The Neuroprotective and Antioxidant Effects of Nanocurcumin Oral Suspension against Lipopolysaccharide-Induced Cortical Neurotoxicity in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of LPS
2.3. Nanocurcumin
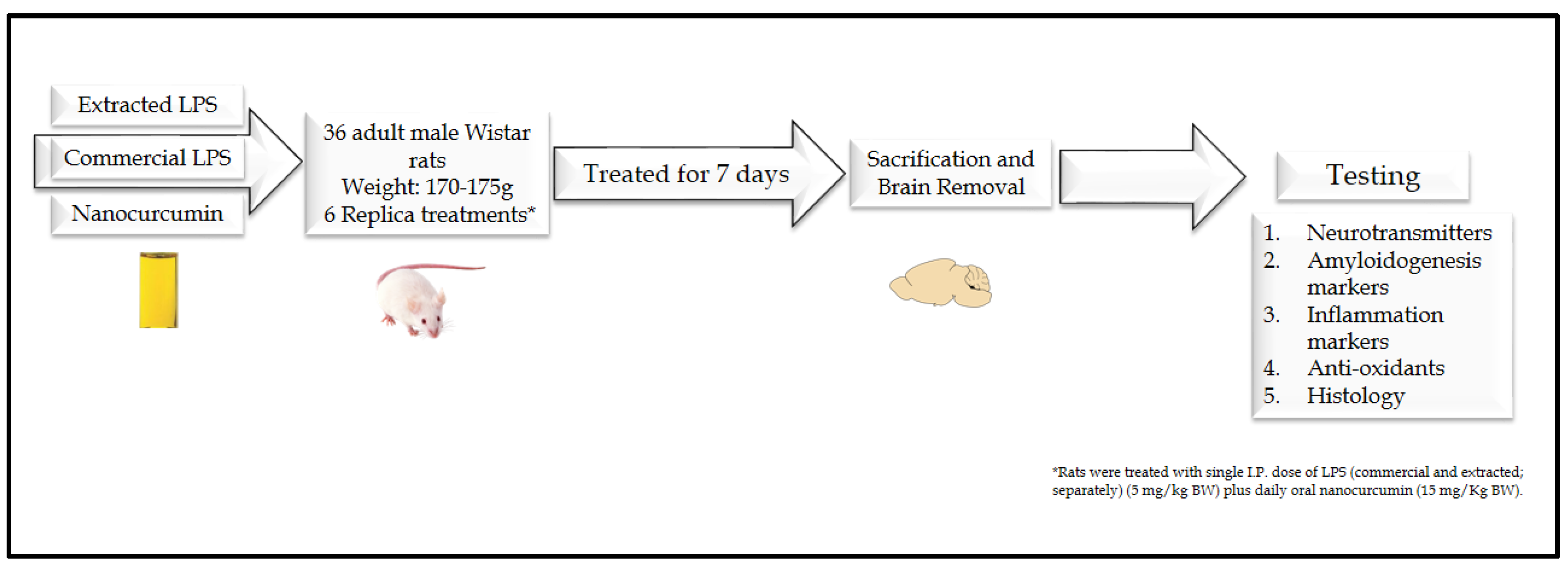
2.4. Animals and Experimental Design
2.4.1. Animals
2.4.2. Experimental Design
2.4.3. Sample Collection and Tissue Preparations
2.5. Assays of Neurotransmitters in Brain Tissues
2.6. Assay of Malondialdehyde (MDA) as Index of Lipid Peroxidation in Brain Tissues
2.7. Assay of Nitric Oxide End Products (NOx) in Brain Tissues
2.8. Assay of Glutathione (GSH) in Brain Tissues
2.9. Assays of Tumor Necrosis Factor-α (TNF-α) and Amyloid Beta Protein 1-42 (Aβ1-42) in Brain Tissues
2.10. Assays of Caspase-3 Activity in Brain Tissues
2.11. Gene Expression of Brain-Derived Neurotropic Factor (BDNF), Mitochondrial Transcription Factor A (mTFA), β-Site APP Cleaving Enzyme 1 (BACE1), and Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)
2.12. Histological Examination
2.13. Statistical Analysis
3. Results
3.1. Effect of LPS and Nanocurcumin on the Brain Neurotransmitters
3.1.1. Acetylcholine (Ach)
3.1.2. Acetylcholine Esterase (AchE)
3.1.3. Serotonin
3.1.4. Dopamine
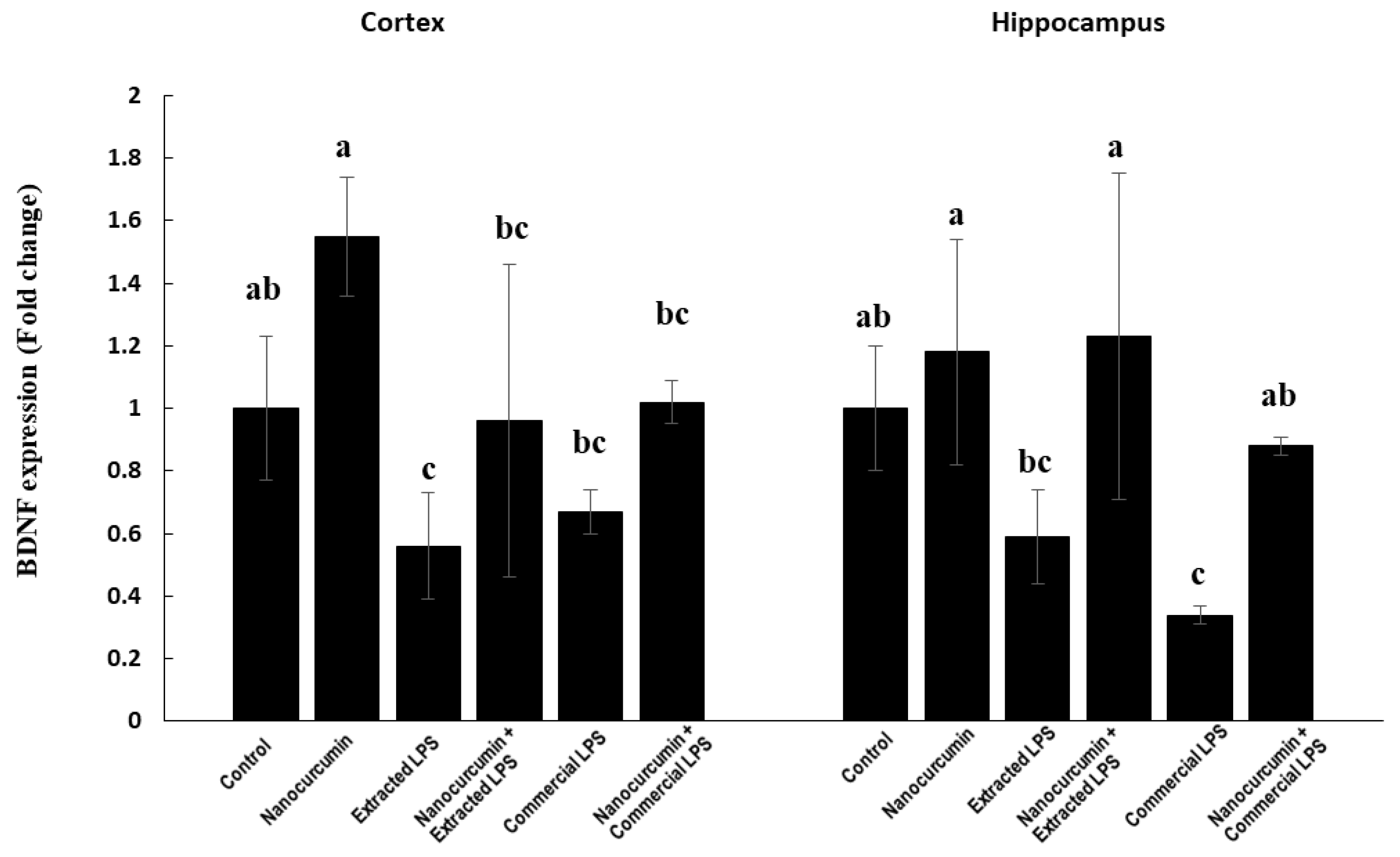
3.1.5. BDNF Expression
3.2. Antioxidant Systems
3.2.1. Glutathione System in Cerebral Cortex
3.2.2. Glutathione System in Hippocampus
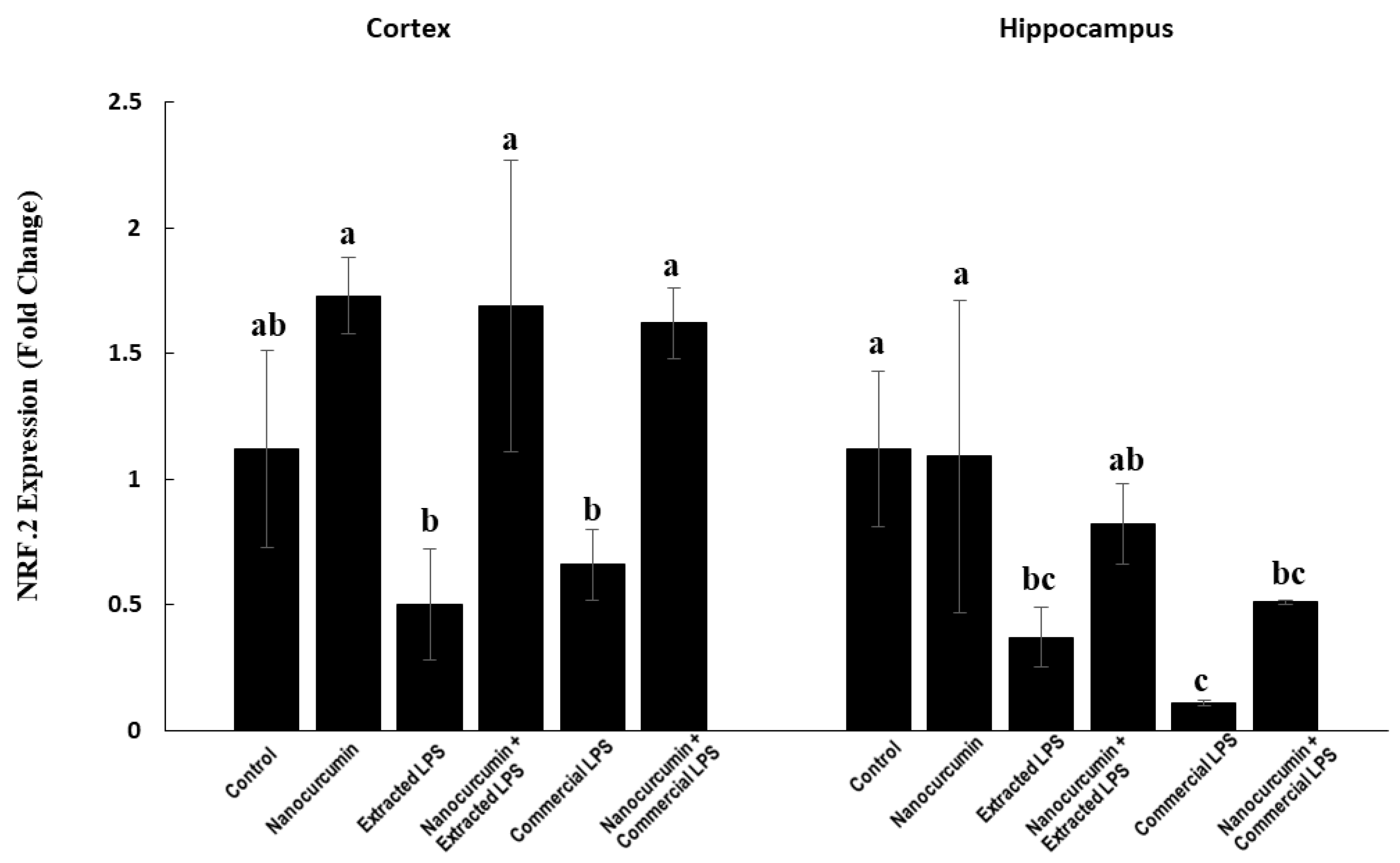
3.2.3. Nuclear Factor-Erythroid Factor 2-Related Factor 2 (NRF2) Expression in Cerebral Cortex and Hippocampus
3.3. Oxidative Stress Markers
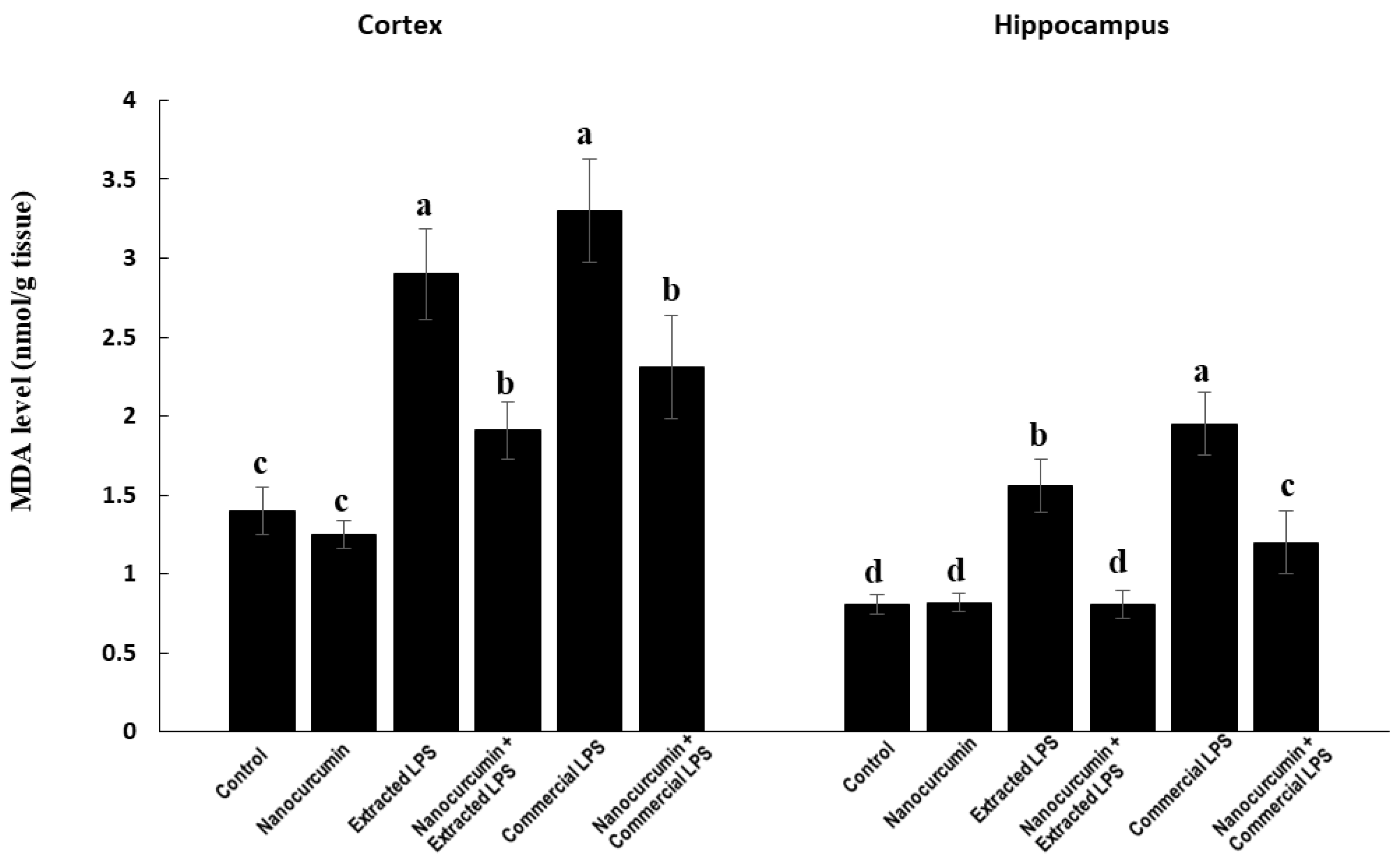
3.3.1. Malondialdehyde (MDA)
3.3.2. Nitric Oxide End Products (NOx)
