic Attacks: The Static and Dynamic Morphology of the Carotid Artery Bifurcation. Br. J. Surg. 1982, 69, S41–S44. [Google Scholar] [CrossRef] [PubMed]
- Takaya, N.; Yuan, C.; Chu, B.; Saam, T.; Polissar, N.L.; Jarvik, G.P.; Isaac, C.; McDonough, J.; Natiello, C.; Small, R.; et al. Presence of Intraplaque Hemorrhage Stimulates Progression of Carotid Atherosclerotic Plaques: A High-Resolution Magnetic Resonance Imaging Study. Circulation 2005, 111, 2768–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takaya, N.; Yuan, C.; Chu, B.; Saam, T.; Underbill, H.; Cai, J.; Tran, N.; Polissar, N.L.; Isaac, C.; Ferguson, M.S.; et al. Association between Carotid Plaque Characteristics and Subsequent Ischemic Cerebrovascular Events: A Prospective Assessment with MRI—Initial Results. Stroke 2006, 37, 818–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, J.T.; Biasiolli, L.; Li, L.; Alkhalil, M.; Galassi, F.; Darby, C.; Halliday, A.W.; Hands, L.; Magee, T.; Perkins, J.; et al. Quantification of Lipid-Rich Core in Carotid Atherosclerosis Using Magnetic Resonance T2 Mapping: Relation to Clinical Presentation. JACC Cardiovasc. Imaging 2017, 10, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Pasterkamp, G. Requiem for the “Vulnerable Plaque”. Eur. Heart J. 2015, 36, 2984–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Toorn, J.E.; Bos, D.; Ikram, M.K.; Verwoert, G.C.; Van Der Lugt, A.; Ikram, M.A.; Vernooij, M.W.; Kavousi, M. Carotid Plaque Composition and Prediction of Incident Atherosclerotic Cardiovascular Disease. Circ. Cardiovasc. Imaging 2022, 15, e013602. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kamm, R.D.; Yang, C.; Zheng, J.; Canton, G.; Bach, R.; Huang, X.; Hatsukami, T.S.; Zhu, J.; Ma, G.; et al. Image-Based Modeling for Better Understanding and Assessment of Atherosclerotic Plaque Progression and Vulnerability: Data, Modeling, Validation, Uncertainty and Predictions. J. Biomech. 2014, 47, 834–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, D.N.; Giddens, D.P.; Zarins, C.K.; Glagov, S. Pulsatile Flow and Atherosclerosis in the Human Carotid Bifurcation. Positive Correlation between Plaque Location and Low and Oscillating Shear Stress. Arteriosclerosis 1985, 5, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, H.; Morikawa, Y.; Mizuno, Y.; Harada, E.; Ito, T.; Matsui, K.; Saito, Y.; Yasue, H. Coronary Spasm Preferentially Occurs at Branch Points. Circ. Cardiovasc. Interv. 2009, 2, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, V.; Sherwin, S.J.; Weinberg, P.D. Does Low and Oscillatory Wall Shear Stress Correlate Spatially with Early Atherosclerosis? A Systematic Review. Cardiovasc. Res. 2013, 99, 242–250. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Hyun, S.; Buchanan, J.R.; Longest, P.W.; Archie, J.P.; Truskey, G.A. Hemodynamic Parameters and Early Intimal Thickening in Branching Blood Vessels. Crit. Rev. Biomed. Eng. 2017, 45, 319–382. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Wischgol, T.; Kassab, G.S. Flow Patterns in Three-Dimensional Porcine Epicardial Coronary Arterial Tree. Am. J. Physiol.—Heart Circ. Physiol. 2007, 293, H2959–H2970. [Google Scholar] [CrossRef] [Green Version]
