A Systematic Review of the Evidence of Hematopoietic Stem Cell Differentiation to Fibroblasts
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Process
2.3. Data Assessment
3. Results
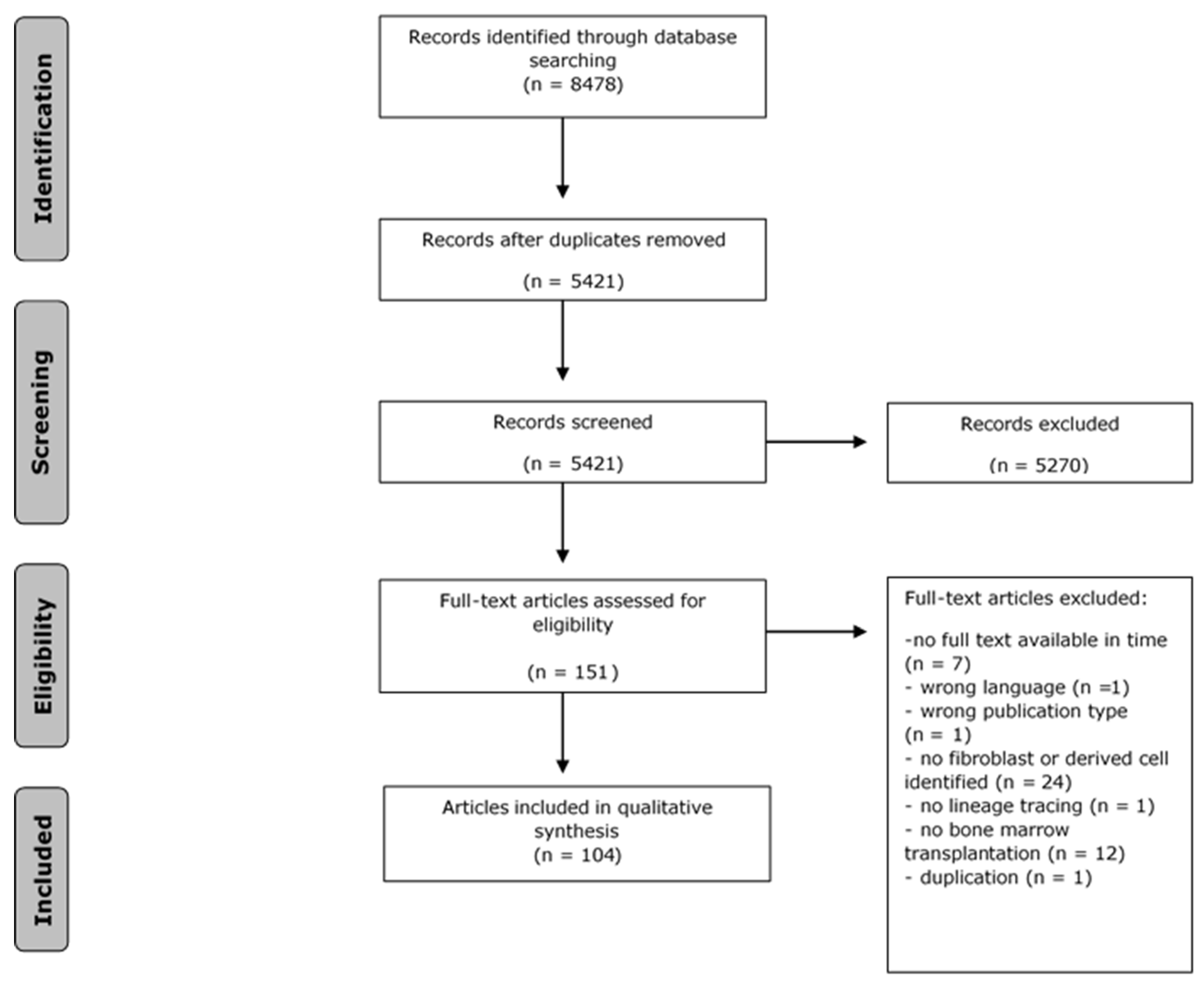
3.1. Search and Selection of Results
3.2. Quality Assessment
3.3. Lineage Tracing and Fibroblast Identification Methods
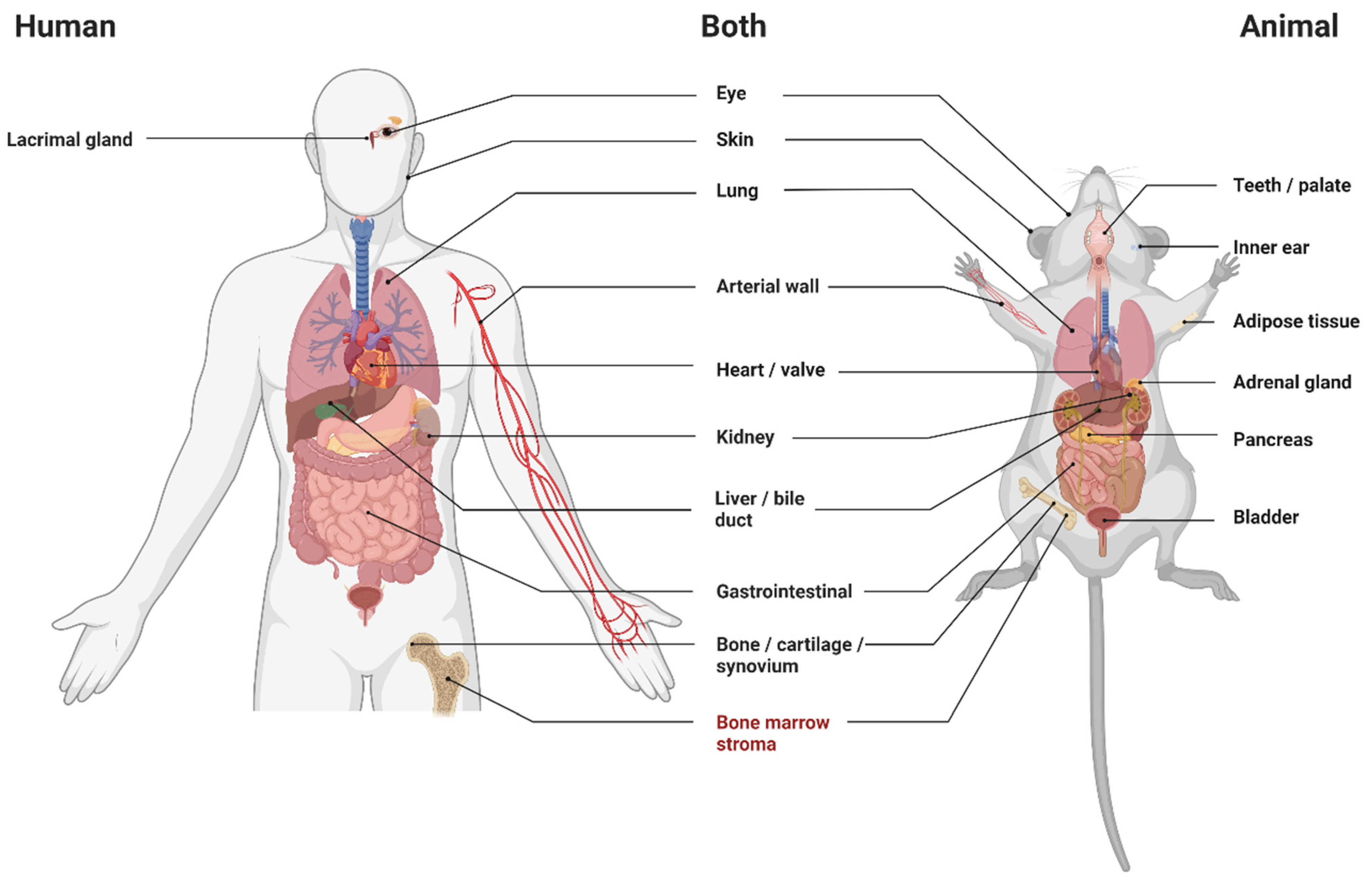
3.4. Animals
3.4.1. Adipose Tissue
3.4.2. Adrenal Gland
3.4.3. Arterial Wall
3.4.4. Bladder
3.4.5. Bone, Cartilage and Synovium
3.4.6. Bone Marrow Stroma
3.4.7. Eye
3.4.8. Gastrointestinal and Peritoneum
3.4.9. Heart and Heart Valves
3.4.10. Inner Ear
3.4.11. Kidney
3.4.12. Liver and Bile Ducts
3.4.13. Lung
3.4.14. Pancreas
3.4.15. Skin
3.4.16. Teeth and Palate
3.5. Human Studies
3.5.1. Arterial Wall
3.5.2. Bone Marrow Stroma
3.5.3. Eye
3.5.4. Lacrimal Gland
3.5.5. Liver and Bile Ducts
3.5.6. Lung
3.5.7. Skin
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; LaRue, A.C.; Drake, C.J. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood 2006, 108, 2893–2896. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.; Duley, J.A.; Dewdney, J.; Allardyce, R.A.; Beard, M.E.; Fitzgerald, P.H. The wound fibroblast and macrophage. II: Their origin studied in a human after bone marrow transplantation. Br. J. Surg. 1981, 68, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Lauby, G.; Boyer, C.; Rennard, S.I.; Sharp, J.G. Transplanted BM and BM side population cells contribute progeny to the lung and liver in irradiated mice. Cytotherapy 2003, 5, 523–533. [Google Scholar] [CrossRef]
- Direkze, N.C.; Forbes, S.J.; Brittan, M.; Hunt, T.; Jeffery, R.; Preston, S.L.; Poulsom, R.; Hodivala-Dilke, K.; Alison, M.R.; Wright, N.A. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells 2003, 21, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Miura, T.; Ikeda, Y.; Matsuda, E.; Saito, K.; Miki, T.; Kobayashi, H.; Nishino, Y.; Ohtani, S.; Shimamoto, K. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc. Pathol. 2005, 14, 241–246. [Google Scholar] [CrossRef]
- Ziegelhoeffer, T.; Fernandez, B.; Kostin, S.; Heil, M.; Voswinckel, R.; Helisch, A.; Schaper, W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ. Res. 2004, 94, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Heart, Lung and Blood Institute. Study Quality Assessment Tools; National Heart, Lung and Blood Institute: Bethesda, MD, USA, 2020. [Google Scholar]
- Guerrero-Juarez, C.F.; Dedhia, P.H.; Jin, S.; Ruiz-Vega, R.; Ma, D.; Liu, Y.; Yamaga, K.; Shestova, O.; Gay, D.L.; Yang, Z.; et al. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat. Commun. 2019, 10, 650. [Google Scholar] [CrossRef]
- Campbell, J.H.; Han, C.L.; Campbell, G.R. Neointimal Formation by Circulating Bone Marrow Cells. Ann. N. Y. Acad. Sci. 2001, 947, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Menocal, L.; St-Pierre, M.; Wei, Y.T.; Khan, S.; Mateu, D.; Calfa, M.; Rahnemai-Azar, A.A.; Striker, G.; Pham, S.M.; Vazquez-Padron, R.I. The origin of post-injury neointimal cells in the rat balloon injury model. Cardiovasc. Res. 2009, 81, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Ren, P.; Zhang, L.; Azares, A.R.; Zhang, S.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. Activation of Bone Marrow-Derived Cells and Resident Aortic Cells During Aortic Injury. J. Surg. Res. 2020, 245, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.H.; Efendy, J.L.; Han, C.; Girjes, A.A.; Campbell, G.R. Haemopoietic origin of myofibroblasts formed in the peritoneal cavity in response to a foreign body. J. Vasc. Res. 2000, 37, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, A.; Yamamoto, S.; Iwai-Kanai, E.; Kanatani, I.; Imamura, M.; Adam, R.M.; Tabata, Y.; Ogawa, O. Induction of smooth muscle cell-like phenotype in marrow-derived cells among regenerating urinary bladder smooth muscle cells. Am. J. Pathol. 2005, 166, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Kanno, Y.; Mitsui, T.; Sano, H.; Kitta, T.; Moriya, K.; Nonomura, K. Contribution of bone marrow-derived mesenchymal stem cells to the morphological changes in the bladder after partial outlet obstruction: A preliminary study. Int. J. Urol. 2014, 21, 714–718. [Google Scholar] [CrossRef]