3.4. Markers of Amyloidogenic Pathway
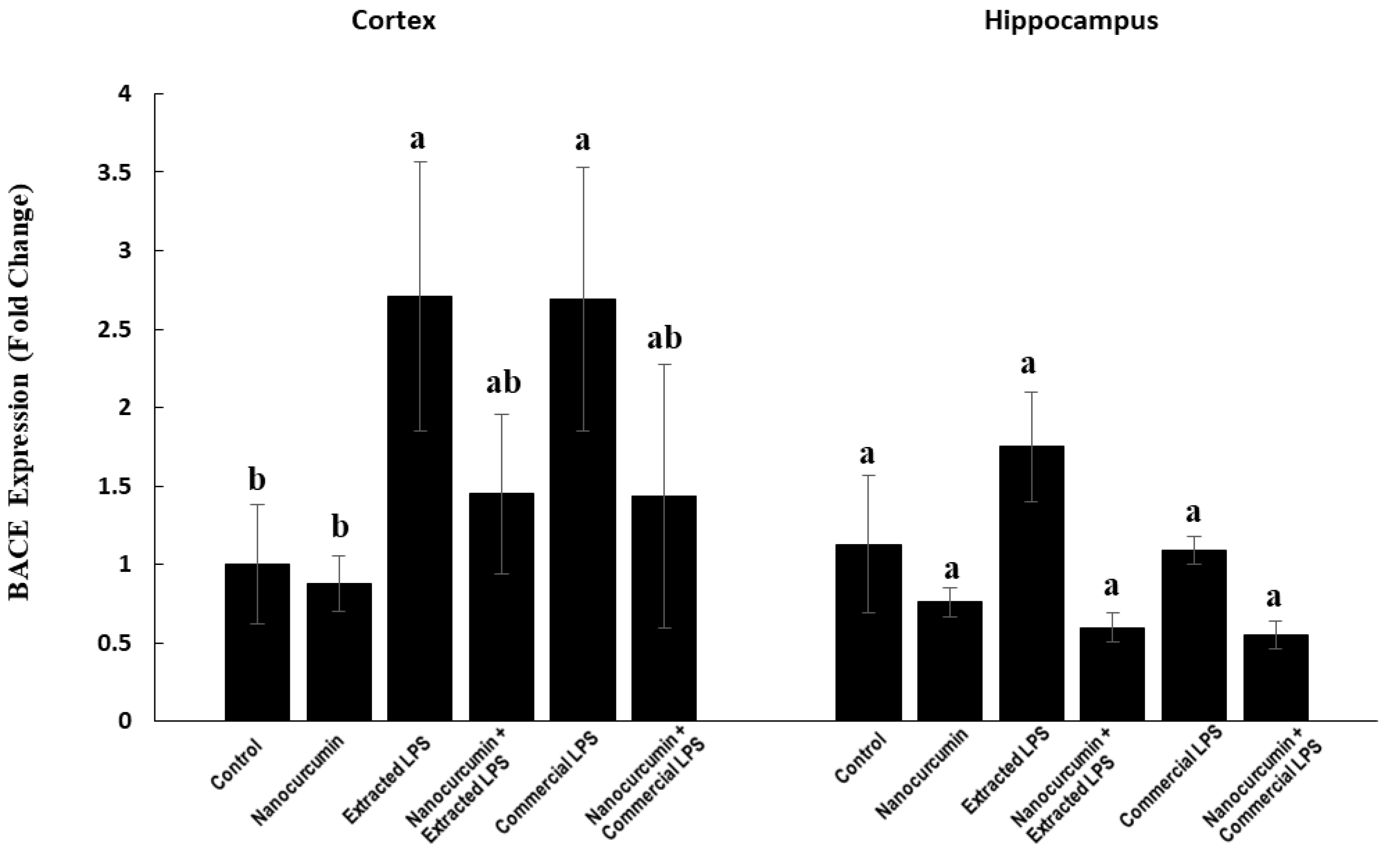
3.4.1. Expression of BACE1
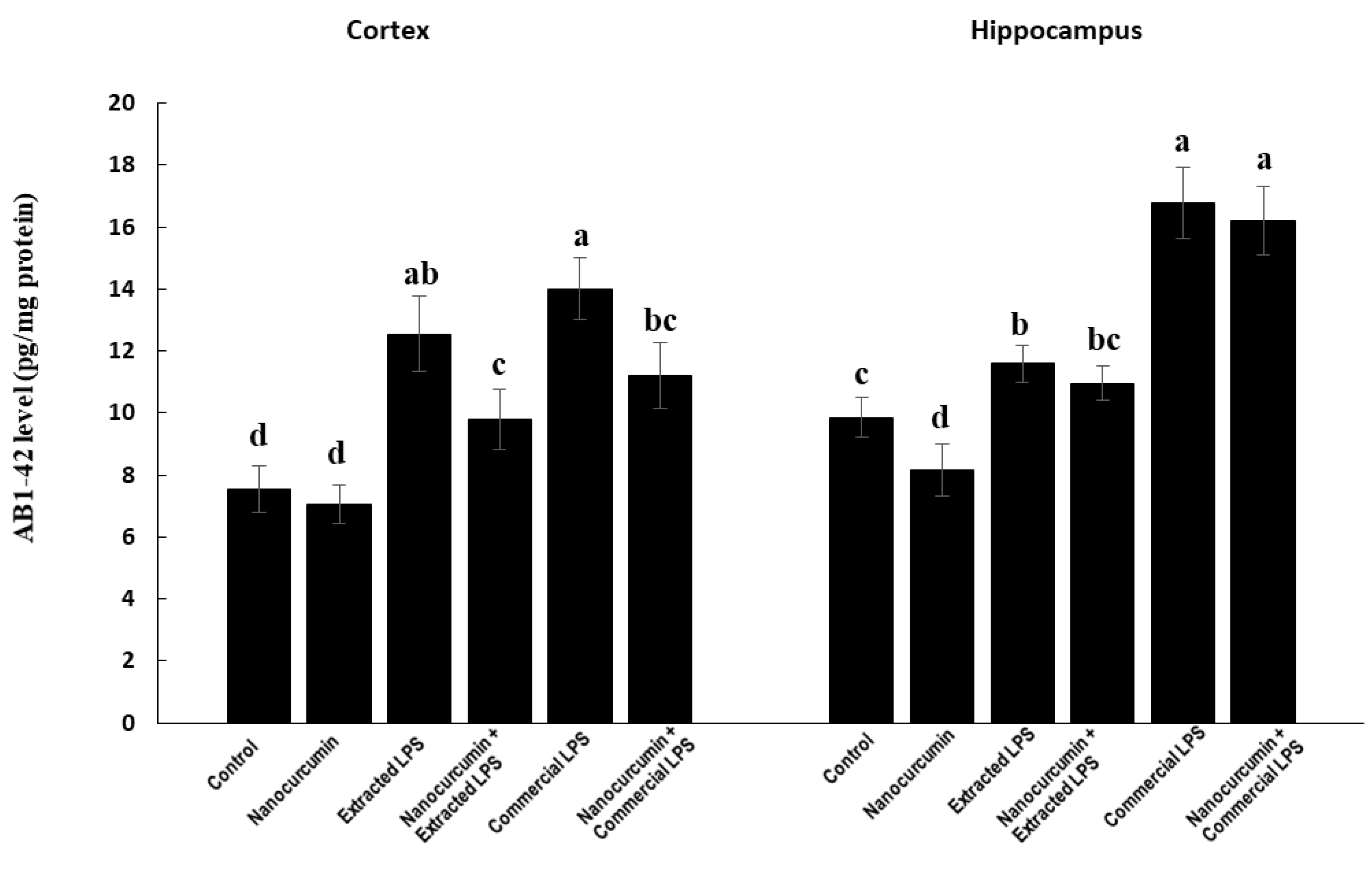
3.4.2. Amyloid Beta Protein 1-42 (Aβ 1-42)
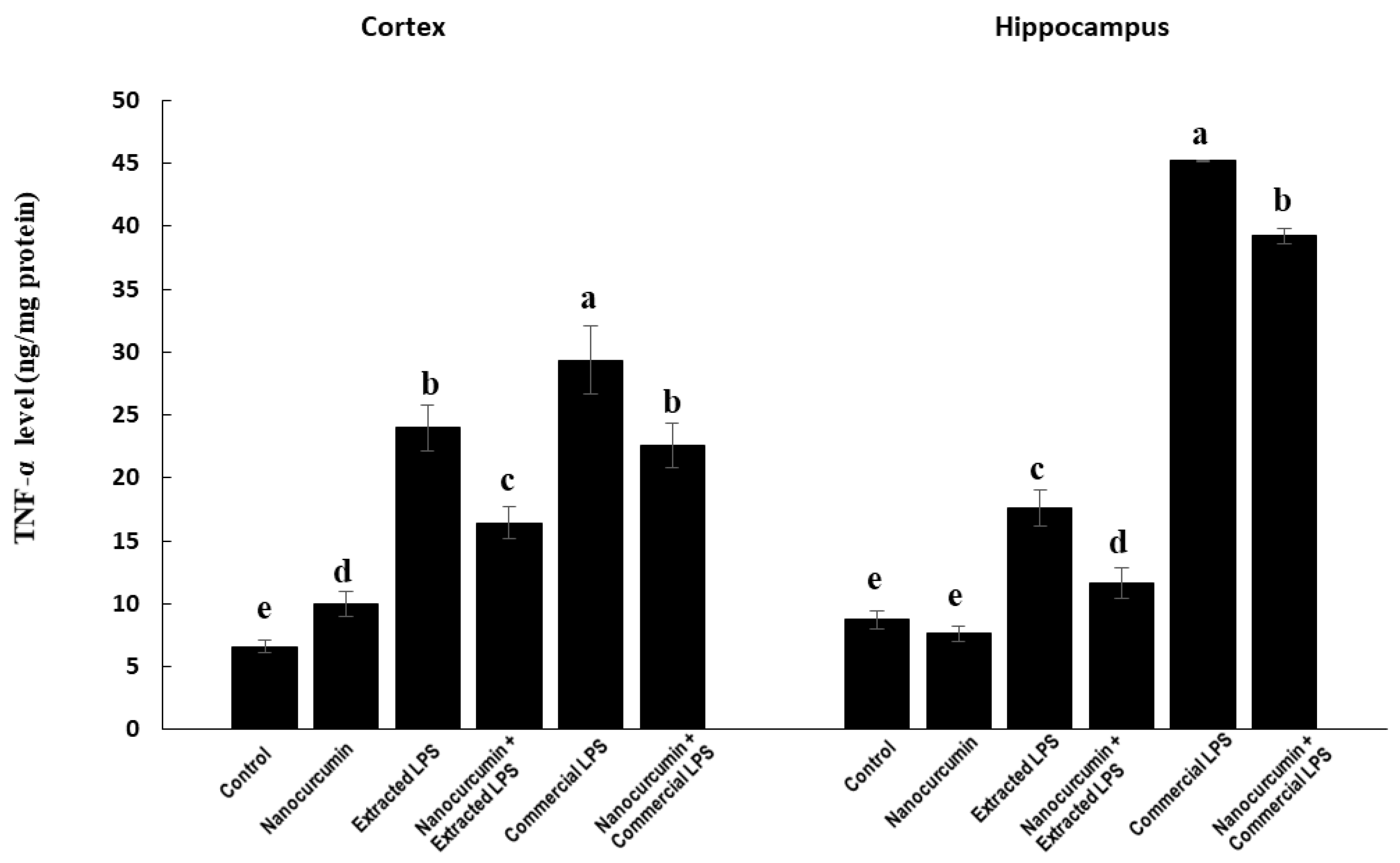
3.4.3. Marker of Neuroinflammation: TNF-α
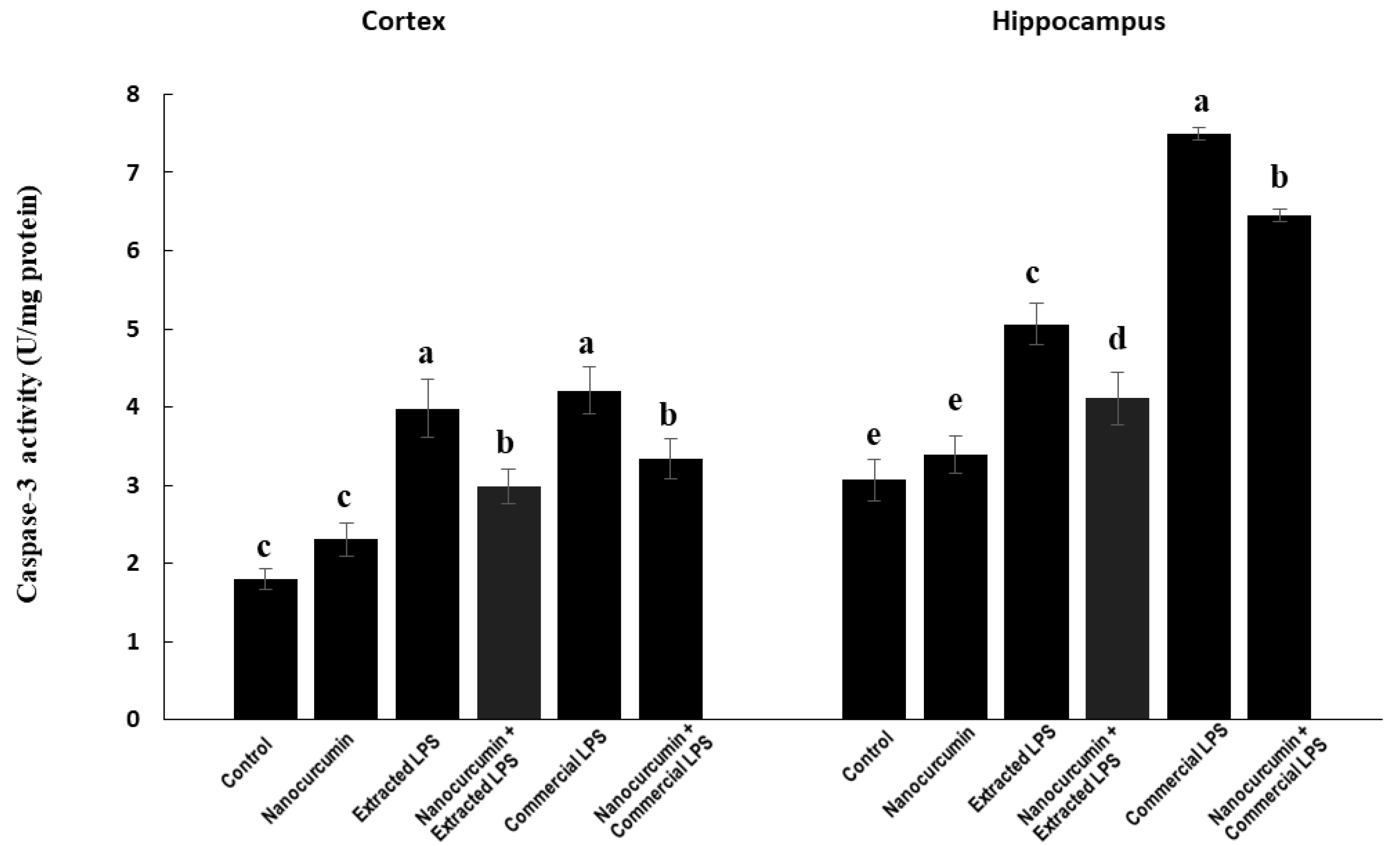
3.4.4. Caspase-3 Activity aa Apoptotic Marker
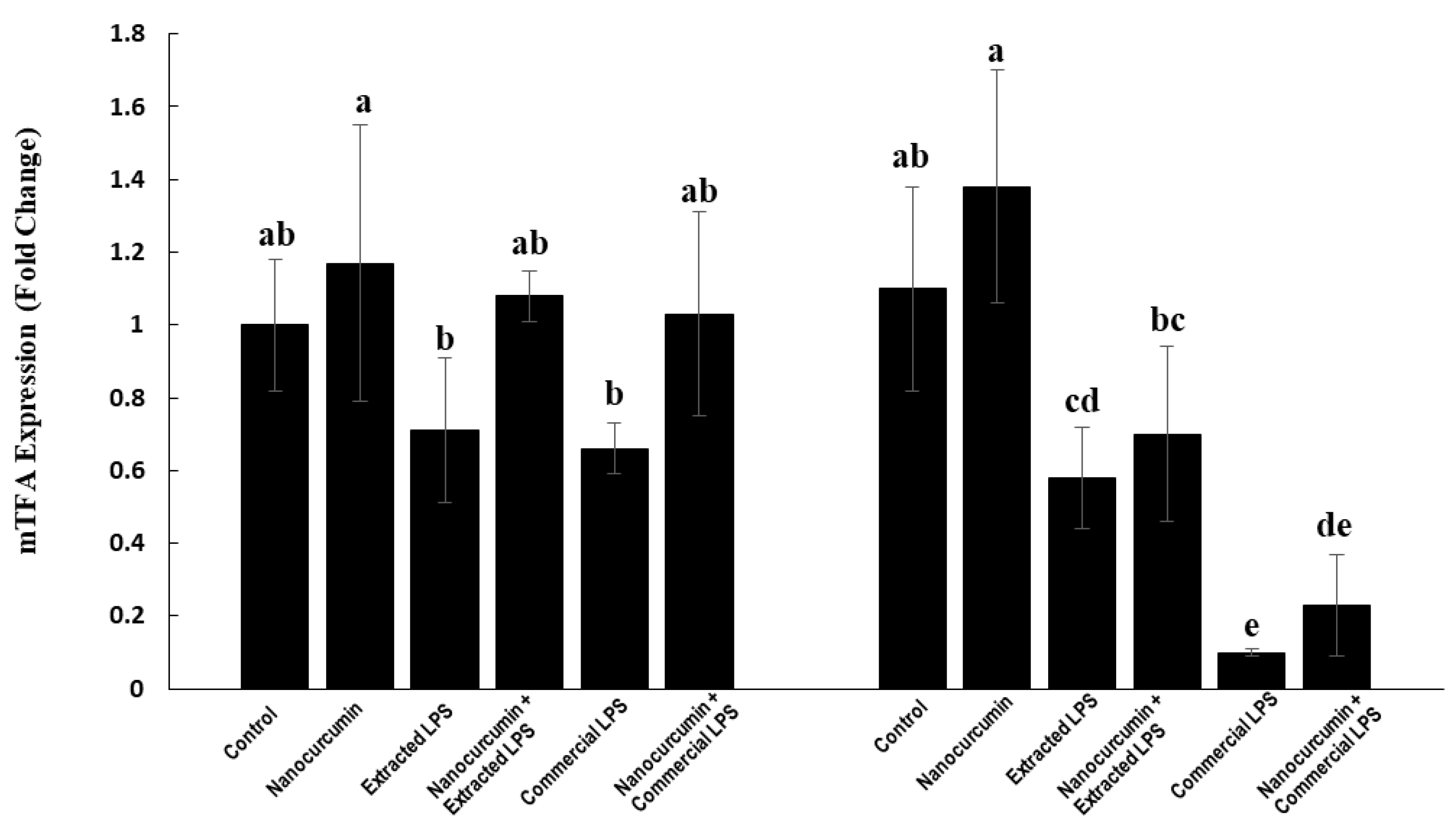
3.4.5. Expression of Mitochondrial Transcription Factor A (mTFA):
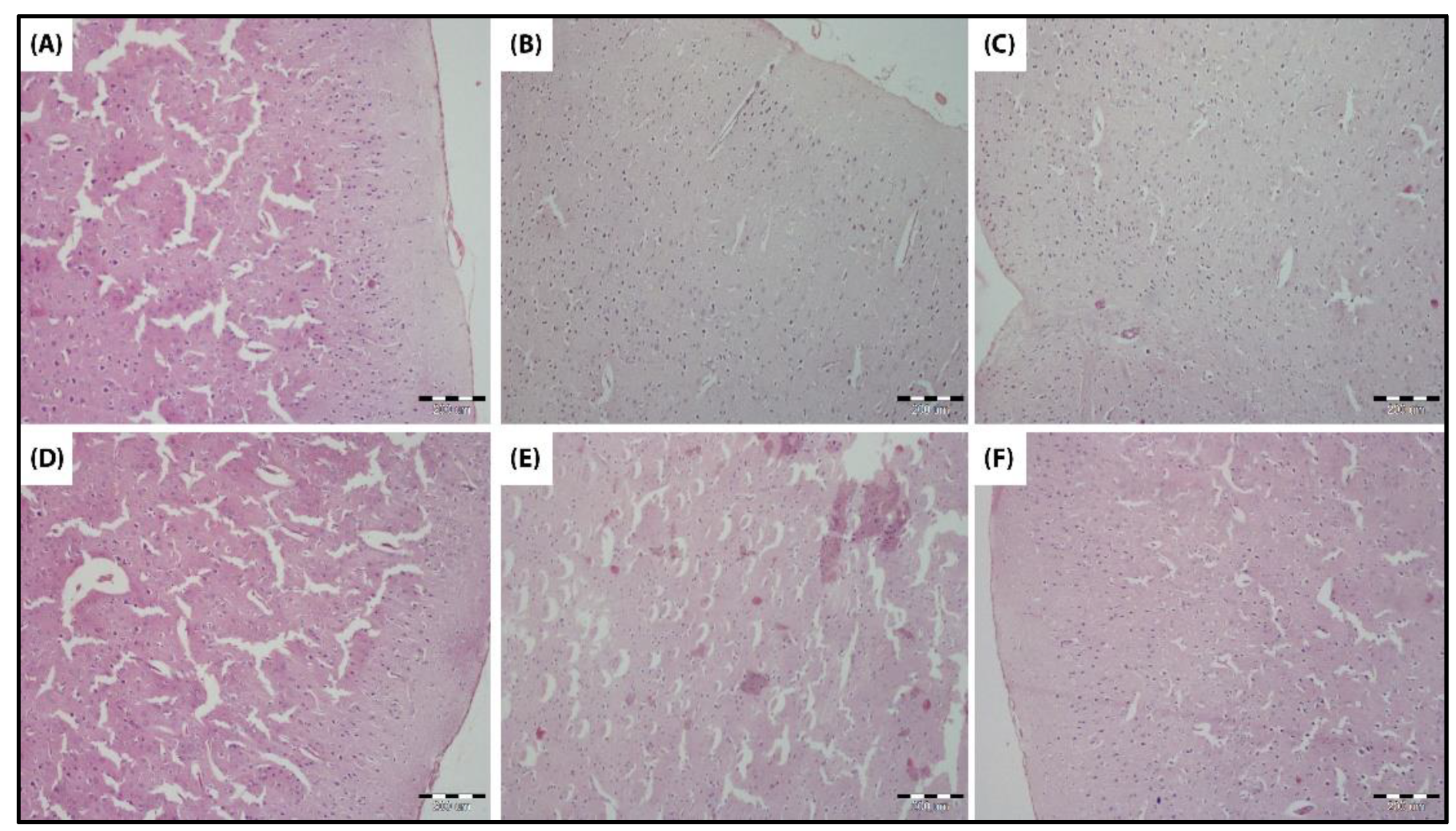
3.4.6. Histological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zähringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Somann, J.P.; Wasilczuk, K.M.; Neihouser, K.V.; Sturgis, J.; Albors, G.O.; Robinson, J.P.; Powley, T.L.; Irazoqui, P.P. Characterization of plasma cytokine response to intraperitoneally administered LPS & subdiaphragmatic branch vagus nerve stimulation in rat model. PLoS ONE 2019, 14, e0214317. [Google Scholar] [CrossRef] [Green Version]
- Bossù, P.; Cutuli, D.; Palladino, I.; Caporali, P.; Angelucci, F.; Laricchiuta, D.; Gelfo, F.; De Bartolo, P.; Caltagirone, C.; Petrosini, L. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J. Neuroinflamm. 2012, 9, 101. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimizadeh, W.; Gargari, S.L.M.; Bazl, R.; Malekshahi, V. Comparison of different LPS extraction methods for isolation of smooth and rough LPS. In Proceedings of the First International & 12th Iranian Congress of Microbiology, Kermanshah, Iran, 23–26 May 2011. [Google Scholar]