- Davies, P.F. Hemodynamic Shear Stress and the Endothelium in Cardiovascular Pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Jäger, W.; Neuss-Radu, M.; Richter, T. Mathematical Modeling and Simulation of the Evolution of Plaques in Blood Vessels. J. Math. Biol. 2016, 72, 973–996. [Google Scholar] [CrossRef] [Green Version]
- Thon, M.P.; Hemmler, A.; Glinzer, A.; Mayr, M.; Wildgruber, M.; Zernecke-Madsen, A.; Gee, M.W. A Multiphysics Approach for Modeling Early Atherosclerosis. Biomech. Model. Mechanobiol. 2018, 17, 617–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschhorn, M.; Tchantchaleishvili, V.; Stevens, R.; Rossano, J.; Throckmorton, A. Fluid–Structure Interaction Modeling in Cardiovascular Medicine—A Systematic Review 2017–2019. Med. Eng. Phys. 2020, 78, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, S.; Redaelli, A.; Vergara, C.; Votta, E.; Zunino, P. Mathematical Modeling and Numerical Simulation of Atherosclerotic Plaque Progression Based on Fluid-Structure Interaction. J. Math. Fluid Mech. 2021, 23, 1–21. [Google Scholar] [CrossRef]
- Zarins, C.K.; Giddens, D.P.; Bharadvaj, B.K.; Sottiurai, V.S.; Mabon, R.F.; Glagov, S. Carotid Bifurcation Atherosclerosis. Quantitative Correlation of Plaque Localization with Flow Velocity Profiles and Wall Shear Stress. Circ. Res. 1983, 53, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Korshunov, V.A.; Schwartz, S.M.; Berk, B.C. Vascular Remodeling: Hemodynamic and Biochemical Mechanisms Underlying Glagov’s Phenomenon. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1722–1728. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic Shear Stress and Its Role in Atherosclerosis. J. Am. Med. Assoc. 1999, 19, 426–434. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ku, D.N. Pulsatile Flow in the Human Left Coronary Artery Bifurcation: Average Conditions. J. Biomech. Eng. 1996, 118, 74–82. [Google Scholar] [CrossRef]
- Eshtehardi, P.; Brown, A.J.; Bhargava, A.; Costopoulos, C.; Hung, O.Y.; Corban, M.T.; Hosseini, H.; Gogas, B.D.; Giddens, D.P.; Samady, H. High Wall Shear Stress and High-Risk Plaque: An Emerging Concept. Int. J. Cardiovasc. Imaging 2017, 33, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Starikov, A.; Hartaigh, B.; Gransar, H.; Kolli, K.K.; Lee, J.H.; Rizvi, A.; Baskaran, L.; Schulman-Marcus, J.; Lin, F.Y.; et al. Relationship between Endothelial Wall Shear Stress and High-Risk Atherosclerotic Plaque Characteristics for Identification of Coronary Lesions That Cause Ischemia: A Direct Comparison with Fractional Flow Reserve. J. Am. Heart Assoc. 2016, 5, e004186. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Steinman, D.A.; Morbiducci, U. Insights into the Co-Localization of Magnitude-Based versus Direction-Based Indicators of Disturbed Shear at the Carotid Bifurcation. J. Biomech. 2016, 49, 2413–2419. [Google Scholar] [CrossRef]
- Guerciotti, B.; Vergara, C. Computational Comparison between Newtonian and Non-Newtonian Blood Rheologies in Stenotic Vessels. In Lecture Notes in Applied and Computational Mechanics; Wriggers, P., Lenarz, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 84, pp. 169–183. [Google Scholar]