- Bilic-Curcic, I.; Kalajzic, Z.; Wang, L.; Rowe, D.W. Origins of endothelial and osteogenic cells in the subcutaneous collagen gel implant. Bone 2005, 37, 678–687. [Google Scholar] [CrossRef]
- Pereira, R.F.; O'Hara, M.D.; Laptev, A.V.; Halford, K.W.; Pollard, M.D.; Class, R.; Simon, D.; Livezey, K.; Prockop, D.J. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA 1998, 95, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Prados, B.; Del Toro, R.; MacGrogan, D.; Gómez-Apiñániz, P.; Papoutsi, T.; Muñoz-Cánoves, P.; Méndez-Ferrer, S.; de la Pompa, J.L. Heterotopic ossification in mice overexpressing Bmp2 in Tie2+ lineages. Cell Death Dis. 2021, 12, 729. [Google Scholar] [CrossRef]
- Sergijenko, A.; Roelofs, A.J.; Riemen, A.H.; De Bari, C. Bone marrow contribution to synovial hyperplasia following joint surface injury. Arthritis Res. Ther. 2016, 18, 166. [Google Scholar] [CrossRef]
- Ch'ang, H.J.; Lin, L.M.; Chang, P.Y.; Luo, C.W.; Chang, Y.H.; Chou, C.K.; Chen, H.H. Bone marrow transplantation enhances trafficking of host-derived myelomonocytic cells that rescue intestinal mucosa after whole body radiation. Radiother. Oncol. 2012, 104, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, Y.; Masuya, M.; Larue, A.C.; Fleming, P.A.; Visconti, R.P.; Minamiguchi, H.; Drake, C.J.; Ogawa, M. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Exp. Hematol. 2006, 34, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gorskaya, Y.F.; Nesterenko, V.G.; Domracheva, E.V.; Aseeva, E.A.; Timofeeva, N.M. Origination of stromal precursor cells replenishing the population of these cells in heterotopic mouse bone marrow transplants after curettage in recipients. Bull. Exp. Biol. Med. 2007, 143, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Kawakami, Y.; Nagai, Y.; Ma, J.X.; Tsai, J.Y.; Kincade, P.W.; Sato, S. Bone marrow lacks a transplantable progenitor for smooth muscle type alpha-actin-expressing cells. Stem Cells 2006, 24, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.L.; Chaurasia, S.S.; Cutler, A.; Asosingh, K.; Kaur, H.; de Medeiros, F.W.; Agrawal, V.; Wilson, S.E. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010, 91, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Harada, Y.; Ishida, W.; Fukuda, K.; Sumi, T.; Kawakita, T.; Taguchi, O.; Fukushima, A. Identification of keratocyte-like cells differentiated from circulating bone marrow-derived cells in the mouse cornea. Med. Mol. Morphol. 2013, 46, 233–238. [Google Scholar] [CrossRef]
- Hisatomi, T.; Sonoda, K.H.; Ishikawa, F.; Qiao, H.; Nakazawa, T.; Fukata, M.; Nakamura, T.; Noda, K.; Miyahara, S.; Harada, M.; et al. Identification of resident and inflammatory bone marrow derived cells in the sclera by bone marrow and haematopoietic stem cell transplantation. Br. J. Ophthalmol. 2007, 91, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, J.; Liu, C.Y.; Hayashi, Y.; Kao, W.W. Bone marrow mesenchymal stem cells can differentiate and assume corneal keratocyte phenotype. J. Cell. Mol. Med. 2012, 16, 1114–1124. [Google Scholar] [CrossRef]