- Rezania, S.; Amirmozaffari, N.; Tabarraei, B.; Jeddi-Tehrani, M.; Zarei, O.; Alizadeh, R.; Masjedian, F.; Zarnani, A.H. Extraction, purification and characterization of lipopolysaccharide from Escherichia coli and Salmonella typhi. Avicenna J. Med. Biotechnol. 2011, 3, 3–9. [Google Scholar]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.W.; Elfving, B.; Lund, S.; Wegener, G. Behavioral and systemic consequences of long-term inflammatory challenge. J. Neuroimmunol. 2015, 288, 40–46. [Google Scholar] [CrossRef]
- Jeong, H.K.; Jou, I.; Joe, E. hye Systemic LPS administration induces brain inflammation but not dopaminergic neuronal death in the substantia nigra. Exp. Mol. Med. 2010, 42, 823–832. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, D.; Yang, Z.; Hu, X.; Qian, S.; Liu, J.; Wilson, B.; Block, M.; Hong, J.S. Curcumin protects dopaminergic neuron against LPS induced neurotoxicity in primary rat neuron/glia culture. Neurochem. Res. 2008, 33, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Anwar, S.K.; Elmonaem, S.N.A.; Moussa, E.; Aboulela, A.G.; Essawy, M.M. Curcumin nanoparticles: The topical antimycotic suspension treating oral candidiasis. Odontology 2022. [Google Scholar] [CrossRef]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef] [Green Version]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Oncotarget 66680 www.impactjournals.com/oncotarget Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-M.; Chien, C.-F.; Lin, L.-C.; Tsai, T.-H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef] [PubMed]
- El-Moslemany, R.M.; El-Kamel, A.H.; Allam, E.A.; Khalifa, H.M.; Hussein, A.; Ashour, A.A. Tanshinone IIA loaded bioactive nanoemulsion for alleviation of lipopolysaccharide induced acute lung injury via inhibition of endothelial glycocalyx shedding. Biomed. Pharmacother. 2022, 155, 113666. [Google Scholar] [CrossRef]