- Yao, X.; Dai, Z.; Zhang, X.; Gao, J.; Xu, G.; Cai, Y.; Li, Z. Carotid Geometry as a Predictor of In-Stent Neointimal Hyperplasia: A Computational Fluid Dynamics Study. Circ. J. 2019, 83, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- Younis, H.F.; Kaazempur-Mofrad, M.R.; Chan, R.C.; Isasi, A.G.; Hinton, D.P.; Chau, A.H.; Kim, L.A.; Kamm, R.D. Hemodynamics and Wall Mechanics in Human Carotid Bifurcation and Its Consequences for Atherogenesis: Investigation of Inter-Individual Variation. Biomech. Model. Mechanobiol. 2004, 3, 17–32. [Google Scholar] [CrossRef]
- Tang, D.; Teng, Z.; Canton, G.; Yang, C.; Ferguson, M.; Huang, X.; Zheng, J.; Woodard, P.K.; Yuan, C. Sites of Rupture in Human Atherosclerotic Carotid Plaques Are Associated With High Structural Stresses. Stroke 2009, 40, 3258–3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennati, L.; Vergara, C.; Domanin, M.; Malloggi, C.; Bissacco, D.; Trimarchi, S.; Silani, V.; Parati, G.; Casana, R. A Computational Fluid-Structure Interaction Study for Carotids with Different Atherosclerotic Plaques. J. Biomech. Eng. 2021, 143, 091002. [Google Scholar] [CrossRef]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.K.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.F.; Vese, L.A. Active Contours without Edges. IEEE Trans. Image Process. 2001, 10, 266–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, L. Minimal Path Algorithms for the Robust Detection of Linear Features in Gray Images. Ismm 1998, 12, 331–338. [Google Scholar]
- Bégin, S.; Dupont-Therrien, O.; Bélanger, E.; Daradich, A.; Laffray, S.; De Koninck, Y.; Côté, D.C. Automated Method for the Segmentation and Morphometry of Nerve Fibers in Large-Scale CARS Images of Spinal Cord Tissue. Biomed. Opt. Express 2014, 5, 4145–4161. [Google Scholar] [CrossRef] [Green Version]
- Adame, I.M.; Van Der Geest, R.J.; Wasserman, B.A.; Mohamed, M.A.; Reiber, J.H.C.; Lelieveldt, B.P.F. Automatic Segmentation and Plaque Characterization in Atherosclerotic Carotid Artery MR Images. Magn. Reson. Mater. Physics Biol. Med. 2004, 16, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Arthur, D.; Vassilvitskii, S. K-Means++: The Advantages of Careful Seeding. In Proceedings of the Eighteenth Annual ACM-SIAM Symposium on Discrete Algorithms, New Orleans, LA, USA, 7–9 January 2007; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2007; pp. 1027–1035. [Google Scholar]
- Paritala, P.K.; Yarlagadda, P.K.D.V.; Wang, J.; Gu, Y.T.; Li, Z. Numerical Investigation of Atherosclerotic Plaque Rupture Using Optical Coherence Tomography Imaging and XFEM. Eng. Fract. Mech. 2018, 204, 531–541. [Google Scholar] [CrossRef]
- Wang, J.; Paritala, P.K.; Mendieta, J.B.; Komori, Y.; Raffel, O.C.; Gu, Y.; Li, Z. Optical Coherence Tomography-Based Patient-Specific Coronary Artery Reconstruction and Fluid–Structure Interaction Simulation. Biomech. Model. Mechanobiol. 2020, 19, 7–20. [Google Scholar] [CrossRef]
- Benitez, J.; Fontanarosa, D.; Wang, J.; Paritala, P.K.; McGahan, T.; Lloyd, T.; Li, Z. Evaluating the Impact of Calcification on Plaque Vulnerability from the Aspect of Mechanical Interaction Between Blood Flow and Artery Based on MRI. Ann. Biomed. Eng. 2021, 49, 1169–1182. [Google Scholar] [CrossRef]