- Ozerdem, U.; Alitalo, K.; Salven, P.; Li, A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3502–3506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayama, T.; Kondo, T.; Kobayashi, M.; Ohta, K.; Ishibashi, Y.; Kanemaru, T.; Shimazu, H.; Ishikawa, F.; Nakamura, T.; Kinoshita, S.; et al. Characteristic Morphology and Distribution of Bone Marrow Derived Cells in the Cornea. Anat. Rec.-Adv. Integr. Anat. Evol. Biol. 2009, 292, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Bamba, S.; Lee, C.Y.; Brittan, M.; Preston, S.L.; Direkze, N.C.; Poulsom, R.; Alison, M.R.; Wright, N.A.; Otto, W.R. Bone marrow transplantation ameliorates pathology in interleukin-10 knockout colitic mice. J. Pathol. 2006, 209, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Brittan, M.; Chance, V.; Elia, G.; Poulsom, R.; Alison, M.R.; MacDonald, T.T.; Wright, N.A. A regenerative role for bone marrow following experimental colitis: Contribution to neovasculogenesis and myofibroblasts. Gastroenterology 2005, 128, 1984–1995. [Google Scholar] [CrossRef]
- Brittan, M.; Hunt, T.; Jeffery, R.; Poulsom, R.; Forbes, S.J.; Hodivala-Dilke, K.; Goldman, J.; Alison, M.R.; Wright, N.A. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut 2002, 50, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Tsuji, S.; Tsujii, M.; Nishida, T.; Ishii, S.; Nakamura, T.; Eguchi, H.; Kawano, S. The transdifferentiation of bone-marrow-derived cells in colonic mucosal regeneration after dextran-sulfate-sodium-induced colitis in mice. Pharmacology 2007, 80, 193–199. [Google Scholar] [CrossRef]
- Komori, M.; Tsuji, S.; Tsujii, M.; Murata, H.; Iijima, H.; Yasumaru, M.; Nishida, T.; Irie, T.; Kawano, S.; Hori, M. Efficiency of bone marrow-derived cells in regeneration of the stomach after induction of ethanol-induced ulcers in rats. J. Gastroenterol. 2005, 40, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Jeffery, R.; Hutchinson, G.; Alison, M.R.; Poulsom, R.; Wright, N.A.; Otto, W.R. Bone marrow cells in murine colitis: Multi-signal analysis confirms pericryptal myofibroblast engraftment without epithelial involvement. PLoS ONE 2011, 6, e26082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.R.; Ranjbarvaziri, S.; Talkhabi, M.; Zhao, P.; Subat, A.; Hojjat, A.; Kamran, P.; Müller, A.M.; Volz, K.S.; Tang, Z.; et al. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ. Res. 2014, 115, 625–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, J.; Sano, M.; Fujita, J.; Hayashida, K.; Yuasa, S.; Aoyama, N.; Takehara, Y.; Kato, O.; Makino, S.; Ogawa, S.; et al. Bone marrow derived cells are involved in the pathogenesis of cardiac hypertrophy in response to pressure overload. Circulation 2007, 116, 1176–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möllmann, H.; Nef, H.M.; Kostin, S.; von Kalle, C.; Pilz, I.; Weber, M.; Schaper, J.; Hamm, C.W.; Elsässer, A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc. Res. 2006, 71, 661–671. [Google Scholar] [CrossRef]
- Moore-Morris, T.; Cattaneo, P.; Guimarães-Camboa, N.; Bogomolovas, J.; Cedenilla, M.; Banerjee, I.; Ricote, M.; Kisseleva, T.; Zhang, L.; Gu, Y.; et al. Infarct Fibroblasts Do Not Derive From Bone Marrow Lineages. Circ. Res. 2018, 122, 583–590. [Google Scholar] [CrossRef]
- Szardien, S.; Nef, H.M.; Troidl, C.; Willmer, M.; Voss, S.; Liebetrau, C.; Hoffmann, J.; Rolf, A.; Rixe, J.; Elsässer, A.; et al. Bone marrow-derived cells contribute to cell turnover in aging murine hearts. Int. J. Mol. Med. 2012, 30, 283–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Amerongen, M.J.; Bou-Gharios, G.; Popa, E.R.; Van Ark, J.; Petersen, A.H.; Van Dam, G.M.; Van Luyn, M.J.A.; Harmsen, M.C. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J. Pathol. 2008, 214, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, Z.; Romeo, S.J.; Fleming, P.A.; Markwald, R.R.; Visconti, R.P.; Drake, C.J. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J. Mol. Cell. Cardiol. 2011, 51, 955–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visconti, R.P.; Ebihara, Y.; LaRue, A.C.; Fleming, P.A.; McQuinn, T.C.; Masuya, M.; Minamiguchi, H.; Markwald, R.R.; Ogawa, M.; Drake, C.J. An in vivo analysis of hematopoietic stem cell potential: Hematopoietic origin of cardiac valve interstitial cells. Circ. Res. 2006, 98, 690–696. [Google Scholar] [CrossRef] [Green Version]