- Nežić, L.; Škrbić, R.; Dobrić, S.; Stojiljković, M.P.; Šatara, S.S.; Milovanović, Z.A.; Stojaković, N. Effect of simvastatin on proinflammatory cytokines production during lipopolysaccharide-induced inflammation in rats. Gen. Physiol. Biophys. 2009, 28, 119–126. [Google Scholar]
- Yadav, A.; Flora, S.J.S.; Kushwaha, P. Nanocurcumin Prevents Oxidative Stress Induced following Arsenic and Fluoride Co-exposure in Rats. Def. Life Sci. J. 2016, 1, 69. [Google Scholar] [CrossRef]
- Annaraj, J.; Dhivya, R.; Vigneshwar, M.; Dharaniyambigai, K.; Kumaresan, G.; Rajasekaran, M. Studies on The Enhanced Biological Applications of PVA Loaded Nanocurcumin. J. Nanosci. Nanotechnol. 2014, 2, 490–495. [Google Scholar]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef]
- Guevara, I.; Iwanejko, J.; Dembińska-Kieć, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Gołabek, I.; Bartuś, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta 1998, 274, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nobakht, M.; Hoseini, S.M.; Mortazavi, P.; Sohrabi, I.; Esmaeilzade, B.; Rooshandel, N.R.; Omidzahir, S. Neuropathological changes in brain cortex and hippocampus in a rat model of Alzheimer’s disease. Iran. Biomed. J. 2011, 15, 51–58. [Google Scholar]
- Soudi, S.A.; Nounou, M.I.; Sheweita, S.A.; Ghareeb, D.A.; Younis, L.K.; El-Khordagui, L.K. Protective effect of surface-modified berberine nanoparticles against LPS-induced neurodegenerative changes: A preclinical study. Drug Deliv. Transl. Res. 2019, 9, 906–919. [Google Scholar] [CrossRef]
- Tyagi, E.; Agrawal, R.; Nath, C.; Shukla, R. Influence of LPS-induced neuroinflammation on acetylcholinesterase activity in rat brain. J. Neuroimmunol. 2008, 205, 51–56. [Google Scholar] [CrossRef]