- Mendieta, J.B.; Fontanarosa, D.; Wang, J.; Paritala, P.K.; McGahan, T.; Lloyd, T.; Li, Z. The Importance of Blood Rheology in Patient-Specific Computational Fluid Dynamics Simulation of Stenotic Carotid Arteries. Biomech. Model. Mechanobiol. 2020, 19, 1477–1490. [Google Scholar] [CrossRef]
- Xu, P.; Liu, X.; Zhang, H.; Ghista, D.; Zhang, D.; Shi, C.; Huang, W. Assessment of Boundary Conditions for CFD Simulation in Human Carotid Artery. Biomech. Model. Mechanobiol. 2018, 17, 1581–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Meng, L.; Zhou, W.; Xin, L.; Xia, X.; Li, S.; Zheng, H.; Niu, L. Computational and Experimental Assessment of Influences of Hemodynamic Shear Stress on Carotid Plaque. Biomed. Eng. Online 2017, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Yang, C.; Zheng, J.; Woodard, P.K.; Saffitz, J.E.; Sicard, G.A.; Pilgram, T.K.; Yuan, C. Quantifying Effects of Plaque Structure and Material Properties on Stress Distributions in Human Atherosclerotic Plaques Using 3D FSI Models. J. Biomech. Eng. 2005, 127, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Howarth, S.; Tang, T.; Graves, M.; U-King-Im, J.; Gillard, J.H. Does Calcium Deposition Play a Role in the Stability of Atheroma? Location May Be the Key. Cerebrovasc. Dis. 2007, 24, 452–459. [Google Scholar] [CrossRef]
- Wong, K.K.L.; Thavornpattanapong, P.; Cheung, S.C.P.; Sun, Z.; Tu, J. Effect of Calcification on the Mechanical Stability of Plaque Based on a Three-Dimensional Carotid Bifurcation Model. BMC Cardiovasc. Disord. 2012, 12, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Virmani, R.; Younis, H.; Burke, A.P.; Kamm, R.D.; Lee, R.T. The Impact of Calcification on the Biomechanical Stability of Atherosclerotic Plaques. Circulation 2001, 103, 1051–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gijsen, F.J.H.; Vis, B.; Barrett, H.E.; Zadpoor, A.A.; Verhagen, H.J.; Bos, D.; Van Der Steen, A.F.W.; Akyildiz, A.C. Morphometric and Mechanical Analyses of Calcifications and Fibrous Plaque Tissue in Carotid Arteries for Plaque Rupture Risk Assessment. IEEE Trans. Biomed. Eng. 2021, 68, 1429–1438. [Google Scholar] [CrossRef]
- Kadry, K.; Olender, M.L.; Marlevi, D.; Edelman, E.R.; Nezami, F.R. A Platform for High-Fidelity Patient-Specific Structural Modelling of Atherosclerotic Arteries: From Intravascular Imaging to Three-Dimensional Stress Distributions. J. R. Soc. Interface 2021, 18, 20210436. [Google Scholar] [CrossRef]
- Walker, C. Advances in Carotid Imaging: Beyond Luminal Stenosis. Vasc. Dis. Manag. 2017, 14, E114. [Google Scholar]
- Fleg, J.L.; Stone, G.W.; Fayad, Z.A.; Granada, J.F.; Hatsukami, T.S.; Kolodgie, F.D.; Ohayon, J.; Pettigrew, R.; Sabatine, M.S.; Tearney, G.J.; et al. Detection of High-Risk Atherosclerotic Plaque: Report of the NHLBI Working Group on Current Status and Future Directions. JACC Cardiovasc. Imaging 2012, 5, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Finet, G.; Ohayon, J.; Rioufol, G. Biomechanical Interaction between Cap Thickness, Lipid Core Composition and Blood Pressure in Vulnerable Coronary Plaque: Impact on Stability or Instability. Coron. Artery Dis. 2004, 15, 13–20. [Google Scholar] [CrossRef]