- Lang, H.; Ebihara, Y.; Schmiedt, R.A.; Minamiguchi, H.; Zhou, D.; Smythe, N.; Liu, L.; Ogawa, M.; Schulte, B.A. Contribution of bone marrow hematopoietic stem cells to adult mouse inner ear: Mesenchymal cells and fibrocytes. J. Comp. Neurol. 2006, 496, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.S.; Kim, J.I.; Jung, K.J.; Kim, J.; Han, K.H.; Park, K.M. Bone marrow-derived cells play a major role in kidney fibrosis via proliferation and differentiation in the infiltrated site. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Lebleu, V.S.; Taduri, G.; O'Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deane, J.A.; Campanale, N.V.; Bertram, J.F.; Ricardo, S.D. The contribution of bone marrow-derived cells to the development of renal interstitial fibrosis. Stem Cells 2007, 25, 697–706. [Google Scholar] [CrossRef]
- Roufosse, C.; Bou-Gharios, G.; Prodromidi, E.; Alexakis, C.; Jeffery, R.; Khan, S.; Otto, W.R.; Alter, J.; Poulsom, R.; Cook, H.T. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J. Am. Soc. Nephrol. 2006, 17, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Asawa, S.; Saito, T.; Satoh, A.; Ohtake, K.; Tsuchiya, T.; Okada, H.; Neilson, E.G.; Gotoh, M. Participation of bone marrow cells in biliary fibrosis after bile duct ligation. J. Gastroenterol. Hepatol. 2007, 22, 2001–2008. [Google Scholar] [CrossRef]
- Azevedo, C.M.; Solano de Freitas Souza, B.; Andrade de Oliveira, S.; Paredes, B.D.; Barreto, E.S.; Neto, H.A.; Ribeiro dos Santos, R.; Pereira Soares, M.B. Bone marrow-derived cells migrate to the liver and contribute to the generation of different cell types in chronic Schistosoma mansoni infection. Exp. Parasitol. 2015, 159, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Bravo, M.; Morán-Jiménez, M.J.; Quintana-Bustamante, O.; Méndez, M.; Gutiérrez-Vera, I.; Bueren, J.; Salido, E.; Segovia, J.C.; Fontanellas, A.; de Salamanca, R.E. Bone marrow-derived cells promote liver regeneration in mice with erythropoietic protoporphyria. Transplantation 2009, 88, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, R.; Moro, T.; Nakao, S.; Mikami, K.; Fukumitsu, H.; Ueda, Y.; Ikeda, K.; Adachi, E.; Bou-Gharios, G.; Okazaki, I.; et al. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology 2009, 137, 1459–1466. [Google Scholar] [CrossRef]
- Jeffery, R.; Poulsom, R.; Alison, M.R. Sources of adult hepatic stem cells: Haematopoietic. Methods Mol. Biol. 2009, 481, 141–154. [Google Scholar] [PubMed]
- Kisseleva, T.; Uchinami, H.; Feirt, N.; Quintana-Bustamante, O.; Segovia, J.C.; Schwabe, R.F.; Brenner, D.A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 2006, 45, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kong, Y.; Wang, H.; Wang, S.; Yu, H.; Liu, X.; Yang, L.; Jiang, X.; Li, L.; Li, L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J. Hepatol. 2009, 50, 1174–1183. [Google Scholar] [CrossRef]
- Russo, F.P.; Alison, M.R.; Bigger, B.W.; Amofah, E.; Florou, A.; Amin, F.; Bou-Gharios, G.; Jeffery, R.; Iredale, J.P.; Forbes, S.J. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 2006, 130, 1807–1821. [Google Scholar] [CrossRef]
- Roderfeld, M.; Rath, T.; Voswinckel, R.; Dierkes, C.; Dietrich, H.; Zahner, D.; Graf, J.; Roeb, E. Bone marrow transplantation demonstrates medullar origin of CD34+ fibrocytes and ameliorates hepatic fibrosis in Abcb4-/- mice. Hepatology 2010, 51, 267–276. [Google Scholar] [CrossRef]
- Angelini, D.J.; Su, Q.; Kolosova, I.A.; Fan, C.; Skinner, J.T.; Yamaji-Kegan, K.; Collector, M.; Sharkis, S.J.; Johns, R.A. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature. PLoS ONE 2010, 5, e11251. [Google Scholar] [CrossRef]
- Deng, C.; Wang, J.; Zou, Y.; Zhao, Q.; Feng, J.; Fu, Z.; Guo, C. Characterization of fibroblasts recruited from bone marrow-derived precursor in neonatal bronchopulmonary dysplasia mice. J. Appl. Physiol. 2011, 111, 285–294. [Google Scholar] [CrossRef]