- Korte-Bouws, G.A.H.; van Heesch, F.; Westphal, K.G.C.; Ankersmit, L.M.J.; van Oosten, E.M.; Güntürkün, O.; Korte, S.M. Bacterial lipopolysaccharide increases serotonin metabolism in both medial prefrontal cortex and nucleus accumbens in male wild type rats, but not in serotonin transporter knockout rats. Pharmaceuticals 2018, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical Implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Guan, Z.; Fang, J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain. Behav. Immun. 2006, 20, 64–71. [Google Scholar] [CrossRef]
- Yan, R. Physiological functions of the β-site amyloid precursor protein cleaving enzyme 1 and 2. Front. Mol. Neurosci. 2017, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Lindholm, K.; Yang, L.B.; Yue, X.; Citron, M.; Yan, R.; Beach, T.; Sue, L.; Sebbagh, M.; Cai, H.; et al. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 2004, 101, 3632–3637. [Google Scholar] [CrossRef] [Green Version]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef]
- Dringen, R.; Hirrlinger, J. Glutathione pathways in the brain. Biol. Chem. 2003, 384, 505–516. [Google Scholar] [CrossRef]
- Rahman, A.; Shuvo, A.A.; Bepari, A.K.; Apu, M.H.; Shill, M.C.; Hossain, M.; Uddin, M.; Islam, R.; Bakshi, M.K.; Hasan, J.; et al. Curcumin improves D-galactose and normal- aging associated memory impairment in mice: In vivo and in silico-based studies. PLoS ONE 2022, 17, e0270123. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslanbaeva, L.; Bisaglia, M. Activation of the Nrf2 Pathway as a Therapeutic Strategy for ALS Treatment. Molecules 2022, 27, 1471. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, A.; Devasena, T. Facile synthesis of Curcumin nanocrystals and validation of its antioxidant activity against circulatory toxicity in wistar rats. J. Nanosci. Nanotechnol. 2015, 15, 4119–4125. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Farid, O.A.H.; Nasr, M.; Ahmed, R.F.; Bakeer, R.M. Beneficial effects of curcumin nano-emulsion on spermatogenesis and reproductive performance in male rats under protein deficient diet model: Enhancement of sperm motility, conservancy of testicular tissue integrity, cell energy and seminal plasma amino a. J. Biomed. Sci. 2017, 24, 66. [Google Scholar] [CrossRef] [Green Version]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N.G. Mitochondrial DNA copy number in human disease: The more the better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Li, X.; Tian, Y. Mitochondrial-to-nuclear communication in aging: An epigenetic perspective. Trends Biochem. Sci. 2022, 47, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Zimmermann, K. Lipopolysaccharides in food, food supplements, and probiotics: Should we be worried? Eur. J. Microbiol. Immunol. 2018, 8, 63–69. [Google Scholar] [CrossRef]

| Gene | Accession No. | Primer Sequence | |
|---|---|---|---|
| BACE1 | NM_019204.2 | F | GCATGATCATTGGTGGTATC |
| R | CCATCTTGAGATCTTGACCA | ||
| BDNF | NM_001270638.1 | F | GAGAAGAGTGATGACCATCCT |
| R | TCACGTGCTCAAAAGTGTCAG | ||
| NRF2 | NM_031789.2 | F | CAAATCCCACCTTGAACACA |
| R | CGACTGACTAATGGCAGCAG | ||
| mTFA | NM_031326.2 | F | CCCTGGAAGCTTTCAGATACG |
| R | AATTGCAGCCATGTGGAGG | ||
| 18s rRNA | NR_046237.2 | F | GTAACCCGTTGAACCCCATT |
| R | CAAGCTTATGACCCGCACTT | ||