- Ohayon, J.; Finet, G.; Gharib, A.M.; Herzka, D.A.; Tracqui, P.; Heroux, J.; Rioufol, G.; Kotys, M.S.; Elagha, A.; Pettigrew, R.I. Necrotic Core Thickness and Positive Arterial Remodeling Index: Emergent Biomechanical Factors for Evaluating the Risk of Plaque Rupture. Am. J. Physiol.—Hear. Circ. Physiol. 2008, 295, H717–H727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.Y.; Tang, T.; U-King-Im, J.; Graves, M.; Sutcliffe, M.; Gillard, J.H. Assessment of Carotid Plaque Vulnerability Using Structural and Geometrical Determinants. Circ. J. 2008, 72, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, S.W.; Fischer, P.F.; Bassiouny, H.S.; Loth, F. Direct Numerical Simulation of Transitional Flow in a Stenosed Carotid Bifurcation. J. Biomech. 2008, 41, 2551–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancellotti, R.M.; Vergara, C.; Valdettaro, L.; Bose, S.; Quarteroni, A. Large Eddy Simulations for Blood Dynamics in Realistic Stenotic Carotids. Int. J. Numer. Method. Biomed. Eng. 2017, 33, e2868. [Google Scholar] [CrossRef]
- Huang, C.; Pan, X.; He, Q.; Huang, M.; Huang, L.; Zhao, X.; Yuan, C.; Bai, J.; Luo, J. Ultrasound-Based Carotid Elastography for Detection of Vulnerable Atherosclerotic Plaques Validated by Magnetic Resonance Imaging. Ultrasound Med. Biol. 2016, 42, 365–377. [Google Scholar] [CrossRef]
- Wang, Q.; Canton, G.; Guo, J.; Guo, X.; Hatsukami, T.S.; Billiar, K.L.; Yuan, C.; Wu, Z.; Tang, D. MRI-Based Patient-Specific Human Carotid Atherosclerotic Vessel Material Property Variations in Patients, Vessel Location and Long-Term Follow Up. PLoS ONE 2017, 12, e0180829. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, S.; Domanin, M.; Forzenigo, L.; Votta, E.; Zunino, P.; Redaelli, A.; Vergara, C. A Surrogate Model for Plaque Modeling in Carotids Based on Robin Conditions Calibrated by Cine MRI Data. Int. J. Numer. Method. Biomed. Eng. 2021, 37, e3447. [Google Scholar] [CrossRef]


| Patient ID | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age | 42 | 77 | 70 |
| Sex | Male | Female | Male |
| Degree of stenosis | 81% | 83% | 82% |
| BMI (kg/m2) | 21.2 | 16 | 22.3 |
| Hypertension | Yes | Yes | Yes |
| Hypercholesterolemia | Yes | No | Yes |
| Diabetes Mellitus | No | No | No |
| Smoking | Former | Current | Current |
| Patient ID | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Calcification volume (mm3) | 3.44 | 77.38 | 10.43 |
| Lipid volume (mm3) | 42.79 | 48.59 | 236.79 |
| Thinnest fibrous cap thickness (mm) | 0.729 | 1.186 | 0.676 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Y.; Huang, X.; Benitez Mendieta, J.; Wang, J.; Paritala, P.K.; Lloyd, T.; Li, Z. The Need to Shift from Morphological to Structural Assessment for Carotid Plaque Vulnerability. Biomedicines 2022, 10, 3038. https://doi.org/10.3390/biomedicines10123038
Xiang Y, Huang X, Benitez Mendieta J, Wang J, Paritala PK, Lloyd T, Li Z. The Need to Shift from Morphological to Structural Assessment for Carotid Plaque Vulnerability. Biomedicines. 2022; 10(12):3038. https://doi.org/10.3390/biomedicines10123038
Chicago/Turabian StyleXiang, Yuqiao, Xianjue Huang, Jessica Benitez Mendieta, Jiaqiu Wang, Phani Kumari Paritala, Thomas Lloyd, and Zhiyong Li. 2022. "The Need to Shift from Morphological to Structural Assessment for Carotid Plaque Vulnerability" Biomedicines 10, no. 12: 3038. https://doi.org/10.3390/biomedicines10123038