- Dupuis, J.; Préfontaine, A.; Villeneuve, L.; Ruel, N.; Lefebvre, F.; Calderone, A. Bone marrow-derived progenitor cells contribute to lung remodelling after myocardial infarction. Cardiovasc. Pathol. 2007, 16, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Jin, H.; Liu, T.; Chensue, S.W.; Phan, S.H. Bone marrow-derived progenitor cells in pulmonary fibrosis. J. Clin. Investig. 2004, 113, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Fujita, J.; Miyake, Y.; Kawada, H.; Ando, K.; Ogawa, S.; Fukuda, K. Bone marrow-derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest 2005, 127, 1793–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, G.; Sangai, T.; Sugiyama, K.; Ito, T.; Hasebe, T.; Endoh, Y.; Magae, J.; Ochiai, A. In vivo characterization of bone marrow-derived fibroblasts recruited into fibrotic lesions. Stem Cells 2005, 23, 699–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, L.T.; Mehrotra, M.; LaRue, A.C. Hematopoietic Origin of Murine Lung Fibroblasts. Stem Cells Int. 2015, 2015, 159713. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, T.; Liu, T.; Yu, H.; Ding, L.; Ullenbruch, M.; Hu, B.; Wu, Z.; Oguro, H.; Phan, S.H. Lung bone marrow-derived hematopoietic progenitor cells enhance pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013, 188, 976–984. [Google Scholar] [CrossRef] [Green Version]
- Ou-Yang, H.F.; Han, X.P.; Zhao, F.; Ti, X.Y.; Wu, C.G. The role of bone marrow-derived adult stem cells in a transgenic mouse model of allergic asthma. Respiration 2012, 83, 74–80. [Google Scholar] [CrossRef]
- Serikov, V.B.; Mikhaylov, V.M.; Krasnodembskay, A.D.; Matthay, M.A. Bone marrow-derived cells participate in stromal remodeling of the lung following acute bacterial pneumonia in mice. Lung 2008, 186, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Spees, J.L.; Pociask, D.A.; Sullivan, D.E.; Whitney, M.J.; Lasky, J.A.; Prockop, D.J.; Brody, A.R. Engraftment of bone marrow progenitor cells in a rat model of asbestos-induced pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Spees, J.L.; Whitney, M.J.; Sullivan, D.E.; Lasky, J.A.; Laboy, M.; Ylostalo, J.; Prockop, D.J. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008, 22, 1226–1236. [Google Scholar] [CrossRef]
- Akita, S.; Kubota, K.; Kobayashi, A.; Misawa, R.; Shimizu, A.; Nakata, T.; Yokoyama, T.; Takahashi, M.; Miyagawa, S. Role of bone marrow cells in the development of pancreatic fibrosis in a rat model of pancreatitis induced by a choline-deficient/ethionine-supplemented diet. Biochem. Biophys. Res. Commun. 2012, 420, 743–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.R.; Inatomi, O.; Lee, C.Y.; Kallis, Y.N.; Otto, W.R.; Jeffery, R.; Poulsom, R.; Alison, M.R. Bone marrow-derived cells contribute to cerulein-induced pancreatic fibrosis in the mouse. Int. J. Exp. Pathol. 2012, 93, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Marrache, F.; Pendyala, S.; Bhagat, G.; Betz, K.S.; Song, Z.; Wang, T.C. Role of bone marrow-derived cells in experimental chronic pancreatitis. Gut 2008, 57, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Sordi, V.; Melzi, R.; Mercalli, A.; Formicola, R.; Doglioni, C.; Tiboni, F.; Ferrari, G.; Nano, R.; Chwalek, K.; Lammert, E.; et al. Mesenchymal cells appearing in pancreatic tissue culture are bone marrow-derived stem cells with the capacity to improve transplanted islet function. Stem Cells 2010, 28, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Masamune, A.; Kikuta, K.; Hirota, M.; Kume, K.; Satoh, K.; Shimosegawa, T. Bone marrow contributes to the population of pancreatic stellate cells in mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 297, G1138–G1146. [Google Scholar] [CrossRef]
- Barisic-Dujmovic, T.; Boban, I.; Clark, S.H. Fibroblasts/myofibroblasts that participate in cutaneous wound healing are not derived from circulating progenitor cells. J. Cell. Physiol. 2010, 222, 703–712. [Google Scholar] [CrossRef]