| Ach (ng/mg Protein) | AchE (ng/mg Protein) | |||
|---|---|---|---|---|
| Cortex | Hippocampus | Cortex | Hippocampus | |
| Control | 6.41 b ± 0.23 | 17.26 a ± 1.52 | 11.73 d ± 0.90 | 20.11 d ± 1.52 |
| Nanocurcumin | 7.46 a ± 0.53 | 16.97 a ± 1.35 | 15.37 c ± 0.90 | 20.79 d ± 1.72 |
| E-LPS | 4.19 c ± 0.29 | 11.21 b ± 1.15 | 22.46 ab ± 2.08 | 28.91 c ± 2.30 |
| E-LPS + Nanocurcumin | 6.33 b ± 0.62 | 15.79 a ± 1.33 | 15.97 c ± 1.20 | 23.24 d ± 2.02 |
| C-LPS | 4.40 c ± 0.18 | 10.73 b ± 0.08 | 24.86 a ± 2.26 | 43.61 a ± 0.08 |
| C-LPS + Nanocurcumin | 6.13 b ± 0.45 | 15.31 a ± 0.33 | 19.49 b ± 1.74 | 37.95 b ± 1.05 |
| Serotonin (ng/mg Protein) | Dopamine (ng/mg Protein) | |||
|---|---|---|---|---|
| Cortex | Hippocampus | Cortex | Hippocampus | |
| Control | 3.85 b ± 0.42 | 4.96 a ± 0.52 | 5.73 a ± 0.44 | 10.27 a ± 0.73 |
| Nanocurcumin | 4.49 a ± 0.33 | 4.92 ab ± 0.43 | 5.06 ab ± 0.36 | 10.66 a ± 0.98 |
| E-LPS | 2.50 d ± 0.23 | 4.25 b ± 0.31 | 4.20 c ± 0.38 | 10.41 a ± 0.98 |
| E-LPS + Nanocurcumin | 3.37 bc ± 0.24 | 4.41 ab ± 0.39 | 5.18 ab ± 0.44 | 10.24 a ± 0.72 |
| C-LPS | 3.01 cd ± 0.23 | 2.82 c ± 0.08 | 4.13 c ± 0.38 | 10.44 a ± 0.08 |
| C-LPS + Nanocurcumin | 3.87 ab ± 0.39 | 2.97 c ± 0.25 | 4.84 bc ± 0.47 | 10.27 a ± 0.62 |
| Total GSH (nmol/mg Protein) | Reduced GSH (nmol/mg Protein) | GSSG (nmol/mg Protein) | GSH/GSSG Ratio | |
|---|---|---|---|---|
| Control | 4.79 ab ± 0.48 | 4.22 a ± 0.44 | 0.28 cd ± 0.03 | 14.93 b ± 1.44 |
| Nanocurcumin | 5.11 a ± 0.42 | 4.64 a ± 0.40 | 0.24 d ± 0.03 | 19.95 a ± 2.58 |
| E-LPS | 4.07 b ± 0.30 | 3.06 b ± 0.24 | 0.51 b ± 0.04 | 6.04 d ± 0.54 |
| E-LPS + Nanocurcumin | 4.14 b ± 0.32 | 3.46 b ± 0.30 | 0.34 c ± 0.03 | 10.14 c ± 1.23 |
| C-LPS | 4.17 b ± 0.42 | 2.95 b ± 0.30 | 0.61 a ± 0.06 | 4.84 d ± 0.48 |
| C-LPS + Nanocurcumin | 4.24 b ± 0.42 | 3.35 b ± 0.30 | 0.49 b ± 0.02 | 8.93 c ± 0.49 |
| Total GSH (nmol/mg Protein) | Reduced GSH (nmol/mg Protein) | GSSG (nmol/mg Protein) | GSH/GSSG Ratio | |
|---|---|---|---|---|
| Control | 5.98 a ± 0.58 | 5.27 ab ± 0.59 | 0.36 c ± 0.04 | 15.04 a ± 2.87 |
| Nanocurcumin | 6.24 a ± 0.66 | 5.58 a ± 0.59 | 0.33 c ± 0.04 | 17.04 a ± 1.62 |
| E-LPS | 4.68 bc ± 0.40 | 3.53 c ± 0.33 | 0.57 b ± 0.04 | 6.15 cd ± 0.54 |
| E-LPS + Nanocurcumin | 5.30 ab ± 0.48 | 4.50 b ± 0.44 | 0.40 c ± 0.04 | 11.19 b ± 1.26 |
| C-LPS | 3.87 c ± 0.39 | 2.43 d ± 0.24 | 0.72 a ± 0.07 | 3.38 d ± 0.34 |
| C-LPS + Nanocurcumin | 4.49 bc ± 0.39 | 3.39 c ± 0.25 | 0.55 b ± 0.07 | 8.42 bc ± 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salah, A.; Yousef, M.; Kamel, M.; Hussein, A. The Neuroprotective and Antioxidant Effects of Nanocurcumin Oral Suspension against Lipopolysaccharide-Induced Cortical Neurotoxicity in Rats. Biomedicines 2022, 10, 3087. https://doi.org/10.3390/biomedicines10123087
Salah A, Yousef M, Kamel M, Hussein A. The Neuroprotective and Antioxidant Effects of Nanocurcumin Oral Suspension against Lipopolysaccharide-Induced Cortical Neurotoxicity in Rats. Biomedicines. 2022; 10(12):3087. https://doi.org/10.3390/biomedicines10123087
Chicago/Turabian StyleSalah, Adham, Mokhtar Yousef, Maher Kamel, and Ahmed Hussein. 2022. "The Neuroprotective and Antioxidant Effects of Nanocurcumin Oral Suspension against Lipopolysaccharide-Induced Cortical Neurotoxicity in Rats" Biomedicines 10, no. 12: 3087. https://doi.org/10.3390/biomedicines10123087