- Bezhaeva, T.; Geelhoed, W.J.; Wang, D.; Yuan, H.; van der Veer, E.P.; Alem, C.; Damanik, F.F.R.; Qiu, X.; Zonneveld, A.V.; Moroni, L.; et al. Contribution of bone marrow-derived cells to in situ engineered tissue capsules in a rat model of chronic kidney disease. Biomaterials 2019, 194, 47–56. [Google Scholar] [CrossRef]
- Boban, I.; Barisic-Dujmovic, T.; Clark, S.H. Parabiosis and transplantation models show no evidence of circulating dermal fibroblast progenitors in bleomycin-induced skin fibrosis. J. Cell. Physiol. 2008, 214, 230–237. [Google Scholar] [CrossRef]
- Fathke, C.; Wilson, L.; Hutter, J.; Kapoor, V.; Smith, A.; Hocking, A.; Isik, F. Contribution of bone marrow-derived cells to skin: Collagen deposition and wound repair. Stem Cells 2004, 22, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Higashiyama, R.; Nakao, S.; Shibusawa, Y.; Ishikawa, O.; Moro, T.; Mikami, K.; Fukumitsu, H.; Ueda, Y.; Minakawa, K.; Tabata, Y.; et al. Differential Contribution of Dermal Resident and Bone Marrow-Derived Cells to Collagen Production during Wound Healing and Fibrogenesis in Mice. J. Investig. Dermatol. 2011, 131, 529–536. [Google Scholar] [CrossRef]
- Mori, L.; Bellini, A.; Stacey, M.A.; Schmidt, M.; Mattoli, S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp. Cell Res. 2005, 304, 81–90. [Google Scholar] [CrossRef]
- Opalenik, S.R.; Davidson, J.M. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005, 19, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.; Giles, N.L.; Webb, S.; Adcroft, K.F.; Evill, L.M.; Strickland, D.H.; Wood, F.M.; Fear, M.W. Bone marrow-derived cells in the healing burn wound-More than just inflammation. Burns 2009, 35, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, J.; van Rheden, R.E.; Katsaros, C.; Torensma, R.; Von den Hoff, J.W. Preferential recruitment of bone marrow-derived cells to rat palatal wounds but not to skin wounds. Arch. Oral Biol. 2012, 57, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhou, L.; Li, C.; Xie, H.; Lu, Y.; Wu, Y.; Liu, H. Bone marrow-derived cells homing for self-repair of periodontal tissues: A histological characterization and expression analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 12379–12389. [Google Scholar] [PubMed]
- Wilson, K.R.; Kang, I.H.; Baliga, U.; Xiong, Y.; Chatterjee, S.; Moore, E.; Parthiban, B.; Thyagarajan, K.; Borke, J.L.; Mehrotra, S.; et al. Hematopoietic Stem Cells as a Novel Source of Dental Tissue Cells. Sci. Rep. 2018, 8, 8026. [Google Scholar] [CrossRef]
- Verstappen, J.; Katsaros, C.; Torensma, R.; Von den Hoff, J.W. Bone marrow-derived cells in palatal wound healing. Oral Dis. 2010, 16, 788–794. [Google Scholar] [CrossRef]
- Kvasnicka, H.M.; Wickenhauser, C.; Thiele, J.; Varus, E.; Hamm, K.; Beelen, D.W.; Schaefer, U.W. Mixed chimerism of bone marrow vessels (endothelial cells, myofibroblasts) following allogeneic transplantation for chronic myelogenous leukemia. Leuk. Lymphoma 2003, 44, 321–328. [Google Scholar] [CrossRef]
- Agematsu, K.; Nakahori, Y. Recipient origin of bone marrow-derived fibroblastic stromal cells during all periods following bone marrow transplantation in humans. Br. J. Haematol. 1991, 79, 359–365. [Google Scholar] [CrossRef]
- Bartsch, K.; Al-Ali, H.; Reinhardt, A.; Franke, C.; Hudecek, M.; Kamprad, M.; Tschiedel, S.; Cross, M.; Niederwieser, D.; Gentilini, C. Mesenchymal stem cells remain host-derived independent of the source of the stem-cell graft and conditioning regimen used. Transplantation 2009, 87, 217–221. [Google Scholar] [CrossRef]
- Cilloni, D.; Carlo-Stella, C.; Falzetti, F.; Sammarelli, G.; Regazzi, E.; Colla, S.; Rizzoli, V.; Aversa, F.; Martelli, M.F.; Tabilio, A. Limited engraftment capacity of bone marrow-derived mesenchymal cells following T-cell-depleted hematopoietic stem cell transplantation. Blood 2000, 96, 3637–3643. [Google Scholar] [CrossRef] [PubMed]
- Golde, D.W.; Hocking, W.G.; Quan, S.G.; Sparkes, R.S.; Gale, R.P. Origin of human bone marrow fibroblasts. Br. J. Haematol. 1980, 44, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Hollings, P.E.; Fitzgerald, P.H.; Heaton, D.C.; Beard, M.E. Host origin of in vitro bone marrow fibroblasts after marrow transplantation in man. Stem Cells 1984, 2, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Laver, J.; Jhanwar, S.C.; O'Reilly, R.J.; Castro-Malaspina, H. Host origin of the human hematopoietic microenvironment following allogeneic bone marrow transplantation. Blood 1987, 70, 1966–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.Y.; Cho, S.W.; Oh, E.S.; Oh, K.W.; Lee, J.M.; Yoon, K.H.; Kang, M.I.; Cha, B.Y.; Lee, K.W.; Son, H.Y.; et al. The effect of bone marrow transplantation on the osteoblastic differentiation of human bone marrow stromal cells. J. Clin. Endocrinol. Metab. 2002, 87, 329–335. [Google Scholar] [CrossRef]
- Ma, D.D.; Da, W.M.; Purvis-Smith, S.; Biggs, J.C. Chromosomal analysis of bone marrow stromal fibroblasts in allogeneic HLA compatible sibling bone marrow transplantations. Leuk. Res. 1987, 11, 661–663. [Google Scholar] [CrossRef]
- Tanaka, J.; Kasai, M.; Imamura, M.; Masauzi, N.; Ohizumi, H.; Matsuura, A.; Morii, K.; Kiyama, Y.; Naohara, T.; Saitoh, M.; et al. Evaluation of mixed chimaerism and origin of bone marrow derived fibroblastoid cells after allogeneic bone marrow transplantation. Br. J. Haematol. 1994, 86, 436–438. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Lu, D.P. Mesenchymal stem cells in stem cell transplant recipients are damaged and remain of host origin. Int. J. Hematol. 2005, 82, 152–158. [Google Scholar] [CrossRef]
- Wilson, F.D.; Konrad, P.N.; Greenberg, B.R.; Klein, A.K.; Walling, P.A. Cytogenetic studies on bone marrow fibroblasts from a male-female hematopoietic chimera. Evidence that stromal elements in human transplantation recipients are of host type. Transplantation 1978, 25, 87–88. [Google Scholar] [CrossRef]
- Eberwein, P.; Faber, P.; Reinhard, T.; Finke, J.; Spyridonidis, A. Conjunctival Epithelial Cells With Donor-Derived Genome After Human Haematopoietic Stem-Cell Transplantation. Transplantation 2009, 87, 915–918. [Google Scholar] [CrossRef]
- Hallberg, D.; Stenberg, K.; Hanson, C.; Stenevi, U.; Brune, M. Conjunctival polyploid cells and donor-derived myofibroblasts in ocular GvHD. Bone Marrow Transpl. 2016, 51, 692–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallberg, D.; Wernstedt, P.; Hanson, C.; Wettergren, Y.; Stenberg, K.; Brune, M.; Stenevi, U. Donor-derived myofibroblasts in the ocular surface after allogeneic haematopoietic stem cell transplantation. Acta Ophthalmol. Scand. 2006, 84, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kodama, H.; Kameyama, K.; Yamazaki, K.; Yasuoka, H.; Okamoto, S.; Inoko, H.; Kawakami, Y.; Kuwana, M. Donor fibroblast chimerism in the pathogenic fibrotic lesion of human chronic graft-versus-host disease. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4519–4527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, S.J.; Russo, F.P.; Rey, V.; Burra, P.; Rugge, M.; Wright, N.A.; Alison, M.R. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology 2004, 126, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Bröcker, V.; Länger, F.; Fellous, T.G.; Mengel, M.; Brittan, M.; Bredt, M.; Milde, S.; Welte, T.; Eder, M.; Haverich, A.; et al. Fibroblasts of recipient origin contribute to bronchiolitis obliterans in human lung transplants. Am. J. Respir. Crit. Care Med. 2006, 173, 1276–1282. [Google Scholar] [CrossRef]
- Goussetis, E.; Spiropoulos, A.; Theodosaki, M.; Stefanaki, K.; Petrakou, E.; Graphakos, S. Myofibroblasts generated in culture from sclerotic skin lesions of a patient with extensive chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation are of recipient origin. Stem Cells Dev. 2010, 19, 1285–1287. [Google Scholar] [CrossRef]
- Wagner, J.E.; Ishida-Yamamoto, A.; McGrath, J.A.; Hordinsky, M.; Keene, D.R.; Riddle, M.J.; Osborn, M.J.; Lund, T.; Dolan, M.; Blazar, B.R.; et al. Bone Marrow Transplantation for Recessive Dystrophic Epidermolysis Bullosa. N. Engl. J. Med. 2010, 363, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Dolado, M.; Pardal, R.; Garcia-Verdugo, J.M.; Fike, J.R.; Lee, H.O.; Pfeffer, K.; Lois, C.; Morrison, S.J.; Alvarez-Buylla, A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 2003, 425, 968–973. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Willenbring, H.; Akkari, Y.; Torimaru, Y.; Foster, M.; Al-Dhalimy, M.; Lagasse, E.; Finegold, M.; Olson, S.; Grompe, M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003, 422, 897–901. [Google Scholar] [CrossRef]
- Sera, Y.; LaRue, A.C.; Moussa, O.; Mehrotra, M.; Duncan, J.D.; Williams, C.R.; Nishimoto, E.; Schulte, B.A.; Watson, P.M.; Watson, D.K.; et al. Hematopoietic stem cell origin of adipocytes. Exp. Hematol. 2009, 37, 1108–1120. [Google Scholar] [CrossRef]
- Broughton, G.I.; Janis, J.E.; Attinger, C.E. The Basic Science of Wound Healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, R.D.; Smilde, B.J.; Pals, G.; Bravenboer, N.; Knaus, P.; Schoenmaker, T.; Botman, E.; Sanchez-Duffhues, G.; Pacifici, M.; Pignolo, R.J.; et al. Fibrodysplasia Ossificans Progressiva: What Have We Achieved and Where Are We Now? Follow-up to the 2015 Lorentz Workshop. Front. Endocrinol. 2021, 12, 732728. [Google Scholar] [CrossRef]
- Smilde, B.J.; Botman, E.; de Ruiter, R.D.; Smit, J.M.; Teunissen, B.P.; Lubbers, W.D.; Schwarte, L.A.; Schober, P.; Eekhoff, E.M.W. Monitoring and Management of Fibrodysplasia Ossificans Progressiva: Current Perspectives. Orthop. Res. Rev. 2022, 14, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cayami, F.K.; Claeys, L.; de Ruiter, R.; Smilde, B.J.; Wisse, L.; Bogunovic, N.; Riesebos, E.; Eken, L.; Kooi, I.; Sistermans, E.A.; et al. Osteogenic transdifferentiation of primary human fibroblasts to osteoblast-like cells with human platelet lysate. Sci. Rep. 2022, 12, 14686. [Google Scholar] [CrossRef] [PubMed]
- De Vries, T.J.; Schoenmaker, T.; Micha, D.; Hogervorst, J.; Bouskla, S.; Forouzanfar, T.; Pals, G.; Netelenbos, C.; Eekhoff, E.M.W.; Bravenboer, N. Periodontal ligament fibroblasts as a cell model to study osteogenesis and osteoclastogenesis in fibrodysplasia ossificans progressiva. Bone 2018, 109, 168–177. [Google Scholar] [CrossRef]
- Schoenmaker, T.; Wouters, F.; Micha, D.; Forouzanfar, T.; Netelenbos, C.; Eekhoff, E.M.W.; Bravenboer, N.; de Vries, T.J. The effect of Activin-A on periodontal ligament fibroblasts-mediated osteoclast formation in healthy donors and in patients with fibrodysplasia ossificans progressiva. J. Cell. Physiol. 2019, 234, 10238–10247. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smilde, B.J.; Botman, E.; de Vries, T.J.; de Vries, R.; Micha, D.; Schoenmaker, T.; Janssen, J.J.W.M.; Eekhoff, E.M.W. A Systematic Review of the Evidence of Hematopoietic Stem Cell Differentiation to Fibroblasts. Biomedicines 2022, 10, 3063. https://doi.org/10.3390/biomedicines10123063
Smilde BJ, Botman E, de Vries TJ, de Vries R, Micha D, Schoenmaker T, Janssen JJWM, Eekhoff EMW. A Systematic Review of the Evidence of Hematopoietic Stem Cell Differentiation to Fibroblasts. Biomedicines. 2022; 10(12):3063. https://doi.org/10.3390/biomedicines10123063
Chicago/Turabian StyleSmilde, Bernard J., Esmée Botman, Teun J. de Vries, Ralph de Vries, Dimitra Micha, Ton Schoenmaker, Jeroen J. W. M. Janssen, and Elisabeth M. W. Eekhoff. 2022. "A Systematic Review of the Evidence of Hematopoietic Stem Cell Differentiation to Fibroblasts" Biomedicines 10, no. 12: 3063. https://doi.org/10.3390/biomedicines10123063




